Abstract
Plectin is a 500-kDa cross-linking protein that plays important roles in a number of cell functions including migration and wound healing. We set out to characterize the role of plectin in mechanical properties of living cells. Plectin−/− cells were less stiff than plectin+/+ cells, but the slopes of the two power laws in response to loading frequencies (0.002–1,000 Hz) were similar. Plectin−/− cells lost the capacity to propagate mechanical stresses to long distances in the cytoplasm; traction forces in plectin−/− cells were only half of those in plectin+/+ cells, suggesting that plectin deficiency compromised prestress generation, which, in turn, resulted in the inhibition of long distance stress propagation. Both plectin+/+ and plectin−/− cells exhibited nonlinear stress-strain relationships. However, plectin+/+ cells, but not plectin−/− cells, further stiffened in response to lysophosphatidic acid (LPA). Dynamic fluorescence resonance energy transfer analysis revealed that RhoA GTPase proteins were activated in plectin+/+ cells but not in plectin−/− cells after treatment with LPA. Expression in plectin−/− cells of constitutively active RhoA (RhoA-V14) but not a dominant negative mutant of RhoA (RhoA-N19) or an empty vector restored the long distance force propagation behavior, suggesting that plectin is important in normal functions of RhoA. Our findings underscore the importance of plectin for mechanical properties, stress propagation, and prestress of living cells, thereby influencing their biological functions.
Keywords: stiffening, prestress, traction, actin, mechanotransduction
plectin is a 500-kDa intermediate filament (IF)-based cytocrosslinker protein that plays a central role in cytoarchitecture (47, 53). Plectin deficiency in mice leads to early death within 1–3 days after birth as a consequence of internal blistering of the oral cavity that interferes with food uptake (1, 3). In addition, mutant mice show myopathies in skeletal muscle and abnormal cytoarchitecture in the heart (3, 25), which has been traced to the disruption of plectin isoform-mediated linkages of desmin IFs to different subcellular structures (24, 25).
Published works have demonstrated that plectin is involved in cell migration and wound healing (2) and resistance to osmotic swelling (34). Eleven alternatively spliced isoforms of plectin contribute to its complexity and versatility in structure and function (13). Recently, one of our groups has demonstrated that plectin isoform 1b mediates mitochondrion-IF network linkage and controls organelle shape (54). However, the mechanical functions of this protein remain unclear.
It is well established that actin microfilaments play critical roles in cell spreading, shape control, and mechanical properties of cells (48, 50). Therefore, the majority of the current research in the field of cell mechanics has been focused on F-actin and its cross-linking proteins regarding their specific contributions to cell mechanical properties. Myosin II is a major actin cross-linking and tension-generating protein in nonmuscle cells and has been the focus of many studies (9). In addition to myosin II, other actin cross-linking proteins play important roles. For example, actin cross-linking protein filamin A-deficient cells have very different cell shapes, exhibit few stress fibers, and have different mechanical properties compared with control cells (21). Focal adhesion protein vinculin-deficient cells also have different cell shapes and different mechanical properties compared with control cells (27). All these reports highlight the strong association between alterations in cell shape and changes in mechanical properties of cells. In contrast, wild-type cells and plectin knockout cells exhibit similar spreading and shape (2). Thus, it is not clear whether plectin plays significant roles in cell mechanical properties. However, there are a few hints from recent findings that plectin might play important roles in mechanical properties of living cells. The elongated and flexible molecular structure of plectin (12, 45) suggests that its organization is similar to that of members of the spectrin family (20) and that its structure may contribute to the elasticity (elastic stiffness) of living cells. In addition, plectin is known to connect integrins at the cell surface with IFs at hemidesmosomes (26, 41) and to connect nesprin-3 on the nuclear envelope with IFs (22), raising the possibility that plectin might mediate direct long distance stress propagation in the cytoplasm and into the nucleus. Keratinocyte fragility and lesional epidermal barrier defects are observed in conditional deletion of plectin in epithelia (1), suggesting that plectin might play an important mechanical role in living cells. In this report, we characterized the mechanical properties of cells in plectin+/+ and plectin−/− cells. We show that plectin plays important roles in cell stiffness, stress propagation, traction generation, and RhoA GTPase activation.
MATERIALS AND METHODS
Cell culture and transfection.
Wild-type (plectin+/+) and plectin-deficient (plectin−/−) mouse skin fibroblasts were isolated following a previous protocol (2). Cells were cultured in 100-mm tissue culture dishes (Nunclon, Roskilde, Denmark) in DMEM supplemented with 10% FCS, 50 U/ml penicillin G sodium, and 50 μg/ml streptomycin sulfate (penicillin-streptomycin, GIBCO-BRL, Life Technologies) at 37°C and 5% CO2 until they reached confluence. Confluent fibroblasts were trypsinized (trypsin-EDTA: 0.05% trypsin and 0.53 mM EDTA-4Na) and split 1:3–1:5. Experiments were performed with cells from passages 5 to 15 after isolation. Cells were serum deprived overnight before being plated on collagen-I-coated (20 μg/ml) rigid dishes or collagen-I-coated (100 μg/ml) polyacrylamide gel substrate (0.1% or 0.3% bis, 5% polyacrylamide, and elastic modulus 4 or 8 kPa) for 4–6 h or overnight. 1-Oleloyl-lysophosphatidic acid (LPA; Sigma, St. Louis, MO) was used at final concentration of 1 μg/ml. PDGF (Sigma) was used at a final concentration of 10 ng/ml (a saturating concentration). Adenovirus-yellow fluorescent protein (YFP) cytochrome c oxidases (mitochondrial inner membrane proteins) (YFP-mito) were used to infect the cells for 2 days (16). YFP-mito was used as a marker to track stress-induced displacements of the cytoskeleton. The cyan fluorescent protein (CFP)-YFP RhoA biosensor was a gift from Dr. M. Matsuda (Kyoto University) and Dr. Jun-ichi Miyazaki (Osaka University). Substantial experiments have been conducted to characterize and confirm the specificity and sensitivity of the RhoA biosensor (55). All plectin−/− cells where we measure RhoA activity expressed RhoA biosensors in the same way as plectin+/+ cells; this was confirmed by the fluorescence of the probe in these cells. Since transfection was not 100% in both types of cells, only the cells (both plectin+/+ and plectin−/−) that expressed RhoA biosensors were chosen for experiments. The Rac biosensor has been improved in its sensitivity by replacing the original fluorescence proteins with enhanced CFP (as a donor for FRET) and YPet [as an acceptor for fluorescence resonance energy transfer (FRET)], which was developed in Dr. Yingxiao Wang's laboratory (35) based on Rac biosensor pRaichu-Rac1/RacCT, a gift from Dr. M. Matsuda (Kyoto University) (19). The probe of pRaichu-Rac1/RacCT has been very well characterized in terms of its specificity (19). Probes were transfected into plectin+/+ and plectin−/− fibroblasts using Lipofectamine following protocols provided by the manufacturer (Invitrogen) and incubated for 24 h. Plectin−/− cells were simultaneously transfected with mCherry-tubulin and a constitutively active RhoA-V14, a dominant negative mutant of RhoA (RhoA-N19), or an empty vector using the Lipofectamine method following the manufacturer's protocol (Invitrogen). Green fluorescent protein (GFP)-zyxin (a gift of Dr. F. Wang) and mCherry-actin or mCherry-tubulin (gifts of Dr. R. Tsien) were transfected according to established protocols.
Polyacrylamide substrate technique.
The technique of the polyacrylamide substrate has been previously described (37). In brief, 0.2-μm-diameter fluorescent submicrobeads were embedded in the polyacrylamide gel (5%) and cross-linked by bis-acrylamide (0.1% or 0.3%). These fluorescent submicrobeads were used to track the deformation of the gel (52).
Traction force microscopy.
To determine the displacement field of the gel beneath individual adherent cells, images of the same region of the gel were taken at different times before or after experimental interventions. The position of the fluorescent beads in the gel in the absence of traction was determined at the end of each experiment by releasing the cells with trypsin; this traction-free image was used as a reference. The displacement field was determined by measuring the changes in the position of corresponding fluorescent beads between the reference (cell free) image and the image containing a cell. The two-dimensional fast Fourier transform algorithm in MATLAB was used to calculate the correlation functions. The traction field was calculated from the displacement field, implementing the solution described by Butler et al. (6). This calculation was based on the Boussinesq solution for the displacement field on the surface of a semi-infinite solid when the distribution of surface traction is known. Writing the displacements as a convolution of tractions and the kernel that maps tractions to displacements and taking the Fourier transform of this relation yield the solution for the traction field on the surface when the surface displacement field and the gel elastic properties are known. The boundary conditions were as follows: 1) zero traction outside of the cell-gel interfacial area and 2) the displacement field within the cell-gel interface that matched the experimentally observed displacements within the cell boundary.
Adenovirus transfection assay.
The adenovirus fluorescent protein assay was developed and used following previously described protocols (32). After the cells had reached 70–80% confluency, adenovirus containing YFP-mitochondria were added at 150 μl/well (6-well dish) for 2 days, and transfection efficiency was examined under a fluorescent microscope.
Microscopy.
A Leica inverted microscope was integrated with a magnetic twisting device and a Dual-View system (Optical Insights, Tucson, AZ) to simultaneously acquire both CFP and YFP emission images in response to stress. For emission ratio imaging, the Dual-View Micro-Imager (Optical Insights) was used. CFP/YFP Dual EX/EM (FRET) (OI-04-SEX2) has the following filter sets: CFP, excitation S430/25 and emission S470/30; and YFP, excitation S500/20 and emission S535/30. The emission filter set uses a 515-nm dichroic mirror to split the two emission images. Cells were illuminated with a 100-W Hg lamp. For FRET imaging, each CFP image (1,344 × 512 pixels) and each YFP image (1,344 × 512 pixels) were simultaneously captured on the same screen using a charge-coupled device camera (Hamamatsu C4742-95-12ERG) and a ×40 (0.55 numerical aperture) air objective or a ×63 (1.32 numerical aperture) oil-immersion objective.
Optical magnetic twisting cytometry.
Optical magnetic cell twisting is an extension of the magnetic cell twisting technique (50) to oscillatory forcing. The technique of applying twisting torques to cells in a dish under a microscope has been described in detail (11). The microscope stage was heated to maintain 37°C for the cells in a dish. The twisting current was driven by a current source controlled by a computer. Ferromagnetic beads (∼4 μm diameter) coated with saturated amount of Arg-Gly-Asp (RGD)-containing peptides (the ligand density on the bead was measured to be ∼1 RGD-peptide per 2 nm2 of bead surface area), ligands for integrin receptors, were bound the surface of adherent cells for 15 min. The binding specificity of bead binding was determined following previously described protocols (50). The magnetic moments of each batch of self-made ferromagnetic beads were calibrated according to previously published methods (50). The beads are magnetized by a strong (1,000 G) and short (<0.1 ms) magnetic field pulse oriented at the horizontal direction using the magnetizing coils. A sinusoidally varying vertical magnetic “twisting” field is then applied, and the resulting bead translational displacements induced by bead rotation are determined by quantifying the bead center movement using an intensity-weighted center of mass algorithm (11). We measured the complex stiffness (G*) as a function of frequency by applying an oscillatory magnetic field and measuring the resultant oscillatory bead motions using the follwing relation: G* = T/d, where T is the oscillatory specific torque resulting from the oscillatory magnetic field of different frequencies and d is the induced horizontal displacement of the beads measured using a charge-coupled device camera attached to an inverted optical microscope. The measured complex stiffness has the units of torque per unit bead volume per unit bead displacement (Pa/nm) and can be separated into elastic (storage) stiffness and dissipative (loss) stiffness. For varying magnitudes, the applied twisting fields were 10, 18, 25, 50, and 75 G. The bead constant was 0.31 Pa/G. Hence, the applied stress was 3.1, 5.58, 7.75, 15.5, or 23.25 Pa, since stress was equal to the bead constant times the twisting field. Two different loading protocols were used to keep the probing time around 10 min for the frequency scan. In the first, the oscillatory frequency sweep points were 0.01, 0.1, 1, 10, 100, and 1,000 Hz; in the second, they were 0.002, 0.04, and 0.2 (the applied stress was fixed at 8.75 Pa). If one assumes a bead-cell contact area (generally ∼10% of bead surface area) and uses a model to convert stiffness (Pa/nm) to modulus (Pa) (28), 1 Pa/nm stiffness is equivalent to 6.8 kPa modulus. F-tests or t-tests were used for statistical analysis.
Analysis of long distance force propagation.
The magnetic bead lateral movement was used to calculate the corresponding dynamic modulus (11, 16). Fluorescent image acquisition of YFP-mitochondria or mCherry-tubulin was phase locked to the twisting field such that 10 images were taken during one twisting cycle of 3.2 s. Therefore, the temporal resolution was 0.32 s. To reduce noise caused by spontaneous cytoskeletal movements, images were taken during the same twisting phase over 3–10 cycles. Averaged images were cropped to a size of 32 μm square. Images were then subdivided into arrays of 11 × 11 pixels (2.2 × 2.2 μm), which were overlapped by 5 pixels. The deformation field was obtained by comparing corresponding arrays between two images taken at different phases during the twisting cycle. We shifted the arrays of the second image by subpixel increments (4 nm) in the Fourier domain until the mean square differences of the pixel intensities between the shifted array and the corresponding array from the first image reached a minimum. The resolution of the displacements was ∼5 nm (17). Stress fields were computed from displacement fields, and the complex modulus was estimated from the magnetic bead using previously published methods (16).
FRET image analysis.
A customized MATLAB (Mathworks) program was used to obtain YFP-to-CFP emission ratios. CFP and YFP images at each time point were first background subtracted, and the YFP image was used to generate a binary mask based on an input threshold so that the pixel value inside the cell was set to 1 and the pixel value outside the cell was set to 0. After multiplication of the original YFP image by the mask image, this updated YFP image and the CFP image were aligned pixel by pixel by maximizing the normalized cross-correlation coefficient of the CFP and YFP images (29). Aligned YFP-to-CFP emission ratios were normalized to the lower emission ratio and displayed as a linear pseudocolor.
Due to the nature of the simultaneous ratiometric FRET imaging approach, the heterogeneous noise engendered from excitation light intensity, cell size/thickness, and biosensor expression levels are canceled out and eliminated by taking the ratio of the donor to acceptor on the same biosensor molecule. Furthermore, the percentile changes of FRET before and after stimulation were obtained from the same live cell, which eliminated the noise engendered from the heterogeneity among different cells. Hence, the ratiometric FRET imaging data are much more reliable than intensity-based information obtained from different groups of cells. It is a generally accepted practice that a large number of cells is not needed for statistical analysis of FRET imaging data.
RESULTS
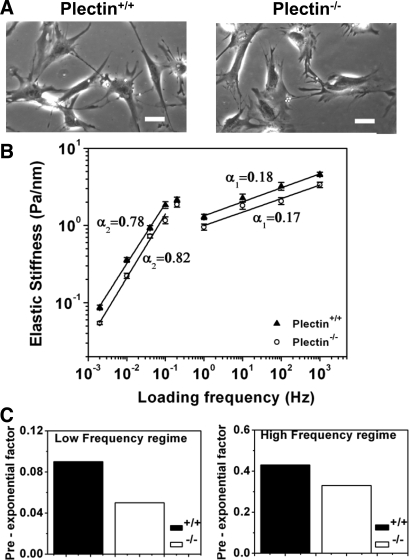
Plectin contributes to cell elastic stiffness but not the frequency response.
To characterize the role of plectin in rheological properties of cells, we compared the frequency responses of plectin−/− skin fibroblasts with those of plectin+/+ cells (2) when local stresses were applied via integrin receptors. Both cell types exhibited similar cell spreading under similar culture conditions (Fig. 1A), but plectin−/− cells were less stiff than plectin+/+ cells in response to the same applied stress (Fig. 1B). Preexponential factors (Fig. 1C) were decreased by ∼30–60% in plectin−/−cells, suggesting that plectin contributes to the magnitude of cell elastic stiffness. By definition, cell stiffness =A × fα, where A is the preexponential factor that is independent of frequency but is dependent on the intrinsic stiffness and prestress of the cell (4), f is the loading frequency, and α is the power law exponent. These differences in elastic stiffness cannot be attributed to the lack of focal adhesions or actin recruitments since GFP-zyxin and mCherry-actin were similarly recruited to the magnetic beads on plectin−/− cells (Supplemental Fig. S1).1 Both cell types exhibited similar power law behaviors at 1–1,000 Hz: the weak power law exponent α1 was 0.18 for plectin+/+ cells and 0.17 for plectin−/− cells. At lower frequencies (0.002–0.1), the strong power law exponent α2 was 0.78 for plectin+/+ cells and 0.82 for plectin−/− cells. The two power laws were separated by a transition region. The power law exponents are a measure of how fluid like a cell behaves: for a pure elastic solid, the power law exponent is 0; for a pure viscous fluid, the exponent is 1. When the exponents are between 0 and 1, the cell behaves viscoelastically. These data suggest that plectin does not contribute to the viscous behavior of the cells in both frequency ranges.
Fig. 1.
Plectin-deficient (plectin−/−) cells are less stiff than wild-type (plectin+/+) cells. A: phase-contrast images of plectin+/+ and plectin−/− cells showing that they are similar in cell spreading after being plated overnight on collagen-I-coated dishes. Scale bars = 40 μm. B: plectin−/− cells are less stiff than plectin+/+ cells, but power law slopes are similar in response to varying forcing frequencies. The applied stress was 8.75 Pa. Elastic stiffness data are shown. The weak power law exponent α1 (equal to 0.18) of plectin+/+ cells was similar to that of plectin−/− cells (α1 = 0.17), although they were statistically different (P < 1 × 10−5); the strong power law α2 (equal to 0.78) of plectin+/+ cells was different from that of plectin−/− cells (α2 = 0.82; P < 0.02). n = 415 beads and ∼300 plectin+/+ cells, and n = 327 beads and ∼250 plectin−/− cells. When α1 was compared with α2 within the same cell type, P < 8.1 × 10−7 for plectin+/+ cells and P < 3.1 × 10−5 for plectin−/− cells. Values are means ± SE. C: plectin+/+ cells exhibited higher preexponential factors than plectin−/− cells. On a log-log scale, the two power law data were fitted with the following equation: G′ = A × (f/f0)α, where G′ is the geometeric mean ± geometric SE, f is the loading frequency, and f0 is the lowest measurable frequency (0.002 Hz). The preexponential factor A (in Pa/nm) can be converted to units of kPa by multiplying by 6.8 (see materials and methods).
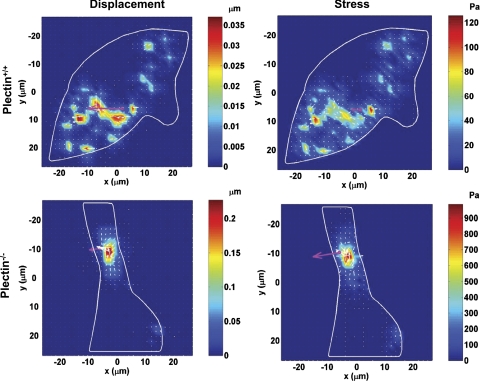
Plectin is necessary in long distance force propagation.
Recently, it has been shown that prestressed actin bundles are necessary for long distance stress propagation (16–18). However, the role of major cross-linking proteins in stress propagation and distribution inside living cells remains elusive. To explore the role of plectin in stress propagation in a living cell, we compared plectin−/− cells with wild-type controls. Wild-type plectin+/+ fibroblast cells, transfected with YFP-mitochondria as a marker of cytoskeletal deformation (16) (Supplemental Fig. S2), exhibited long distance stress propagation (Fig. 2), consistent with previous findings in normal smooth muscle cells. In contrast, plectin−/− cells did not. This is somewhat surprising since it has been shown that these plectin−/− cells have more actin bundles and more focal adhesions than wild-type cells after the first several hours of plating and similar numbers after longer plating times (2). If one reasoned that the presence of actin bundles and focal adhesions was necessary and sufficient for long distance propagation, one would expect that plectin−/− cells should also exhibit long distance force propagation.
Fig. 2.
Living cells failed to propagate mechanical stresses to long distances in the absence of plectin. Top: a wild-type skin fibroblast transfected with yellow fluorescent protein (YFP)-mitochondria exhibited numerous sites of strain (left) and stress focusing (right) at the remote cytoplasm in response to a localized mechanical loading [50 G (17.5 Pa) at 0.3 Hz]. Cells were plated for ∼4 h in serum-free medium before mechanical measurements. Supplemental Fig. S2 shows a bright-field image of the cell with the bead and the fluorescent image of YFP-mitochondria. Bottom: a plectin−/− cell only exhibited local deformation (left) and local stresses (right) in response to the same mechanical loading. White arrows and colors represent the directions and magnitudes of the displacements or stresses. The pink arrow represents the direction and magnitudes of the bead displacement. Note that the maximum displacement in the plectin+/+ cell was only ∼0.035 μm, whereas the maximum displacement in the plectin−/− cell was ∼0.2 μm, indicating that the peak cytoskeletal deformation in the plectin+/+ cell is much less than that in the plectin−/− cell, consistent with the results showing that plectin+/+ cells are more stiff than plectin−/− cells. The stress map was computed from the displacement map and the assumed average complex modulus of the cell. Since the input energy (the total input torque) was the same for both types of cells and there was long distance force propagation in the plectin+/+ cell, the peak stress must be much lower in the plectin+/+ cell (∼120 Pa) than that in the plectin−/− cell (∼900 Pa), where the stress is localized only around the site of torque application. Two other cells each exhibited similar behaviors in two separate experiments.
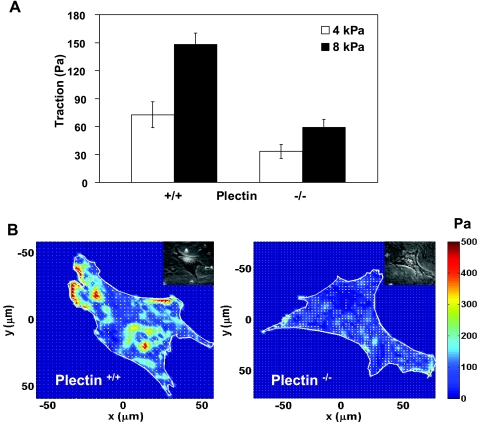
Plectin−/− cells exert lower traction forces.
Previous work has shown that the cytoskeletal tension (prestress) is important in mediating long distance stress propagation (16). We reasoned that in the absence of plectin, the abolishment of IF connections with focal adhesions and with myosins might reduce the tension in the cytoskeleton. To explore if this was the case, we measured traction forces in both cell types. Traction measurements show that root mean square traction forces in plectin−/− cells were much less than those in plectin+/+ cells (Fig. 3). Differences in the two cell types became greater on stiffer substrates (Fig. 3, A and B). These results suggest that the failure of plectin−/− cells to mediate long distance force propagation may be due to a reduction of prestress in actin bundles, since the distribution and structure of microtubules (MTs) in both plectin−/− and plectin+/+ cells are similar (2).
Fig. 3.
Plectin−/− cells exerted lower tractions than plectin+/+ cells. A: tractions (root mean square) of plectin+/+ cells and plectin−/− cells cultured under identical serum-free conditions for 4–6 h (means ± SE, P < 0.005). The substrate stiffness was 4 kPa (n = 6 cells each) or 8 kPa (n = 9 cells each). B: representative cell traction map of either a plectin+/+ cell or a plectin−/− cell on a substrate stiffness of 8 kPa. Insets show phase-contrast images.
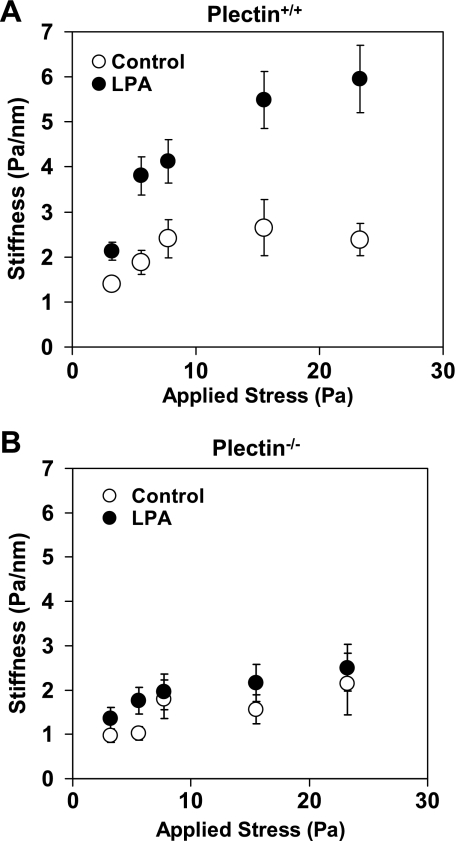
Plectin−/− cells do not stiffen in response to LPA.
To further explore how mechanical signaling pathways in plectin−/− cells might have changed, we examined how they responded to LPA, a drug that activates the Rho pathway to increase contractility of the cell (23, 39). It has been established that wild-type and plectin−/− cells have functional LPA receptors on their surface (2). Both plectin−/− and plectin+/+ cells stiffened in response to increasing applied stresses, i.e., exhibited a nonlinear stress-strain relationship (Fig. 4, A and B), although plectin−/− cells appeared to stiffen somewhat less than plectin+/+ cells. Interestingly, plectin−/− cells did not stiffen further when treated with LPA (1 μg/ml for up to 2 min; Fig. 4B), whereas plectin+/+ cells stiffened dramatically in response to LPA (Fig. 4A). These results are consistent with previous findings on the lack of a reorganization response in plectin−/− cells in response to serum starvation (2).
Fig. 4.
Different stiffening responses in plectin+/+ and plectin−/− cells to loading magnitudes and lysophosphatidic acid (LPA). A: plectin+/+ cells stiffened in response to increases in applied stresses at loading frequency of 0.75 Hz (controls). Complex stiffness data are shown. Plectin+/+ cells further stiffened dramatically in response to treatment of LPA (1 μg/ml for 2 min). B: plectin−/− cells stiffened slightly to the applied stresses (controls). However, they did not stiffen further in response to LPA. n = 80 beads (∼60 cells) for plectin+/+ cells, and n = 89 beads (∼70 cells) for plectin−/− cells. Cells were plated 4–6 h before mechanical measurements. Results are presented as means ± SE.
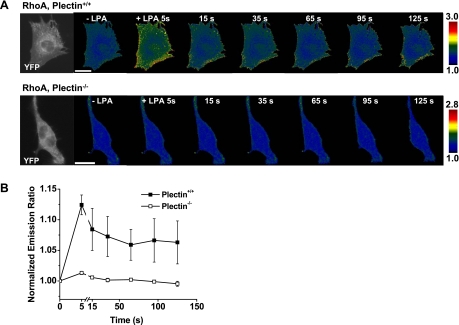
RhoA GTPases are not activated in plectin−/− cells in response to LPA.
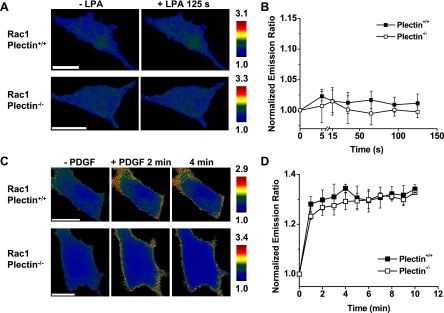
To further determine why plectin+/+ but not plectin−/− cells stiffen after LPA treatment, we quantified the activation of RhoA in cells transfected with a CFP-YFP RhoA cytosolic sensor by measuring temporal changes of FRET ratios (13a). FRET changes were detected as early as 5 s (the earliest possible time point after the addition of LPA to the medium) in the periphery of plectin+/+ cells (Fig. 5). In contrast, there were no observable changes of FRET ratios of RhoA in plectin−/− cells in response to LPA up to several minutes (Fig. 5), suggesting that the Rho signaling pathway was compromised in the absence of plectin. Since Rac proteins play an antagonistic role against Rho proteins (33), we wondered if the lack of stiffening in plectin−/− cells could also be partially due to the activation of Rac by LPA in these cells. However, after treatment with LPA, no FRET ratio changes were observed in either plectin+/+ or plectin−/− cells transfected with CFP-YFP Rac1 cytosolic biosensors (Fig. 6, A and B). The unresponsiveness of these cells to LPA was not due to general defects in Rac1 in these cells, since both plectin+/+ and plectin−/− cells activated Rac1 in response to PDGF (Fig. 6, C and D), a soluble growth factor known to activate Rac (42). Taken together, these findings strongly suggest that the lack of a stiffening response to LPA in plectin−/− cells is mostly due to an inability to activate RhoA in these cells.
Fig. 5.
Plectin+/+ but not plectin−/− cells activated RhoA in response to LPA. A, top: time-lapse images of a representative plectin+/+ cell transfected with the RhoA biosensor. Fluorescence resonance energy transfer (FRET) changes were detected as early as 5 s after LPA (1 μg/ml) treatment in plectin+/+ cells. Bottom, time-lapse images of a representative plectin−/− cell transfected with the RhoA biosensor. No FRET changes were detected in plectin−/− cells after LPA treatment. The far left images in both the top and bottom show YFP-RhoA biosensor fluorescence in the plectin+/+ and plectin−/− cell. Note that both cells expressed biosensors well. B: normalized YFP-to-cyan fluorescent protein (CFP) emission ratio of plectin+/+ (n = 8) and plectin−/− (n = 9) cells from three separate experiments as a function of time after LPA treatment. Cells were plated for 24 h for transfection and then serum was withdrawn 4 h before experiments. Results are presented as means ± SE. Scale bars = 20 μm.
Fig. 6.
Rac was activated by PDGF but not by LPA. A: time-lapse images of representative plectin+/+ and plectin−/− cells transfected with the Rac1 biosensor. No FRET changes were detected from 5 s up to 125 s after LPA treatment (1 μg/ml). For clarity, only images before and 125 s after LPA are shown. B: normalized YFP-to-CFP emission ratio of plectin+/+ (n = 7) and plectin−/− (n = 6) cells treated with LPA. C: time-lapse images of Rac1 activation in representative PDGF-treated plectin+/+ and plectin−/− cells. D: normalized YFP-to-CFP emission ratios of plectin+/+ and plectin−/− cells treated with PDGF (10 ng/ml). Culture conditions were the same as those in Fig. 5. Results are presented as means ± SE. Scale bars = 20 μm.
Expression of constitutively active RhoA in plectin−/− cells restores long distance force propagation behavior.
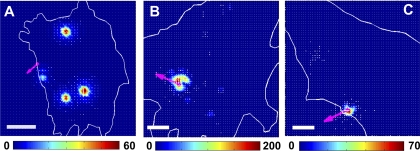
To further determine the role of active RhoA in mechanical behaviors of living cells, we measured stress propagation in plectin−/− cells when different forms of RhoA and mCherry-tubulin were cotransfected in the same cells. Recently, we (49) have shown that the structural basis for the stress propagation observed in YFP-mitochondria-transfected cells originates from the deformation of MTs. Therefore, we measured MT deformation directly in response to a local stress application. Expression in plectin−/− cells of a constitutively active RhoA (RhoA-V14), but not a dominant negative mutant of RhoA (RhoA-N19) or an empty vector, restored the long distance force propagation behavior (Fig. 7 and Supplemental Fig. S3), suggesting that plectin is important in the proper activation of RhoA, which, in turn, facilitates the long distance force propagation behavior in living cells, possibly by elevating the tension in actin bundles/stress fibers.
Fig. 7.
Long distance force propagation behavior depended on RhoA activity. A–C: deformation maps of representative plectin−/− cells transfected with a constitutively active RhoA (RhoA-V14; A) (4 different other cells showed similar effects), a dominant mutant of RhoA (RhoA-N19; B) (3 different other cells showed similar effects), or an empty vector (C) (2 different other cells showed similar effects). The same local stress was applied each cell at 17.6 Pa at 0.3 Hz to integrin receptors. mCherry-tubulin (Supplemental Fig. S3) deformation was quantified using previously published methods (49). Pink arrows represent the bead movement direction. Note that the expression of RhoA-V14 but not RhoA-N19 or the empty vector in plectin−/− cells restored long distance stress propagation. Color bar units are in nanometers. Scale bars = 10 μm. The transfection of these RhoA mutants was confirmed by the transfection of mCherry-tubulin into the same cell. Only cells that expressed mCherry-tubulin and thus the RhoA mutants were chosen for further experiments. There were no biases in this selection criterion since active RhoA, negative dominant RhoA, and empty vector were all selected the same way on the basis of expression the same amount of fluorescence (Supplemental Fig. S3). Cells were plated for 24 h for transfection and serum was then withdrawn 2–3 h before mechanical measurements.
DISCUSSION
We have shown that plectin plays important mechanical roles in cell stiffness, stress propagation in the cytoplasm, traction generation, and RhoA GTPase activation in response to LPA. All these findings in living cells in response to mechanical or chemical stimulation significantly extend previously published work on the structural and biological roles of plectin (2, 33) and provide a mechanical basis for the observed biological functions of plectin.
Cell shear stiffness is a fundamental measure of how much a living cell resists external mechanical deformation (52). The fact that plectin−/− cells are less stiff than plectin+/+ cells under similar culture conditions shows that plectin significantly contributes to cell shape stability. This finding is consistent with the recent result that plectin−/− cells are less resistant to osmotic swelling (34). At this time, we do not know the exact mechanism of how plectin contributes to cell stiffness. However, there is increasing evidence that plectin binds to IFs and anchors them at peripheral junctions, such as hemidesmosomes (41) and focal adhesions (5, 15, 44), thereby contributing to cell stiffness by stabilization of cytoskeletal structures and focal adhesions.
In addition, we speculate that the appropriate positioning of IFs at focal adhesions to bear some of the myosin-dependent tension might be important for mechanical/biochemical feedback loops in living cells. This possibility is consistent with our finding that plectin−/− cells generate half as much traction forces as plectin+/+ cells on substrates whose rigidity is similar in magnitude to that of the cells; it is known that prestress in the cell plays a major role in determining cell shear stiffness (52). The substrate stiffness in our study was 4 or 8 kPa, which is within the range of soft tissues (1–20 kPa). It has been shown that sizes and tractions of focal adhesions increase with elastic moduli of the substrate (14). Therefore, our data that the differences in tractions between plectin+/+ and plectin−/− cells are greater on stiffer substrates are consistent with published results.
Our data of higher actin recruitments (within ∼15 min) to the bead on plectin−/− cells are consistent with previously published data showing that during early adhesion, there are more focal adhesions and more stress fibers in plectin−/− cells than in plectin+/+ cells (2). The higher number of focal adhesions and stress fibers in plectin−/− cells might be due to the compensatory response of the cytoskeletal remodeling to the lack of plectin. There is a distinction between cell adhesion and cell stiffness. For example, it is known that in vimentin knockout cells, there is an increase in cell adhesion but cell stiffness is much lower (∼30–40%) than in wild-type cells (10, 51). It is also known that it is the tension in the stress fibers, not just the stress fiber number, that dictates the magnitude of cell stiffness (16, 17). There is also a difference between cell adhesion and cell traction. Traction generation depends not only on F-actin but, more importantly, on myosin II, which, in turn, depends largely on RhoA activity and myosin light chain activity. The lower stiffness and lower tractions (prestress) in plectin−/− cells are consistent with published data showing that prestress and stiffness are tightly coupled in normal cells and that cell prestress dictates cell stiffness (52).
It is interesting that both plectin−/− and plectin+/+ cells exhibited a similar weak power law (exponent α1 = 0.17–0.18) at frequencies higher than 1 Hz. At frequencies lower than 0.1 Hz, plectin−/− cells exhibited a higher power law (exponent α2 ≈ 0.82) than plectin+/+ cells (exponent α2 ≈ 0.78), suggesting that the presence of plectin influences only the slow dynamics of living cells. The existence of two power laws in our cells is consistent with previously published results in other cell types (46) and is not consistent with the timescale free hypothesis, which states that there should be only one power law (8). Importantly, we (7) have recently shown that the molecular mechanism of the two power laws originates from a nonequilibrium to an equilibrium transition of noncovalent bonds. These findings suggest that plectin does not contribute to the nonequilibrium to equilibrium transition of noncovalent bonds. Our data of preexponential factors of the power laws in wild-type cells ranged from 0.5 to 2.9 kPa, consistent with previously published results using different techniques in living cells (0.16–3.66 kPa) (4) and in nuclei (0.15–0.83 kPa) (36). It is interesting that the absence of plectin reduces these preexponential factors by ∼30% (Fig. 1C), suggesting that plectin does play an important role in the elastic stiffness of the cell, consistent with the implications from the identified molecular structures of plectin (45).
We found that Rho activation by LPA in wild-type cells is mostly cortical, consistent with previously published data (38). We are not clear on how plectin deficiency interferes with the RhoA signaling pathway, but it may be related to the lack of/misguided anchoring sites for these RhoA proteins in the absence of plectin that prevents them to be activated by LPA. It is possible that RhoA proteins need the presence of plectin in anchoring at the focal adhesions and/or assuming proper conformation to contribute to traction generation by myosin II or long distance force propagation. No matter what exact molecular roles plectins might play in affecting cell mechanical functions, the fact that several important cell mechanical properties are changed in the absence of plectin suggests that plectin protein contributes significantly to cell mechanical and biological functions. Future study is needed in elucidating the molecular pathways between plectin and RhoA.
Recently, we (30) have demonstrated that rapid signal transduction by a local mechanical stress is a unique feature of mechanotransduction. This behavior is fundamentally different from that of soluble molecule-induced signal transduction (29). It is now clear that it is essential for a living cell to possess the capacity of long distance force propagation to exhibit this unique mechanotransduction behavior. Our present findings that plectin−/− cells exert lower tractions and thus do not propagate forces to remote sites in the cytoplasm suggest that plectin is also important in mediating rapid mechanotransduction.
Since cytoskeletal cross-linking proteins play important roles in the structure and function of living cells, it is interesting to compare the mechanical roles of different cross-linking proteins. The lower traction and lower stiffness features exhibited by plectin−/− cells are similar to those by filamin A (an actin cross-linking protein)-deficient cells compared with their respective controls (21). However, there are several major differences. First, filamin A-deficient cells exhibit numerous membrane blebbings, whereas plectin−/− cells do not. Second, the overall cell shape is very different for filamin A-deficient cells and their controls. In contrast, plectin−/− cells have very similar overall cell shapes as wild-type cells. Our present results are consistent with a recent report (34) from one of our groups on elevated susceptibility of plectin−/− keratinocytes to osmotic stress challenge. The present mechanical data of plectin−/− cells are also in line with the previous work (2) showing that these plectin−/− cells exhibited lower migration rates and slower wound healing; similar reductions in migration rates and wound healing have also been observed in vimentin-deficient cells (10). It is also known that vimentin-deficient cells exhibit lower contractile forces (10), lower cell stiffness, and lower stiffening responses (51). The fact that plectin−/− cells behave mechanically and biologically in a manner similar to vimentin-deficient cells suggests that they may share a common mechanism: a compromised cytoskeletal tension in these deficient cells may partly be the cause for their abnormal biological functions. Myosin-dependent feedback loops appear to be critical for living cells to sense the rigidity of their substrate microenvironment (9) and to achieve appropriate cell shape stability (i.e., cell shear stiffness) (52). Currently, the exact mechanism for this feedback response is not clear. However, several recent reports (9, 30) have suggest that it may be related to both inside-out and outside-in stress propagation across the cell membrane via the cytoskeleton and the activation of key signaling proteins for cell remodeling. Future studies are needed to understand how plectin might help in the regulation of the myosin-dependent feedback loop.
GRANTS
This work was supported by National Institute of General Medical Sciences Grant GM-072744 (to N. Wang), by the University of Illinois at Urbana-Champaign (to N. Wang), by Wallace H. Coulter Foundation and Beckman Laser Institute, Incorporated (to Y. Wang), and by the Austrian Science Research Fund (to G. Wiche).
Supplementary Material
Acknowledgments
We thank Dr. Michiyuki Matsuda (Kyoto University) and Dr. Jun-ichi Miyazaki (Osaka University) for the gifts of RhoA and the original Rac1 biosensors, Dr. Fei Wang for GFP-zyxin, and Dr. R. Tsien for the gifts of mCherry-actin and mCherry-tubulin. We also thank Y. C. Poh for technical assistance.
Part of these results was presented at the China-Overseas Joint Workshop on Biomechanics in Guangzhou, China, on July 4–8, 2007.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Supplemental material for this article is available online at the American Journal of Physiology-Cell Physiology website.
REFERENCES
- 1.Ackerl R, Walko G, Fuchs P, Fischer I, Schmuth M, Wiche G. Conditional targeting of plectin in prenatal and adult mouse stratified epithelia causes keratinocyte fragility and lesional epidermal barrier defects. J Cell Sci 120: 2435–2443, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Andrä K, Nikolic B, Stocher M, Drenckhahn D, Wiche G. Not just scaffolding: plectin regulates actin dynamics in cultured cells. Genes Dev 12: 3442–3451, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrä K, Lassmann H, Bittner R, Shorny S, Fässler R, Propst F, Wiche G. Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture. Genes Dev 11: 3143–56, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balland M, Desprat N, Icard D, Féréol S, Asnacios A, Browaeys J, Hénon S, Gallet F. Power laws in microrheology experiments on living cells: comparative analysis and modeling. Phys Rev E Stat Nonlin Soft Matter Phys 74: 021911, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bershadsky AD, Tint IS, Svitkina TM. Association of intermediate filaments with vinculin-containing adhesion plaques of fibroblasts. Cell Motil Cytoskeleton 8: 274–28, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Estimating traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282: C595–C605, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury F, Na S, Collin O, Tay B, Li F, Tanaka T, Leckband DE, Wang N. Is cell rheology governed by nonequilibrium to equilibrium transition of noncovalent bonds? Biophys J 95: 719–727, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng L, Trepat X, Butler JP, Millet E, Morgan KG, Weitz DA, Fredberg JJ. Fast and slow dynamics of the cytoskeleton. Nat Mater 5: 636–640, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber DE, Merckling A, Langa F, Aumailley M, Delouvee A, Koteliansky V, Babinet C, Krieg T. Impaired mechanical stability, migration, and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci 111: 1897–1907, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Fabry B, Maksym GM, Shore SA, Moore PE, Panettieri RA Jr, Butler JP, Fredberg JJ. Time course and heterogeneity of contractile responses in cultured human airway smooth muscle cells. J Appl Physiol 91: 986–994, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Foisner R, Wiche G. Structure and hydrodynamic properties of plectin molecules. J Mol Biol 198: 515–531, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs P, Zörer M, Rezniczek GA, Spazierer D, Oehler S, Castañón MJ, Hauptmann R, Wiche G. Unusual 5′ transcript complexity of plectin isoforms: novel tissue-specific exons modulate actin binding activity. Hum Mol Genet 8: 2461–2472, 1999. [DOI] [PubMed] [Google Scholar]
- 13a.Giepmans BN, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science 312: 217–224, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol 172: 259–268, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzales M, Weksler B, Tsuruta D, Goldman RD, Yoon KJ, Hopkinson SB, Flitney FW, Jones JC. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol Biol Cell 12: 85–100, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu S, Chen J, Fabry B, Numaguchi Y, Gouldstone A, Ingber DE, Fredberg JJ, Butler JP, Wang N. Intracellular stress tomography reveals stress focusing and structural anisotropy in the cytoskeleton of living cells. Am J Physiol Cell Physiol 285: C1082–C1090, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Hu S, Eberhard L, Chen J, Love JC, Butler JP, Fredberg JJ, Whitesides GM, Wang N. Mechanical anisotropy of adherent cells probed by a three dimensional magnetic twisting device. Am J Physiol Cell Physiol 287: C1184–C1191, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Hu S, Chen J, Butler JP, Wang N. Prestress mediates force propagation into the nucleus. Biochem Biophys Res Commun 329: 423–428, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol 22: 6582–6591, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson CP, Tang HY, Carag C, Speicher DW, Discher DE. Forced unfolding of proteins within cells. Science 317: 663–666, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasza KE, Nakamura F, Hu S, Kollmannsberger P, Bonakdar N, Fabry B, Stossel TP, Wang N, Weitz DA. Filamin A is essential for active cell stiffening but not passive stiffening under external force. Biophys J. In press. [DOI] [PMC free article] [PubMed]
- 22.Ketema M, Wilhelmsen K, Kuikman I, Janssen H, Hodzic D, Sonnenberg A. Requirements for the localization of nesprin-3 at the nuclear envelope and its interaction with plectin. J Cell Sci 120: 3384–3394, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Kole TP, Tseng Y, Huang L, Katz JL, Wirtz D. Rho kinase regulates the intracellular micromechanical response of adherent cells to rho activation. Mol Biol Cell 15: 3475–3484, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konieczny P, Wiche G. Muscular integrity–a matter of interlinking distinct structures via plectin. In: The Sarcomere and Skeletal Muscle Disease, edited by Laing NG. Berlin: Landes Bioscience-Springer, 2007.
- 25.Konieczny P, Fuchs P, Reipert S, Kunz WS, Zeöld A, Fischer I, Paulin D, Schröder R, Wiche G. Myofiber integrity depends on desmin network targeting to Z-disks and costameres via distinct plectin isoforms. J Cell Biol 181: 667–81, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koster J, van Wilpe S, Kuikman I, Litjens SH, Sonnenberg A. Role of binding of plectin to the integrin beta4 subunit in the assembly of hemidesmosomes. Mol Biol Cell 15: 1211–1223, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mierke CT, Kollmannsberger P, Zitterbart DP, Smith J, Fabry B, Goldmann WH. Mechano-coupling and regulation of contractility by the vinculin tail domain. Biophys J 94: 661–670, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mijailovich SM, Kojic M, Zivkovic M, Fabry B, Fredberg JJ. A finite element model of cell deformation during magnetic bead twisting. J Appl Physiol 93: 1429–1436, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Na S, Wang N. Application of fluorescence resonance energy transfer and magnetic twisting cytometry to quantify mechanochemical signaling activities in a living cell. Sci Signal 1: l1, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci USA 105: 6626–6631, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Numaguchi Y, Huang S, Polte TR, Eichler GS, Wang N, Ingber DE. Caldesmon-dependent switching between capillary endothelial cell growth and apoptosis through modulation of cell shape and contractility. Angiogenesis 6: 55–64, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodeling. Nat Cell Biol 8: 803–814, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Osmanagic-Myers S, Gregor M, Walko G, Burgstaller G, Reipert S, Wiche G. Plectin-controlled keratin cytoarchitecture affects MAP kinases involved in cellular stress response and migration. J Cell Biol 174: 557–568, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang M, Sun J, Chien S, Wang Y. Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors. Proc Natl Acad Sci USA 105: 14353–14358, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci USA 104: 15619–15624, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelham RJ Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94: 13661–13665, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440: 1069–1072, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J 18: 578–585, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci 113: 3673–3678, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Rezniczek GA, de Pereda JM, Reipert S, Wiche G. Linking integrin α6β4-based cell adhesion to the intermediate filament cytoskeleton: direct interaction between the β4 subunit and plectin at multiple molecular sites. J Cell Biol 141: 209–225, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70: 401–410, 1992. [DOI] [PubMed] [Google Scholar]
- 43.Roy P, Petroll WM, Cavanagh HD, Jester JV. Exertion of tractional force requires the coordinated up-regulation of cell contractility and adhesion. Cell Motil Cytoskeleton 43: 23–34, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Seifert GJ, Lawson D, Wiche G. Immunolocalization of the intermediate filament-associated protein plectin at focal contacts and actin stress fibers. Eur J Cell Biol 59: 138–147, 1992. [PubMed] [Google Scholar]
- 45.Sonnenberg A, Rojas AM, de Pereda JM. The structure of a tandem pair of spectrin repeats of plectin reveals a modular organization of the plakin domain. J Mol Biol 368: 1379–1391, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Stamenovic D, Rosenblatt N, Montoya-Zavala M, Matthews BD, Hu S, Suki B, Wang N, Ingber DE. Rheological behavior of living cells is timescale-dependent. Biophys J 93: L39–L41, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svitkina TM, Verkhovsky AB, Borisy GG. Plectin sidearms mediates interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J Cell Biol 135: 991–1007, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stossel TP On the crawling of animal cells. Science 260: 1086–1094, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Wang N Structural basis of stress concentration in the cytoskeleton. Mol Cell Biomech 141: 1–12, 2009. [PubMed] [Google Scholar]
- 50.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260: 1124–1127, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Wang N, Stamenovic D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am J Physiol Cell Physiol 279: C188–C194, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Wang N, Tolic-Norrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol 282: C606–C616, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Wiche G Plectin: general overview and appraisal of its potential role as a subunit protein of the cytomatrix. Critic Rev Biochem Mol Biol 24: 41–67, 1989. [DOI] [PubMed] [Google Scholar]
- 54.Winter L, Abrahamsberg C, Wiche G. Plectin isoform 1b mediates mitochondrion-intermediate filament network linkage and controls organelle shape. J Cell Biol 181: 903–911, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N, Nagashima K, Matsuda M. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol 162: 223–232, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol 9: 858–867, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.