Abstract
The human sodium-dependent multivitamin transporter (hSMVT) mediates sodium-dependent uptake of biotin in renal and intestinal epithelia. To date, however, there is nothing known about the structure-function relationship or targeting sequences in the hSMVT polypeptide that control its polarized expression within epithelia. Here, we focused on the role of the COOH-terminal tail of hSMVT in the targeting and functionality of this transporter. A full-length hSMVT-green fluorescent protein (GFP) fusion protein was functional and expressed at the apical membrane in renal and intestinal cell lines. Microtubule disrupting agents disrupted the mobility of trafficking vesicles and impaired cell surface delivery of hSMVT, which was also prevented in cells treated with dynamitin (p50), brefeldin, or monensin. Progressive truncation of the COOH-terminal tail impaired the functionality and targeting of the transporter. First, biotin transport decreased by approximately 20–30% on deletion of up to 15 COOH-terminal amino acids of hSMVT, a decrease mimicked solely by deletion of the terminal PDZ motif (TSL). Second, deletions into the COOH-terminal tail (between residues 584-612, containing a region of predicted high surface accessibility) resulted in a further drop in hSMVT transport (to ∼40% of wild-type). Third, apical targeting was lost on deletion of a helical-prone region between amino acids 570-584. We conclude that the COOH tail of hSMVT contains several determinants important for polarized targeting and biotin transport.
Keywords: biotin, epithelial polarity, vitamin, microtubule
biotin (vitamin H) is a water-soluble micronutrient required for normal cellular function, growth, and development in humans owing to its catalytic role in many biochemical reactions (6, 44). Biotin deficiency may result from inborn errors of biotin metabolism, long-term use of anticonvulsants, chronic alcoholism, or inflammatory bowel disease, in infants with seborrhoeic dermatitis and Leiner's disease, and can occur during pregnancy (4, 5, 11, 21, 22, 27, 29, 33, 45). Humans have lost the capacity for de novo biosynthesis of biotin and thus must obtain the vitamin from dietary sources via intestinal absorption. In addition, systemic elimination of biotin is regulated via reabsorption of filtered biotin in the renal glomeruli. Thus renal and intestinal epithelial cells play pivotal roles in regulating biotin homeostasis. Biotin transport occurs via a carrier-mediated mechanism, mediated by the human sodium-dependent multivitamin transporter [hSMVT, the product of the SLC5A6 gene (36, 48)], the functional properties of which have been demonstrated in different epithelia (2, 3, 36, 41, 48, 49).
To date, however, nothing is known about 1) the structure-activity relationship of domains within the hSMVT polypeptide, or 2) the mechanisms that control hSMVT trafficking and targeting to the apical membrane in polarized cells. Molecular determinants that dictate apical targeting of transporters encompass a vast variety of motifs/signals, including posttranslational modifications (glycosylation and glycosylphosphatidylinositol linkages) or specific sequence determinants within the polypeptide (24, 39, 42, 46). Notably, for several transmembrane proteins including vitamin transporters, specific targeting determinants have been identified within the COOH-terminal cytoplasmic tail (8, 18, 19, 31, 40, 43). Therefore, as a first step toward identifying regions of hSMVT important for physiological function, we used [3H]biotin uptake and live cell imaging methods in polarized epithelial cell lines to resolve the functionality and targeting of a series of 10 truncations into the cytoplasmic COOH-terminal tail of hSMVT. Our results map several domains within the cytoplasmic tail region that are important for hSMVT functionality and trafficking to the apical cell membrane, including a truncation that results in mispolarization of hSMVT to the basolateral domain. Finally, we used both real-time and steady-state approaches to gain insight into the cell biological mechanisms underpinning the delivery of hSMVT to the cell surface and show that impairment of microtubule-dependent processes disrupted vesicular motility and cell surface targeting of hSMVT.
MATERIALS AND METHODS
Materials.
[3H]biotin (30 Ci/mmol, radiochemical purity >98%) was obtained from American Radiolabeled Chemical (St. Louis, MO). The enhanced green fluorescent protein (GFP-N3) and red fluorescent protein (DsRed) vectors and DsRed-endoplasmic reticulum (DsRed2-ER) and DsRed-Golgi plasmids were from BD Biosciences (Palo Alto, CA). Geneticin (G418) was from Invitrogen (Carlsbad, CA). Cytoskeletal disrupting agents were from Calbiochem (La Jolla, CA). Madin-Darby canine kidney (MDCK, NBL-2), human-derived duodenal adenocarcinoma cell line (HuTu-80), and human adenocarcinoma (Caco-2) cells were from American Type Culture Collection (ATCC, Manassas, VA). pCB6-HA-p50 was a generous gift from Prof. T. A. Schroer (Johns Hopkins University). All other reagents were from Sigma (St. Louis, MO).
Western blot analysis.
Western blot analysis was performed on apical and basolateral membrane vesicles isolated from the human colon of organ donors using established procedures [kindly provided by Dr. Pradeep K. Dudeja, University of Illinois, Chicago, IL (10)]. Apical and basolateral membrane vesicles (30 μg) were heated with loading buffer (Invitrogen) for 5 min at 70°C and resolved onto premade 4–12% Bis-Tris Mini gel (Invitrogen). All samples were simultaneously run on the same gel. After electrophoresis, proteins were electroblotted onto polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA). The membranes were washed with PBS containing 0.1% Tween 20 (Sigma) and then blocked with a PBS solution containing 5% dried milk (Bio-Rad) for 1 h at room temperature or overnight at 4°C. After blocking, membranes were incubated either with hSMVT polyclonal antibody raised in rabbit [Alpha Diagnostics, San Antonio, TX (32)] or with antigenic peptide (Alpha Diagnostics) pretreated antibodies for 1 h at room temperature. Subsequently, membranes were washed twice and incubated with secondary antibodies [goat anti-rabbit conjugated to horseradish peroxidase (HRP; Santa Cruz Biotechnology, Santa Cruz, CA)]. Next, membranes were washed and incubated with enhanced chemiluminescent substrate (Amersham, Arlington Heights, IL) and exposed to radiography films (Kodak, Rochester, NY). Finally, exposed films were developed using an automated film-developing machine (Procare Imaging Systems, Paramount, CA). The same membranes were stripped using reblotting stripping solution (Chemicon, Temecula, CA) and blocked as mentioned above. The stripped membranes were then incubated with human β-actin antibodies raised in goat (Santa Cruz Biotechnology) for 1 h at room temperature and then washed and incubated with bovine anti-goat conjugated HRP secondary antibodies (Santa Cruz Biotechnology). Immunodetection was performed as described above.
Generation of hSMVT constructs.
The open reading frame (ORF) of hSMVT (1,905 bp) was amplified by RT-PCR from human small intestinal total RNA using gene-specific primers (Table 1). The full-length hSMVT-GFP and truncated constructs were generated by PCR using the primer combinations shown in Table 1 and conditions previously described (40, 42). The PCR products and the GFP-N3 vectors were digested with the restriction enzymes EcoRI and Sal I, and the products were gel-separated and then ligated together to generate in-frame fusion proteins with the GFP fused to the COOH terminus of each construct. The nucleotide sequence of each construct was verified by sequencing (Laragen).
Table 1.
Gene-specific primers used for generating hSMVT truncations
| Construct (Amino Acid) | Forward and Reverse Primers (5′–3′) | Positions, bp | Fragment, bp |
|---|---|---|---|
| hSMVT-GFP | CCGGAATTCATGAGTGTAGGGGTGAGC; | 1-1905 | 1905 |
| ACGCGTCGACCAGGGAGGTCTCCTGGAG | |||
| hSMVT[1-632]-GFP | CCGGAATTCATGAGTGTAGGGGTGAGC; | 1-1896 | 1896 |
| ACGCGTCGACCTCCTGGAGGATGCAGGT | |||
| hSMVT[1-624]-GFP | CCGGAATTCATGAGTGTAGGGGTGAGC; | 1-1872 | 1872 |
| ACGCGTCGACGCTCCCCTGATAGGCTGTG | |||
| hSMVT[1-620]-GFP | CCGGAATTCATGAGTGTAGGGGTGAGC; | 1-1860 | 1860 |
| ACGCGTCGACGGCTGCGGCAGCCAGGG | |||
| hSMVT[1-616]-GFP | CCGGAATTCATGAGTGTAGGGGTGAGC; | 1-1848 | 1848 |
| ACGCGTCGACCGCGGCCATGGCCTCCT | |||
| hSMVT[1-612]-GFP | CCGGAATTCATGAGTGTAGGGGTGAGC; | 1-1836 | 1836 |
| ACGCGTCGACCTCCTTGTCTCTGCTGTCCC | |||
| hSMVT[1-600]-GFP | CCGGAATTCATGAGTGTAGGGGTGAGC; | 1-1800 | 1800 |
| ACGCGTCGACCGGCTTCTCAGGAAACAGGC | |||
| hSMVT[1-584]-GFP | CCGGAATTCATGAGTGTAGGGGTGAGC; | 1-1752 | 1752 |
| ACGCGTCGACCCTGCAGTGGAGCCGCT | |||
| hSMVT[1-575]-GFP | CCGGAATTCATGAGTGTAGGGGTGAGC; | 1-1725 | 1725 |
| ACGCGTCGACCAACGGAAGGAGGGACA | |||
| hSMVT[1-570]-GFP | CCGGAATTCATGAGTGTAGGGGTGAGC; | 1-1710 | 1710 |
| ACGCGTCGACCAGGAGCTTTGGCAACACTG | |||
| hSMVT[1-567]-GFP | CCGGAATTCATGAGTGTAGGGGTGAGC; | 1-1701 | 1701 |
| ACGCGTCGACTGGCAACACTGGGTAAAGG | |||
| hSMVT[ΔC]-GFP | CCGGAATTCATGAGTGTAGGGGTGAGC; | 1-1656 | 1656 |
| ACGCGTCGACCATTCTCCCAGTGAGTAGACT |
Shown is the combination of primer sequence used to generate full-length and truncated human sodium-dependent multivitamin transporter (hSMVT) constructs by PCR. Restriction sites for EcoRI (underlined) and Sal I (boldface) were added to the hSMVT primers to allow subsequent subcloning into the enhanced green fluorescent protein (GFP-N3) vector. hSMVT[ΔC]-truncation of COOH terminus of hSMVT.
Cell culture, transient, and stable transfection.
MDCK and HuTu-80 cells were maintained in MEM, and Caco-2 cells were maintained in DMEM. All media were supplemented with 10–20% fetal bovine serum, glutamine (0.29 g/l), sodium bicarbonate (2.2 g/l), penicillin (100,000 U/l), and streptomycin (10 mg/l). For transient transfection on sterile glass-bottomed petri dishes (MatTek), cells were grown and transfected at 95% confluency with 2 μg of plasmid DNA using Lipofectamine 2000 (Invitrogen). For transient transfection on filters, MDCK or Caco-2 cells were seeded onto collagen-coated filters (Corning Costar) and grown until confluency. Individual filter dishes were transfected ∼5 days postconfluency in serum-free medium containing 2 μg of plasmid DNA using Lipofectamine 2000. Transiently transfected MDCK cells were imaged 24–48 h later. For stable expression, MDCK cell clones were selected with G418 (0.8 mg/l) for 6–8 wk. Brefeldin A (BFA) and monensin treatments were performed using the hSMVT-GFP-expressing stable MDCK cell line, transiently transfected with either DsRed2-ER or DsRed-Golgi, and subsequently treated with BFA (5 μg/ml) or monensin (5 μM) for 14 h at 37°C to block transport from ER to Golgi or Golgi to cell surface, respectively.
Uptake analyses.
For [3H]biotin uptake experiments on filters, stable hSMVT-GFP MDCK cells were seeded onto collagen-coated filters and grown until 5 days postconfluency (39, 40). Cell monolayers were incubated [3 min at 37°C in Krebs-Ringer buffer (pH 7.4)] in the presence of [3H]biotin (9 nM) added to either the apical or basolateral chamber or to culture medium for assays using solid supports. Uptake assays were terminated by addition of 5 ml of ice-cold Krebs-Ringer buffer, and accumulated radioactivity was determined using scintillation counting.
Live cell imaging.
Cells cultured on petri dishes were imaged using a Nikon C1 confocal scanner head attached to a Nikon inverted phase-contrast microscope. For imaging cells grown on filters, a Bio-Rad MRC 1024 confocal scanner attached to an Olympus Provis AX70 upright microscope equipped with a ×60 water immersion objective was used. Fluorophores were excited using the 488-nm line from an argon ion-laser, and emitted fluorescence was monitored with a 530- ± 20-nm band-pass (GFP) or a 620-nm long-pass filter (RFP). Total internal reflection fluorescence (TIRF) imaging of vesicular trafficking was performed using an Olympus TIRF illuminator attached to an IX70 inverted microscope. Images were captured using an EMCCD, Roper Cascade II, and the motion of individual vesicles tracked using a frame-to-frame tracking function in MetaMorph (Universal Imaging, Downingtown, PA). Videos are provided as Supplemental Movies S1–4 available in the data supplement online at the AJP-Cell Physiology web site.
Flow cytometry.
Flow cytometry was performed using a FACSCalibur bench-top cytometer (BD Biosciences). hSMVT wild-type and mutants transiently expressing MDCK cells were grown within T-25 tissue culture flasks. The monolayer was trypsinized, and cells were pelleted and resuspended in 1-ml aliquots of Ca2+- and Mg2+-containing HBSS as described previously (39). In all flow cytometry experiments, samples of untransfected and GFP alone transfected MDCK cells were run in parallel with experimental samples to calibrate optical parameters for identifying the intact, transfected cell population.
RESULTS
hSMVT-GFP targeting and functionality in polarized epithelia.
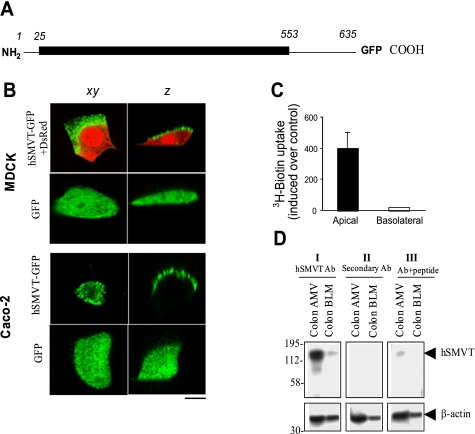
A schematic representation of the full-length hSMVT-GFP fusion construct is shown in Fig. 1A. This representation depicts the overall organization of the polypeptide (635 amino acids), which includes a short cytoplasmic NH2-terminal domain (residues 1-24), a transmembrane domain with 12 predicted membrane spanning regions (residues 25-552), and a cytoplasmic COOH-terminal domain (83 amino acids, residues 553-635) to which GFP was attached (Fig. 1A). To examine the targeting and functionality of the full-length hSMVT protein, a stable hSMVT-GFP-expressing MDCK cell line was generated (see materials and methods). Semiquantitative RT-PCR demonstrated hSMVT-mRNA expression was ∼9-fold higher in the stable cell line than mock-transfected MDCK cells (data not shown). Confocal analysis of confluent monolayers grown on filter supports revealed apical targeting of hSMVT-GFP (Fig. 1B). Similar results were obtained with filter-grown confluent Caco-2 cells (Fig. 1B). In contrast, expression of GFP alone (as a control) resulted in cytoplasmic fluorescence in both MDCK and Caco-2 cells (Fig. 1B). To determine the functional consequences of the asymmetrical distribution of hSMVT-GFP, [3H]biotin was introduced either to the apical or basolateral compartment. Apical [3H]biotin uptake increased ∼40-fold in hSMVT-GFP-expressing stable MDCK cells compared with mock-transfected cells, whereas there was no significant change in basolateral uptake of [3H]biotin in stable compared with mock-transfected MDCK cells (Fig. 1C). Finally, expression of hSMVT was assessed in native human colon by Western blotting, using enriched colonic apical and basolateral membrane preparations isolated from organ donors by established procedures (10) and well-characterized polyclonal anti-hSMVT antibodies (32). The hSMVT protein was predominantly expressed in the apical membrane preparation (Fig. 1D). Taken together, the asymmetry in [3H]biotin uptake was consistent with confocal imaging and immunoblotting data, underscoring the concept that hSMVT mediates biotin transport across the apical domain of polarized epithelia.
Fig. 1.
Apical targeting of the human sodium-dependent multivitamin transporter (hSMVT)-green fluorescent protein (GFP) fusion protein in renal and intestinal epithelial cells. A: schematic representation of the full-length hSMVT protein (1-635 residues) with GFP fused to the COOH terminus (hSMVT-GFP). B: distribution of hSMVT-GFP and GFP alone in lateral (xy, left) and axial (z, right) section in renal [top; Madin-Darby canine kidney (MDCK)] and intestinal [bottom; human adenocarcinoma (Caco-2)] cell lines. MDCK cells were cotransfected with red fluorescent protein (DsRed) vector to allow resolution of cellular volume. Scale bar is 5 μm. C: [3H]biotin uptake assays in a stable hSMVT-GFP-expressing MDCK cell line grown 5–7 days after confluence on permeable filter supports after introduction of [3H]biotin to the apical (solid) or basolateral (open) chamber. D: expression of the hSMVT protein in native human colon apical membrane vesicles (AMV) and basolateral membrane vesicles (BLM) by Western blotting. All samples were run simultaneously on the same gels (but lanes were grouped for clear presentation), and a representative blot is shown. I: membranes were incubated with primary polyclonal anti-hSMVT antibodies (Ab) raised in rabbits and horseradish peroxidase (HRP)-conjugated secondary antibodies (goat anti-rabbit). II: membranes were incubated with only secondary antibodies. III: anti-hSMVT antibodies pretreated first with antigenic peptide and then incubated with secondary antibodies. Bottom are the same membranes stripped and incubated with human β-actin antibodies. Molecular mass estimations (in kilodaltons) are shown.
Cytoplasmic COOH-terminal truncations impact [3H]biotin uptake.
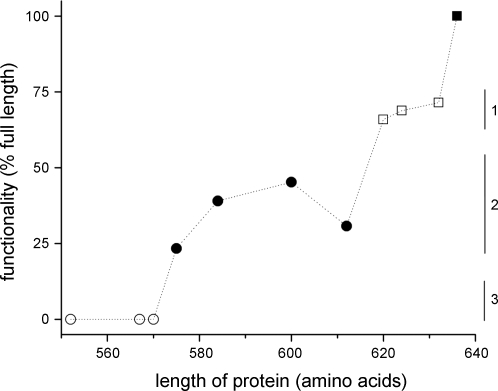
To assess the role of the cytoplasmic COOH tail of hSMVT, we generated a series of 10 truncation constructs in the tail region (Table 1). Each construct was analyzed in terms of 1) functionality ([3H]biotin uptake), and 2) population expression level of the truncated construct (>10,000 cells, flow cytometry analyses). A crude way of interpreting the functionality of each of these constructs was obtained by calculating the level of [3H]biotin transport normalized to the expression level of each construct (judged by means of population fluorescence), i.e., the rate of transport relative to the amount of hSMVT expressed (Fig. 2). Each construct was then further analyzed at the single cell level to resolve the subcellular localization of each mutant. These analyses are discussed, in turn, below.
Fig. 2.
Functionality of hSMVT COOH-terminal tail truncations. Functionality of 10 cytoplasmic tail truncations relative to that of full-length hSMVT-GFP was calculated by measuring the amount of [3H]biotin uptake in a defined period (3 min) corrected for the construct expression level (judged by the mean population fluorescence by flow cytometry). The length of the construct is indicated on the x-axis (amino acids). Data are corralled into 3 groups relative to wild-type (▪, 635 amino acids): ∼75% wild-type functionality (□, group 1), approximately 25–50% wild-type functionality (•, group 2), and transport-null constructs (group 3).
From the collated results shown in Fig. 2, it is clear that truncation of the entire cytoplasmic COOH tail [amino acids 553-635, hSMVT[ΔC]-GFP (truncation of COOH terminus of hSMVT)] abrogated hSMVT functionality, as no enhancement of [3H]biotin uptake was observed relative to controls. However, functionality was recovered in constructs with shorter deletions. Truncation mutants fell into 2 broad groupings, exhibiting ∼75% or approximately 25–50% of wild-type activity. The grouping of higher activity (group 1) was associated with deletions up to 15 residues (up to residue 620, i.e., 621-635 deleted), encompassing constructs hSMVT[632]-GFP, hSMVT[624]-GFP, and hSMVT[620]-GFP. The next broad grouping (group 2) encompassed truncations of up to 60 residues (up to residue 575), encompassing hSMVT[612]-GFP, hSMVT[600]-GFP, hSMVT[584]-GFP, and hSMVT[575]-GFP. Further deletions resulted in a more progressive loss of functionality, and constructs hSMVT[ΔC]-GFP, hSMVT[567]-GFP, and hSMVT[570]-GFP were all transport-null (group 3). Therefore, these experiments implicated 3 broad regions in the COOH tail of hSMVT that progressively impacted cellular [3H]biotin accumulation (Fig. 2).
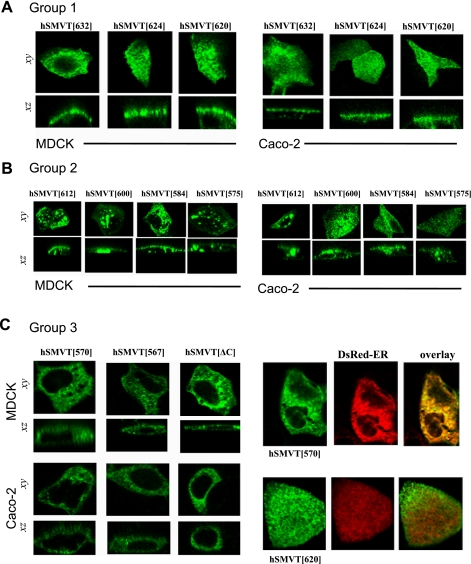
We proceeded to investigate the polarized targeting of these 10 constructs in both MDCK and Caco-2 cells. Each truncated construct was transiently transfected into MDCK and Caco-2 cells, and the resulting cellular distribution was resolved by confocal imaging (Fig. 3). The 3 constructs that displayed slight impaired functionality (group 1; Fig. 2), namely hSMVT[632]-GFP, hSMVT[624]-GFP, and hSMVT[620]-GFP, all targeted to the apical cell surface, just like the full-length transporter (Fig. 3A). The next group of deletions associated with a further impairment in [3H]biotin transport (group 2; Fig. 2), namely hSMVT[612]-GFP, hSMVT[600]-GFP, hSMVT[584]-GFP, and hSMVT[575]-GFP, targeted to the apical cell surface but displayed a progressively increased punctuate intracellular fluorescence suggestive of an increased redistribution to trafficking vesicles (Fig. 3B). Finally, no cell surface expression was observed with hSMVT[570]-GFP, hSMVT[567]-GFP, and hSMVT[ΔC]-GFP, rather fluorescence expression was confined within intracellular membranes (Fig. 3C), consistent with their behavior as transport-null constructs (group 3; Fig. 2). In cells cotransfected with hSMVT[570]-GFP and an ER-targeted red fluorescent protein construct (DsRed2-ER), significant fluorescence overlap was observed. This contrasted with cells cotransfected with DsRed2-ER and, for example, hSMVT[620]-GFP (Fig. 3D). In summary, group 1 constructs displayed impaired functionality but normal apical targeting, group 2 constructs displayed progressively impaired apical targeting and decreased functionality, whereas group 3 constructs were retained within the ER.
Fig. 3.
COOH-terminal truncations disrupt the apical cell surface targeting of hSMVT. A: distribution of indicated hSMVT truncation constructs in MDCK (left) and Caco-2 cells (right) in lateral (xy, top) and axial (xz, bottom) section. All group 1 constructs (Fig. 2) targeted to the apical cell surface. B: group 2 constructs showed progressively impaired apical cell surface targeting in both MDCK (left) and Caco-2 cells (right) shown in lateral (xy, top) and axial (xz, bottom) section. C: left, group 3 constructs were retained intracellularly in both MDCK (left) and Caco-2 cells (right); right, cotransfection of hSMVT[570]-GFP (top) or hSMVT[620]-GFP (bottom) together with DsRed-endoplasmic reticulum (ER) (middle), shown as overlay (right).
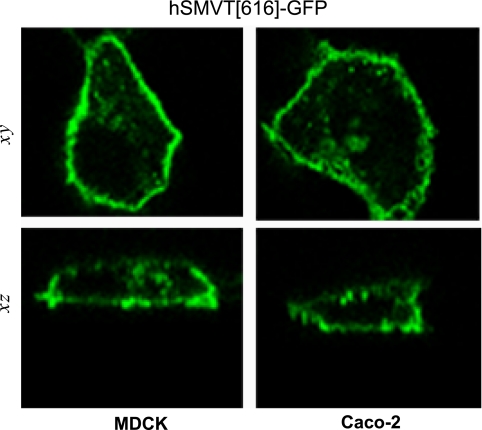
Altered targeting of the COOH tail of hSMVT.
As presented above, truncation of the COOH-terminal tail of hSMVT alters cell surface expression. Surprisingly, the serial truncation analysis resulted in the identification of one construct (hSMVT[616]-GFP) that displayed a mispolarized cell surface expression profile with marked basolateral expression in both MDCK and Caco-2 cells (Fig. 4). Additionally, [3H]biotin uptake by confluent monolayers expressing this truncated construct, i.e., uptake across the apical membrane domain, was decreased compared with wild-type (226 ± 7 vs. 86 ± 8 fmol/mg protein/3 min for wild-type and hSMVT[616]-GFP, respectively), which is most likely due to the decrease in apical expression of the truncated protein in transfected cell monolayers.
Fig. 4.
Diverse targeting potential of hSMVT tail truncation. Basolateral targeting of hSMVT[616]-GFP in MDCK (left) and Caco-2 cells (right) shown in lateral (xy, top) and axial (z, bottom) section.
Microtubule-dependent apical expression of hSMVT.
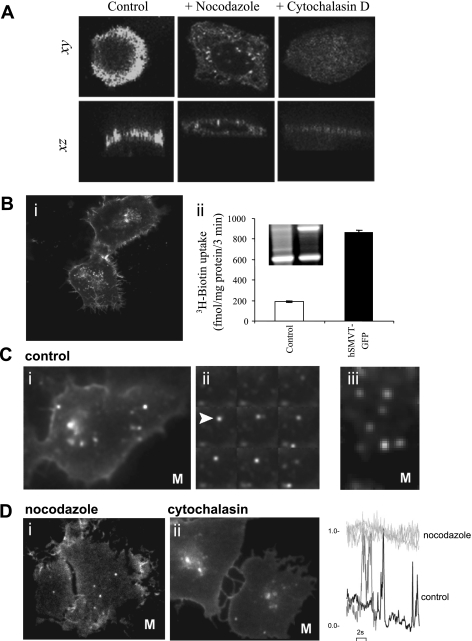
The role of the microtubule network in the apical targeting of hSMVT was investigated. To examine the effect of microtubule disruption on steady-state expression of hSMVT, confluent MDCK cells were treated with nocodazole (20 μM, 30 min), after which cells were transfected with hSMVT-GFP cDNA before imaging 18–24 h later (as described previously in Ref. 23). Nocodazole treatment impaired the apical localization of hSMVT, yielding a nonpolarized distribution of hSMVT-GFP and a significant increase in intracellular fluorescence resolved in axial confocal sections (Fig. 5A). In contrast, treatment with cytochalasin did not impair the apical targeting of hSMVT-GFP (10 μM, 30 min). These results suggested that apical expression/retention of hSMVT in MDCK cells was dependent on an intact microtubule network.
Fig. 5.
Effect of cytoskeletal disruption on hSMVT trafficking and targeting. A: MDCK cells treated with nocodazole (20 μM, 30 min; middle) or cytochalasin D (10 μM, 30 min; right) before transient transfection were imaged after 18–24 h and compared with transfected control cells (left). Lateral (xy, top) and axial (xz, bottom) confocal planes are shown. B: i, distribution of hSMVT-GFP trafficking vesicles in a stable human-derived duodenal adenocarcinoma cell line (HuTu-80) cell line grown on cover glass-bottomed petri dishes for 5–7 days; ii, [3H]biotin uptake and RT-PCR of hSMVT mRNA (inset) in the stable HuTu-80 cell line. C: i, total internal reflection fluorescence (TIRF) images of hSMVT vesicular dynamics in HuTu-80 cells (at 37°C; Supplemental Movie S1); ii, montage of single frames (taken at 200-ms intervals) of hSMVT vesicles (e.g., arrow) in control cells; iii, lateral motion of hSMVT containing vesicles (Supplemental Movie S2). D: image stills in cells treated with nocodazole (i; Supplemental Movie S3) and cytochalasin (ii; Supplemental Movie S4). Right: examples of fluorescence profile of vesicles in a nocodazole-treated cell (light gray) to illustrate immobility in the TIRF field relative to time 0 and in a control cell (black) and cytochalasin-treated cell (gray) to illustrate transitions into/out of the TIRF field. M, movie.
To resolve more acute effects of cytoskeletal disruption on the real-time trafficking of full-length hSMVT-GFP, we used TIRF microscopy to image the behavior of individual vesicles near the cell surface. For the TIRF experiments, we used a human duodenally derived cell line (HuTu-80). Usage of this particular cell line facilitated trafficking studies as 1) vesicles are numerous, and 2) the cell line does not show polarized transporter expression under our culture conditions, enabling vesicle tracking at cell/cover glass interface.
A stable hSMVT-GFP-expressing HuTu-80 cell line displayed fluorescence both at the cell surface as well as within numerous cytoplasmic structures (Fig. 5B, i). Functional measurements of [3H]biotin accumulation indicated that [3H]biotin uptake was ∼5-fold greater in the stable cell line, confirming the functionality of the full-length fusion protein (Fig. 5B, ii). Densitometric quantification of RT-PCR products demonstrated that hSMVT expression was ∼4.5-fold greater in the stable cell line compared with mock-transfected cells (Fig. 5B, ii, inset).
TIRF imaging of the near-membrane dynamics of hSMVT-GFP resolved considerable vesicular motility within the evanescent field (Fig. 5C, i; Supplemental Movie S1). Examples of motion comprised transitions into and out of the TIRF field associated with changes in fluorescence intensity that were most evident in montages (Fig. 5C, ii) of individual frames selected at constant intervals from a continual recording (20-ms frames, 6-s recording). Lateral movements beneath the cell surface through the plane of the field were also evident (Fig. 5C, iii; Supplemental Movie S2). Therefore, trafficking vesicles containing hSMVT show considerable motility in the juxtamembrane region.
Consistent with the steady-state results observed with cytoskeletal inhibitors, nocodazole (10 μM, 30 min) and cytochalasin (10 μM, 30 min) produced distinct effects on hSMVT-GFP vesicular trafficking (Fig. 5D). Nocodazole treatment abrogated vesicle movement (Fig. 5D, i; Supplemental Movie S3), such that vesicles in the TIRF field remained largely immobile during recording (Fig. 5D, iii). In control and cytochalasin D treated cells, however, vesicles were still observed transitioning the TIRF field (Fig. 5D, ii; Supplemental Movie S4).
Role of dynein in apical targeting of hSMVT.
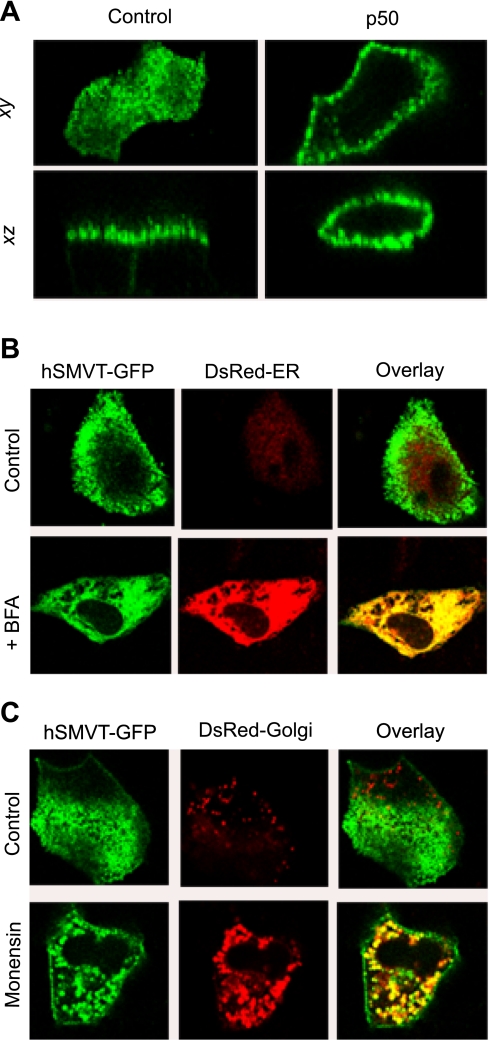
In light of the data implicating microtubules in the apical delivery of hSMVT, we proceeded to investigate the role of microtubule-associated motors. Microtubules in well-differentiated epithelial cells are arranged longitudinally with their minus ends oriented toward the apical plasma membrane. Cytoplasmic dynein is a minus end-directed motor protein that drives membrane vesicles toward the periphery of polarized epithelial cells. We used the dominant-negative functional effects of dynamitin (p50, a subunit of the dynactin complex) to disrupt cytoplasmic dynein. Dynamitin plays an important role in holding the dynactin complex together; when overexpressed, it causes p150glued and p24/22 to disintegrate from the dynactin complex, thereby affecting subsequent dynein association with microtubules (9, 42, 47). MDCK cell monolayers were either transfected with hSMVT-GFP or cotransfected with p50 and hSMVT-GFP. After 24 h, confocal images showed that, in cells overexpressing p50, hSMVT-GFP was distributed across the cell surface in the apical, lateral, and basal membranes compared with polarized apical expression in control cells (Fig. 6B).
Fig. 6.
Disruption of hSMVT trafficking. A: effect of dynamitin (p50) overexpression on hSMVT-GFP polarity. Lateral (xy, top) and axial (xz, bottom) confocal images of MDCK cells transfected with hSMVT-GFP (left) or hSMVT-GFP and p50 (right) after 24 h. B: lateral confocal images (xy) showing the effect of brefeldin A (BFA; 5 μg/ml) treatment (14 h, 37°C) on the cellular distribution of hSMVT-GFP in MDCK cells cotransfected with DsRed-ER. C: confocal images (xy) reveal the effect of monensin (5 μM) treatment (14 h, 37°C) on the cellular distribution of hSMVT-GFP in MDCK cells cotransfected with DsRed-Golgi.
Apical hSMVT targeting occurs via a BFA- and monensin-sensitive pathway.
BFA is a fungal metabolite known to cause the Golgi to fuse with ER and block intracellular vesicular transport to the cell surface (8, 20, 25, 34, 43). Monensin also blocks the delivery of newly synthesized membrane protein to the cell membrane (13, 30, 34, 43). Both drugs disrupted apical expression of hSMVT in the stable MDCK cell line. BFA (5 μg/ml, 14 h at 37°C) caused hSMVT-GFP to accumulate in the ER as demonstrated by colocalization with DsRed2-ER in the majority of cells (Fig. 6A). In a minority of cells (approximately 10–15%), a nonpolarized distribution across the cell surface was evident (data not shown). Monensin (5 μM, 14 h at 37°C) blocked the delivery of hSMVT to the apical membrane, and hSMVT remained within intracellular structures (Fig. 6C). Again, in a small fraction of cells (approximately 10–15%), a nonpolarized distribution at the cell surface was observed. Taken together, these results suggest that the apical targeting of hSMVT-GFP is through a classic BFA- and monensin-sensitive pathway.
DISCUSSION
hSMVT is a Na+-dependent, electrogenic biotin transporter that is responsible for apical biotin uptake in polarized epithelia (26, 36). Expression of hSMVT is subject to adaptive regulation under scenarios of biotin deficiency (3, 37). Here, we used live cell imaging and [3H]biotin uptake approaches to investigate the cellular mechanisms governing the trafficking and targeting of this transporter in a variety of epithelial cell lines.
Serial truncations into the COOH terminus of hSMVT delineated several domains within the cytoplasmic tail important for biotin transport and ultimately export of the transporter from the ER to the apical cell surface. First, deletions of up to 15 amino acids (group 1) resulted in ≤25% decrease in the rate of biotin transport (Fig. 2), without impairment of plasma membrane targeting in polarized renal and intestinal epithelial cells. This region of the cytoplasmic tail is predicted to terminate with an extended confirmation ending with a consensus PDZ motif as seen in many transporters (1, 7, 12, 28). This is of interest given that deletion of only the 3 terminal amino acids of hSMVT (TSL) comprising the PDZ motif (28) was sufficient to decrease the rate of [3H]biotin accumulation. The next set of truncations (group 2) contains a region predicted in silico to have high surface accessibility (35); deletions into this region resulted in a further ∼25% decrease in biotin transport, possibly attributable to the decreased localization of transporters at the cell surface. Interestingly, the region between these domains (groups 1 and 2) contained the localization of 1 construct (hSMVT[616]-GFP) observed to mispolarize to the basolateral domain in both MDCK and Caco-2 cells, suggesting the presence of a multiplicity of targeting determinants within the hSMVT COOH-terminal tail. Such an observation is consistent with previous observations of a hierarchy of sorting determinants within other polypeptides (14). As the COOH-terminal sequence of hSMVT[616]-GFP does not conform to any known basolateral targeting sequences, the possibility of a conformational motif functioning as a basolateral routing signal is possible. Further truncations upstream of residue 584 resulted in a rapid elimination of biotin accumulation (Fig. 2) and a corresponding marked retention of fluorescence with intracellular vesicles (hSMVT[575]-GFP) and ultimately the ER (Fig. 3). The elimination of transport on removal of the 5 residues between hSMVT[575]-GFP and hSMVT[570]-GFP corresponds to deletions into a predicted helical region, which extends upstream to encompass a putative endocytosis motif (YXXL) as well as a polyproline core (PXXP) and 2 sequential dileucine (LL) motifs that have been implicated as signals important for targeting in several other transporters (14, 16, 18). Future studies will examine the role of these regions in the context of the full-length transporter.
Equilibrium and real-time trafficking analyses implicated a crucial role for microtubule-based processes in cell surface delivery of hSMVT-GFP. Acute and chronic treatment with nocodazole impaired vesicular motility and cell surface delivery, respectively, whereas microfilament disruption had little apparent effect. The dynamics of hSMVT-containing structures observed in epithelial cells is of obvious interest in connection with potential regulation of transporter levels at the cell surface that may take place under certain conditions. One of several mechanisms that can be envisaged to impact overall nutrient uptake capacity by a given cell would be differential rates of transporter insertion/retrieval from the apical membrane domain as has been shown for a variety of substrates (15, 17, 38). The ability to resolve individual hSMVT vesicle trafficking events (Fig. 5) will allow future studies to evaluate whether regulation of transporter insertion and retrieval occurs in cells under specific conditions. Apical membrane localization of hSMVT is mediated at least in part by BFA-sensitive pathway consistent with other apical membrane targeting proteins (8, 25, 43).
In conclusion, we have demonstrated that domains within the COOH-terminal tail of hSMVT are essential for functionality of the hSMVT transporter and for expression at the apical plasma membrane domain in both renal and intestinal epithelia.
GRANTS
This study is supported by the Department of Veterans Affairs and National Institutes of Health (NIH) Grants DK-058057 and DK-056061 (to H. M. Said) and DK-71538 (to V. S. Subramanian). Work in the Marchant laboratory is supported by NIH Grant NS-046783 and National Science Foundation (NSF) CAREER Fellowship no. 0237946 (to J. S. Marchant).
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anzai N, Miyazaki H, Nohiro R, Khamdang S, Chairoungdua A, Enomoto A, Hirata T, Shin HJ, Sakamoto S, Tomita K, Kanai Y, Endou H. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C-terminal. J Biol Chem 279: 45942–45950, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Balamurugan K, Ortiz A, Said HM. Biotin uptake by human intestinal and liver epithelial cells: role of the SMVT system. Am J Physiol Gastrointest Liver Physiol 285: G73–G77, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Balamurugan K, Vaziri ND, Said HM. Biotin uptake by human proximal tubular epithelial cells: cellular and molecular aspects. Am J Physiol Renal Physiol 288: F823–F831, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Banares FF, Lacruz AA, Gine JJ, Esteve M, Gassull MA. Vitamin statues in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989. [PubMed] [Google Scholar]
- 5.Bonjour JP Vitamins and alcoholism. Int J Vitam Nutr Res 50: 321–38, 1980. [PubMed] [Google Scholar]
- 6.Bonjour JP Biotin. In: Handbook of Vitamins: Nutritional, Biochemical, and Clinical Aspects, edited by Machlin LJ. New York: Dekker, 1984, p. 403–435.
- 7.Brone B, Eggermont J. PDZ proteins retain and regulate membrane transporters in polarized epithelial cell membranes. Am J Physiol Cell Physiol 288: C20–C29, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Chuang JZ, Sung CH. The cytoplasmic tail of rhodopsin acts as a noval apical sorting signal in polarized MDCK cells. J Cell Biol 142: 1245–1256, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deacon SW, Serpinskaya AS, Vaughan PS, Fanarraga ML, Vernos I, Vaughan KT, Gelfand VI. Dynactin is required for bidirectional organelle transport. J Cell Biol 160: 297–301, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudeja PK, Torania SA, Said HM. Evidence for the existence of a carrier-mediated folate uptake mechanism in human colonic luminal membranes. Am J Physiol Gastrointest Liver Physiol 272: G1408–G1415, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Fennelly J, Frank O, Baker H, Leevy CM. Peripheral neuropathy of the alcoholics: I. Aetiological role of aneurin and other B-complex vitamins. Br Med J 2: 1290–1292, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gisler SM, Stgljar I, Traebert M, Bacic D, Biber J, Murer H. Interaction of the type IIa Na/Pi cotransporter with PDZ proteins. J Biol Chem 276: 9206–9213, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths G, Quinn P, Warren G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J Cell Biol 96: 835–850, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He C, Hobert M, Friend L, Carlin C. The epidermal growth factor receptor juxtamembrane domain has multiple basolateral plasma membrane localization determinants, including a dominant signal with a polyproline core. J Biol Chem 277: 38284–38293, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Hernando N, Forster IC, Biber J, Murer H. Molecular characterization of phosphate transporters and their regulation. Exp Nephrol 8: 366–375, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Hitchcock IS, Chen MM, King JR, Kaushansky K. YRRL motifs in the cytoplasmic domain of the thrombopoietin receptor regulator internalization and degradation. Blood 112: 2222–2231, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kandror KV, Pilch PF. Compartmentalization of protein traffic in insulin-sensitive cells. Am J Physiol Endocrinol Metab 271: E1–E14, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Karim-Jimenez Z, Hernando N, Biber J, Murer H. Requirement of a leucine residue for (apical) membrane expression of type IIb NaPi cotransporters. Proc Natl Acad Sci USA 97: 2916–2921, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klapper M, Daniel H, Doring F. Cytosolic COOH terminus of the peptide transporter PEPT2 is involved in apical membrane localization of the protein. Am J Physiol Cell Physiol 290: C472–C483, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol 116: 1071–1080, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krause KH, Berlit P, Bonjour JP. Impaired biotin status in anticonvulsant therapy. Ann Neurol 12: 485–486, 1982. [DOI] [PubMed] [Google Scholar]
- 22.Krause KH, Bonjour J, Berlit P, Kochen W. Biotin status of epileptics. Ann NY Acad Sci 447: 297–313, 1985. [DOI] [PubMed] [Google Scholar]
- 23.Kreitzer G, Marmorstein A, Okamoto P, Vallee R, Rodriguez-Bolan E. Kinesin and dynamin are required for post-Golgi transport of a plasma membrane protein. Nat Cell Biol 2: 125–127, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Lin S, Naim HY, Rodriguez AC, Roth MG. Mutations in the middle of the transmembrane domain reverse the polarity of transport of the influenza virus hemagglutinin in MDCK epithelial cells. J Cell Biol 142: 51–57, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Low SH, Tang BL, Wong SH, Hong W. Selective inhibition of protein targeting to the apical domain of MDCK cells by brefeldin A. J Cell Biol 118: 51–62, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo S, Kansara VS, Zhu X, Mandava NK, Pal D, Mitra AK. Functional characterization of sodium-dependent multivitamin transporter in MDCK-MDR1 cells and its utilization as a target for drug delivery. Mol Pharm 3: 329–339, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messaritakis J, Katlamis C, Karabula C, Matsaniotis N. Generalized seborrhoeic dermatitis: clinical and therapeutic data of 25 patients. Arch Dis Child 50: 871–874, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazaki H, Anzai N, Ekaratanawong S, Sakata T, Shin HJ, Jutabha P, Hirata T, He X, Nonoguchi H, Tomita K, Kanai Y, Endou H. Modulation of renal apical organic anion transporter 4 function by two PDZ domain-containing proteins. J Am Soc Nephrol 16: 3498–3506, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Mock DM, Stadler DD, Stratton SL, Mock NI. Biotin status assessed longitudinally in pregnant women. J Nutr 127: 710–716, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Mollenhauer HH, Morre DJ, Rowe LD. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim Biophys Acta 1031: 225–246, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muth TR, Ahn J, Caplan MJ. Identification of sorting determinants in the C-terminal cytoplasmic tails of the gamma-aminobutyric acid transporters GAT-2 and GAT-3. J Biol Chem 40: 25616–25627, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Nabokina SM, Subramanian VS, Said HM. Comparative analysis of ontogenic changes in renal and intestinal biotin transport in the rat. Am J Physiol Renal Physiol 284: F737–F742, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Nisenson A Seborrhoeic dermatitis of infants and Leiner's disease: a biotin deficiency. J Pediatr 51: 537–548, 1957. [DOI] [PubMed] [Google Scholar]
- 34.Neufeld EB, Remaley AT, Demosky SJ, Stonik JA, Cooney AM, Comly M, Dwyer NK, Zhang M, Blanchette-Mackie J, Santamarina-Fojo S, Brewer HB. Cellular localization and trafficking of the human ABCA1 transporter. J Biol Chem 276: 27584–27590, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31: 3635–3641, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad PD, Wang H, Huang W, Fei YJ, Leibach FH, Devoe LD, Ganapathy V. Molecular and functional characterization of the intestinal Na+-dependent multivitamin transporter. Arch Biochem Biophys 366: 95–106, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Reidling JC, Nabokina SM, Said HM. Molecular mechanisms involved in the adaptive regulation of human intestinal biotin uptake: a study of the hSMVT system. Am J Physiol Gastrointest Liver Physiol 292: G275–G281, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Snyder PM The epithelial Na+ channel: cell surface insertion and retrival in Na+ homeostasis and hypertension. Endocr Rev 23: 258–275, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian VS, Marchant JS, Said HM. Apical membrane targeting and trafficking of the human proton-coupled folate transporter in polarized epithelia. Am J Physiol Cell Physiol 294: C233–C240, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Subramanian VS, Marchant JS, Boulware MJ, Said HM. A C-terminal region dictates the apical plasma membrane targeting of the human sodium-dependent vitamin C transporter-1 in polarized epithelia. J Biol Chem 279: 27719–27728, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Subramanian VS, Marchant JS, Said HM. Biotin-responsive basal ganglia disease-linked mutations inhibit thiamine transport via hTHTR-2: biotin is not a substrate for hTHTR2. Am J Physiol Cell Physiol 291: C851–C859, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Subramanian VS, Marchant JS, Said HM. Targeting and trafficking of the human thiamine transporter-2 in epithelial cells. J Biol Chem 281: 5233–5245, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Sun AQ, Salkar R, Sachchidanand Xu S, Zeng L, Zhou MM, Suchy FJ. A 14-amino acid sequence with a β-turn structure is required for apical membrane sorting of the rat ileal bile acid transporter. J Biol Chem 278: 4000–4009, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Sweetman L, Nyhan WL. Inheritable biotin-treatable disorders and associated phenomena. Annu Rev Nutr 6: 314–343, 1986. [DOI] [PubMed] [Google Scholar]
- 45.Urabe K, Fujita K, Okabe N, Yamamoto T, Yao T, Doi S. Decreased plasma biotin levels in patients with Crohn's disease. Jpn J Gastroenterol 83: 307–308, 1986. [PubMed] [Google Scholar]
- 46.Vagin O, Turdikulova S, Sachs G. The H,K-ATPase β subunit as a model to study the role of N-glycosylation in membrane trafficking and apical sorting. J Biol Chem 279: 39026–39034, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Valetti C, Wetzel DM, Schrader M, Hasbani MJ, Gill SR, Kreis TE, Schroer TA. Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol Biol Cell 10: 4107–4120, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Huang W, Fei YJ, Xia H, Yang-Feng T, Leibach FH, Devoe LD, Ganapathy V, Prasad PD. Human placental Na+-dependent multivitamin transporter. Cloning, functional expression, gene structure, and chromosomal localization. J Biol Chem 274: 14875–14883, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Zempleni J Uptake, localization, and noncarboxylase roles of biotin. Annu Rev Nutr 25: 175–196, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.