Abstract
The ubiquitously expressed G protein α-subunit Gsα is required for receptor-stimulated intracellular cAMP responses and is an important regulator of energy and glucose metabolism. We have generated skeletal muscle-specific Gsα-knockout (KO) mice (MGsKO) by mating Gsα-floxed mice with muscle creatine kinase-cre transgenic mice. MGsKO mice had normal body weight and composition, and their serum glucose, insulin, free fatty acid, and triglyceride levels were similar to that of controls. However, MGsKO mice were glucose intolerant despite the fact that insulin sensitivity and glucose-stimulated insulin secretion were normal, suggesting an insulin-independent mechanism. Isolated muscles from MGsKO mice had increased basal glucose uptake and normal responses to a stimulator of AMP-activated protein kinase (AMPK), which indicates that AMPK and its downstream pathways are intact. Compared with control mice, MGsKO mice had reduced muscle mass with decreased cross-sectional area and force production. In addition, adult MGsKO mice showed an increased proportion of type I (slow-twitch, oxidative) fibers based on kinetic properties and myosin heavy chain isoforms, despite the fact that these muscles had reduced expression of peroxisome proliferator-activated receptor coactivator protein-1α (PGC-1α) and reduced mitochondrial content and oxidative capacity. Therefore Gsα deficiency led to fast-to-slow fiber-type switching, which appeared to be dissociated from the expected change in oxidative capacity. MGsKO mice are a valuable model for future studies of the role of Gsα signaling pathways in skeletal muscle adaptation and their effects on whole body metabolism.
Keywords: G protein, atrophy
gsα is a ubiquitously expressed G protein α-subunit that couples many receptors to adenylyl cyclase and is required for the intracellular cAMP response to hormones, neurotransmitters, and other extracellular signals (44). Genetic defects in Gsα signaling lead to several diseases, including some (e.g., pseudohypoparathyroidism type 1A) that are associated with metabolic disorders such as obesity (25). The Gsα gene Gnas is a complex gene with multiple gene products (45). We have generated mice with a germline Gnas haploinsufficiency that specifically disrupts Gsα expression and showed that widespread Gsα deficiency leads to obesity, glucose intolerance, and insulin resistance (4). However, it is difficult to evaluate the contributions of Gsα deficiency in specific tissues on metabolic regulation using this model. We have therefore created Gsα-floxed mice with loxP recombination sites surrounding Gsα exon 1 to be able to generate tissue-specific Gsα-knockout (KO) lines (5). Using Gsα-floxed mice, we have previously generated liver-specific Gsα-KO mice and showed them to have increased glucose tolerance and insulin sensitivity and reduced adiposity (5), indicating that changes in glucose and energy metabolism observed in the germline KOs result from Gsα deficiency in one or more tissues besides the liver.
Skeletal muscle is an important tissue in glucose and energy metabolism due to its large requirement for nutrients and is a major site of glucose disposal after a glucose load (53). The function of skeletal muscle is determined by muscle mass and fiber composition. In vertebrates, skeletal muscles are composed of various muscle fiber types, differing with respect to their contractile and metabolic properties. Muscle fibers can be classified as type I, IIa, IIx, or IIb based on the presence of myosin heavy chain (MHC) isoforms. Slow-twitch type I muscle fibers are rich in mitochondria and possess high oxidative capacity and are resistant to fatigue. Muscles enriched in type I fibers, such as soleus, perform sustained and tonic contractile activities, like postural tension. Conversely, fast-twitch type II muscle fibers have high glycolytic metabolism and fatigue easily. Muscle enriched in type II fibers, such as quadriceps and extensor digitorum longus (EDL), are typically involved in intense and rapid activities of short duration. Skeletal muscle can adapt to functional and metabolic demands by remodeling with fiber-type switches. Changes in muscle fiber composition are often associated with altered glucose metabolism, diabetes, and obesity with increased fast-twitch glycolytic fibers and decreased slow-twitch oxidative fibers (36). Studies in animal models have also shown a strong relationship between muscle fiber type and the development of diabetes and obesity (39, 43). Endurance training is beneficial to obese and diabetic patients through switching of muscle fibers to a more favorable metabolic composition (more type I fibers) (7, 19). In addition, exercise stimulates insulin-independent glucose uptake in muscle through stimulation of AMP-activated kinase (AMPK) (11).
In the present study, we generated skeletal muscle-specific Gsα-KO (MGsKO) mice to examine the effects of complete Gsα deficiency on glucose and energy metabolism and on muscle composition and function. Our results show that muscle Gsα deficiency led to glucose intolerance despite no evidence for a defect in insulin secretion or sensitivity. Muscle Gsα deficiency also led to reduced skeletal muscle mass, which may play a causative role in the impaired acute glucose clearance. In addition, MGsKO mice showed an increase in the relative number of slow-twitch type I muscle fibers as they aged, despite the fact that these muscles had reduced mitochondrial content and oxidative capacity, features more typical of type II muscle fibers.
METHODS
Generation of MGsKO mice.
Mice with loxP sites surrounding Gsα exon 1 (E1fl/fl) (5) were mated with muscle creatine kinase-promoter-cre-transgenic (Mck-cre) mice (Taconic, Hudson, NY) to generate MGsKO mice (genotype E1flox/flox:Mck-cre+). Specificity and efficiency of the Gsα deletion were examined by immunoblotting using a Gsα-specific antibody (40). Because the E1flox allele had no effect on Gsα expression or phenotype, all E1+/+ or Mck-cre− littermates were used as controls. Mice were genotyped by PCR on mouse tail DNA as previously described (5). Animals were maintained on a 12-h light-dark cycle (6 AM/6 PM) and a standard pellet diet (NIH-07, 11% calories from fat by weight). All experiments were performed on male mice at age 12–16 wk unless indicated otherwise. Animal experiments were approved by the National Institute of Diabetes and Digestive and Kidney Diseases Animal Care and Use Committee.
Measurements of body composition, blood pressure, and metabolism.
Body composition was measured in nonanesthetized mice using the Bruker Minispec NMR analyzer mq10 (Bruker Optics, Woodlands, TX). Blood pressure was measured by a BP-2000 Specimen platform (Visitech System, Apex, NC). Oxygen consumption was measured by indirect calorimetry using a four-chamber Oxymax system with one mouse per chamber (Columbus Instruments, Columbus, OH; 2.5-liter chambers with wire mesh floors, using 0.6 l/min flow rate, 90-s purge, and 60-s measure). Total and ambulating motor activities were determined by infrared beam interruption (Opto-Varimex mini; Columbus Instruments).
Biochemical assays.
Serum insulin, leptin, adiponectin, IL-6, and TNF-α levels were measured by radioimmunoassay kits (Linco, St Charles, MO). Free fatty acids and triglycerides were measured using reagents purchased from Thermo DNA (Waltheram, MA) and Roche (Indianapolis, IN), respectively. Measurement of tissue glycogen or triglyceride content was performed as previously described (6). Fatty acid oxidation rates in isolated soleus muscles (18) and in whole body in vivo (12) were measured as previously described. Urinary catecholamine levels, corrected for creatinine, were measured as previously described (5). Serum lactate was measured using a kit from BioAssay Systems (Hayward, CA). For measurement of ATP, gastrocnemius muscle from randomly fed control and MGsKO mice were homogenized and precipitated with 1% trichloracetic acid. ATP was extracted with ether twice and dried to powder in a speed vacuum. ATP levels were measured with a kit from BioAssay Systems kit using a luminometer (CentroLB 960, Berthold Technologies, Oak Ridge, TN).
Glucose and insulin tolerance tests and hyperinsulinemic-euglycemic clamp studies.
For glucose tolerance test, overnight-fasted mice were given intraperitoneal (ip) glucose (2 mg/g body wt), and tail blood was collected before (time 0) and at indicated times after injection for measurement of glucose (Glucometer Elite, Bayer, Elkhart, IN) and insulin. To examine insulin secretion, the study was repeated using glucose (3 mg/g body wt ip), and earlier time points were measured. For insulin tolerance tests, insulin (Humulin, 0.75 mIU/g ip) was administered to overnight-fasted mice, and glucose was measured at indicated time points. Hyperinsulinemic-euglycemic clamp studies were performed in awake, overnight-fasted mice as previously described (52), with modification. Experiments used primed-continuous infusion of 3-[3H]glucose (2.5 μCi bolus, 0.05 μCi/min during basal period, and 0.1 μCi/min during clamp period). Insulin was infused at the rate of 2.5 mU·kg−1·min−1 (without bolus). In vivo glucose fluxes were calculated as previously described (6).
Measurement of glucose uptake in skeletal muscle.
Glucose uptake in isolated muscles was measured using [3H]2-deoxyglucose, with [14C]mannitol to correct for extracellular fluid. Muscle tissues were freshly isolated and equilibrated for 3 h in Krebs-Henseleit buffer containing 6 mM glucose, 18 mM mannitol, and 2 mM pyruvate in a 95% O2-5% CO2 atmosphere and were then incubated in the presence or absence of 10 mIU/ml insulin (Humulin) or 2 mM aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR; Sigma) for 20 min, followed by addition of 100 mM (2.4 mCi) 1,2-[3H]2-deoxyglucose and 0.7 mCi of 1-[14C]mannitol. After 20 min at 30°C, the tissue 3H and 14C were measured, and the 3H uptake of muscle fibers was determined after adjustment for 14C uptake into extracellular spaces.
Quantitative real-time RT-PCR.
Gene expression levels were measured by quantitative RT-PCR in a real-time PCR machine (MxP3000, Stratagene, La Jolla, CA). Total RNA was isolated using TRIzol reagent (Gibco-BRL, Gaithersburg, MD) and treated with DNase I at room temperature for 15 min. cDNA was synthesized by reverse transcription using random primers (Roche, Branchburg, NJ). PCR was performed in a mixture (20–40 μl) containing 20–40 ng target cDNA, 50–100 nM primers and 1× SYBR (Applied Biosystems, Foster City, CA). The expression of each gene examined was normalized to β-actin expression, which was simultaneously measured in each sample by comparative quantification using the MxP3000 software. Specificity of each RT-PCR product was indicated by its dissociation curve and the presence of a single band of expected size on acrylamide gels. Primer sequences are available on request.
Glycerol-SDS-PAGE.
Skeletal muscle MHC isoforms were separated by glycerol-SDS-PAGE and quantified as previously described (50). Total protein was directly extracted from muscle tissues by homogenization in SDS-PAGE sample buffer. MHC isoforms were resolved using 8% polyacrylamide gel with a 50:1 ratio of acrylamide-to-bisacrylamide containing 30% glycerol prepared in 200 mM Tris·HCl, 100 mM glycine (pH 8.8), and 0.4% SDS. The upper running buffer was composed of 100 mM Tris base, 150 mM glycine, and 0.1% SDS. The lower running buffer was a 50% dilution of the upper running buffer. The gels were run in an icebox at 72 V for 18 h, followed by 83 V for 6 h. Resolved protein bands were visualized by staining with Coomassie Blue R250. The SDS-gel and Western blots were quantified by densitometry using ImageJ software (version 1.61; National Institutes of Health, Bethesda, MD).
Measurements of muscle function.
To measure postural tension, mice were placed on a metal grid plate that was then slowly turned upside down. The duration that mice were held in this position before dropping from the plate was determined as position holding time. Muscle contractile velocity was measured as previously described (50). Briefly, mice were anesthetized with pentobarbital sodium (1 mg/10 g body wt ip), and intact soleus muscles were rapidly dissected and kept in a modified Krebs solution (118 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM KH2PO4, 2.25 mM MgSO4, 2.25 mM CaCl2, and 11 mM d-glucose, pH 7.4) equilibrated with 95% O2-5% CO2 at room temperature (21–23°C). Twitch contractions were elicited by electrical stimulation (model 701B, Aurora Scientific) at supramaximal constant voltage pulses (0.1 ms, 15 V). Isometric contraction was analyzed at optimal muscle length predetermined by a serial testing of preloads with a dual-mode lever arm force transducer (model 300B, Aurora Scientific Inc). Data were collected by a digital controller A/D interface (model 604C, Aurora Scientific) and recorded by the Chart software (Aurora Scientific). Peak of twitch force was calculated as the difference between baseline and maximum force.
Immunohistochemistry.
Muscles were rapidly frozen in liquid nitrogen-cooled isopentane and sectioned at a thickness of 10 μm. The cryosections were fixed in 4% formaldehyde for 10 min, washed with PBS and placed in 3% H2O2 for 10 min. After blocking in 10% normal goat serum, the sections were incubated with an anti-MHC I monoclonal Ab FA2 (1:100 dilution) (20), or mouse anti-cytochrome-c oxidase subunit IV Ab CoxIV (1:500 dilution, Abcam, Cambridge, MA), at 4°C overnight. Sections were then incubated with biotinylated sheep anti-mouse secondary Ab in blocking solution containing 10% sheep serum and 1% BSA in PBS at room temperature for 30 min. The binding of FA2 to MHC I was detected and amplified using the avidin-biotin complex peroxidase method and visualized with 3,3′-diaminobenzidine. The sections were counterstained with methyl green before microscopic documentation.
Statistical analysis.
Data are expressed as means ± SE. Statistical significance among groups was determined using nonpaired Student's t-test, and differences were considered significant at P < 0.05 (one- or two-tailed).
RESULTS
Generation and characterization of MGsKO mice.
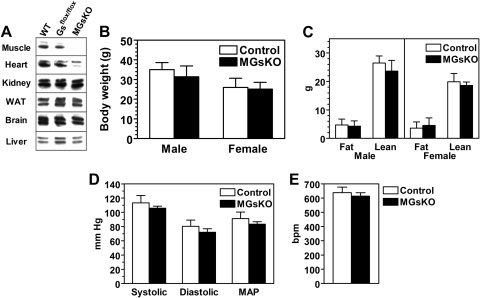
MGsKO mice were generated by mating mice with loxP sites flanking Gsα exon 1 (E1fl/fl) (5) with muscle Mck-cre mice. Immunoblotting showed Gsα protein to be virtually absent in skeletal muscle with no change in Gsα expression in kidney, white adipose tissue, brain, or liver (Fig. 1A). As there was no effect on Gsα expression in E1flox/flox Mck-cre− (Fig. 1A) and no effect on phenotype, E1+/+ and Mck-cre− littermates were used as controls.
Fig. 1.
Characterization of skeletal muscle-specific Gsα-knockout (KO) mice (MGsKO). A: immunoblotting analysis of tissue protein extracts from wild-type (E1+/+), E1flox/flox:Mck-cre− (Gsflox/flox), and MGsKO mice using a Gsα-specific antibody. The presence of long and short forms of Gsα produced by alternative splicing of exon 3 results in a doublet. WAT, white adipose tissue. B and C: body weight (B) and body composition (fat and lean mass) (C) of male and female MGsKO (closed bars) and control (open bars) mice (n = 10–16/group). D and E: systolic, diastolic, and mean arterial pressure (MAP) (D) and heart rate (E) in MGsKO mice and controls (bpm, beats/min; n = 5–6/group).
Gsα protein levels were also significantly reduced in the heart, although not to the same extent as in skeletal muscle (Fig. 1A). This reduction in Gsα expression in heart did not have severe effects on cardiac function in the basal state because MGsKO mice had normal heart rates and blood pressure (Fig. 1, D and E). While a severe decrease in cardiac function is generally associated with an increase in sympathetic nervous system (SNS) activity, we did not find evidence for such an increase in SNS activity in MGsKO mice based on urine catecholamine excretion, indicating well-compensated in vivo cardiac function (Fig. 3C). MGsKO mice had normal survival and no differences in either body weight or composition as compared with controls (Fig. 1, B and C).
Glucose intolerance with normal insulin secretion and sensitivity in MGsKO mice.
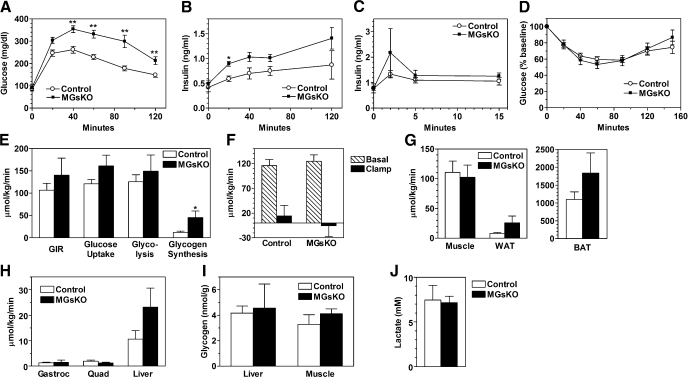
Skeletal muscle is the main site of insulin-stimulated glucose uptake, and therefore we first determined the effects of muscle Gsα deficiency on glucose metabolism. MGsKO and control mice had similar levels of glucose, insulin, free fatty acids, triglycerides, leptin, adiponectin, and IL-6 when measured in randomly fed mice (Table 1). Serum IL-2 and TNF-α levels were undetectable in both control and MGsKO mice. There were also no differences in IL-6 or TNF-α mRNA levels in quadriceps muscle samples from MGsKO and control mice (Fig. 4G). Intraperitoneal glucose tolerance tests showed MGsKO mice to be significantly glucose intolerant compared with controls (Fig. 2A). Simultaneously measured insulin levels showed them to be higher in MGsKO mice than controls at all postinjection time points (Fig. 2B), suggesting that the glucose intolerance is not due to an obvious deficiency in insulin secretion.
Table 1.
Serum chemistry in 16-wk-old control and MGsKO mice
|
Males |
Females
|
|||
|---|---|---|---|---|
| Controls | MGsKO | Controls | MGsKO | |
| Glucose, mg/dl | 169±10 | 173±8 | 162±6 | 159±11 |
| Insulin, ng/ml | 1.43±0.31 | 1.15±0.11 | 0.89±0.15 | 1.06±0.15 |
| Free fatty acids, mM | 0.59±0.09 | 0.61±0.12 | 0.60±0.07 | 0.54±0.08 |
| Triglycerides, mg/dl | 144±19 | 157±15 | 180±22 | 205±23 |
| Leptin, ng/ml | 11.4±1.9 | 11.0±1.9 | ND | ND |
| Adiponectin, μg/ml | 7.17±0.82 | 8.08±0.43 | ND | ND |
| IL-6, pg/ml | 57±24 | 67±11 | 19±6 | 30±16 |
Values are means ± SE (n = 5–7/group). MGsKO, muscle-specific Gsα−knockout mice. ND, not determined.
Fig. 2.
Glucose metabolism in MGsKO mice. Glucose (A) and insulin (B) levels were measured in MGsKO (▪) and control (○) mice at indicated times before and after intraperitoneal (ip) glucose (2 mg/g body wt; n = 20–25/group from 6 separate experiments). C: insulin levels measured before and after glucose administration (3 mg/g ip; n = 8–13/group). D: blood glucose levels measured at indicated times before and after intraperitoneal insulin (n = 12–13 /group). E: glucose infusion rate (GIR) and whole body glucose uptake, glycolysis, and glycogen synthesis rates in MGsKO (closed bars) and control (open bars) mice during euglycemic-hyperinsulinemic clamp studies (n = 4–8/group). F: basal (hatched bars) and clamp (closed bars) endogenous glucose production rate in both groups of mice during the clamp study (n = 7–8/group). G: glucose uptake in gastrocnemius muscle, WAT, and brown adipose tissue (BAT) during the clamp study (n = 7–8/group). H: glycogen synthesis rates in gastrocnemius muscle (Gastroc), quadriceps muscle (Quad), and liver during the clamp studies (n = 3–8/group). I: liver and muscle glycogen content in randomly fed MGsKO and control mice obtained during the AM period (n = 4–5/group). J: serum lactate levels in randomly fed male MGsKO and control mice (n = 5–6/group). *P < 0.05 or **P < 0.01 vs. controls.
To further evaluate the early and late phases of insulin secretion, a glucose tolerance test was repeated using a higher glucose dose (3 mg/g) and measuring insulin at earlier time points (Fig. 2C). Postinjection glucose levels were higher in MGsKO mice than controls (data not shown). At 2 min postinjection, MGsKO mice had insulin levels twofold higher than in controls, although the difference was not significant due to variability in the data. In later time points, insulin levels were similar in MGsKO mice and controls. These results were consistent with an intact early phase of glucose-stimulated insulin release. In addition, there was no abnormal pancreatic islet histology observed in MGsKO mice (data not shown). Taken together, these results indicated that insulin deficiency or defect in glucose-stimulated insulin secretion is an unlikely explanation for the glucose intolerance of MGsKO mice. Although muscle-specific peroxisomal proliferator-activated receptor-γ coactivator 1α (PGC-1α) knockout mice were shown to have impaired β-cell function mediated by excess IL-6 secretion from muscle (14), our results provide no evidence for a similar phenomenon in MGsKO mice.
To determine whether MGsKO mice had reduced insulin sensitivity, we next performed insulin tolerance tests and euglycemic-hyperinsulinemic clamp studies. Insulin tolerance tests showed that MGsKO mice had normal hypoglycemic responses to insulin (Fig. 2D). This observation was confirmed by the euglycemic-hyperinsulinemic clamp studies (Fig. 2, E–G). Before the clamp, control and MGsKO mice had similar fasting insulin levels (control 0.94 ± 0.21 vs. MGsKO 0.81 ± 0.18 ng/ml). Fasting glucose levels tended to be higher in MGsKO mice (control 161 ± 9 vs. MGsKO 207 ± 23 mg/dl). During the clamp, insulin was infused at the rate of 2.5 mIU·kg−1·min−1 to maintain insulin levels within physiological range (∼2 ng/ml). Glucose infusion was adjusted to maintain the same levels of plasma glucose (∼160 ng/ml). Glucose infusion rate, whole body glucose uptake, whole body glycolysis, and suppression of the endogenous glucose production during the clamp were similar in control and MGsKO mice (Fig. 2, E and F), suggesting comparable whole body insulin sensitivity. Glucose uptake in skeletal muscle (gastrocnemius) was similar between control and MGsKO mice, while MGsKO mice had a tendency toward increased glucose uptake in white and brown adipose tissue (WAT, BAT) (Fig. 2G). Whole body glycogen synthesis rates were higher in MGsKO mice (Fig. 2E). This may be related to increased glycogen synthesis in liver because we found that this tended be higher in livers of MGsKO mice, although this did not reach statistical difference due to large variability in the data (Fig. 2H). However, we found no differences in glycogen synthesis rate in either gastrocnemius or quadriceps muscle (Fig. 2H). Liver and muscle glycogen levels measured in the morning (∼16 h after the beginning of the last dark cycle) were also similar in MGsKO and control mice (Fig. 2I). Consistent with the similar rates of whole body glycolysis during the clamp study, serum lactate levels were also similar in randomly fed control and MGsKO mice (Fig. 2J), suggesting that there is no defect in glycolytic glucose metabolism in muscles of MGsKO mice. Taken together, these results indicate that MGsKO mice had similar insulin sensitivity in peripheral tissues as compared with control mice.
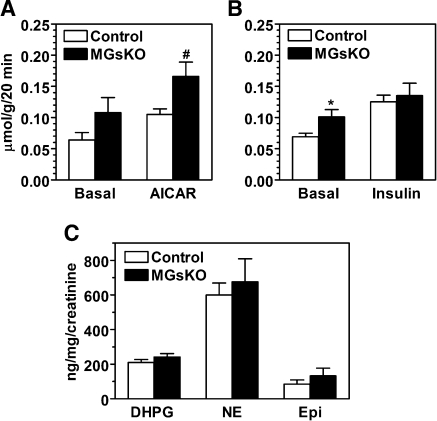
The phenotype of glucose intolerance despite normal insulin secretion and responsiveness suggests that skeletal muscle deficiency of Gsα may impair an insulin-independent pathway that also stimulates muscle glucose uptake, such as AMPK (2). To test this possibility, we measured the effect of the AMP analog AICAR on glucose uptake in isolated EDL muscles. Under basal conditions, glucose uptake tended to be higher in EDL muscles of MGsKO mice than in controls, and the increase in glucose uptake in the presence of AICAR was similar in muscles from both MGsKO and control mice (Fig. 3A). These results show that the ability of AMPK to stimulate glucose uptake was not altered in MGsKO muscle, although it did not rule out the possibility that upstream stimulators of AMPK were reduced in MGsKO mice. Immunoblotting analysis to determine the extent of AMPK stimulation did not find a significant difference between control and MGsKO mice in AMPK phosphorylation at Thr172 in hind limb muscles of randomly fed mice (data not shown). In parallel, we measured insulin-stimulated glucose uptake in isolated soleus muscles. Similar to the result obtained from EDL muscles, soleus muscles of MGsKO mice showed higher basal glucose uptake than controls. The response to insulin, when defined as rise over the basal level, was reduced in soleus muscles from MGsKO mice compared with controls, although total glucose uptake in the presence of insulin was similar in the two groups (Fig. 3B).
Fig. 3.
Glucose uptake in isolated muscles and sympathetic nervous activity. A: basal and aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR)-stimulated glucose uptake in isolated extensor digitorum longus muscle from MGsKO (closed bars) and control (open bars) mice (#P < 0.05 vs. basal MGsKO rate; n = 5–7/group). B: basal and insulin-stimulated glucose uptake in isolated soleus muscles (*P < 0.05 vs. basal control rate; n = 5–7/group). C: urinary excretion of dihydroxyphenylglycol (DHPG), norepinephrine (NE), and epinephrine (Epi) normalized to creatinine in MGsKO and control mice (n = 9/group).
Because Gsα deficiency would be expected to disrupt β-adrenergic signal pathway in muscle, we examined urine catecholamine excretion, normalized to urine creatinine, to rule out the possibility that disruption of this pathway would lead to an alteration of overall SNS activity (Fig. 3C). Excretion of norepinephrine and its metabolite dihydroxyphenylglycol, which mainly reflect SNS activity, and epinephrine, which mainly reflects adrenomedullary secretion, was similar between MGsKO and control mice, consistent with MGsKO mice having no major alterations in overall SNS activity.
MGsKO mice had reduced fatty acid oxidation in skeletal muscle but normal whole body energy expenditure and lipid metabolism.
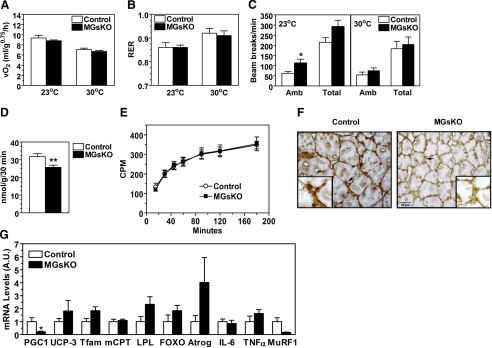
To determine whether muscle-specific Gsα deficiency may also affect energy balance and lipid metabolism, we measured metabolic rate and oxidative capacity in MGsKO and control mice. There were no differences in either total oxygen consumption or respiratory exchange ratio (V̇co2/V̇o2) between MGsKO and control mice at either ambient temperature (23°C) or thermoneutral temperature (30°C) when SNS is minimized (Fig. 4, A and B). MGsKO mice had significantly increased ambulatory activity levels at 23°C, although activity levels were not different at 30°C (Fig. 4C).
Fig. 4.
Energy expenditure and lipid metabolism in MGsKO mice. A–C: total energy expenditure (A), respiratory exchange ratios (RER) (B), and ambulatory (Amb) and total activity levels (C) at 23°C (left) and 30°C (right) in 3-mo-old male mice (n = 6–8 mice/group). D: fatty acid oxidation rate in isolated soleus muscles from control (open bars) and MGsKO (closed bars) mice (n = 7 mice/group). E: whole body in vivo fatty acid oxidation rate in MGsKO (▪) and control (○) mice (n = 5–6/group); cpm, counts per minute. F: immunohistochemistry of soleus muscle from control and MGsKO mice using a COXIV antibody showing perinuclear mitochondria (arrows). Original magnification ×40 and ×100 (insets). G: gene expression in quadriceps muscle of MGsKO and control mice measured by quantitative real-time RT-PCR and normalized to β-actin mRNA (n = 5–8/group). PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; UCP-3, uncoupling protein 3; Tfam, transcription factor A mitochondrial; mCPT, carnitine palmitoyltransferase 1, muscle isoform; LPL, lipoprotein lipase; FOXO, forkhead transcription factor 1; Atrog, atrogin-1/MAFbx; MuRF1, muscle-specific ring finger protein 1; AU, arbitrary units. *P < 0.05 or **P < 0.01 vs. controls.
Isolated soleus muscles from MGsKO mice had reduced in vitro fatty acid oxidation rates (Fig. 4D). Although we did not observe differences in expression of genes related to thermogenesis (uncoupling protein 3), lipid metabolism (muscle carnitine palmitoyltransferase 1; lipoprotein lipase), and mitochondrial function (transcription factor A mitochondrial) in quadriceps muscle (Fig. 4G), immunohistochemistry using an antibody against cytochrome-c oxidase subunit IV demonstrated that soleus muscles of MGsKO mice had reduced perinuclear mitochondria (Fig. 4F), which is consistent with the lower fatty acid oxidation in these muscles. Moreover, DNA microarray in soleus and quantitative RT-PCR in soleus and quadriceps showed significantly reduced mRNA levels of PGC-1α, a cAMP-inducible transcription factor that promotes mitochondrial biogenesis. Because there were no differences in serum or liver and muscle tissue triglyceride levels between MGsKO and control mice (Table 1 and data not shown), the reduced fatty acid oxidation did not result in increased lipid accumulation in tissues. Consistent with the presence of normal triglyceride levels and energy expenditure, MGsKO mice had similar rates of whole body fatty acid oxidation as controls (Fig. 4E), suggesting the presence of compensation in other tissue(s).
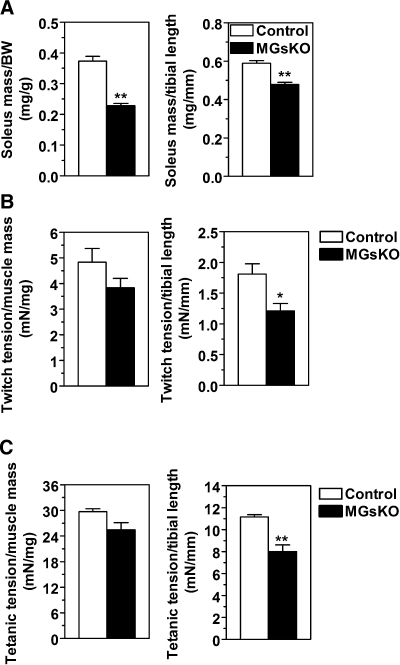
MGsKO mice had reduced muscle mass and force production.
Tibial bone length of MGsKO mice did not differ from that of controls (18.9 ± 0.1 mm in MGsKO vs. 18.7 ± 0.2 mm in controls), indicating no gross abnormalities in linear growth. However, soleus muscles of MGsKO mice had significantly reduced muscle mass, as evidenced by decreases in the ratios of muscle-to-body weight and muscle mass-to-tibial length (Fig. 5A). Position holding time in mice suspended on an upside down grate, a measure of postural tension, was significantly lower in MGsKO than control mice (MGsKO 13.8 ± 1.8 vs. control 32.3 ± 6.2 s, P < 0.05). This reduced capacity of postural holding was not related to a difference in body weight, indicating that along with reduced muscle mass, MGsKO mice had reduced overall muscle power.
Fig. 5.
Muscle mass and force production in soleus muscles of MGsKO mice. A: soleus muscle weight of female MGsKO (closed bars) or control (open bars) mice normalized to body weight (BW; left) or tibial length (right). **P < 0.01; n = 3/group. B and C: twitch tension (B) and titanic tension (C) in soleus muscles normalized to muscle mass (left) or tibial length (right) (n = 3/group). *P < 0.05 or **P < 0.01 vs. control.
More quantitative measurements of muscle force showed that soleus muscles of MGsKO mice tended to have lower twitch tension than that of controls when normalized by muscle weight, and significantly lower twitch tension when normalized by the tibial length (Fig. 5B). Tetanic force, which is more representative of skeletal muscle function in vivo, was also decreased in soleus muscles of MGsKO mice when normalized by either muscle mass or tibial length (Fig. 5C).
Changes in muscle fiber type composition in MGsKO mice.
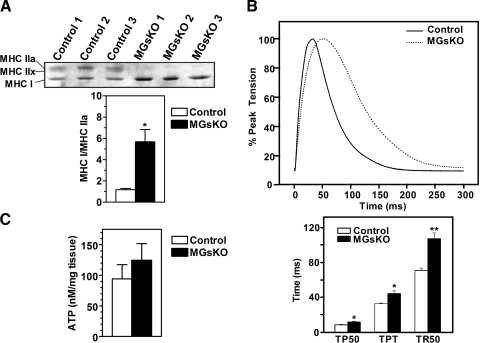
Muscle fiber type composition was assessed by measuring the relative amounts of MHC isoforms in soleus muscle by glycerol-SDS-PAGE (Fig. 6A, top). MHC I, MHC IIa, and MHC IIx were clearly detectable in muscles of control mice, and MHC I, an isoform specifically expressed in type I fibers, was significantly increased in muscles of MGsKO mice, while the MHC IIa and MHC IIx isoforms associated with type II fibers were virtually undetectable. Quantification of MHC isoforms confirmed that muscles of MGsKO mice had a switch toward higher presence of more type I fibers (Fig. 6A, bottom).
Fig. 6.
Fiber-type changes in muscles of MGsKO mice. A, top: glycerol-SDS-PAGE analysis of soleus muscle from control and MGsKO mice with bands showing the indicated myosin heavy chain (MHC) isoforms. A, bottom: densitometric quantification of the ratio of MHCI to MHCIIa in control (open bars) and MGsKO (closed bars) mice (n = 3/group). B, top: representative twitch contraction curves of soleus muscle from MGsKO (dotted line) and control (solid line) mice normalized to peak tension. B, bottom: time to 50% of peak tension (TP50), time to peak tension (TPT), and time to relax to 50% of peak tension (TR50) in soleus muscle from MGsKO and control mice (n = 3/group). C: ATP levels in gastrocnemius muscles from randomly fed control and MGsKO mice (n = 7/group). *P < 0.05 or **P < 0.01 vs. control.
Consistent with the observed fiber-type switch based on MHC isoform analysis, studies of twitch contraction showed soleus muscles from MGsKO mice to have decreased contractile and relaxation velocities with prolonged contractile and relaxation periods (Fig. 6B), which are characteristic of muscles primarily composed of type I fibers. Therefore soleus muscle of MGsKO underwent a fiber-type switch toward an increase in type I fibers based on both MHC isoform ratios and functional characteristics. To rule out the possibility that this fiber switch is secondary to an impaired energetic state due to decreased mitochondrial content, we measured ATP levels in gastrocnemius muscles of randomly fed MGsKO and control mice and found, if anything, these levels to be slightly higher in MGsKO mice, although the difference was not significant (Fig. 6C).
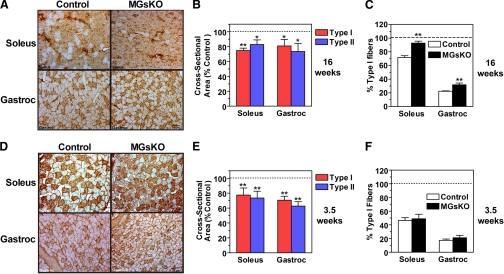
To determine whether the switch of contractile proteins toward a more slow fiber phenotype in muscles of MGsKO mice was due to an increase in the size and/or number of type I fibers, we performed immunohistochemistry using a MHC I-specific monoclonal antibody on both soleus and gastrocnemius muscles, the latter being a mixed fiber type muscle. Compared with control muscles, both soleus and gastrocnemius muscles of 16-wk-old MGsKO mice had a greater proportion of MHC I-positive fibers (Fig. 7, A and C). This fiber switch was acquired during the postweaning period, because similar experiments performed in 3.5-wk-old mice showed that there were no differences between control and MGsKO mice in either muscle, although the proportion of type I fibers was lower in both sets of animals at the younger age (Fig. 7, D and F). In addition, both type I and II muscle fibers in the soleus and quadriceps had reduced cross-sectional area in 16-wk-old MGsKO mice (Fig. 7, A and B). This finding is consistent with our observation of MGsKO having reduced muscle mass (Fig. 5A). Reduced muscle fiber size was not acquired during adult life because we observed similar changes in type I and II muscle fiber size in 3.5-wk-old MGsKO mice (Fig. 7, D and E). We examined the expression of two ubiquitin ligase genes [atrogin 1/MAFbx and muscle-specific ring finger protein 1 (MuRF1)] known to be overexpressed in skeletal muscles undergoing atrophy (34). Atrogin 1/MAFbx tended to be overexpressed, whereas MuRF1 tended to be underexpressed in quadriceps muscle of MGsKO mice, although the differences for both genes was not significant (Fig. 4G). Because these genes are regulated by FOXO1, we performed immunoblots on gastrocnemius and soleus muscle extracts from control and MGsKO mice and found no differences in either phosphorylated or total FOXO1 protein levels (data not shown) or in FOXO1 mRNA expression (Fig. 4G). Therefore the reduction in fiber size in MGsKO mice occurs early in development and the rate of growth of fiber cross-sectional area during early adult life appears to be similar in control and MGsKO mice.
Fig. 7.
Muscle fiber switch and atrophy in muscles from MGsKO mice. A: immunohistochemistry of soleus (top) and gastrocnemius (bottom) muscles of 16-wk-old female control and MGsKO mice using a MHCI-specific antibody (brown stain). B: quantification of cross-sectional area of muscle fibers (type 1, red; type 2, blue) in soleus and gastrocnemius from 16-wk-old MGsKO mice expressed as % of control (n = 3–4/group). C: percentage of type I (MHC1-positive) muscle fibers in soleus and gastrocnemius from 16-wk-old MGsKO (closed bars) and control (open bars) mice (n = 3–4/group). D: immunohistochemistry as in A in 3.5-wk-old female mice. E: cross-sectional area of type 1 and 2 muscle fibers in soleus and gastrocnemius muscles from 3.5-wk-old female MGsKO expressed as % of control (n = 4–5/group). F: percentage of type I muscle fibers in soleus and gastrocnemius muscles in 3.5-wk-old control and MGsKO mice (n = 4–5/group). *P < 0.05 or **P < 0.01 vs. control.
DISCUSSION
Our previous mouse model with germline Gnas mutation revealed that partial Gsα deficiency causes diabetes and obesity (4), indicating the important role of Gsα signaling pathways in glucose and lipid metabolism. Since muscle is a major organ in glucose homeostasis, we have generated MGsKO mice to investigate its tissue-specific phenotypes. We found that MGsKO mice exhibited reduced skeletal muscle mass and altered myofiber composition with a switch toward type I oxidative fibers based upon MHC subtypes and kinetic properties although these skeletal muscles had reduced oxidative capacity. Metabolic studies revealed that MGsKO mice had normal body weight and composition, and normal baseline blood glucose and insulin levels as compared with their control littermates. However, the ability of MGsKO mice to clear an acute glucose load was markedly impaired. Results of insulin tolerance tests and euglycemic-hyperinsulinemic clamp studies showed that glucose intolerance in MGsKO mice was not a consequence of impaired insulin sensitivity. A similar phenotype of glucose intolerance with normal insulin sensitivity was reported by Handschin et al. (14) in skeletal muscle-specific PGC-1α-KO mice. In this model, glucose intolerance was the result of increased muscle secretion of IL-6 and other cytokines leading to reduced insulin secretion from pancreatic β-cells. Glucose intolerance in MGsKO mice appears to result from a different mechanism, because these mice had normal IL-6 expression in muscle, normal serum IL-6 levels, and no evidence for a defect in insulin secretion. Therefore an alternative insulin-independent pathway might be responsible for the decreased glucose clearance in MGsKO mice.
The role of insulin-independent pathways in muscle glucose uptake has been emphasized in light of the presence of normal glucose tolerance in muscle-specific insulin receptor KO mice (1). Indeed, contraction- or hypoxia-induced glucose uptake in muscle requires activation of AMPK (29, 33, 47). Administration of AICAR, an AMPK activator, enhances translocation of the glucose transporter 4 (GLUT-4) to the plasma membrane and glucose uptake in muscle (29). Wortmannin, a phosphatidylinositol 3-kinase inhibitor, completely blocks insulin-stimulated glucose uptake but does not inhibit AICAR- or contraction-stimulated glucose uptake (16), suggesting that AMPK induces glucose uptake in an insulin-independent manner. We did not observe significant differences in AMPK protein content or phosphorylation in muscle from MGsKO mice (data not shown). In addition, AICAR stimulation of glucose uptake in isolated muscles from MGsKO mice was similar to that of controls, indicating that intracellular AMPK remains intact in the muscle of MGsKO mice, and therefore, is capable of being normally activated, although we have not ruled out the possibility that metabolic factors or signaling molecules upstream of AMPK are altered in MGsKO mice.
Evidence has demonstrated that intertissue communication plays an important role in the maintenance of normal glucose homeostasis. Changes in glucose concentration can be sensed by a variety of cellular mechanisms involving glucose sensors that constantly monitor blood glucose concentration to regulate food intake and glucose utilization (17). One of the identified glucose sensors is located in the hepatoportal vein that can be activated when a glucose gradient is established between the portal vein and the hepatic artery (3). Increased portal vein glucose levels stimulate muscle glucose uptake through mechanisms requiring AMPK and GLUT-4 but not insulin and are presumed to be mediated via SNS output from the CNS (2). Several studies have shown that the glucose uptake in skeletal muscle and other tissues such as brown adipose tissue is stimulated by the SNS in response to various factors, such as leptin and increased food intake (15, 30, 31, 35). Some studies have implicated β-adrenergic receptors as mediators of SNS-stimulated glucose uptake in skeletal muscle (15, 32). If this is the case, then one would predict that these effects would be disrupted by Gsα deficiency. It is likely that reduced muscle mass, particularly the marked reduction in fast-twitch type II muscle fibers that rapidly utilize glucose, also significantly contributes to reduced acute glucose clearance observed in MGsKO mice.
Skeletal muscle atrophy is a debilitating response to aging, starvation, physical inactivity, and many systemic diseases including malnutrition and diabetes. In efforts to identify therapeutic agents to reverse muscle wasting conditions, β-adrenoceptor (AR) agonists, traditionally used for treating asthma, were found to also lead to increased muscle mass in human and animals (10, 26, 28). Since skeletal muscles contain predominantly β2-ARs with <10% β1-ARs and a smaller population of α-ARs (23, 46), it is rational to believe that Gsα, which couples β-ARs to increase intracellular cAMP levels, plays an important role in the anabolic effect of β-AR agonists on skeletal muscle. Consistent with this, muscle Gsα deficiency in our mice resulted in reduced muscle mass with reductions in fiber cross-sectional area, muscle mass, and muscle force.
In the present study, muscles of MGsKO mice had low levels of PGC-1α expression, which may potentially contribute to the reduced muscle mass in MGsKO mice because PGC-1α overexpression has been shown to prevent loss of muscle mass (38). The reduced PGC-1α expression is likely secondary to impaired Gsα/cAMP signaling because cAMP is known to induce the PGC-1α gene via phosphorylation of the transcription factor cAMP-responsive binding protein (CREB) (37) and the induction of PGC-1α expression in muscle by exercise is mediated through the β-adrenergic pathway (32). Transducer of regulated CREB binding protein 1 also stimulates PGC-1α gene expression and mitochondrial biogenesis in muscle through CREB (49). PGC-1α promotes mitochondrial biogenesis in skeletal muscle (24), and therefore, in the present study, the reduced mitochondrial content in muscle of MGsKO mice was consistent with the low PGC-1α expression and lower fatty acid oxidation rates.
Skeletal muscle displays enormous plasticity, and its fiber composition can be regulated to meet physiological and environmental demands. Exercise, electrical activity, and calcium signaling all play important roles in the regulation of muscle fiber composition. Chronic administration of β-agonists have been shown to produce slow-to-fast muscle fiber transitions (27, 51). Consistent with this, we found that muscle Gsα deficiency, which would block β-adrenergic signaling, caused a fast-to-slow fiber-type switch in both soleus and gastrocnemius muscle. Various transcriptional factors are involved in the control of muscle fiber-type switching (21, 24, 39). Muscle atrophy and downregulation of slow-twitch type I fibers have been observed in transgenic mice overexpressing FOXO1 in skeletal muscle (21), but we find no changes in FOXO1 expression or phosphorylation in skeletal muscles of MGsKO mice. Surprisingly, although studies have shown PGC-1α to be preferentially expressed in muscles enriched with slow-twitch type 1 fibers and to be required for the formation and maintenance of type I fibers (13, 24), MGsKO mice had a significant shift toward slow-twitch type I fibers concomitant with lower PGC-1α expression levels and lower oxidative capacity. These findings implicate a pathway independent of PGC-1α in the switch to type I fibers and show that the fiber type phenotype (e.g., MHC isoforms and twitch velocities) may not always correlate with oxidative capacity. We found no reduction in ATP levels in muscle of MGsKO mice, indicating that the fiber-type switch is not likely to be a response to an impaired energetic state. Increased locomotor activity (Fig. 4C) found in MGsKO mice may activate other signaling pathways to promote type I fiber formation, such as the calcineurin/calmodulin kinase pathway (48). The increased activity levels, adaptive changes in switching toward type I fibers, and possible compensatory changes in other tissues may account for the fact that MGsKO mice were able to maintain normal whole body fatty acid oxidation rates and normal overall energy balance.
Muscle adaptation occurs under various physiological and pathophysiological conditions. A decrease in slow-twitch type I fibers is associated with increased glycolytic capacity and reduced oxidative capacity in skeletal muscle of type 2 diabetes patients (36, 41). Conversely, both physical training (42) and short-term high-fat diet (8) lead to an increase of type I fibers relative to type II fibers. In the present study, we showed that muscle-specific Gsα deficiency resulted in a phenotype of reduced muscle mass and force production, decreased contractile and relaxation velocities, and impaired muscle mitochondrial function, a phenotype similar with aging-related sarcopenia (9, 22). Since muscle is a main site of glucose uptake, reduced muscle mass may directly cause the impaired acute glucose utilization. In addition, the fast-to-slow fiber-type switching in MGsKO mice may be an adaptive response to maintain normal energy balance and nutrient utilization. Thus, our results provide evidence that Gsα plays an important role in preserving normal muscle growth and function such as contractility, fatigue tolerance, and glucose metabolism. Therefore, MGsKO mice provide a valuable model for future studies of the role of Gsα signal pathways in skeletal muscle adaptation to stress conditions as well as in the aging progress and may provide insights into therapeutic targets for metabolic and skeletal muscle disorders.
GRANTS
This work was supported by the Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH), U.S. Department of Health and Human Services, and by NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases Extramural Research Grant AR-048816 (to J.-P. Jin).
Acknowledgments
We thank K. Pacak and E. Lai for assistance in measurements of urine catecholamines and R. Vinitsky for technical support.
Present addresses: D. Gupta, Duke University School of Medicine, Durham, NC 27705; K. E. Dickerson, Indiana University School of Medicine, Indianapolis, IN 46202; D. Hunt, Department of Standards Development, U.S. Pharmacopeia, Rockville, MD 20852; J. Kelleher, New Jersey Medical School, Newark, NJ 07101-1709.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2: 559–569, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Burcelin R, Crivelli V, Perrin C, Da Costa A, Mu J, Kahn BB, Birnbaum MJ, Kahn CR, Vollenweider P, Thorens B. GLUT4, AMP kinase, but not the insulin receptor, are required for hepatoportal glucose sensor-stimulated muscle glucose utilization. J Clin Invest 111: 1555–1562, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burcelin R, Dolci W, Thorens B. Portal glucose infusion in the mouse induces hypoglycemia: evidence that the hepatoportal glucose sensor stimulates glucose utilization. Diabetes 49: 1635–1642, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Gavrilova O, Liu J, Xie T, Deng C, Nguyen AT, Nackers LM, Lorenzo J, Shen L, Weinstein LS. Alternative Gnas gene products have opposite effects on glucose and lipid metabolism. Proc Natl Acad Sci USA 102: 7386–7391, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Gavrilova O, Zhao WQ, Nguyen A, Lorenzo J, Shen L, Nackers L, Pack S, Jou W, Weinstein LS. Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gsα deficiency. J Clin Invest 115: 3217–3227, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M, Haluzik M, Wolf NJ, Lorenzo J, Dietz KR, Reitman ML, Weinstein LS. Increased insulin sensitivity in paternal Gnas knockout mice is associated with increased lipid clearance. Endocrinology 145: 4094–4102, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Daugaard JR, Richter EA. Relationship between muscle fibre composition, glucose transporter protein 4 and exercise training: possible consequences in non-insulin-dependent diabetes mellitus. Acta Physiol Scand 171: 267–276, 2001. [DOI] [PubMed] [Google Scholar]
- 8.De Wilde J, Mohren R, van den Berg S, Boekschoten M, Willems-Van Dijk K, De Groot P, Muller M, Mariman EC, Smit E. Short-term high fat-feeding results in morphological and metabolic adaptations in the skeletal muscle of C57BL/6J mice. Physiol Genomics 32: 360–369, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev 5: 179–195, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Emery PW, Rothwell NJ, Stock MJ, Winter PD. Chronic effects of β2-adrenergic agonists on body composition and protein synthesis in the rat. Biosci Rep 4: 83–91, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Fujii N, Jessen N, Goodyear LJ. AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab 291: E867–E877, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Gautam D, Gavrilova O, Jeon J, Pack S, Jou W, Cui Y, Li JH, Wess J. Beneficial metabolic effects of M3 muscarinic acetylcholine receptor deficiency. Cell Metab 4: 363–375, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1α muscle-specific knock-out animals. J Biol Chem 282: 30014–30021, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest 117: 3463–3474, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiuchi M, Shimazu T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes 48: 1706–1712, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47: 1369–1373, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Invest 116: 1767–1775, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heron-Milhavet L, Haluzik M, Yakar S, Gavrilova O, Pack S, Jou WC, Ibrahimi A, Kim H, Hunt D, Yau D, Asghar Z, Joseph J, Wheeler MB, Abumrad NA, LeRoith D. Muscle-specific overexpression of CD36 reverses the insulin resistance and diabetes of MKR mice. Endocrinology 145: 4667–4676, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Hickey MS, Carey JO, Azevedo JL, Houmard JA, Pories WJ, Israel RG, Dohm GL. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am J Physiol Endocrinol Metab 268: E453–E457, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Jin JP, Brotto MA, Hossain MM, Huang QQ, Brotto LS, Nosek TM, Morton DH, Crawford TO. Truncation by Glu180 nonsense mutation results in complete loss of slow skeletal muscle troponin T in a lethal nemaline myopathy. J Biol Chem 278: 26159–26165, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 279: 41114–41123, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Kamel HK Sarcopenia and aging. Nutr Rev 61: 157–167, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Sainz RD, Molenaar P, Summers RJ. Characterization of β1- and β2-adrenoceptors in rat skeletal muscles. Biochem Pharmacol 42: 1783–1789, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL. Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Gαs in the development of human obesity. J Clin Endocrinol Metab 92: 1073–1079, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol 535: 591–600, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maltin CA, Delday MI, Reeds PJ. The effect of a growth promoting drug, clenbuterol, on fibre frequency and area in hind limb muscles from young male rats. Biosci Rep 6: 293–299, 1986. [DOI] [PubMed] [Google Scholar]
- 28.Maltin CA, Delday MI, Watson JS, Heys SD, Nevison IM, Ritchie IK, Gibson PH. Clenbuterol, a β-adrenoceptor agonist, increases relative muscle strength in orthopaedic patients. Clin Sci (Lond) 84: 651–654, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol Endocrinol Metab 273: E1107–E1112, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes 48: 287–291, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Minokoshi Y, Okano Y, Shimazu T. Regulatory mechanism of the ventromedial hypothalamus in enhancing glucose uptake in skeletal muscles. Brain Res 649: 343–347, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, Minokoshi Y, Ezaki O. An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) mRNA in response to exercise is mediated by β-adrenergic receptor activation. Endocrinology 148: 3441–3448, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Mu J, Brozinick JT Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Nader GA Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int J Biochem Cell Biol 37: 1985–1996, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Nevzorova J, Evans BA, Bengtsson T, Summers RJ. Multiple signalling pathways involved in β2-adrenoceptor-mediated glucose uptake in rat skeletal muscle cells. Br J Pharmacol 147: 446–454, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, Schon MR, Bluher M, Punkt K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 29: 895–900, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev 24: 78–90, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103: 16260–16265, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, Desvergne B, Wahli W, Chambon P, Metzger D. PGC1α expression is controlled in skeletal muscles by PPARβ, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab 4: 407–414, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Simonds WF, Goldsmith PK, Woodard CJ, Unson CG, Spiegel AM. Receptor and effector interactions of Gs. Functional studies with antibodies to the αs carboxyl-terminal decapeptide. FEBS Lett 249: 189–194, 1989. [DOI] [PubMed] [Google Scholar]
- 41.Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 83: 166–171, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Staron RS, Karapondo DL, Kraemer WJ, Fry AC, Gordon SE, Falkel JE, Hagerman FC, Hikida RS. Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. J Appl Physiol 76: 1247–1255, 1994. [DOI] [PubMed] [Google Scholar]
- 43.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol 2: e294, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinstein LS, Liu J, Sakamoto A, Xie T, Chen M. Minireview: GNAS: normal and abnormal functions. Endocrinology 145: 5459–5464, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Weinstein LS, Xie T, Zhang QH, Chen M. Studies of the regulation and function of the Gsα gene Gnas using gene targeting technology. Pharmacol Ther 115: 271–291, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams RS, Caron MG, Daniel K. Skeletal muscle β-adrenergic receptors: variations due to fiber type and training. Am J Physiol Endocrinol Metab 246: E160–E167, 1984. [DOI] [PubMed] [Google Scholar]
- 47.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 88: 2219–2226, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, Bassel-Duby R, Williams RS. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J 20: 6414–6423, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, Bare O, Labow M, Spiegelman B, Stevenson SC. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1α transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci USA 103: 14379–14384, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu ZB, Gao F, Feng HZ, Jin JP. Differential regulation of myofilament protein isoforms underlying the contractility changes in skeletal muscle unloading. Am J Physiol Cell Physiol 292: C1192–C1203, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeman RJ, Ludemann R, Easton TG, Etlinger JD. Slow to fast alterations in skeletal muscle fibers caused by clenbuterol, a β2-receptor agonist. Am J Physiol Endocrinol Metab 254: E726–E732, 1988. [DOI] [PubMed] [Google Scholar]
- 52.Zhao H, Yakar S, Gavrilova O, Sun H, Zhang Y, Kim H, Setser J, Jou W, LeRoith D. Phloridzin improves hyperglycemia but not hepatic insulin resistance in a transgenic mouse model of type 2 diabetes. Diabetes 53: 2901–2909, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, Wojtaszewski JF, Hirshman MF, Virkamaki A, Goodyear LJ, Kahn CR, Kahn BB. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 6: 924–928, 2000. [DOI] [PubMed] [Google Scholar]