Abstract
We previously demonstrated that fibrinogen (Fg) binding to the vascular endothelial intercellular adhesion molecule-1 (ICAM-1) leads to microvascular constriction in vivo and in vitro. Although a role of endothelin-1 (ET-1) in this Fg-induced vasoconstriction was suggested, the mechanism of action was not clear. In the current study, we tested the hypothesis that Fg-induced vasoconstriction results from ET-1 production by vascular endothelial cells (EC) and is mediated by activation of extracellular signal-regulated kinase -1/2 (ERK-1/2). Confluent, rat heart microvascular endothelial cells (RHMECs) were treated with one of the following: Fg (2 or 4 mg/ml), Fg (4 mg/ml) with ERK-1/2 kinase inhibitors (PD-98059 or U-0126), Fg (4 mg/ml) with an antibody against ICAM-1, or medium alone for 45 min. The amount of ET-1 formed and the concentration of released von Willebrand factor (vWF) in the cell culture medium were measured by ELISAs. Fg-induced exocytosis of Weibel-Palade bodies (WPBs) was assessed by immunocytochemistry. Phosphorylation of ERK-1/2 was detected by Western blot analysis. Fg caused a dose-dependent increase in ET-1 formation and release of vWF from the RHMECs. This Fg-induced increase in ET-1 production was inhibited by specific ERK-1/2 kinase inhibitors and by anti-ICAM-1 antibody. Immunocytochemical staining showed that an increase in Fg concentration enhanced exocytosis of WPBs in ECs. A specific endothelin type B receptor blocker, BQ-788, attenuated the enhanced phosphorylation of ERK-1/2 in ECs caused by increased Fg content in the culture medium. The presence of an endothelin converting enzyme inhibitor, SM-19712, slightly decreased Fg-induced phosphorylation of ERK-1/2, but inhibited production of Fg-induced ET-1 production. These results suggest that Fg-induced vasoconstriction may be mediated, in part, by activation of ERK-1/2 signaling and increased production of ET-1 that further increases EC ERK-1/2 signaling. Thus, an increased content of Fg may enhance vasoconstriction through increased production of ET-1.
Keywords: exocytosis, extracellular signal-regulated kinase, intercellular adhesion molecule-1, von Willebrand factor, Weibel-Palade bodies
fibrinogen (Fg) is a soluble plasma glycoprotein that is synthesized by the liver and plays a key role in hemostasis, being involved in blood coagulation and thrombogenesis (43). An elevated plasma level of Fg is a risk factor for coronary heart disease (3, 12) and a strong predictor of stroke, including intracerebral hemorrhagic stroke (45). Individuals with Fg levels in the highest quartile were almost seven times more likely to suffer a hemorrhagic stroke and more than twice as likely to die from a stroke (3). Fg binds intercellular adhesion molecule-1 (ICAM-1) and, by this interaction, appears to regulate various physiological and pathophysiological processes, such as cardiomyocyte contractility (4, 11, 36), leukocyte adhesion and transendothelial migration (21), mitogenesis (9), and endothelial cell survival (36). We showed that Fg binding to endothelial ICAM-1 caused vascular constriction, which was abolished by an endothelin type A (ETA) receptor blocker (26). These results suggested a role of Fg in production of endothelin-1 (ET-1), the most potent vasoconstrictor from endothelial cells (ECs) (26), but the precise molecular mechanisms of this process were not clear.
ICAM-1 is a transmembrane glycoprotein that is upregulated in various diseases, including cardiovascular diseases (8, 53). Endothelial cells express ICAM-1, which has a major role in the adhesion of leukocytes to the endothelium (20, 21) and their subsequent transmigration to the site of inflammation (36, 44). Interestingly, one of the signaling events of Fg interaction with ICAM-1 is the phosphorylation of extracellular signal-regulated kinases-1 and 2 (ERK-1/2) in ECs that regulate cell survival (36). A recent study showed that ET-1 induced contraction of rat aortic smooth muscle strips by activating the mitogen-activated protein kinase (MAPK) pathway, of which ERK-1/2 is a member (16). Therefore, MAPK signaling plays a very important role in ET-1- and Fg-induced vascular events. Nevertheless, the physiological role of MAPK signaling in Fg-induced ET-1 production and vascular constriction has not been demonstrated.
We showed that Fg binding to ECs through ICAM-1 causes constriction of arterioles in rat cremaster muscle (26). This vasoconstriction was mediated by ETA receptor activity, suggesting a direct link between Fg binding to ICAM-1 on the surface of ECs and production of ET-1 from the ECs. It is well established that Big ET-1, an inactive form of ET, is stored in Weibel-Palade bodies (WPBs) with endothelin-converting enzyme (ECE) bound to intracellular membranes (37–39). Upon induction, regulated production of ET-1, through release of Big ET-1 and its conversion to ET-1, occurs during exocytosis of WPBs (39). Therefore, Fg-induced vasoconstriction may occur by inducing WPB exocytosis.
Because Fg is markedly increased in most inflammatory states (27), it is of particular interest to determine whether Fg is able to influence vascular reactivity through interaction with vascular endothelial ICAM-1, and to define its mechanism. In the present study, we tested the hypothesis that an elevated content of Fg causes production of ET-1 from the ECs. Furthermore, we explored the possibility that this process is mediated by interaction between Fg and ICAM-1 that activates downstream MAPK cascade and leads to a regulated production of ET-1.
MATERIALS AND METHODS
Reagents and antibodies.
Human plasma Fg (FIB 3, plasminogen, fibronectin, and von Willebrand factor depleted) was purchased from Enzyme Research Laboratories (Lafayette, IN). The purity of the protein was confirmed as described previously (26). The following chemicals or reagents were purchased: specific inhibitors of the MAPK family member MEK (MAP/ERK-1/2 kinase), PD-98059 (2′-amino-3′-methoxyflavone) and U-0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene], were from Calbiochem (La Jolla, CA). Mouse polyclonal antibodies to ERK-1/2 and ERK-1 were from Cell Signaling (Danvers, MA). Mouse monoclonal function-blocking antibody to ICAM-1, horseradish peroxidase (HRP)-linked anti-mouse, and anti-rabbit secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse IgG (polyclonal) was from SEROTEC (Raleigh, NC), antibody against von Willebrand factor (vWF) was from Novus Biologicals (Littleton, CO), vWF-specific ELISA kit was from Helena Laboratories (Beaumont, TX), human endothelin (1–21) ELISA kit was from ALPCO Diagnostics (Salem, NH), and polyvinylidene difluoride (PVDF) membrane was from Bio-Rad (Hercules, CA). Specific endothelin type B receptor (ETB) receptor blocker, BQ-788; endothelin type A (ETA) receptor blocker, BQ-123; a nonpeptide, potent, and selective ECE inhibitor, SM-19712 {4-chloro-N-[[(4-cyano-3-methyl-1-phenyl-1H-pyrazol-5-yl)amino]carbonyl]benzenesulfonamide, sodium salt}; and all other analytical reagents were from Sigma-Aldrich (St. Louis, MO). Rat heart microvascular endothelial cells (RHMECs) were purchased from Vec Technologies (Rensselaer, NY).
Cell culture.
The endothelial nature of the RHMECs was verified by uptake of acylated low-density lipoprotein and positive staining for CD-31 (24). The RHMECs were grown in MCDB-131 complete medium (Vec Technologies). Cells were grown at 37°C with 5% CO2 in a humidified chamber. RHMECs were used at the 6th to 8th passages. Before experimentation, the cells were serum deprived for 1 h using MCDB-131C medium without serum.
Detection of ET-1 and vWF content by ELISA.
RHMECs were grown in six-well plates until confluent. Cells were treated with one of the following: Fg 2 mg/ml, Fg 4 mg/ml, Fg 4 mg/ml with PD-98059 (50 μM), Fg 4 mg/ml with U-0126 (50 μM), Fg 4 mg/ml with anti-ICAM-1 antibody (100 μg/ml), Fg 4 mg/ml with SM-19712 (0.2 mM), or SM-19712 (0.2 mM). The dose of SM-19712 was selected on the basis of a report that bolus injection of 10 mg/kg body wt SM-19712 significantly inhibited function of ECE in Sprague-Dawley rats (30). Estimating that blood volume of a Sprague-Dawley rat used in the above study was ∼100 ml/kg body wt, the rats were given ∼0.1 mg of SM-19712 per each ml of blood. Accordingly, in our in vitro study, we used 0.1 mg/ml (0.2 mM) of SM-19712. Cells treated with serum-free medium alone were used as control. PD-98059, U-0126, ICAM-1, or SM-19712 was added to the medium bathing the cells 1 h before adding 4 mg/ml of Fg. To detect content of ET-1 produced by ECs in response to Fg treatment, after each experiment, cell culture medium was collected and centrifuged at 5000 g for 5 min at 4°C to remove cell debris. Supernatants were collected and ELISA assays were done using an endothelin (1–21) ELISA kit according to the manufacturer's instructions. Briefly, 50 μl of 10-times diluted cell culture supernatant and 50 μl of standard/control were placed in duplicate into wells in a 96-well plate, except the blank. Then, 200 μl of primary antibody (detection antibody) was added into each well, except the blank, and swirled gently. The plate was covered tightly and incubated for 24 h at room temperature (21 ± 2°C). After incubation, contents of the wells were aspirated and the wells were washed 5 times with 300 μl of washing buffer. Then, HRP conjugate (200 μl) was added to each well. The plate was covered tightly and incubated for 1 h at room temperature. Contents of the wells were aspirated and the cells were washed 5 times with 300 μl washing buffer. Substrate (200 μl) was added to each well and the plate was incubated for 30 min at room temperature in the dark. The reaction was stopped by adding 50 μl of stopping solution to each well. Absorbance was measured immediately at 450 nm in a spectrophotometer (Spectramax M2, Molecular Devices, Sunnyvale, CA). ET-1 concentrations from the unknown samples were calculated from a standard curve.
To detect content of vWF released from ECs in response to Fg treatment, vWF detecting ELISA kit was used according to the manufacturer's instructions. Cell culture medium was diluted as suggested and incubated in the wells. This incubation allowed available vWF:Ag (von Willebrand factor antigen) to bind the vWF antibody to the plastic. Plates were rinsed to remove unbound vWF:Ag. Bound vWF:Ag was quantitated by a HRP-conjugated vWF detection antibody. Unbound conjugated vWF was washed, and the chromogenic substrate tetramethylbenzidine and HRP were added to develop color. The intensity of the color (integrated optical density, IOD) was measured by a spectrophotometer at 450 nm. Relative percentage of vWF:Ag concentrations in experimental groups was determined by using a standard curve made from the reference sample provided by the manufacturer.
It is well known that thrombin converts Fg to fibrin. To determine whether the Fg-induced effects described in the present study could be due to conversion of Fg to fibrin, the results from experiments where cells were treated with Fg in the presence or absence of the thrombin activity inhibitor, huridin (0.1 U/ml), were compared. The results of these experiments were not different (data not shown), suggesting absence of functionally active thrombin, and therefore absence of fibrin formation in the Fg-treated groups. Consequently, the experiments in the present study were performed in the absence of huridin.
Assessment of Fg-induced exocytosis of WPBs.
Exocytosis of WPBs induced by Fg was determined by a method described elsewhere (49). RHMECs were grown in eight-well, chambered glass coverslips coated with fibronectin until confluent. Cells were serum starved for 1 h and incubated with either Fg 2 mg/ml, Fg 4 mg/ml, Fg 4 mg/ml with PD-98059 (50 μM), Fg 4 mg/ml with anti-ICAM-1 antibody (100 μg/ml), or Fg 4 mg/ml with IgG (100 μg/ml). Cells treated with serum-free medium alone were used as a control. PD-98059, U-0126, ICAM-1, or IgG was added to the cells 1 h before adding of 4 mg/ml of Fg. Incubation was continued for 45 min at 37°C. After incubation, cells were washed with phosphate-buffered saline (PBS) and fixed in 3.7% paraformaldehyde (in PBS) solution then permeabilized with lysophosphatidylcholine (100 μg/ml). Cells were washed and blocked with 1% fetal calf serum in PBS for 30 min at room temperature. After three washes with PBS, they were incubated with anti-vWF antibody for 2 h at room temperature, then washed three times with PBS. Cells were stained for 1 h in the dark at room temperature with a secondary antibody conjugated with Alexa 555 fluorescent dye, then counterstained with anti-CD31 antibody followed by FITC-labeled secondary antibody according to procedure described above. In addition, 4′,6-diamidino-2-phenylindole (DAPI) was used to stain cell nuclei. Coverslips were mounted on glass slides, and digital images were taken with a laser scanning confocal microscope (Olympus IV1000, objective ×100). Cell nuclei were visualized using a HeNe-G laser (596 nm) to excite the dye, and emission was observed above 620 nm. CD-31 (Alexa 488), an EC marker, was visualized using a multiline Argon laser (495 nm) to excite the dye, and emission was observed above 519 nm. vWF (Alexa 555) was visualized using a HeNe-R laser (578 nm) to excite the dye, and emission was observed above 603 nm.
In four experiments that were done in duplicate (two wells per experimental group), Fg-induced exocytosis of WPBs (total red fluorescence intensity) was assessed for each experimental group by analyzing the total fluorescence intensity in five random fields (in each well) with image analysis software (Image-Pro Plus, Media Cybernetics). Fluorescence intensity values for each experimental group were averaged and are presented as a percentage of control.
Fg-induced ERK-1/2 phosphorylation in ECs.
Phosphorylation of ERK-1/2 induced by Fg interaction with ECs was assessed by Western blot analysis. RHMECs were grown in 12-well cell culture plates (Techno Plastic Products, Trasadingen, Switzerland). Confluent cells were serum starved for 1 h before they received one of the following treatments: Fg 2 mg/ml, Fg 4 mg/ml, Fg 4 mg/ml with BQ-788 (1 μM), Fg 4 mg/ml with BQ-123 (1 μM), Fg 4 mg/ml with SM-19712 (0.2 mM), ET-1 (10 nM), or ET-1 (10 nM) with BQ-788 (1 μM). The dose of ET-1 (10 nM) was based on results of a study demonstrating that 10 nM ET-1 caused constriction of third-order arterioles (55).
Cells treated with medium alone were used as a control. BQ-788 was added in the appropriate wells 1 h before addition of Fg or ET-1. The cells were incubated for 45 min at 37°C. After incubation, cells were washed with PBS and lysed with RIPA lysis buffer containing proteinase inhibitors to extract total protein according to a method described previously (41). Protein content in each sample was measured by a BCA protein assay kit (Pierce Biotechnology, Rockford, IL). Equal amounts of protein from each of the samples were separated by SDS-PAGE, transferred to a PVDF membrane, and immunochemically detected as described (42). The membranes were stripped and reprobed for total ERK-1 as a loading control. The blots were analyzed with Gel-Pro Analyzer software (Media Cybernetics, Silver Spring, MD) as described previously (25). Protein expression intensity was assessed by IOD, i.e., the area of the band in the lane profile. To account for possible differences in the protein load, the results of the measurements are presented as the ratio of IOD of each band (protein of interest) to the IOD of the respective total ERK-1 band.
Statistical analysis.
Values are reported as means ± SE. Differences between groups were tested by one-way ANOVA. If ANOVA indicated a significant difference (P < 0.05), Tukey's multiple-comparison test was used to compare group means, and differences were considered significant if P < 0.05.
RESULTS
Fg-induced ET-1 release through ICAM-1-dependent ERK-1/2 phosphorylation pathway.
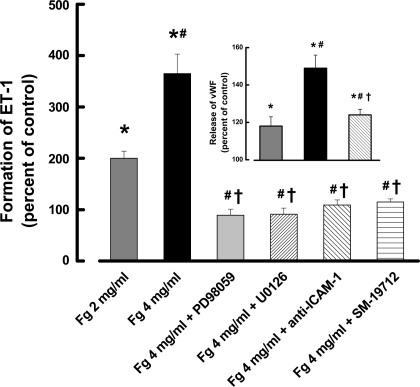
In this series of experiments, Fg-induced ET-1 production from cultured EC was evaluated using an ELISA method. Production of ET-1 in ECs treated with 2 mg/ml of Fg was significantly increased compared with that from the cells treated with medium alone (Fig. 1). Treatment with 4 mg/ml of Fg resulted in a significant increase in ET-1 content in the medium collected from these cells compared with that found in the medium from the cells treated with 2 mg/ml of Fg or treated with medium alone (Fig. 1). Fg (4 mg/ml)-induced production of ET-1 was inhibited by SM-19712 (Fig. 1). Production of ET-1 in the presence of SM-19712 alone (109 ± 5% of control) was not different from control. Similarly, Fg induced a dose-dependent increase in secretion of vWF from ECs (Fig. 1, inset). These results suggest that Fg is involved in production of ET-1 and release of vWF from WPBs of the ECs.
Fig. 1.
Fibrinogen (Fg)-induced production of endothelin-1 (ET-1) from endothelial cells (ECs) is mediated by binding of Fg to intercellular adhesion molecule-1 (ICAM-1) and subsequent ERK-1/2 signaling. *P < 0.05 vs. control (medium alone), #P < 0.05 vs. 2 mg/ml Fg, †P < 0.05 vs. 4 mg/ml Fg; n = 6 for all groups. Inset: Fg-induced release of von Willebrand factor (vWF) from ECs is mediated by Fg binding to ICAM-1. *P < 0.05 vs. control (medium alone), #P < 0.05 vs. 2 mg/ml Fg, †P < 0.05 vs. 4 mg/ml Fg; n = 4 for all groups.
To determine whether ERK-1/2 signaling is involved in production of ET-1 by the higher dose (4 mg/ml) of Fg, MEK inhibitors (PD-98059 or U-0126) were added to the groups of cells that were treated with 4 mg/ml Fg. The specific MEK/ERK-1 inhibitor PD-98059 or MEK/ERK-1/2 inhibitor U-0126 attenuated the Fg-induced increased production of ET-1 (Fig. 1). These results suggest that Fg-induced production of ET-1 and release of vWF are mediated though ERK-1/2 activation.
Since Fg is a ligand for ICAM-1, we tested the involvement of ICAM-1 in Fg-induced ET-1 production in the ECs. Production of ET-1 in cells treated with 4 mg/ml of Fg was attenuated by anti-ICAM-1 antibody (Fig. 1). Furthermore, secretion of vWF from the cells treated with 4 mg/ml of Fg in the presence of anti-ICAM-1 antibody was significantly less compared with that in the cells treated with 4 mg/ml of Fg alone, and it was greater than in the control and 2 mg/ml Fg groups (Fig. 1, inset).
Fg-induced exocytosis of WPBs.
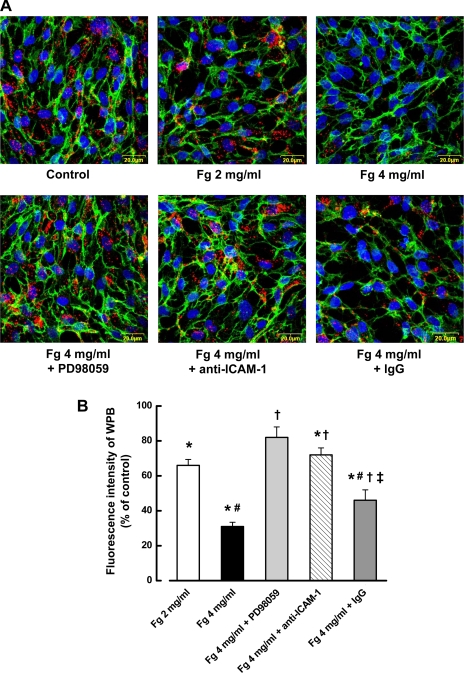
Since WPBs contain vWF, to observe exocytosis of WPBs, we immunocytochemically stained the ECs with a specific antibody against vWF in various treatment groups (Fig. 2). Fg (4 mg/ml) induced exocytosis of WPBs as evidenced by a decrease in the number of fluorescently (red color) labeled granules (Fig. 2). The number of the fluorescently labeled granules in the cells treated with ERK-1 inhibitor was similar to that in the control group (Fig. 2, A and B). Similar results were observed when function blocking anti-ICAM-1 antibody was present in the group of cells treated with 4 mg/ml of Fg (Fig. 2). In contrast, although addition of IgG (a control for anti-ICAM-1) with 4 mg/ml Fg slightly inhibited Fg-induced exocytosis of WPBs, it still resulted in greater exocytosis of WPBs than in the control, Fg 2 mg/ml, or Fg 4 mg/ml + anti-ICAM-1 antibody groups (Fig. 2). As we showed earlier, increased gap formation in the EC monolayer is a result of incubation with increased concentration of Fg (48). However, slight effect of the fixation process should not be ruled out.
Fig. 2.
Fg-induced exocytosis of Weibel-Palade bodies (WPBs) from ECs is mediated by ICAM-1 and ERK-1/2 signaling. A: images show examples of significant decreases in staining (red) of von Willebrand factor (indicator of WPBs) in ECs in response to Fg treatment. ECs are identified by their marker CD-31 (green) and nuclei (blue). B: fluorescence (red) intensity changes in ECs are shown in the histogram. *P < 0.05 vs. control, #P < 0.05 vs. 2 mg/ml Fg, †P < 0.05 vs. 4 mg/ml Fg; ‡P < 0.05 vs. Fg + anti-ICAM-1; n = 4 for all groups.
Fg-induced ERK-1/2 phosphorylation.
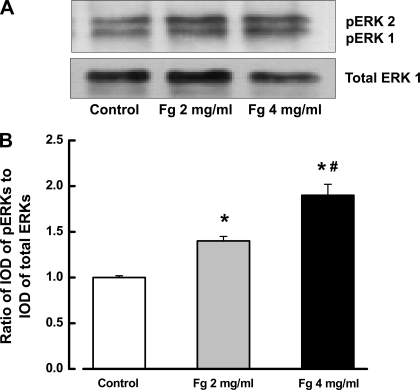
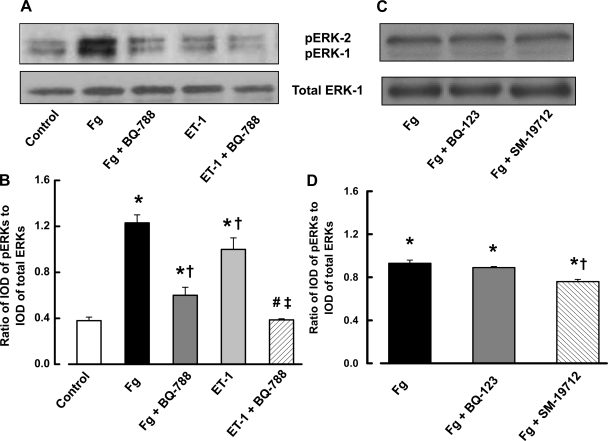
The physiological dose of Fg (2 mg/ml) caused a significantly higher level of ERK-1/2 activation compared with the group without Fg (Fig. 3, A and B). This activation of ERK-1/2 was further enhanced by increasing the Fg content (4 mg/ml). Since ET-1 causes ERK-1/2 phosphorylation (54), to determine whether Fg or ET-1 stimulates ERK-1/2 phosphorylation, ECs were treated with 4 mg/ml of Fg and ET-1 with or without the specific ETB receptor blocker BQ-788. Fg and ET-1 both increased phosphorylation of ERK-1/2 compared with the control group (Fig. 4, A and B). The presence of BQ-788 significantly decreased phosphorylation of ERK-1/2 induced by 4 mg/ml of Fg (Fig. 4, A and B). However, phosphorylation of ERK-1/2 induced by ET-1 was completely abolished by BQ-788 as shown by the significant difference between the groups treated with Fg or ET-1 in the presence of BQ-788 (Fig. 4, A and B). The specific ETA receptor blocker BQ-123 did not affect 4 mg/ml Fg-induced phosphorylation of ERK-1/2 in the cultured ECs (Fig. 4, C and D). To determine whether increased content of Fg alone can enhance ERK-1/2 phosphorylation, ECs were treated with 4 mg/ml of Fg with or without the specific ECE inhibitor SM-19712. This nonpeptide compound blocks conversion of Big ET-1 to active form of ET-1. SM-19712 decreased Fg-induced ERK-1/2 phosphorylation (Fig. 4, C and D), although it was still significantly higher than in control (Fig. 4).
Fig. 3.
Fg induces phosphorylation of ERK-1/2 (pERK) in a dose-dependent manner. A: examples of Fg-induced phosphorylation of ERK-1/2 shown by Western blot analysis. B: densitometry analysis of the bands. Data (n = 5) are presented as a ratio of the total integrated optical density (IOD) of phosphorylated ERK-1/2 bands to the IOD of bands of the corresponding total ERK-1 protein. *P < 0.05 vs. control, #P < 0.05 vs. 2 mg/ml Fg.
Fig. 4.
Fg-induced ERK-1/2 activation is partially a result of endothelin-1 (ET-1) production (mediated by endothelin-converting enzyme), which binds to endothelin type B receptors on ECs. A and C: examples of Fg (4 mg/ml)- and ET-1 (10−8 M)-induced phosphorylation of ERK-1/2 shown by Western blot analysis. The endothelin type B receptor blocker BQ-788 (1 μM) decreased Fg-induced ERK-1/2 phosphorylation and abolished ET-1-induced phosphorylation of ERK-1/2; n = 9 (A). The endothelin-converting enzyme inhibitor SM-19712 (0.2 mM) decreased Fg-induced ERK-1/2 phosphorylation, whereas endothelin type A receptor blocker BQ-123 (1 μM) did not; n = 3 (C). B and D: densitometric analysis of ERK-1/2 signal. Data are presented as a ratio of IOD of phosphorylated ERK-1/2 bands to the IOD of bands of the corresponding total ERK-1 protein. *P < 0.05 vs. control, †P < 0.05 vs. Fg, #P < 0.05 vs. ET-1, ‡P < 0.05 vs. Fg + BQ-788.
DISCUSSION
In the present study, an increased content of Fg led to enhanced production of ET-1 and secretion of vWF from cultured ECs. These results suggest a direct involvement of Fg in production of ET-1. It was initially believed that ET-1, a 21-amino acid peptide, is not stored in secretory granules of ECs (34). Stimuli such as hypoxia, ischemia, thrombin, epinephrine, angiotensin II, growth factors, cytokines, and free radicals were proposed to induce transcription of ET-1 messenger RNA (mRNA), synthesis and secretion of ET-1 within minutes (22, 29). However, later findings showed that a number of cellular components, including cytokines and ET-1, are present in secretory granules (37, 38). These studies showed that regulated production of ET-1 is associated with exocytosis of WPBs and therefore implicate a greater role of WPB exocytosis in inflammation, hemostasis, regulation of vascular tone, and angiogenesis. The present investigation demonstrates that Fg can dose dependently induce ET-1 formation, which was attenuated by ECE inhibitor or specific function blocking antibody against ICAM-1. Previously, we showed that Fg binding to endothelial cells was significantly inhibited by anti-ICAM-1 antibody (26). Combined, these results indicate that Fg induces ET-1 production and release of vWF through binding of Fg to endothelial ICAM-1.
The MAPK pathway is a major signaling cascade. Upon activation, it mediates a number of cellular events such as cell activation, proliferation, or adhesion (23, 46, 47). The MAPK family consists of three major groups: ERK-1/2, c-Jun NH2-terminal kinases (JNK), and p38 MAP. In cardiac, renal, and vascular tissue from hypertensive rats, activation of tyrosine kinases, ERK-1/2, JNK, and p38 MAP were augmented by exogenous stimulation (11, 47). These activation processes contribute to arterial remodeling through enhanced cell growth, inflammation, fibrosis, and vascular constriction (47). Stimulation of ERK-1/2 was also implicated in expression of ET-1 (13, 33).
Fg:ICAM-1 interaction leads to phosphorylation of ERK-1/2 (36). Expression of ICAM-1 is increased during hypertension (51). Hypertension is accompanied by an increased plasma Fg content (18, 19, 52). Since Fg is a ligand for ICAM-1, an increase in Fg content may lead to enhanced binding of Fg to ICAM-1 during hypertension, which may result in greater ERK-1/2 phosphorylation. Therefore, it is plausible that an increased Fg content could cause ERK-1/2 activation. Previously, we demonstrated that Fg dose dependently induced ERK-1/2 activation, which was attenuated by a specific inhibitor of ERK-1 phosphorylation, PD-98059, or specific ERK-1/2 inhibitor, U-0126 (48). The present findings that 1) PD-98059 and U-0126 abolished production of ET-1; 2) PD-98059 decreased exocytosis of WPBs; and 3) anti-ICAM-1 antibody decreased Fg-induced vWF release and WPB exocytosis suggest that Fg is involved in ET-1 production through binding to endothelial ICAM-1 and the resultant activation of ERK-1/2.
Although the release of vWF from ECs treated with Fg 4 mg/ml + anti-ICAM-1 antibody was greater than that in control, it was only slightly higher than that treated with Fg 2 mg/ml. This suggests that blocking of ICAM-1 function did not completely inhibit the release of vWF induced by Fg, indicating the possible involvement of other pathways where Fg binding to another receptor, besides ICAM-1, may stimulate vWF release. For example, it has been shown that Fg binding to endothelial αvβ3-and α5β1-receptors serves as a key step in multiple signaling cascades including ERK-1/2 phosphorylation (1, 10). Furthermore, α5β1-integrin is involved in vasoconstriction (32) and in Fg-induced increased EC layer permeability (48). Therefore, it is possible that Fg-induced ERK-1/2 phosphorylation through the α5β1-integrin-dependent pathway may also, in part, trigger vWF release, and therefore, ET-1 production from EC cells. These conclusions are supported by the findings of our parallel study of Fg-induced exocytosis of WPBs. Nevertheless, our data show that Fg:ICAM-1 interaction may be a main determinant in mediating ERK-1/2 signaling cascade that regulates release of vWF and production of ET-1 from the EC granules. Although blockade of ICAM-1 function abolished production of ET-1 induced by 4 mg/ml Fg, release of vWF was only decreased to the level produced by 2 mg/ml Fg. This discrepancy may result from interference by the anti-ICAM-1 antibody in the ET-1 ELISA kit used in the present study.
Direct observation of Fg-induced exocytosis of WPBs confirmed the conclusion stated above. Fg dose dependently increased exocytosis of WPBs, which was inhibited by PD-98059 and the anti-ICAM-1 antibody. These data agree with other results of the present study suggesting that Fg may be involved in regulated production of ET-1 through binding to endothelial ICAM-1 and activating ERK-1/2 signaling, which leads to exocytosis of WPBs. Again, partial abolishment of Fg-induced exocytosis of WPBs by anti-ICAM-1 antibody suggests that yet another Fg endothelial receptor can have a role in Fg-regulated WPB exocytosis.
ET-1 causes activation of ERK-1/2 and thus triggers cell activation (54). Therefore Fg-induced activation of ERK-1/2 may be a combination of two effects: 1) Fg:ICAM-1 interaction-induced ERK-1/2 activation and 2) activation of ERK-1/2 by ET-1 produced as a result of Fg to EC binding. These effects were confirmed in the present study: Fg-induced ERK-1/2 activation was attenuated by BQ-788, but this ETB receptor blocker completely abolished activation of ERK-1/2 induced by ET-1 alone. The significant difference between the effects suggests that Fg may directly activate ERK-1/2, while the production of ET-1 as a result of Fg binding to ECs may cause the further activation of ERK-1/2. In addition, Fg-induced activation of ERK-1/2 was decreased by the ECE inhibitor SM-19712. On the other hand, Fg-induced production of ET-1 was almost abolished by SM-19712. The difference in ERK-1/2 phosphorylation induced by 4 mg/ml of Fg and induced by Fg (4 mg/ml) in the presence of SM-19712 indicate that Fg:ICAM-1 binding may cause ERK-1/2 phosphorylation through a feedback mechanism. The dose of ET-1 used in the present study was based on experiments where this dose caused constriction of third-order arterioles by almost 90% (55). The higher dose of Fg used in the present study caused constriction of third-order arterioles by only 40% (26). Our data may indicate that 4 mg/ml of Fg caused production of less ET-1 from cultured ECs than the dose of ET-1 we used for EC treatment to observe the resultant activation of ERK-1/2.
It is well known that microvascular ECs express ETB but not ETA receptors (40). To our knowledge, only one group has reported the existence of ETA receptors on ECs of the aortic valve (35). The data presented here indicate that an ETA receptor inhibitor had no effect on Fg-induced ERK-1/2 phosphorylation. Since it was not our goal to define the presence or absence of ETA receptor on the EC surface, we can only infer that RHMECs may not express the ETA receptor. This conclusion concurs with the results of publications related to endothelial ET receptors (6, 14, 40). Thus, the results of the present study suggest that an increase in Fg level enhances exocytosis of WPBs, causing an increased production of ET-1 and release of vWF from the vesicles by binding of Fg to endothelial ICAM-1 and subsequent activation of ERK-1/2, which may be further stimulated by ET-1.
Increased blood content of Fg, an inflammatory plasma adhesion protein, is associated with development of different cardiovascular diseases including hypertension, diabetes, stroke, hemorrhagic stroke, and heart failure (7, 26). Previously, we showed direct correlation of Fg concentration and arteriolar constriction in vivo and in vitro (26). Although a role of Fg-induced ET-1-mediated vascular diameter changes was suggested, the mechanism of action was not clear (26). Although controversy exists whether an increased blood content of Fg is a consequence or a cause of a cardiovascular disease, in epidemiological studies, both perspectives consider Fg to predict high cardiovascular risk (5). A number of effects have been investigated to explain the association between elevated levels of Fg and cardiovascular diseases that are typically accompanied by increased vasoconstriction (17). These include subintimal hemorrhage (15), inflammatory vascular changes and endothelial dysfunction (31, 31), atherosclerosis and thrombosis (28), and plaque formation (2, 28).
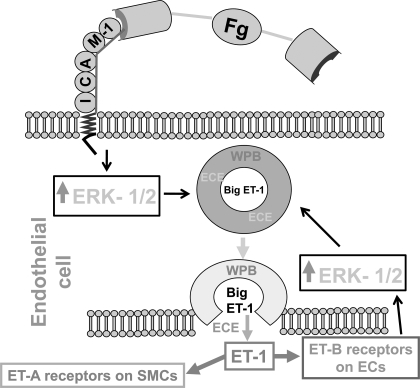
The results of the present study clarify the mechanisms for increased arteriolar constriction during pathologically high levels of Fg observed in our previous study (26). The schematic representation of this mechanism is presented in Fig. 5 of the present study. Enhanced blood content of Fg leads to increased binding of Fg to its endothelial surface receptor ICAM-1, which, in turn, causes activation of ERK-1/2 signaling and increased exocytosis of WPBs from vascular ECs. During exocytosis of WPBs, which contain Big ET-1 and ECE, Big ET-1 is released and converted to ET-1 by ECE. The newly formed ET-1 binds to ETB receptors on ECs. Binding of ET-1 to ETB receptors causes further activation of endothelial ERK-1/2, leading to an increased production of ET-1. Since exocytosis of WPBs from ECs occurs mainly in the direction of smooth muscle cells (SMCs) (50), the ET-1 easily binds to endothelin type A receptors on vascular SMCs and causes their constriction, leading to a decrease in vascular diameter. Thus, Fg-induced regulated production of ET-1, at least in part, may be the mechanism for increased vasoconstriction seen during diseases such as hypertension, diabetes, and stroke that are accompanied by an increased blood content of Fg.
Fig. 5.
Schematic representation of the proposed hypothesis for Fg-induced production of ET-1 from endothelial cells. At pathologically high levels of Fg, its binding to endothelial ICAM-1 is increased. This leads to activation of ERK-1/2. Activated ERK-1/2 induces enhanced exocytosis of WPBs that contain Big ET-1 and endothelin-converting enzyme (ECE). During exocytosis of WPB, Big ET-1 is released from the WPBs and comes into contact with ECE, which converts Big ET-1 to ET-1. This newly formed ET-1 binds to endothelin type A (ET-A) receptors on vascular smooth muscle cells (SMCs) and endothelin type B (ET-B) receptors on ECs. Binding of ET-1 to ET-B causes further activation of endothelial ERK-1/2, leading to an increase in ET-1 production. Binding of ET-1 to ET-A receptors on SMCs causes vascular constriction.
GRANTS
This study was supported in part by National Institutes of Health Grants HL-80394 (to D. Lominadze) and HL-71010 and NS-051568 (to S. C. Tyagi).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Belkin AM, Tsurupa G, Zemskov E, Veklich Y, Weisel JW, Medved L. Transglutaminase-mediated oligomerization of the fibrin(ogen) alphaC domains promotes integrin-dependent cell adhesion and signaling. Blood 105: 3561–3568, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett J Platelet-fibrinogen interactions. Ann NY Acad Sci 936: 340–354, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Bots ML, Elwood PC, Salonen JT, Freire de Concalves A, Sivenius J, Di Carlo A, Nikitin Y, Benetou V, Tuomilehto J, Koudstaal PJ, Grobbee DE. Level of fibrinogen and risk of fatal and non-fatal stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health 56: i14–i18, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd J, Chau E, Tokunanga C, Bateman R, Haljan G, Davani E, Wang Y, Walley K. Fibrinogen decreases cardiomyocyte contractility through an ICAM-1-dependent mechanism. Crit Care 12: R2, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner E, Davey Smith G, Marmot M, Canner R, Beksinska M, O'Brien J. Childhood social circumstances and psychosocial and behavioural factors as determinants of plasma. Lancet 347: 1008–1013, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Deng LY, Li JS, Schiffrin EL. Endothelin receptor subtypes in resistance arteries from humans and rats. Cardiovasc Res 29: 532–535, 1995. [PubMed] [Google Scholar]
- 7.Engström G, Hedblad B, Tydén P, Indgärde F. Inflammation-sensitive plasma proteins are associated with increased incidence of heart failure: a population-based cohort study. Atherosclerosis 202: 617–622, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Fornoni A, Ijaz A, Tejada T, Lenz O. Role of inflammation in diabetic nephropathy. Curr Diabetes Rev 4: 10–17, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Gardiner EE, D'Souza SE. A mitogenic action for fibrinogen mediated through intercellular adhesion molecule-1. J Biol Chem 272: 15474–15480, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol 19: 173–206, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 61: 448–460, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Heinrich J, Balleisen L, Schulte H, Assmann G, van de Loo J. Fibrinogen and factor VII in the prediction of coronary risk. Results from the PROCAM study in healthy men. Arterioscler Thromb 14: 54–59, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Juan S, Chen J, Chen C, Lin H, Cheng C, Liu J, Hsieh M, Chen Y, Chao H, Chen T, Chan P, Cheng T. 17β-Estradiol inhibits cyclic strain-induced endothelin-1 gene expression within vascular endothelial cells. Am J Physiol Heart Circ Physiol 287: H1254–H1261, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Just A, Olson A, Arendshorst W. Dual constrictor and dilator actions of ETB receptors in the rat renal microcirculation: interactions with ETA receptors. Am J Physiol Renal Physiol 286: F660–F668, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB, D'Agostino RB, Belanger AJ, Silbershatz H, Tofler GT. Long-term influence of fibrinogen on initial and recurrent cardiovascular events in men and women. Am J Cardiol 78: 90–92, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Kim B, Kim J, Bae YM, Cho SI, Kwon SC, Jung JY, Park J, Ahn HY. p38 mitogen-activated protein kinase contributes to the diminished aortic contraction by endothelin-1 in DOCA-salt hypertensive rats. Hypertension 43: 1086–1091, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Klein RL, Hunter SJ, Jenkins AJ, Zheng D, Semler AJ, Clore J, Garvey WT. Fibrinogen is a marker for nephropathy and peripheral vascular disease in Type 1 diabetes: studies of plasma fibrinogen and fibrinogen gene polymorphism in the DCCT/EDIC cohort. Diabetes Care 26: 1439–1448, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Koenig W Fibrin(ogen) in cardiovascular disease: an update. Thromb Haemost 89: 601–609, 2003. [PubMed] [Google Scholar]
- 19.Landin K, Tengborn L, Smith U. Elevated fibrinogen and plasminogen activator inhibitor (PAI-1) in hypertension are related to metabolic risk factors for cardiovascular disease. J Intern Med 227: 273–278, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Languino L, Plescia J, Duperrray A, Brian A, Plow E, Geltosky J, Alteri D. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell 73: 1423–1434, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Languino LR, Duperray A, Joganic KJ, Fornaro M, Thornton GB, Altieri DC. Regulation of leukocyte-endothelium interaction and leukocyte transendothelial migration by intercellular adhesion molecule 1-fibrinogen recognition. Proc Natl Acad Sci USA 92: 1505–1509, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin E Endothelins. N Engl J Med 333: 356–363, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Theus M, Wei L. Role of ERK 1/2 signaling in neuronal differentiation of cultured embryonic stem cells. Dev Growth Differ 46: 513–523, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Lincoln DW, Larsen AM, Phillips PG, Bove K. Isolation of murine aortic endothelial cells in culture and the effects of sex steroids on their growth. In Vitro Cell Dev Biol Anim 39: 140–145, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Lominadze D, Schuschke D, Joshua I, Dean W. Increased ability of erythrocytes to aggregate in spontaneously hypertensive rats. Clin Exp Hypertens 24: 397–406, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lominadze D, Tsakadze N, Sen U, Falcone J, D'Souza S. Fibrinogen- and fragment D-induced vascular constriction. Am J Physiol Heart Circ Physiol 288: H1257–H1264, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Lowe GDO Circulating inflammatory markers and risks of cardiovascular and non-cardiovascular disease. J Thromb Haemost 3: 1618–1627, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Lowe G, Rumley A. Fibrinogen and its degradation products as thrombotic risk factors. Ann NY Acad Sci 936: 560–565, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Marasciulo FL, Montagnani M, Potenza MA. Endothelin-1: the yin and yang on vascular function. Curr Med Chem 13: 1655–1665, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Matsumura Y, Kuro T, Obayashi Y, Ekawa K, Hashi N, Akaoka M. Protective effect of SM-19712, a novel and potent endothelin converting enzyme inhibitor, on ischemic acute renal failure in rats. Jpn J Pharmacol 84: 16–24, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Mendall MA, Patel P, Ballam L, Strachan D, Northfield TC. C reactive protein and its relation to cardiovascular risk factors: a population based cross sectional study. BMJ 312: 1061–1065, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mogford J, Davis G, Meininger G. RGDN peptide interaction with endothelial alpha5beta1 integrin causes sustained endothelial-dependent vasoconstriction to rat skeletal muscle arterioles. J Clin Invest 100: 1647–1653, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morey A, Razandi M, Pedram A, Hu R, Prins B, Levin E. Oestrogen and progesterone inhibit the stimulated production of endothelin-1. Biochem J 15: 1097–1105, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura S, Naruse M, Naruse K, Demura H, Uemura H. Immunocytochemical localization of endothelin in cultured bovine endothelial cells. Histochemistry 94: 475–477, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura J, Aoki H, Chen X, Shikasho T, Kobayashi S, Kanaide H. Evidence for the presence of endothelin ETA receptors in endothelial cells in situ on the aortic side of porcine aortic valve. Br J Pharmacol 115: 1369–1376, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pluskota E, D'Souza S. Fibrinogen interactions with ICAM-1 (CD54) regulate endothelial cell survival. Eur J Biochem 267: 4693–4704, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Rondaij MG, Bierings R, Kragt A, van Mourik JA, Voorberg J. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol 26: 1002–1007, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Russell F, Skepper J, Davenport A. Evidence using immunoelectron microscopy for regulated and constitutive pathways in the transport and release of endothelin. J Cardiovasc Pharmacol 31: 424–430, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Russell F, Skepper J, Davenport A. Human endothelial cell storage granules: a novel intracellular site for isoforms of the endothelin-converting enzyme. Circ Res 83: 314–321, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Schiffrin E, Lariviere R, Touyz R. ETA and ETB receptors on vascular smooth muscle cells from mesenteric vessels of spontaneously hypertensive rats. Clin Exp Pharmacol Physiol Suppl 22: S193–S194, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Sen U, Moshal K, Tyagi N, Kartha G, Tyagi S. Homocysteine-induced myofibroblast differentiation in mouse aortic endothelial cells. J Cell Physiol 209: 767–774, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Sen U, Tyagi N, Kumar M, Moshal KS, Rodriguez WE, Tyagi SC. Cystathionine-β-synthase gene transfer and 3-deazaadenosine ameliorate inflammatory response in endothelial cells. Am J Physiol Cell Physiol 293: C1779–C1787, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Soria JM, Almasy L, Souto JC, Buil A, Lathrop M, Blangero J, Fontcuberta J. A genome search for genetic determinants that influence plasma fibrinogen levels. Arterioscler Thromb Vasc Biol 25: 1287–1292, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Springer T Adhesion receptors of the immune system. Nature 346: 425–434, 1990. [DOI] [PubMed] [Google Scholar]
- 45.Sturgeon JD, Folsom AR, Longstreth W Jr, Shahar E, Rosamond WD, Cushman M. Hemostatic and inflammatory risk factors for intracerebral hemorrhage in a pooled cohort. Stroke 39: 2268–2273, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Touyz RM Recent advances in intracellular signalling in hypertension. Curr Opin Nephrol Hypertens 12: 165–174, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Touyz R Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol 90: 449–455, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Tyagi N, Roberts A, Dean W, Tyagi S, Lominadze D. Fibrinogen induces endothelial cell permeability. Mol Cell Biochem 307: 13–22, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vischer U, Wagner D. CD63 is a component of Weibel-Palade bodies of human endothelial cells. Blood 82: 1184–1191, 1993. [PubMed] [Google Scholar]
- 50.Wagner OF, Christ G, Wojta J, Vierhapper H, Parzer S, Nowotny PJ, Schneider B, Waldhausl W, Binder BR. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem 267: 16066–16068, 1992. [PubMed] [Google Scholar]
- 51.Waki H, Liu B, Miyake M, Katahira K, Murphy D, Kasparov S, Paton JFR. Junctional adhesion molecule-1 is upregulated in spontaneously hypertensive rats: evidence for a prohypertensive role within the brain stem. Hypertension 49: 1321–1327, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Wong LYF, Leung RYH, Ong KL, Cheung BMY. Plasma levels of fibrinogen and C-reactive protein are related to interleukin-6 gene -572C>G polymorphism in subjects with and without hypertension. J Hum Hypertens 21: 875–882, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Yamazaki T, Seko Y, Tamatani T, Miyasaka M, Yagita H, Okumura K, Nagai R, Yazaki Y. Expression of intercellular adhesion molecule-1 in rat heart with ischemia-reperfusion and limitation of infarct size by treatment with antibodies against cell adhesion molecules. Am J Pathol 143: 410–418, 1993. [PMC free article] [PubMed] [Google Scholar]
- 54.Yogi A, Callera G, Montezano A, Aranha A, Tostes R, Schiffrin E, Touyz R. Endothelin-1, but not Ang II, activates MAP kinases through c-Src-independent Ras-Raf-dependent pathways in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 27: 1960–1967, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Zhao H, Joshua I, Porter J. Microvascular responses to endothelin in deoxycorticosterone acetate-salt hypertensive rats. Am J Hypertens 13: 819–826, 2000. [DOI] [PubMed] [Google Scholar]