Abstract
Hypertension provokes differential trafficking of the renal proximal tubule Na+/H+ exchanger 3 (NHE3) to the base of the apical microvilli and Na+-Pi cotransporter 2 (NaPi2) to endosomes. The resultant diuresis and natriuresis are key to blood pressure control. We tested the hypothesis that this differential trafficking of NHE3 vs. NaPi2 was associated with partitioning to distinct membrane domains. In anesthetized rats, arterial pressure was increased (104 ± 2 to 142 ± 4 mmHg, 15 min) by arterial constriction and urine output increased 23-fold. Renal membranes were fractionated by cold 1% Triton X-100 extraction then centrifugation through OptiPrep flotation gradients. In controls, 84 ± 9% of NHE3 localized to flotillin-enriched lipid raft domains and 69 ± 5% of NaPi2 localized to transferrin receptor-enriched nonrafts. MyosinVI and dipeptidyl peptidase IV, associated with NHE3 regulation, coenriched in lipid rafts with NHE3, while NHE regulatory factor-1 coenriched in nonrafts with NaPi2. Partitioning was not altered by hypertension. Detergent insoluble membranes were pelleted after detergent extraction. NHE3 detergent insolubility decreased as it redistributed from body (80 ± 10% detergent insoluble) to base (75 ± 3%) of the apical microvilli, while NaPi2 partitioned into more insoluble domains as it moved from the microvilli (45 ± 7% detergent insoluble) to endosomes (82 ± 1%). In conclusion, NHE3 and NaPi2, while both localized to apical microvilli, are segregated into domains: NHE3 to lipid rafts and NaPi2 to nonrafts. These domain properties may play a role in the distinct trafficking patterns observed during elevated pressures: NHE3 remains in rafts and settles to the base of the microvilli while NaPi2 is freely endocytosed.
Keywords: subcellular fractionation, hypertension, lipid raft, kidney, proximal tubule
although the cellular and molecular mechanisms responsible for determining the blood pressure set point are not completely understood, it is clear that the kidney plays a central role (9, 16). An acute or chronic increase in blood pressure triggers a compensatory increase in fluid and salt excretion that normalizes blood pressure, a phenomenon known as pressure diuresis and natriuresis (16, 17). About two-thirds of sodium reabsorption along the nephron occurs in the proximal tubule where the apical membrane facing the lumen is highly differentiated into tall and densely packed microvilli, providing a large surface area for reabsorption. An acute increase in blood pressure causes a significant decrease in Na+ and water reabsorption in the proximal tubule, resulting in a 50% increase in volume flow out of this region (7, 8). The Na+/H+ exchanger isoform 3 (NHE3) is the main transporter mediating sodium reabsorption in the proximal tubule (27, 39). The sodium phosphate cotransporter 2 (NaPi2), another major sodium transporter in this region, is essential for the reabsorption of filtered phosphate (32). We have reported that during acute high blood pressure the decrease in proximal tubule salt and volume reabsorption is associated with the differential trafficking of NHE3 from the body to the base of the microvilli (i.e., not endocytosed) and NaPi2 from the body of the microvilli to subapical endosomes (50). This differential redistribution suggests the hypothesis that these sodium transporters and their associated proteins are localized to distinct membrane domains, such as ordered lipid rafts vs. nonraft domains.
Lipid rafts are liquid-ordered membrane microdomains that are enriched in sphingolipids and cholesterol (4). Many membrane proteins are known to preferentially partition into these domains, facilitating clustering and protein-protein interaction. Lipid rafts are characterized by their relative insolubility in nonionic detergents (e.g., Triton X-100). Therefore, detergent extraction followed by centrifugation is commonly used to study these domains. However, strong cytoskeletal attachment will also confer resistance of a protein to detergent solubilization. Consequently, the presence of a protein in the detergent insoluble fraction can be interpreted as either its localization in a lipid raft domain and/or a strong attachment to the cytoskeleton (31). Because of their high lipid-to-protein ratio, the density of lipid rafts is lower than other types of membrane domains, allowing them to be isolated on the basis of their flotation to the top of density gradients (4, 18), a preferred method of isolation.
Studies in the rabbit ileal brush border indicate that 50% of NHE3 is Triton X-100 insoluble and that one-third of this detergent-insoluble NHE3 is associated with lipid raft domains (25). In addition, studies in cultured renal proximal tubule cells suggest that both NHE3 activity and trafficking are dependent on NHE3 localization to lipid raft domains (33). To our knowledge, the in vivo lipid domain properties of renal NHE3 have not been analyzed; however, the properties of renal NaPi2 have: ∼80% of NaPi2 is found in low density cholesterol-, sphingomyelin-, and GM1-enriched fractions characterized as “lipid raft” fractions, and in animals deprived of potassium, that proportion increases, associated with reduced NaPi2 lateral diffusion and decreased NaPi2 transport activity (18).
A number of proteins have been shown to influence NHE3 and NaPi2 activity and/or distribution. Regarding NHE3, dipeptidyl peptidase IV (DPPIV) associates with NHE3 (13) and its inhibition decreases NHE3 abundance in isolated microvillar membrane vesicles (14). Hypertension or treatment with angiotensin-converting enzyme inhibitors provokes the rapid and coordinated redistribution of the unconventional molecular motor myosin VI along with NHE3 from the body to the base of the microvilli, supporting the hypothesis that this motor may play a role in directing NHE3 trafficking down the microvilli (51). A study in cultured kidney cells indicates that NHE3 binds to the actin cytoskeleton via a direct interaction with the actin-binding protein ezrin and that when this interaction is compromised, NHE3 activity is drastically reduced (6). NHE regulatory factor-1 (NHERF-1) was originally isolated on the basis of its requirement for cAMP-mediated inhibition of NHE3 in cultured cells (46, 47) and has recently been shown to be critical for parathyroid hormone (PTH) regulation of NaPi2 by anchoring the transporter to the cytoskeleton via interactions with ezrin (10, 29, 41). Interestingly, in NHERF-1 null mice there is no difference in proximal tubule NHE3 activity vs. wild-type mice but the null mice lost PKA inhibition of NHE3 activity, indicating that NHERF-1 is not necessary for baseline NHE3 activity or distribution but is required for cAMP regulation (46). It became clear that NHERF-1 is a major regulator of NaPi2 when NHERF-1 knockout mice were found to exhibit both phosphate wasting and mislocalization of NaPi2 in the renal proximal tubule (36). Like NHE3, NaPi2 trafficking is key to its regulation. NHERF-1-bound NaPi2 indirectly attaches the actin cytoskeleton via ezrin, and the formation of this complex is essential for normal NaPi2 activity (29). Our laboratory has shown that NaPi2 partially colocalizes with the clathrin adaptor AP2 as it is internalized during acute hypertension (50).
The aims of this study were to first test the hypothesis that the differential trafficking of NHE3 (to the base of the microvilli) and NaPi2 (to endosomes) during a rapid increase in blood pressure (acute hypertension) reflects distribution of these sodium cotransporters and their associated proteins in distinct membrane domains, and secondly, to determine whether the domain properties of NHE3 and/or NaPi2 and their associated proteins are altered during acute hypertension.
MATERIALS AND METHODS
Animal protocols.
All animal experiments were approved by the University of Southern California Keck School of Medicine Institutional Animal Care and Use Committee and conducted in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were performed on male Sprague-Dawley rats (∼250 g; Harlan, San Diego, CA) that were kept under diurnal light conditions and had free access to food and water. The animals were anesthetized intraperitoneally with Inactin (100 mg/kg) and a small dose of intramuscular ketamine (100 mg/kg). Body temperature was maintained thermostatically at 37°C. Polyethylene catheters (PE-50) were inserted into the carotid artery to monitor blood pressure and into the jugular vein for constant infusion of 4.0% BSA in 0.9% saline at 50 μl/min to maintain euvolemia. Glomerular filtration rate was measured using FITC-inulin as a marker as previously described (23). Mean arterial pressure was acutely increased by ∼40 mmHg for 15 min by constriction of the superior mesenteric artery, celiac artery, and abdominal aorta below the renal arteries with silk ligatures as previously described (50). Because low temperature has been shown to block membrane trafficking (21), at the end of each experiment, the abdominal cavity was packed with ice and flushed with ice-cold PBS before removal of the kidneys. We did not measure the final kidney temperature, but the kidneys were very cold to the touch.
Confocal microscopy.
Kidneys from Sprague-Dawley rats (sham operated or 15-min acute hypertension) were perfusion fixed via the dorsal aorta with paraformaldehyde lysine periodate (PLP) fixative (2% paraformaldehyde, 75 mM lysine, and 10 mM Na-periodate, pH 7.4) and then postfixed in PLP for another 2–3 h. The perfusion rate did not alter blood pressure. The fixed tissue was cryoprotected by incubation overnight in 30% sucrose in PBS, embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA), and frozen in liquid nitrogen. Cryosections (5 μm) were cut and transferred to Fisher Superfrost Plus-charged glass slides and air dried. For immunofluorescence labeling, the sections were rehydrated in PBS, followed by a 10-min wash in 50 mM NH4Cl in PBS and then with 1% SDS in PBS for 4 min for antigen retrieval. Dual labeling was performed by incubating with polyclonal antiserum NHE3-C00 at 1:100 dilution or McNaPi2 at 1:50 dilution with monoclonal antibody against villin (Immuntech, Chicago, IL) at 1:100. A set was also dual labeled with antiserum McNaPi2 at 1:50 dilution and monoclonal antibody against NHE3 (4F5 clone, Millipore) at 1:50 dilution for 1.5–2 h. The sections were then incubated with a mixture of FITC-conjugated goat-anti-rabbit (Cappel Research Products, Durham, NC) and Alexa 568-conjugated goat-anti-mouse (Molecular Probes) secondary antibodies diluted 1:100 for 1 h, mounted in Prolong Antifade containing the nuclear dye DAPI (Molecular Probes). Slides were viewed with a Zeiss LSM 510 microscope with differential interference contrast overlay.
Preparation of 2,000-g supernatant and total membranes.
After the kidneys were collected, renal cortices were dissected, diced, suspended in an ∼5 ml isolation buffer (5% sorbitol, 0.5 mM disodium EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 9 μg/ml aprotinin, and 5 mM histidine/imidazole buffer, pH 7.5), homogenized for 10 min at a low setting with an Ultra-Turrax T25 (IKA-Labortechnik), and centrifuged at 2,000 g for 10 min. The supernatant was saved, the pellet was rehomogenized in 5 ml isolation buffer and recentrifuged, and the two supernatants were pooled (pellets, containing <10% of NHE3, were discarded). Total membranes were isolated by centrifuging the 2,000-g supernatant at 200,000 g for 75 min and resuspending the pellet in TNE buffer (50 mM Tris·HCl pH 7.4, 150 mM NaCl, 5 mM EDTA, and Complete Protease Inhibitor Cocktail; Roche Diagnostics) through a 25-G needle on ice.
Isolation of lipid raft fractions by flotation gradient.
Lipid raft fractions were isolated using a modification of methods previously reported (18, 26). Total membranes were incubated in TNE buffer containing 1% Triton X-100 on ice for 30 min with occasional vortexing. The extract was adjusted to 50% OptiPrep (Sigma Chemicals). A volume of 600 μl was loaded at the bottom of an ultracentrifuge tube and overlaid with 1 ml each of 40, 30, and 20% OptiPrep in TNE and 400 μl of 10% OptiPrep in TNE. After centrifugation at 170,000 g at 4°C for 4 h, a continuous density gradient was formed (see Fig. 2A). Six 250-μl fractions were collected from the top followed by collection of five 500-μl fractions.
Sorbitol density gradient fractionation.
In one series, the crude 2,000-g supernatant, which is essentially a cellular homogenate, was subjected to subcellular fractionation on sorbitol density gradients to assess density distribution of proteins, i.e., distribution in plasma membrane vs. intracellular membrane enriched fractions in response to an acute increase in blood pressure as described previously (55). In brief, 4 ml of the 2,000-g supernatant were mixed with 6 ml of 87.4% sorbitol buffer, loaded between two hyperbolic sorbitol gradients, and centrifuged at 100,000 g for 5 h. Twelve fractions were collected from the top, pelleted at 250,000 g for 75 min, resuspended in 1 ml isolation buffer, and stored at −80°C pending assay. To assess density distribution, 8 μl of each of the 12 fractions were assayed by immunoblot (described below) and results were expressed as the percentage of the total on the gradient defined as 100%.
Isolation of detergent-soluble and detergent-insoluble fractions.
To analyze detergent soluble vs. insoluble fractions as a function of membrane protein density, the 12-fraction sorbitol density gradient was separated into three “windows”: window I (WI; fractions 4–5), window II (WII; fractions 6–7), and window III (WIII; fractions 8–10), and the protein concentration (measured by BCA) of each was adjusted to 3 mg/ml. On ice, samples were treated with 0% or 1% Triton X-100 in extraction buffer (50 mM MES pH 6.4, 60 mM NaCl, 3 mM EGTA, and 5 mM MgCl2 and Complete Protease Inhibitor Cocktail; Roche Diagnostics at 1 pill/1 ml buffer) with a final protein concentration of 2.7 mg/ml. After a 1-h incubation on ice with frequent vortexing, the samples were centrifuged at 100,000 g for 30 min. Supernatants (detergent soluble) and pellets (detergent insoluble) were collected, and pellets were resuspended in the same volume as the supernatants in a 10/90 mixture of extraction buffer and 5% sorbitol. Equal volumes of detergent soluble and insoluble fractions were assayed side by side by immunoblot to calculate the percentage of the total that was detergent soluble vs. insoluble in each window.
Immunoblot analysis.
For all immunoblots, a constant volume of sample was denatured in SDS-PAGE sample buffer for 20 min at 60°C, resolved on either a 7.5 or a 5–15% SDS-polyacrylamide gel according to Laemmli (22), and transferred to polyvinylidene difluoride membranes (Millipore Immobilon-P). Blots were blocked and incubated with one of the following: rabbit polyclonal antisera against NHE3 (1:2,000; NHE3-COO; Ref. 48), NaPi2 (1:500; McNaPi2 from McDonough Laboratory), DPPIV (1:1,500; αDPPIV 95 1/6; M. Farquhar, University of California, San Diego), NHERF-1 (R-1046; 1:2,000; E. Weinman, Univ. of Maryland), myosin VI (K-15; 1:2,000; Sigma), megalin (459; 1:5,000; M. Farquhar, University of California, San Diego), goat polyclonal antisera against ezrin (C-19; 1:1,000; Santa Cruz Biotechnology), flotillin (K-19; 1:500; Santa Cruz Biotechnology,), mouse monoclonal antisera against AP2 (1:1,000; α- adaptin, Sigma), transferrin receptor (TfR, 1:500; Zymed Laboratories), and villin (1:2,000; Immunotech). Secondary antibodies were all used at 1:5,000 dilution: Alexa 680-labeled goat anti-rabbit, Alexa 680-labeled goat anti-mouse, Alexa 680-labeled donkey anti-goat (Molecular Probes, Eugene, OR), and IRDye 800 labeled goat anti-mouse (LI-COR, Lincoln, NB). Signals were detected and quantitated with the Odyssey Infrared Imaging System (LI-COR) and accompanying LI-COR software. For each antibody, multiple amounts of proteins were loaded to assess linearity of the detection system.
Quantitation and statistical analysis.
Data are expressed as means ± SE. Differences were regarded significant at P < 0.05. Control and acute hypertension rats were treated in a paired fashion through all fractionations and assays. In OptiPrep flotation gradient assays and sorbitol density gradient assays, two-way ANOVA was applied to determine whether there was a significant effect of treatment on the overall density distribution pattern. After significance was established, the location of the difference in the pattern was assessed by two-tailed Student's t-test, assuming equal variance with Bonferroni adjustments for multiple comparisons.
RESULTS
Renal response to acute hypertension.
Raising blood pressure from 104 ± 2 to 142 ± 4 mmHg leads to a significant increase in urine output from 1.8 ± 0.5 to 41.7 ± 15.5 mg/min without a change in the glomerular filtration rate (Table 1), evidence for pressure natriuresis in the face of autoregulation (16, 17, 37).
Table 1.
Effect of an increase in mean arterial pressure on GFR and urine output in control rats and rats subjected to acute hypertension
| Measurement | Control | High BP |
|---|---|---|
| Mean arterial pressure, mmHg | 104±2 | 142±4* |
| GFR, ml·min−1·kg−1 | 3.3±0.4 | 5.6±1.3 |
| Urine output, mg/min | 1.8±0.5 | 41.7±15.5* |
Values are mean ± SE; n = 5.GFR: glomerular filtration rate from 1 kidney, measured using FITC-inulin. Urine output: for each experiment, values are the average of 3 measurements taken every 10 min for control and every 5 min for acute hypertension (High BP) from 1 ureter.
P < 0.05, compared with control values.
Differential redistribution of NHE3 and NaPi2 during acute hypertension.
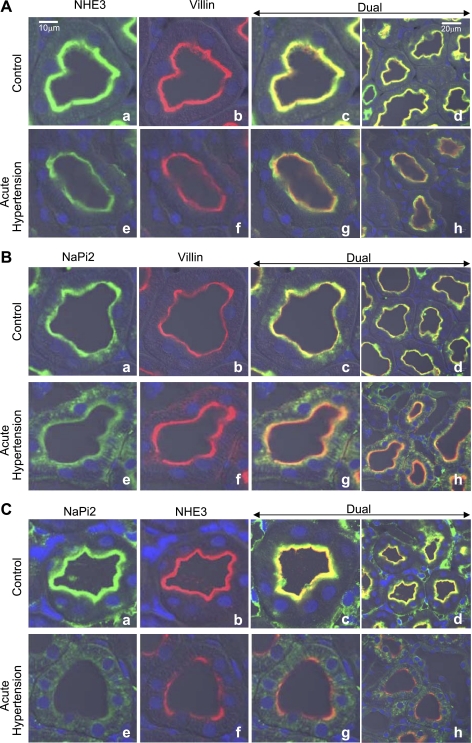
Kidneys from animals at baseline blood pressure and those subjected to acute hypertension were perfusion fixed with PLP fixative at a rate that did not alter basal blood pressure, they were frozen, and cryosections were stained for either NHE3 and villin (Fig. 1A), NaPi2 and villin (Fig. 1B), or NaPi2 and NHE3 (Fig. 1C). As shown before by our laboratory (50), at baseline blood pressure both NHE3 (Fig. 1A, a–d) and NaPi2 (Fig. 1B, a–d) are colocalized (yellow stain) with the actin-bundling protein villin, used as a marker for the apical brush border of the proximal tubule. When blood pressure is elevated, both sodium transporters redistribute. NHE3 redistributes to the base of the microvilli revealing the green-stained NHE3 at the base separate from the red-stained villin in the microvilli (Fig. 1A, e–h). In contrast, NaPi2 is redistributed into the cell-revealing green-stained NaPi2 in the cytoplasm below the red-stained villin in the microvilli (Fig. 1B, e–h). To emphasize the differential redistribution of these two transporters, we costained for NHE3 and NaPi2 (Fig. 1C). At baseline blood pressure, NHE3 and NaPi2 colocalized in the microvilli (Fig. 1C, a–d), and then when blood pressure was increased, they went to separate locations revealing green-stained NaPi2 in the cytoplasm and red-stained NHE3 at the base of the microvilli (Fig. 1C, e–h).
Fig. 1.
Effect of acute hypertension on the subcellular distribution of Na+/H+ exchanger 3 (NHE3) and Na+-Pi cotransporter 2 (NaPi2). Paired kidneys from animals at baseline blood pressure and after 15-min arterial hypertension were fixed by perfusion without changing arterial pressure as described in materials and methods. These paired samples were placed on the same slide and processed identically. A: NHE3 was costained with villin using an anti-NHE3 polyclonal detected with FITC-conjugated goat anti-rabbit secondary antibody and a monoclonal anti-villin detected with Alexa 568-conjugated goat anti-mouse secondary. B: NaPi2 was costained with villin using an anti-NaPi2 polyclonal detected with FITC-conjugated goat anti-rabbit secondary antibody and a monoclonal anti-villin detected with Alexa 568-conjugated goat anti-mouse secondary. C: NHE3 was costained with NaPi2 using an anti-NHE3 monoclonal detected with Alexa 568-conjugated goat anti-mouse secondary and anti-NaPi2 polyclonal detected with FITC-conjugated goat anti-rabbit secondary antibody. Bars in a and d indicate magnification scale; a–d, control; e–h, acute hypertension.
Fractionation of lipid raft associated proteins on flotation gradients.
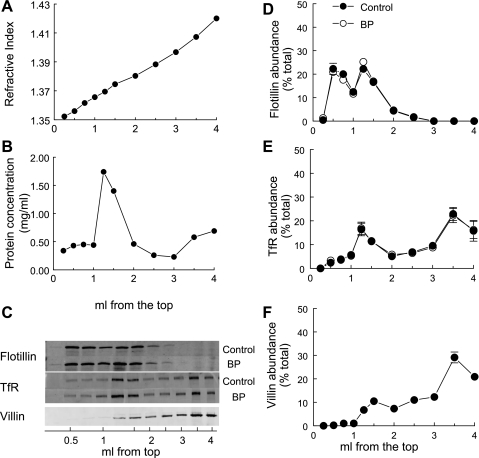
Given the marked differential redistribution of NHE3 vs. NaPi2 during acute hypertension, we investigated the domain properties of these two transporters. Fifteen minutes of acute hypertension did not change the total pool size of NHE3 or NaPi2 measured in the homogenates (Supplemental Fig. S1; supplemental data for this article are available online at the Am J Physiol Cell Physiol website). Renal cortex homogenates from animals at baseline blood pressure and those with acutely elevated blood pressure were first subjected to cold Triton X-100 extraction, as described. Both proteins in lipid rafts and cytoskeletal proteins are resistant to Triton solubilization and nonraft proteins are solubilized. The total extract was then resolved on OptiPrep gradients which allow separation of membranes based on their buoyancy: membranes with a high lipid-to-protein ratio “float” in the low-density portion of the gradient while nonraft proteins distribute to higher densities and cytoskeletal elements will distribute differentially based on whether they associate with proteins that partition into raft or nonraft domains (5, 35). The gradient characteristics are shown in Fig. 2. For improved resolution of the ordered lipid domains at the top of the gradient, six 250-μl fractions were collected followed by five 500-μl fractions. The protein distribution has a sharp peak ∼1.25 ml from the top of the 4-ml gradient (Fig. 2B). The lipid raft marker flotillin distributed in the top 1.5 ml and was unaffected by raising the blood pressure (Fig. 2C). The TfR, a nonraft marker, distributed primarily in the bottom 2 ml of the gradient (Fig. 2D), but there was also a minor peak at 1.5 ml that coincided with the major protein peak, indicating that there are likely some nonraft membranes in that region. The microvillar actin bundling protein villin, highly enriched in the proximal tubule with NHE3 and NaPi2 (Fig. 1), is >85% insoluble after cold Triton X-100 extraction, as expected of a cytoskeletal protein (not shown). Although both the cytoskeleton and lipid raft proteins are detergent insoluble, they can be separated on density gradients: villin partitions to heavy densities with the nonraft marker TfR rather than with the lipid raft marker flotillin (Fig. 2E), illustrating that the OptiPrep gradient fractionates proteins based on density rather than detergent solubility.
Fig. 2.
Flotation gradient characteristics. Renal cortex total membranes treated with Triton X-100 were fractionated on OptiPrep gradients. The top 1.5 ml was collected as six 250-μl samples to delineate the lipid raft domain, and the remainder of the gradient was collected as five 500-μl aliquots. A constant volume of each fraction was assayed. A: representative refractive index as a function of volume (ml) from the top of the gradient after centrifugation. B: protein concentration as a function of volume (ml) from the top of the gradient. C: representative immunoblots of a constant volume of each fraction. D: distribution of the lipid raft marker flotillin. E: distribution of transferrin receptor (TfR), a nonraft marker. F: distribution of the actin bundling protein villin (average for 3 gradients). D, E: samples from control rats (•; n = 5) and samples from rats with acute hypertension (○; BP; n = 5). Data are expressed as the percentage of the total signal in all 11 fractions (means ± SE).
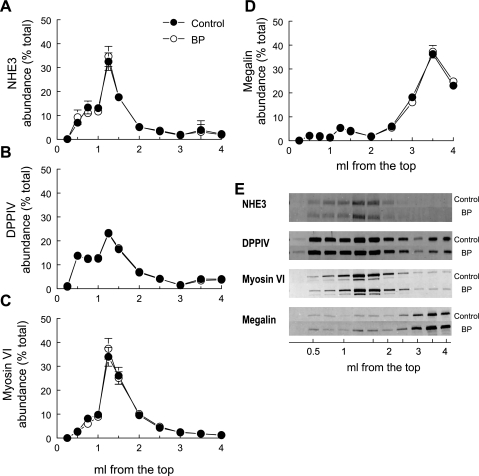
Table 2 summarizes the percentage of each of the proteins in lipid raft enriched fractions in the top 1.5 ml of the gradient. Most of the NHE3 (84%) distributed in the top 1.5 ml of the flotation gradient with the lipid raft marker flotillin (Fig. 3, A and E; Table 2). DPPIV, a protein known to regulate NHE3 activity (13–15), and myosin VI, a molecular motor postulated to participate in NHE3 redistribution along the microvilli (51), codistributed preferentially into flotillin-enriched fractions (Fig. 3, B, C, and E). Megalin, an endocytic receptor in the renal proximal tubule found along the microvilli, has also been reported to associate with NHE3 (2), yet it distributes almost entirely to the nonraft domain in the bottom 1.5 ml of the gradient (Fig. 3, D and E). The PDZ domain protein NHERF-1 has been reported to coimmunoprecipitate with NHE3 as well as with ezrin, suggesting that it tethers the cotransporter to the cytoskeleton via association with ezrin (41). Interestingly, only ∼30% of NHERF-1 and ezrin are cofractionated with NHE3 and flotillin into the top 1.5 ml of the gradient, while ∼70% of NHERF-1 and ezrin partition to the nonraft domains (Fig. 4, B, C, and E; Table 2). This is not necessarily surprising given the fact that NHERF-1 and ezrin also associate with other membrane proteins, including NaPi2, which is not primarily localized to raft enriched fractions.
Table 2.
Protein partitioning into lipid raft-enriched fractions in control vs. acute hypertension treated rats
| Protein |
%Total in Lipid Rafts |
|
|---|---|---|
| Control | High BP | |
| Flotillin | 94±0 | 94±1 |
| NHE3 | 84±9 | 84±8 |
| Myosin VI | 81±5 | 80±4 |
| DPPIV | 80±1 | 80±1 |
| TfR | 40±6 | 41±6 |
| Ezrin | 33±7 | 33±5 |
| NaPi2 | 31±5 | 38±5 |
| NHERF-1 | 31±5 | 30±2 |
| AP2 | 25±1 | 23±1 |
| Megalin | 15±2 | 15±3 |
Values are mean ± SE; n = 5. Percentage of each protein in lipid raft fractions was calculated by summing the percentage localized to the top 1.5 ml of the gradient (Figs. 1–3), based on the localization of the lipid raft marker flotillin listed from highest to lowest %total in lipid rafts. NHE3, Na+/H+ exchanger 3; DPPIV, dipeptidyl peptidase IV; TfR, transferrin receptor; NaPi2, Na+-Pi cotransporter 2; NHERF-1, NHE regulatory factor-1.
Fig. 3.
Flotation gradient profiles of NHE3 and associated proteins are not altered by elevated blood pressure. Renal cortex total membranes treated with Triton X-100 were fractionated on OptiPrep gradients. The top 1.5 ml was collected as six 250-μl samples to delineate the lipid raft domain, and the remainder of the gradient was collected as five 500-μl aliquots. A constant volume of each fraction from control rats (•; n = 5) and rats with acute hypertension (○; n = 5) was assayed by immunoblot. A: NHE3; B: dipeptidyl peptidase IV (DPPIV); C: myosin VI; D: megalin; E: typical immunoblots. Data are expressed as the percentage of the total signal in all 11 fractions (means ± SE).
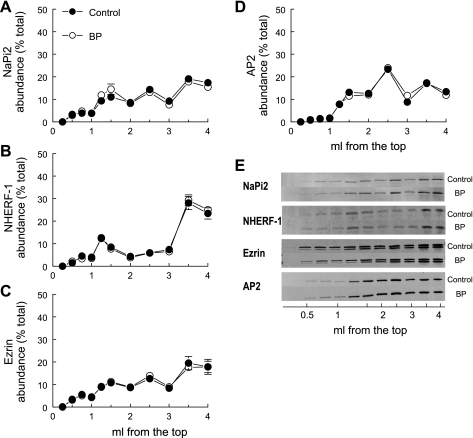
Fig. 4.
Flotation gradient profiles of NaPi2 and associated proteins are not altered by elevated blood pressure. Renal cortex total membranes treated with Triton X-100 were fractionated on OptiPrep gradients. The top 1.5 ml was collected as six 250-μl samples to delineate the lipid raft domain, and the remainder of the gradient was collected as five 500-μl aliquots. A constant volume of each fraction from control rats (•; n = 5) and rats with acute hypertension (○; n = 5) was assayed by immunoblot. A: NaPi2; B: NHERF-1; C: ezrin; D: AP2; E: typical immunoblots. Data are expressed as the percentage of the total signal in all 11 fractions (means ± SE).
Most of the NaPi2 (65%) distributes in the bottom half of the gradient with the nonraft marker TfR (Fig. 4, A and E; Table 2), demonstrating that NHE3 and NaPi2 are in distinct membrane domains in the proximal tubule. NHERF-1, which has been shown to be important for NaPi2 localization in the apical membrane (10, 36, 44), associates with ezrin, and both have flotation gradient patterns similar to NaPi2 (Fig. 4, B, C, and E). This suggests that they are in a common domain and that NaPi2 is tethered to the cytoskeleton via association with NHERF-1 and ezrin. Since NaPi2 is internalized during hypertension, we examined the flotation properties of the clathrin adaptor AP2 and found that it is also enriched in nonraft domains along with NaPi2. Two groups are evident: 80% or more of NHE3, DPPIV, myosin VI, and flotillin are found in lipid raft enriched domains, while 40% or less of NaPi2, NHERF-1, ezrin, AP2, and TfR are localized to lipid rafts, that is, 60% or more of these are enriched in nonraft domains.
Detergent solubility of NHE3, NaPi2, and their associated proteins.
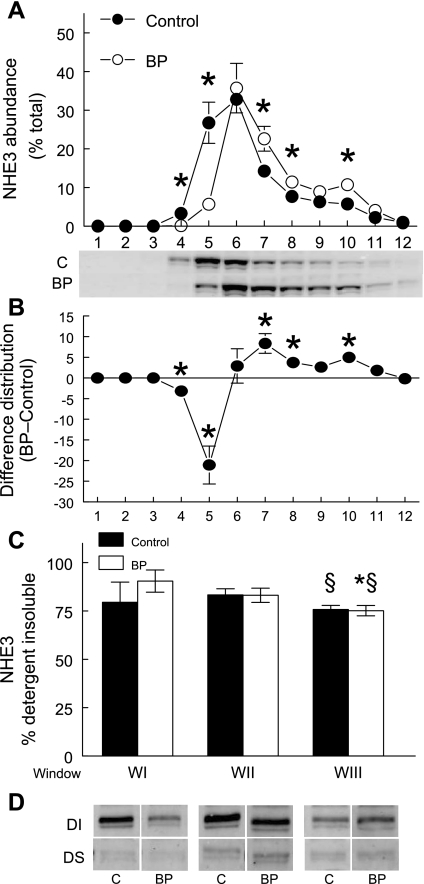
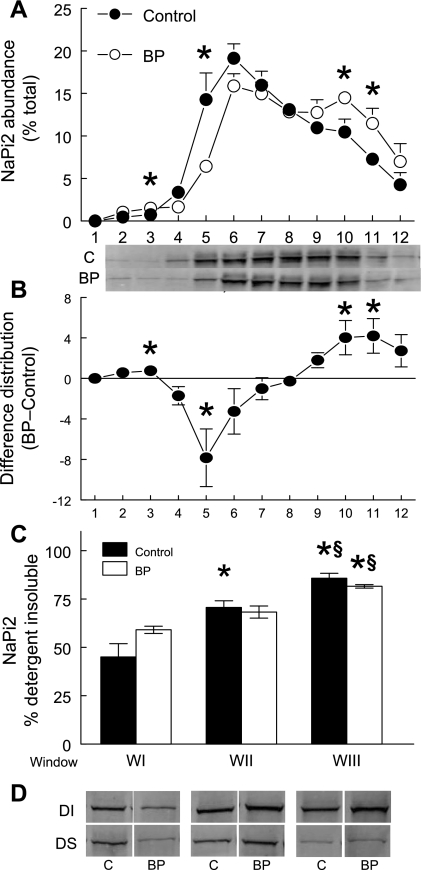
We and others have used microscopy techniques to show that NHE3 and NaPi2 are trafficked out of the body of the microvilli very rapidly when blood pressure is elevated (12, 50, 52). In addition, we have shown this trafficking is evident as a redistribution to higher density membranes on sorbitol density gradients (54–56). Hypertension-provoked redistribution patterns on sorbitol density gradients are shown for NHE3 in Fig. 5 and for NaPi2 in Fig. 6. We (28, 48, 54–56) previously characterized the gradient characteristics with classic membrane markers: WI (fractions 4–5) contains the plasma membrane markers alkaline phosphatase, NHE3, DPPIV, and sodium pump subunits; WII (fractions 6–7) contains the peak of megalin, a marker for the cleft region located at the base of the microvilli as well as plasma membrane markers found in WI; WIII (fractions 8–10) is enriched in endosomal rab5a and lysosomal β-hexoseaminidase as well as the intermicrovillar marker. To assess detergent solubility of NHE3 and NaPi2 in the body of the microvilli at baseline blood pressure (WI) and after blood pressure is increased, which traffics NHE3 to the base of the microvilli (WII) and NaPi2 to subapical endosomes (WIII), we first pooled the gradient fractions into these three “windows,” normalized total protein levels then subjected each to cold Triton X-100 extraction and centrifugation to separate detergent soluble from detergent insoluble components enriched in lipid-ordered rafts or cytoskeleton-attached membranes (without OptiPrep flotation gradient analysis). The percentage of NHE3 in the detergent insoluble fraction was 80–90% in WI and WII and slightly but, significantly, lower (75%) in WIII (Fig. 5; Table 3). WIII is the region of the gradient significantly increased during acute hypertension, likely representing NHE3 at the base of the microvilli (Fig. 5). Since the fraction of NHE3 in lipid raft domains was not altered by acute hypertension, one interpretation is that there is less or weaker attachment of NHE3 to the cytoskeleton at the base of the microvilli (WIII) than in the body of the microvilli (WI, WII). The percentage of NaPi2 in the detergent insoluble fraction was also a function of location on the density gradient and distinct from the pattern observed for NHE3. In the microvilli-enriched WI NaPi2 was 45% detergent insoluble at baseline, while NaPi2 in endosome-enriched WIII was more than 80% detergent insoluble (Fig. 6; Table 3). The results suggest that as NaPi2 moves out of WI into WII-WIII during acute hypertension its membrane domain becomes more associated with the cytoskeleton and thus less detergent soluble. The alternative explanation, that NaPi2 redistributes to raft enriched domains, is not supported by the results shown in Fig. 4, which indicate that the NaPi2 remains in nonraft regions of the flotation gradient when blood pressure is increased.
Fig. 5.
Detergent insolubility of NHE3 decreases as it traffics to higher density membranes in response to hypertension. A: renal cortex total membranes from control rats (•; n = 5) and rats subjected to acute hypertension (○; n = 5) were fractionated on sorbitol density gradients. During acute hypertension NHE3 redistributes to higher density fractions as reported previously, *P < 0.05 vs. control (50). B: difference in density distribution of renal cortical NHE3 between control and acute hypertension. C: to assess detergent solubility, fractions were first pooled into three windows: WI (fractions 4–5), WII (fractions 6–7), and WIII (fractions 8–10), adjusted to constant protein, treated with 1% Triton X-100 and centrifuged to separate detergent insoluble (DI; pellet resuspended in the same volume as supernatant) from detergent soluble (DS; supernatant) as described in materials and methods. A constant volume of DS and DI were assayed by immunoblot to calculate the DI percentage. The percent NHE3 in the detergent insoluble fraction was significantly lower in WIII compared with WI or WII. D: representative immunoblots of NHE3 in the DS and DI fractions from control (C) and acute hypertension treated rats. Values are means ± SE; n = 5. *P < 0.05 compared with WI; §P < 0.05 compared with WII within the same treatment.
Fig. 6.
Detergent insolubility of NaPi2 increases as it traffics to higher density membranes in response to hypertension. A, renal cortex total membranes from control (•; n = 5) and rats subjected to acute hypertension (○; n = 5) were fractionated on sorbitol density gradients. During acute hypertension NaPi2 redistributes to higher density fractions corresponding to endosomes, as reported previously, *P < 0.05 vs. control (50). B: difference in density distribution of renal cortical NaPi2 between control and acute hypertension. C: to assess detergent solubility, fractions were first pooled into three windows: WI (fractions 4–5), WII (fractions 6–7), and WIII (fractions 8–10), adjusted to constant protein, treated with 1% Triton X-100, and centrifuged to separate DI (pellet resuspended to the same volume as supernatant) from DS (supernatant) as described in materials and methods. A constant volume of DS and DI were assayed by immunoblot to calculate the DI percentage. The percent NaPi2 in the detergent insoluble fraction was higher in the intracellular membrane enriched WII and WIII compared with the microvillar enriched WI. C: representative immunoblots of NaPi2 in the DS and DI fractions from control (C) and acute hypertension treated rats. Values are means ± SE; n = 5. *P < 0.05 compared with WI; §P < 0.05 compared with WII within the same treatment.
Table 3.
Percentage of NHE3, NaPi2, and associated proteins in the detergent-insoluble fraction
| Protein |
Window I |
Window II
|
Window III
|
|||
|---|---|---|---|---|---|---|
| Control | High BP | Control | High BP | Control | High BP | |
| NHE3 | 80±10 | 90±6 | 83±3 | 83±4 | 76±2§ | 75±3*† |
| DPPIV | 29±0 | 38±7 | 28±4 | 20±1 | 24±4 | 20±3 |
| Myosin VI | 100 | 100 | 100 | 100 | 100 | 100 |
| Megalin | 38±1 | 51±4 | 28±1* | 31±3* | 24±5 | 23±5* |
| NaPi2 | 45±7 | 59±2 | 71±3* | 68±3 | 86±4*† | 82±1*† |
| NHERF-1 | 59±4 | 76±8 | 57±6 | 64±3 | 58±4 | 63±3 |
| AP2 | 78±11 | 91±7 | 94±1 | 93±1 | 94±1 | 94±1 |
| Ezrin | 52±19 | 49±14 | 63±4 | 60±3 | 70±14 | 76±5 |
Values are means ± SE; n = 5. Fractions after sorbitol density gradient centrifugation from control and acutely hypertensive animals were pooled into 3 windows, adjusted to the same protein concentration and then treated with cold 1% Triton X-100 and centrifuged to separate detergent insoluble pellet from detergent soluble supernatant. The pellet was resuspended in same volume as supernatant. A constant amount of supernatant and resuspended pellet was assayed by immunoblot to determine percentage in detergent insoluble fraction. WI, pooled fractions 4–5; WII, pooled fractions 6–7; WIII, pooled fractions 8–10.
P < 0.05 compared with WI;
P < 0.05 compared with WII within the same treatment. There was no myosin VI detected in detergent soluble fraction so no error could be calculated.
Table 3 summarizes the detergent solubility of NHE3, NaPi2, and their associated proteins in the three windows. NHE3-associated DPPIV and myosin VI have very similar flotation gradient profiles (Fig. 3), yet they have very distinct detergent insolubility (Table 3), evidence for distinct cytoskeletal attachment properties. Not surprisingly, myosin VI is 100% detergent insoluble in all windows, evidence for cytoskeletal attachment of this actin-associated motor protein. DPPIV is only 20–30% detergent insoluble, despite 80% distribution in lipid raft domains (Table 2). The NaPi2-associated NHERF-1 and ezrin were ∼60% detergent insoluble despite the observation that only ∼30% were localized to lipid raft domains (Table 2), providing evidence for attachment of NHERF-1 and ezrin to the actin cytoskeleton (40–42, 45). The clathrin adaptor AP2 was 80–90% detergent insoluble, evidence of NaPi2 association with the clathrin-coated vesicles (38). The endocytic receptor megalin, which partitioned entirely into nonraft domains (Fig. 4), has a variable percent detergent insoluble that perhaps reflects changes in trafficking during hypertension.
DISCUSSION
Pressure natriuresis is an important homeostatic mechanism that involves a decrease in sodium reabsorption (and consequent natriuresis) in response to an increase in blood pressure. This response is central to blood pressure regulation, which motivates understanding the molecular mechanisms involved. Pressure natriuresis is a rapid and reversible response (8, 54) and trafficking of renal sodium transporters and associated proteins may be a key to the decrease in Na+ reabsorption in the proximal tubule as well as further along the nephron. During acute hypertension, both NHE3 and NaPi2 traffic down the microvilli laterally within the plane of the membrane (50), suggesting that they bind the cytoskeleton, likely indirectly through tethering proteins, and are shuttled down along the actin filaments, potentially via molecular motors (51). When NHE3 and NaPi2 reach the bottom of the microvilli, they part ways, as NHE3 stops near the base, above the intermicrovillar cleft, and NaPi2 goes into the cleft where it becomes internalized (50). This differential targeting suggests that the two proteins either move down the microvilli via the same route and are then sorted to different routes when they reach the base of the microvilli or, alternatively, that they take distinct routes along the microvilli, one leading to the base, the other to the intermicrovillar cleft and the endocytic apparatus. The results of this study suggest the latter: that NHE3 traffics via lipid rafts to the base of the microvilli and NaPi2 traffics via nonrafts to the intermicrovillar clefts.
The results of this study demonstrate that the apical plasma membrane of the renal proximal tubule microvilli is not homogenous but rather is composed of domains of membranes with distinct properties. NHE3, DPPIV, and myosin VI are preferentially localized to lipid raft domains, segregated from NaPi2, which interacts with NHERF-1 and ezrin, or AP2 in nonraft domains. Specifically, we propose that an increase in blood pressure activates signaling cascades that alter the tethering of NHE3- and DPPIV-containing protein complexes in lipid rafts to the microvillar cytoskeleton in a fashion that activates their redistribution, driven by lipid raft associated myosin VI, within the plane of the microvillar membrane, to the base where these complexes remain in a new steady state as long as blood pressure is increased (49). The previously described tethering of NHE3 to the cytoskeleton via NHERF-1 and ezrin (11) is not ruled out by our findings, as a significant fraction (∼30%) of these proteins is localized to lipid raft enriched fractions (Table 2). Recently, it has been reported that NHE3 located at the base of the microvilli is phosphorylated, suggesting that covalent modification of NHE3 may play a role in the trafficking as well (19, 20). In parallel, we propose that the increase in blood pressure alters the tethering of NaPi2-, NHERF-1-, and ezrin-containing protein complexes in nonraft domains to the cytoskeleton in a fashion that activates their redistribution to the intermicrovillar cleft where NaPi2 is now free to interface with the endocytic machinery, including megalin and AP2, localized to the same domains. A recent relevant study implicates phosphorylation of NHERF-1 in the regulated endocytosis of NaPi2 (43). Specifically, in cultured kidney cells, PTH and other activators of PKC and PKA dissociate NHERF-1 from NaPi2, facilitated by phosphorylation at serine 77. Mutating this residue not only prevented the dissociation of NHERF-1 from NaPi2 but also prevented the PTH-mediated decrease in NaPi2 transport activity normally mediated by endocytosis.
The presence of membrane domains with distinct lipid content facilitates the clustering and segregation of distinct sets of proteins. Even though the renal cortex is a very mixed cell tissue, the distribution of NHE3 and NaPi2 is for the most part restricted to proximal tubule cells and the availability of specific antibodies allows analysis of their domain properties in mixed tissues. The Donowitz group (25) analyzed in vivo NHE3 domain properties in rabbit ileal brush border and concluded that about one-half of the NHE3 was Triton X-100 insoluble and about one-third of this pool was in lipid raft domains. They also concluded that rapid stimulation of NHE3 activity increased the lipid raft pool of NHE3. More recently, the same group conducted a study in opossum kidney cells that determined that half of the apical membrane NHE3 vs. only 11% of intracellular NHE3 was localized to lipid raft domains and that disruption of lipid rafts with methyl-β-cyclodextrin significantly decreased both NHE3 activity and NHE3 endocytosis (33). Adding to these studies, our analysis of NHE3 in vivo in the renal cortex finds 84% of NHE3 in lipid raft domains and about the same percentage in the detergent insoluble fraction. Additionally, we did not detect any change in NHE3 domain properties with acute regulation by hypertension (Table 2), which we know involves trafficking of NHE3 to the base of the microvilli (50) and a decrease in NHE3 activity (53). Given the morphological differences between proximal tubule cells in vivo and opossum kidney cells (30), and given the lack of endocytosis of NHE3 in vivo, it is probably not surprising that there is a much higher fraction of NHE3 in lipid raft domains and no change in lipid domain pool size with acute regulation in vivo. All studies indicate that NHE3 localization to lipid raft domains is essential to its normal functioning and regulation.
The fact that DPPIV and myosin VI also partition into lipid raft domains is consistent with their importance for NHE3 regulation. DPPIV associates with NHE3 (13) and DPPIV activity increases NHE3 activity via a tyrosine kinase signaling pathway (15). Our laboratory (23, 24, 50, 51, 56) has highlighted the potential role for myosin VI in the trafficking of NHE3 down the microvilli during such signals as hypertension, ACE inhibition, or PTH treatment. The fact that we found myosin VI to be 100% detergent insoluble corresponds with the nature of the protein as an actin-based motor. In contrast, the endocytic receptor megalin partitions entirely into heavy density nonraft domains, not overlapping with NHE3-containing domains. The separation between NHE3 and the endocytic receptor megalin is also consistent with the fact that NHE3 does not become internalized. Our immunoelectron microscopy results suggest that when NHE3 redistributes to the base of the microvilli, it does not enter the intermicrovillar cleft where megalin is enriched (57) but rather stops on top of the clefts (50). A similar pattern of distribution has been reported for the vacuolar H-ATPase, also by immunoelectron microscopy (3). Interestingly, we found that H-ATPase-β2 partitions into lipid raft domains like NHE3 (data not shown). We hypothesize that the intermicrovillar cleft domain may be enriched in nonraft membranes, which exclude the lipid raft membranes containing NHE3 and H-ATPase. In contrast, the nonraft protein NaPi2 is able to move all the way down to the cleft where it can be internalized.
A previous study of the membrane domain properties of rat renal cortex NaPi2 used the density gradient flotation technique used in this study, but the sample membranes were not pretreated with cold Triton X-100, a protocol referred to as detergent-free density gradient flotation (18). The investigators reported that 80% of NaPi2 partitioned into low-density cholesterol-, sphingomyelin-, and GM1-enriched fractions, which they labeled “lipid raft” fractions. In contrast, we found by using Triton X-100 treatment followed by the same density gradient flotation technique that only 35% of the NaPi2 was found in the flotillin/lipid raft-enriched fractions (Fig. 4; Table 2). To resolve this discrepancy, we investigated the behavior of our membranes when fractionated by detergent-free density gradient flotation as described previously (18). The behavior of the markers and transporters on the gradient was very distinct from the distributions that were observed after we treated the same membranes with Triton X-100 (Figs. 2–4). In particular, the distributions of the lipid domain marker flotillin, the nonraft marker TfR, NHE3, and NaPi2 were almost identical: all were enriched between 1 and 2 ml below the top of the 4-ml gradient (data not shown). We propose that the Triton X-100 extraction is necessary to physically separate the lipid raft domains from the nonraft domains and that this separation is not accomplished by homogenization of the renal cortex alone. In the absence of this detergent extraction, the distribution of the membranes on the gradient likely reflects the heterogeneity of the membranes containing both lipid raft and nonraft domains.
In our study, we found that the fraction of NaPi2 that is detergent insoluble was significantly higher in the membranes enriched in subapical endosomes (WIII, 82–86% detergent insoluble) compared with membranes enriched in apical microvilli (WI, 45–59% detergent insoluble; Fig. 5; Table 3). Since NaPi2 is localized in nonraft domains, this difference in detergent solubility is likely attributable to changes in how strongly NaPi2 binds the cytoskeleton: it appears that NaPi2 may be more loosely connected to the cytoskeleton when it is in the brush border than when it moves to the base and becomes internalized. As discussed above, NHERF-1 and ezrin play a role in tethering NaPi2 to the cytoskeleton and they also appear to be enriched in nonraft domains (Fig. 4; Table 3). We found that the detergent solubility of NHERF-1, ezrin, and NaPi2 in microvilli-enriched WI were overlapping, consistent with the known interaction of these proteins (29, 34). In contrast, the clathrin adaptor AP2 is almost completely detergent insoluble (Table 3). We propose that as NaPi2 is transported to the intermicrovillar cleft regions containing clathrin-coated vesicles (enriched in WIII), it associates with the AP2-containing membranes, which increases the percentage of NaPi2 that is detergent insoluble. This is consistent with our previous results demonstrating colocalization of the cotransporter with the clathrin adaptor protein during acute hypertension (50).
In summary, it appears that sodium transporters in the renal proximal tubule apical membrane are localized with their interacting binding partners and regulators to distinct domains that likely play a role in their distinct redistribution routes observed in response to acute hypertension or PTH (1, 50). The signaling cascades that connect the stimulus to the redistribution and ultimately the natriuresis likely involve phosphorylation of NHE3 and NHERF-1 (19, 20, 43), which could change protein-protein and protein-cytoskeleton associations in a fashion that triggers the trafficking via lipid raft vs. nonraft domains.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-34316 Histology and Microscopy was performed with the help of the Cell and Tissue Imaging Core of the University of Southern California Research Center for Liver Diseases (National Institute of Diabetes and Digestive and Kidney Diseases Grant P30-DK-048522).
Supplementary Material
Acknowledgments
We thank Curtis Okamoto, the University of Southern California School of Pharmacy, and Moshe Levi, University of Colorado, for very helpful advice and direction.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bacic D, Lehir M, Biber J, Kaissling B, Murer H, Wagner CA. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int 69: 495–503, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Biemesderfer D, Nagy T, DeGray B, Aronson PS. Specific association of megalin and the Na+/H+ exchanger isoform NHE3 in the proximal tubule. J Biol Chem 274: 17518–17524, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Brown D, Hirsch S, Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest 82: 2114–2126, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown DA Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 21: 430–439, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68: 533–544, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Cha B, Tse M, Yun C, Kovbasnjuk O, Mohan S, Hubbard A, Arpin M, Donowitz M. The NHE3 juxtamembrane cytoplasmic domain directly binds ezrin: dual role in NHE3 trafficking and mobility in the brush border. Mol Biol Cell 17: 2661–2673, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou CL, Marsh DJ. Role of proximal convoluted tubule in pressure diuresis in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 251: F283–F289, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Chou CL, Marsh DJ. Time course of proximal tubule response to acute arterial hypertension in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 254: F601–F607, 1988. [DOI] [PubMed] [Google Scholar]
- 9.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham R, Steplock D, Wang F, Huang H, EX, Shenolikar S, Weinman EJ. Defective parathyroid hormone regulation of NHE3 activity and phosphate adaptation in cultured NHERF-1−/− renal proximal tubule cells. J Biol Chem 279: 37815–37821, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Donowitz M, Li X. Regulatory binding partners and complexes of NHE3. Physiol Rev 87: 825–872, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Dos Santos EA, Dahly-Vernon AJ, Hoagland KM, Roman RJ. Inhibition of the formation of EETs and 20-HETE with 1-aminobenzotriazole attenuates pressure natriuresis. Am J Physiol Regul Integr Comp Physiol 287: R58–R68, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Girardi AC, Degray BC, Nagy T, Biemesderfer D, Aronson PS. Association of Na(+)-H(+) exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule. J Biol Chem 276: 46671–46677, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Girardi AC, Fukuda LE, Rossoni LV, Malnic G, Reboucas NA. Dipeptidyl peptidase IV inhibition downregulates Na+-H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol 294: F414–F422, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Girardi AC, Knauf F, Demuth HU, Aronson PS. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am J Physiol Cell Physiol 287: C1238–C1245, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Guyton AC Blood pressure control–special role of the kidneys and body fluids. Science 252: 1813–1816, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Guyton AC Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension 19: I2–I8, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Inoue M, Digman MA, Cheng M, Breusegem SY, Halaihel N, Sorribas V, Mantulin WW, Gratton E, Barry NP, Levi M. Partitioning of NaPi cotransporter in cholesterol-, sphingomyelin-, and glycosphingolipid-enriched membrane domains modulates NaPi protein diffusion, clustering, and activity. J Biol Chem 279: 49160–49171, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Kocinsky H, Girardi ACC, Orlowski J, Aronson PS. Generation and use of phosphospecific antibodies to determine the phosphorylation of NHE3 at PKA consensus sites (Abstract). FASEB J 14: A539, 2003. [Google Scholar]
- 20.Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phosphospecific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol 289: F247–F248, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kuismanen E, Saraste J. Low temperature-induced transport blocks as tools to manipulate membrane traffic. Methods Cell Biol 32: 257–274, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 23.Leong PK, Devillez A, Sandberg MB, Yang LE, Yip DK, Klein JB, McDonough AA. Effects of ACE inhibition on proximal tubule sodium transport. Am J Physiol Renal Physiol 290: F854–F863, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Leong PK, Yang LE, Lin HW, Holstein-Rathlou NH, McDonough AA. Acute hypotension induced by aortic clamp vs. PTH provokes distinct proximal tubule Na+ transporter redistribution patterns. Am J Physiol Regul Integr Comp Physiol 287: R878–R885, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Galli T, Leu S, Wade JB, Weinman EJ, Leung G, Cheong A, Louvard D, Donowitz M. Na+-H+ exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3. J Physiol 537: 537–552, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang X, Nazarian A, Erdjument-Bromage H, Bornmann W, Tempst P, Resh MD. Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. J Biol Chem 276: 30987–30994, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol Renal Physiol 277: F447–F453, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Magyar CE, Zhang Y, Holstein-Rathlou NH, McDonough AA. Proximal tubule Na transporter responses are the same during acute and chronic hypertension. Am J Physiol Renal Physiol 279: F358–F369, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Mahon MJ Ezrin promotes functional expression and parathyroid hormone-mediated regulation of the sodium-phosphate co-transporter 2a in LLC-PK1 cells. Am J Physiol Renal Physiol 294: F667–F675, 2008. [DOI] [PubMed] [Google Scholar]
- 30.McDonough AA, Biemesderfer D. Does membrane trafficking of NHE3 play a role in regulating Na+/H+ exchanger in the proximal tubule? Curr Opin Nephrol Hypertens 12: 533–541, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Munro S Lipid rafts: elusive or illusive? Cell 115: 377–388, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Murer H, Hernando N, Forster I, Biber J. Molecular aspects in the regulation of renal inorganic phosphate reabsorption: the type IIa sodium/inorganic phosphate co-transporter as the key player. Curr Opin Nephrol Hypertens 10: 555–561, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Murtazina R, Kovbasnjuk O, Donowitz M, Li X. Na+/H+ exchanger NHE3 activity and trafficking are lipid Raft-dependent. J Biol Chem 281: 17845–17855, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Reczek D, Bretscher A. The carboxyl-terminal region of EBP50 binds to a site in the amino-terminal domain of ezrin that is masked in the dormant molecule. J Biol Chem 273: 18452–18458, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol 122: 789–807, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shenolikar S, Voltz JW, Minkoff CM, Wade JB, Weinman EJ. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci USA 99: 11470–11475, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shipley RE, Study RS. Changes in renal blood flow, extraction of inulin, glomerular filtration rate, tissue pressure and urine flow with acute alterations of renal artery blood pressure. J Clin Invest 167: 676–688, 1951. [DOI] [PubMed] [Google Scholar]
- 38.Smythe E Regulating the clathrin-coated vesicle cycle by AP2 subunit phosphorylation. Trends Cell Biol 12: 352–354, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Vallon V, Schwark JR, Richter K, Hropot M. Role of Na(+)/H(+) exchanger NHE3 in nephron function: micropuncture studies with S3226, an inhibitor of NHE3. Am J Physiol Renal Physiol 278: F375–F379, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Voltz JW, Brush M, Sikes S, Steplock D, Weinman EJ, Shenolikar S. Phosphorylation of PDZ1 domain attenuates NHERF-1 binding to cellular targets. J Biol Chem 282: 33879–33887, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Wade JB, Liu J, Coleman RA, Cunningham R, Steplock DA, Lee-Kwon W, Pallone TL, Shenolikar S, Weinman EJ. Localization and interaction of NHERF isoforms in the renal proximal tubule of the mouse. Am J Physiol Cell Physiol 285: C1494–C1503, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Wade JB, Welling PA, Donowitz M, Shenolikar S, Weinman EJ. Differential renal distribution of NHERF isoforms and their colocalization with NHE3, ezrin, and ROMK. Am J Physiol Cell Physiol 280: C192–C198, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Weinman EJ, Biswas RS, Peng Q, Shen L, Turner CL, EX, Steplock D, Shenolikar S, Cunningham R. Parathyroid hormone inhibits renal phosphate transport by phosphorylation of serine 77 of sodium-hydrogen exchanger regulatory factor-1. J Clin Invest 117: 3412–3420, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Weinman EJ, Boddeti A, Cunningham R, Akom M, Wang F, Wang Y, Liu J, Steplock D, Shenolikar S, Wade JB. NHERF-1 is required for renal adaptation to a low-phosphate diet. Am J Physiol Renal Physiol 285: F1225–F1232, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Weinman EJ, Cunningham R, Wade JB, Shenolikar S. The role of NHERF-1 in the regulation of renal proximal tubule sodium-hydrogen exchanger 3 and sodium-dependent phosphate cotransporter 2a. J Physiol 567: 27–32, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinman EJ, Steplock D, Shenolikar S. NHERF-1 uniquely transduces the cAMP signals that inhibit sodium-hydrogen exchange in mouse renal apical membranes. FEBS Lett 536: 141–144, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Weinman EJ, Steplock D, Wang Y, Shenolikar S. Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na(+)-H+ exchanger. J Clin Invest 95: 2143–2149, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang L, Leong PK, Chen JO, Patel N, Hamm-Alvarez SF, McDonough AA. Acute hypertension provokes internalization of proximal tubule NHE3 without inhibition of transport activity. Am J Physiol Renal Physiol 282: F730–F740, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Yang LE, Leong PK, McDonough AA. Reducing blood pressure in SHR with enalapril provokes redistribution of NHE3, NaPi2, and NCC and decreases NaPi2 and ACE abundance. Am J Physiol Renal Physiol 293: F1197–F1208, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol Renal Physiol 287: F896–F906, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Redistribution of myosin VI from top to base of proximal tubule microvilli during acute hypertension. J Am Soc Nephrol 16: 2890–2896, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Yip KP, Tse CM, McDonough AA, Marsh DJ. Redistribution of Na+/H+ exchanger isoform NHE3 in proximal tubules induced by acute and chronic hypertension. Am J Physiol Renal Physiol 275: F565–F575, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Yip KP, Wagner AJ, Marsh DJ. Detection of apical Na(+)/H(+) exchanger activity inhibition in proximal tubules induced by acute hypertension. Am J Physiol Regul Integr Comp Physiol 279: R1412–R1418, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Magyar CE, Norian JM, Holstein-Rathlou NH, Mircheff AK, McDonough AA. Reversible effects of acute hypertension on proximal tubule sodium transporters. Am J Physiol Cell Physiol 274: C1090–C1100, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Mircheff AK, Hensley CB, Magyar CE, Warnock DG, Chambrey R, Yip KP, Marsh DJ, Holstein-Rathlou NH, McDonough AA. Rapid redistribution and inhibition of renal sodium transporters during acute pressure natriuresis. Am J Physiol Renal Fluid Electrolyte Physiol 270: F1004–F1014, 1996. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Norian JM, Magyar CE, Holstein-Rathlou NH, Mircheff AK, McDonough AA. In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. Am J Physiol Renal Physiol 276: F711–F719, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D. Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem 279: 34302–34310, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.