Abstract
Extracellular inorganic pyrophosphate (PPi) is a potent suppressor of physiological calcification in bone and pathological calcification in blood vessels. Ectonucleotide pyrophosphatase/phosphodiesterases (eNPPs) generate PPi via the hydrolysis of ATP released into extracellular compartments by poorly understood mechanisms. Here we report that cultured vascular smooth muscle cells (VSMC) from rat aorta generate extracellular PPi via an autocrine mechanism that involves ATP release tightly coupled to eNPP activity. The nucleotide analog β,γ-methylene ATP (MeATP or AMPPCP) was used to selectively suppress ATP metabolism by eNPPs but not the CD39-type ecto-ATPases. In the absence of MeATP, VSMC generated extracellular PPi to accumulate ≥600 nM within 2 h while steadily maintaining extracellular ATP at 1 nM. Conversely, the presence of MeATP completely suppressed PPi accumulation while increasing ATP accumulation. Probenecid, which inhibits PPi efflux dependent on ANK, a putative PPi transporter or transport regulator, reduced extracellular PPi accumulation by approximately twofold. This indicates that autocrine ATP release coupled to eNPP activity comprises ≥50% of the extracellular PPi-generating capacity of VSMC. The accumulation of extracellular PPi and ATP was markedly attenuated by reduced temperature but was insensitive to brefeldin A, which suppresses constitutive exocytosis of Golgi-derived secretory vesicles. The magnitude of extracellular PPi accumulation in VSMC cultures increased with time postplating, suggesting that ATP release coupled to PPi generation is upregulated as cultured VSMC undergo contact-inhibition of proliferation or deposit extracellular matrix.
Keywords: adenosine 5′-triphosphate release, vascular calcification, ectonucleotide pyrophosphatase, ectoadenosine 5′-triphosphatase
extracellular atp acts as an agonist for the P2X (44) and P2Y (8, 29, 61) families of nucleotide receptors. To prevent activation of these receptors in resting conditions, concentrations of ATP in extracellular compartments are maintained within a narrow nanomolar range through the coordinated coupling of ATP release mechanisms and metabolism of released ATP via various ectonucleotidases (28, 29). Recent studies indicate that extracellular ATP metabolism also contributes to the regulation of cardiovascular calcification in blood vessels and valves through generation of inorganic pyrophosphate (PPi), a potent inhibitor of calcification (22, 51).
At micromolar levels, extracellular PPi inhibits the ability of Ca2+ and phosphate (Pi) to form insoluble hydroxyapatite crystals in both in vitro solutions (60) and intact tissues such as aortic rings cultured ex vivo (37). The importance of extracellular PPi homeostasis in human cardiovascular function is highlighted by the often fatal syndrome of idiopathic infantile arterial calcification (51, 52) that has been linked to inactivating mutations in the ENPP1 gene, which encodes an ectonucleotide pyrophosphatase/phosphodiesterase (eNPP1) also known as PC-1 (57). Calcification of large-capacitance arteries also markedly disrupts normal cardiovascular hemodynamics in more common human diseases such as renal failure and Type II diabetes (10, 45, 56).
The suppressive effect of PPi on pathological calcification in soft tissues, such as blood vessels, requires its regulated accumulation within local extracellular compartments, and three distinct plasma membrane proteins regulate this local accumulation. These include eNPP1 (59), which catabolizes extracellular ATP to produce PPi, and tissue nonselective alkaline phosphatase (TNAP), which hydrolyzes PPi into phosphate and thereby opposes the action of eNPP1 (40). The progressive ankylosis disease susceptibility gene product (ANK) is a plasma membrane protein that either directly mediates efflux of cytosolic PPi or regulates the activity of another as-yet-undefined PPi transporter (17). Thus, sustained activities of NPP1 and/or ANK are required for the active, steady-state suppression of pathological calcification in soft tissues. TNAP is a marker protein for osteoblasts and, via its ability to clear locally generated PPi, facilitates normal mineralization of bone (15). Similarly, aberrant accumulation of TNAP-expressing cells within vascular lesions can promote arterial calcification (10, 45, 56).
Calcification of large arteries in rodents can be induced by experimental manipulations that induce renal failure or conditions, such as hyperphosphatemia and hyperparathyroidism, which accompany chronic kidney disease (38, 42). The capacity of PPi-regulatory proteins to counteract vascular calcification is also contingent on local extracellular matrix proteins and the ambient levels of Ca2+ and Pi (13, 33). While deletion of eNPP1 or inactivation of ANK predisposes mice to aortic calcification (22), the rate and severity of calcification is greatly potentiated when enpp1−/− or ank mice are fed a high-phosphate diet (42).
That loss of either eNPP1 or ANK activity potentiates vascular calcification suggests that these proteins act in nonredundant, parallel pathways for the maintenance of appropriate extracellular PPi homeostasis. Johnson et al. (22) observed that steady-state levels of PPi in the conditioned media of cultured aortic vascular smooth muscle cells (VSMC) from either enpp1−/− or ank mice were similarly reduced by ∼50%, relative to wild-type cultures, during induction of in vitro calcification by elevated phosphate. However, increased phosphate also alters expression of both eNPP1 and ANK (18). Thus, the relative contribution of the eNPP1 versus ANK pathway to extracellular PPi generation by VSMC in the basal state is unclear.
ENPP1 is part of a broader purinergic signaling network in blood vessels that regulates not only calcification but also vascular tone, inflammation, and remodeling (8). Notably, the source and mechanism for delivery of the extracellular ATP that drives eNPP1-mediated generation of PPi in vascular compartments remains undefined. Here we characterize an autocrine pathway of ATP release that is tightly coupled to the production of extracellular PPi in a rat aortic VSMC model. Our data indicate that eNPP1-mediated hydrolysis of endogenous ATP released from VSMC operates in parallel with another pathway involving direct efflux of intracellular PPi to maintain extracellular PPi levels in the micromolar range.
EXPERIMENTAL PROCEDURES
Materials.
Low-glucose (1 g/l) Dulbecco's modified Eagle's medium (LG-DMEM), firefly luciferase assay mix (FL-AAM), ATP standards (FL-AAS), sodium pyrophosphate, adenosine 5′-phosphosulfate (APS), adenosine-5′-triphosphate sulfurylase (ATP-sulfurylase), β,γ-methylene ATP (MeATP or AMPPCP), probenecid (PB), brefeldin A (BFA), levamisole, methylene diphosphonate (PCP), and TRIzol were from Sigma-Aldrich (St. Louis, MO); 1,N6-ethenoadenosine 5′-triphosphate (ɛ-ATP) was from Invitrogen/Molecular Probes (Eugene, OR). Fetal bovine serum, newborn calf serum (CS), and penicillin-streptomycin (P/S) were from Hyclone (Logan, UT). Oligo(dT)15 primer and RNase inhibitor were purchased from Promega (Madison, WI). Avian myeloblastosis virus (AMV) reverse transcriptase, Taq polymerase, and PCR nucleotide mix were from Roche (Indianapolis, IN) while the dNTP mix was from Stratagene. PCR primers were obtained from Operon Biotechnologies (Huntsville, AL). Anti-human eNPP1 (L-20), anti-green fluorescent protein (GFP) (FL), and anti-actin (C-11) primary antibodies and secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). A plasmid expression vector encoding human eNPP1 was provided by Dr. Robert Terkeltaub (University of California, San Diego).
VSMC isolation and cell culture.
Sprague-Dawley rats were maintained and handled in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Science, National Research Council; published by National Institutes of Health, Publication No. 85-23, Revised 1996), as approved by the Institutional Animal Use and Care Committee of Case Western Reserve University. Rats (250 g, male) were anesthetized by intraperitoneal injection of a saturated pentobarbital sodium solution (40 mg/kg body wt). Following achievement of deep anesthesia, the chest was opened and the thoracic aorta was rapidly removed under aseptic surgical conditions. Rat aortic VSMC were isolated using an explanted aortic ring model previously described (11). All subsequent steps were performed using a dissection microscope in a cell culture hood under sterile airflow. The adventitial and endothelial layers were gently removed, and the aorta was sectioned into 2- to 3-mm rings and placed onto the prescratched (by a scalpel blade) and washed surfaces of wells within a six-well culture plate. Three rings were placed per well together with 2 ml growth media (LG-DMEM supplemented with 10% fetal bovine serum, 1% P/S, and 0.11 g/l sodium-pyruvate) and maintained at 37°C in a 10% CO2 atmosphere. After initial culture for 2 days, the rings were transferred to the wells of a fresh six-well plate and cultured for an additional 5 days during which significant outgrowth of VSMC was observed. These primary VSMC were then passed weekly by trypsinization and 1:4 splits for subculture. Unless otherwise noted, experiments were performed using VSMC at passages 2-4. The cells were ≥95% positive for smooth muscle α-actin as measured by immunofluorescence (data not shown).
HEK-293 cells were stably transfected with expression plasmids encoding either GFP or human eNPP1. The transfected HEK-293 lines were selected and maintained in the presence of G418 (400 μg/ml) in DMEM supplemented with 10% CS and antibiotics. Mouse L-cells differentiated by culture in the presence of 1 mM 8-chlorophenylthio-cyclic AMP (CPT-cAMP) for 2 days were used as positive control cells that express TNAP at high levels (5).
RT-PCR analysis of ectonucleotidase subtypes.
Semiquantitative RT-PCR was used to compare the expression profiles of ectonucleotidases and PPi homeostatic proteins in the cultured VSMC. Total RNA was isolated using TRIzol following the manufacturer's protocol. First-strand cDNA synthesis was accomplished using 1–2 μg total RNA, primed with 1 μg oligo(dT) primer, and incubated with 2 units AMV reverse transcriptase, 8 mM dNTPs, and 1 unit RNase inhibitor at 42°C for 1 h. The following cDNA transcripts were analyzed: eNPTDase1/CD39, eNTPDase2, eNTPDase3, eNPP1/PC1, eNPP3/B10/gp130, CD73, TNAP, and ANK. The PCR primer pairs and amplicon sizes for the various ectonucleotidases have been previously reported (25). The primer pair (5′-GCCGCTGCTCATCCCCATCC, antisense, 5′-GGCCGAGGTGACCGTGTTGTTC) used to analyze ANK cDNA transcripts was designed using DNAStar v.5 (DNASTAR) to produce a 400-bp ANK amplicon. Glyceraldehyde phosphate dehydrogenase was used as a high-copy housekeeping gene product. PCR reactions were performed in 20-μl reaction volumes containing 10-fold dilutions of RT reaction product (1:10 to 1:10,000) with 10 mM Tris·HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, 250 μM dNTPs, 1 μM primer mix, and 2.5 U/μl Taq DNA polymerase. PCR running conditions were as follows: 94°C for 2 min, followed by 35 cycles of 94°C for 40 s, 58°C for 40 s, and 74°C for 3 min with a final extension step for 5 min at 37°C. PCR products were electrophoretically separated on 1.5% agarose gels containing ethidium bromide (1 μg/ml), and the fluorescent bands were visualized and recorded with a Bio-Rad GelDoc 1000 detector.
Ectonucleotidase assays.
Ecto-ATPase activities of VSMC (as adherent monolayers) or HEK-293 cells (as trypsin-detached suspensions) were monitored by luciferase-based methods. HEK-293 cells (106) were resuspended in 200 μl basal salt solution (BSS) containing (in mM) 130 NaCl, 5 KCl, 1.5 CaCl2, 1 MgCl2, 25 Na-HEPES (adjusted to pH 7.5 at room temperature, 22–24°C), 5 glucose, and 0.1% BSA, supplemented with 8 μl from a concentrated luciferase/luciferin stock solution (FL-AAM). The cell suspensions were then pulsed with 100–500 nM exogenous ATP; decreases in ATP-dependent luminescence over the next 20–30 min were continuously monitored using a Turner Designs (TD 20/20) luminometer. Where indicated, the cell suspensions were supplemented with 300 μM MeATP immediately before addition of the ATP pulse. Ecto-ATPase activities of adherent VSMC were performed using cell monolayers (0.3–0.6 × 106 cells/well of 6-well plates) bathed in 1 ml BSS. These experiments were performed at 37°C in the absence or presence of 300 μM MeATP. The cells were preincubated for 20–30 min to clear endogenously released ATP before the addition of exogenous ATP. Extracellular samples (100 μl) taken at various times after ATP addition were boiled for 5 min (to inactivate any shed ectonucleotidase activity) and then stored on ice before analysis of ATP and PPi levels as described below.
Enzyme assays for quantitation of ATP and PPi levels in extracellular medium samples.
To quantitate extracellular PPi we adapted an enzyme-linked bioluminescence assay originally developed to detect liberated PPi from RNA reverse transcription reactions (27, 50). In this assay system, PPi reacts with APS in the presence of ATP-sulfurylase to generate ATP which is measured by a coupled luciferin/luciferase reaction. Our adaptation of this assay facilitated quantitative measurements of both the ATP and PPi content within the same sample of extracellular medium. Briefly, 75 μl of heat-inactivated (100°C, 5 min) extracellular sample was mixed with 20 μl of 25 μM APS, and 4 μl concentrated FL-AAM to yield a final reaction volume of 99 μl (with 5 μM APS). The initial steady-state bioluminescence, measured at room temperature with the TD 20/20 luminometer, was an indicator of the ATP content in the extracellular medium sample. When this initial ATP-dependent bioluminescence reached a steady value, ATP-sulfurylase (0.01 units in 1 μl) was added to drive conversion of PPi to ATP, and bioluminescence was continuously measured until a new steady state was reached. This value was recorded and subtracted from the original ATP-dependent bioluminescence value to yield PPi-dependent bioluminescence. Calibration curves were generated for each experiment using identical coupled enzymes reactions with BSS containing ATP and PPi standards (1 nM to 1 μM) in place of the cell-conditioned media samples. For extracellular samples containing pharmacological inhibitors of ATP release pathways or ectonucleotidases, additional calibration curves were generated in the presence of the indicated inhibitor to assess possible inhibitory effects on the ATP-sulfurylase or luciferase reactions.
This assay system was also used to measure extracellular ATP and PPi concentrations produced endogenously by rat aortic VSMC. For these experiments, unless otherwise stated, adherent monolayers of rat aortic VSMC (0.3–0.6 × 106 cells/well) were used between passages 2 and 4 and 3–9 days postplating on six-well plates at 37°C. For these experiments, the cell monolayers were rapidly washed twice with PBS (after aspiration of tissue culture medium) and then transferred to 1.2 ml fresh BSS to initiate the test incubations at 37°C in the absence or presence of the indicated inhibitor(s). An initial 100-μl aliquot of extracellular media was immediately taken (∼1- to 3-min time point), and additional 100-μl samples were taken at various intervals (5–60 min as indicated in specific experiments) for up to 2 h after transfer of the cells to the BSS test medium. Each extracellular medium sample was heated (100°C, 5 min) and centrifuged before quantitative analysis of ATP and PPi content.
Alkaline phosphatase activity in lysates of VSMC or CPT-cAMP-differentiated L-cells was assayed in the absence or presence of levamisole by previously described methods (5, 38).
Ectonucleotidase assays by high-performance liquid chromatography.
Reverse-phase high-performance liquid chromatography (HPLC) was used to measure the extracellular metabolism of fluorescent, etheno-derivitized, adenine nucleotide analogs ɛ-ATP (Molecular Probes) and ɛ-MeATP (25, 31). Cell monolayers were grown in six-well tissue culture plates, washed twice with 1 ml PBS pH 7.4, and equilibrated for 30 min in 1 ml BSS at room temperature. Cells (HEK-293 or VSMC) were then pulsed with indicated concentrations of either ɛ-ATP or ɛ-MeATP. At indicated time points, 100 μl of extracellular medium were removed and boiled for 5 min. Adenine nucleotide derivatives were resolved using an Alltech C18 Ad-sorbosphere column eluted at 1.3 ml/min. A methanol gradient was formed by mixing buffer A (0.1 M KH2PO4, pH 6, 5% methanol) with buffer B (0.1 M KH2PO4, pH 6, 15% methanol) using the following protocol: 0–2 min (100% buffer A); 2–3 min (ramp to 75% buffer A/25% buffer B); 3–26 min (ramp to 35% buffer A/65% buffer B); 26–30 min (ramp to 100% buffer A). Fluorescent adenine analogs were detected with a Linear LC305 fluorescence detector using 270 nm excitation and 410 nm emission wavelengths. Alternatively, isocratic reverse-phase HPLC was used to assay the extracellular metabolism of 300 μM MeATP by VSMC monolayers grown in six-well dishes. Extracellular samples were processed as described before injection onto the Alltech C18 column. Nucleotides were resolved by isocratic elution (1.3 ml/min) with buffer A and detected by absorbance at 254 nm.
Western blot analysis.
Adherent cultures of HEK-293 cells or VSMC were extracted and analyzed by SDS-PAGE and Western blotting using previously described methods (3).
Statistical analysis.
All experiments with VSMC were repeated at least three times using different preparations of cultured VSMC. All data, unless otherwise stated, represent means ± SE, and statistical significance was defined as P < 0.05 using two- or three-way ANOVA with the Bonferroni post hoc test.
RESULTS
Clearance of extracellular ATP is coupled to PPi accumulation in rat aortic VSMC.
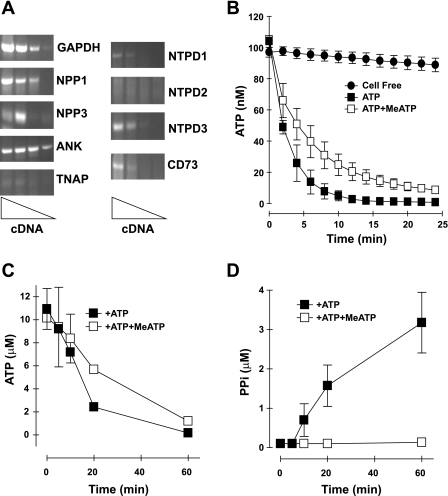
We used rat aortic VSMC at low passage number (2–4) as a tissue culture model for quantitatively assaying extracellular ATP release and metabolism and coupled PPi accumulation. These cultures expressed smooth muscle-specific α-actin in >95% of the cells for at least six passages, as well as mRNA for multiple VSMC gene markers, including smooth muscle myosin-1 heavy chain, SM22-α, caldesmon, and calponin (data not shown). Analysis of mRNA transcripts for different ectonucleotidase subtypes indicated significant expression of eNPP1, NTPDase3, and CD73, while TNAP, NTPDase1 (CD39), and eNPP3 were present at lower levels (Fig. 1A); mRNA for the ANK PPi transporter/regulator was also abundant. The intact VSMC expressed robust ecto- ATPase activity as indicated their ability to completely hydrolyze an exogenously added pulse of 100 nM ATP within 10 min (Fig. 1B). Notably, the rate at which the VSMC hydrolyzed extracellular ATP was reduced twofold in the presence of MeATP (also known as AMPPCP). We previously reported that MeATP selectively suppresses the catabolism of extracellular ATP by eNPP-family, but not CD39-family, ecto- ATPases in several rat and human cell types (3, 24, 25). Ecto-ATPase measurements in HEK-293 cells stably transfected with human eNPP1 verified this selectivity of MeATP (supplemental Fig. 1; supplemental data and online article are available at the American Journal of Physiology-Cell Physiology website). To demonstrate that eNPP1 in VSMC functions as a PPi-generating ecto-ATPase, we pulsed monolayers of adherent cells (∼5 × 105 cells·ml−1·35-mm dish−1) with 10 μM ATP in the absence or presence of MeATP (300 μM) and measured extracellular levels of both ATP and PPi over a 60-min test period. The 10 μM pulse of exogenous ATP was almost completely cleared by the VSMC monolayers within 60 min (Fig. 1C) and correlated with the progressive accumulation of extracellular PPi (Fig. 1D). However, the extracellular PPi concentration ([PPi]) at 60 min was only 3 μM, indicating that only 30% of the hydrolyzed ATP was coupled to PPi generation or that PPi itself was further metabolized. MeATP modestly retarded clearance of the 10 μM ATP pulse (Fig. 1C) but completely suppressed the accumulation of PPi (Fig. 1D). This indicates that rat aortic VSMC express MeATP-sensitive eNPP-family ectonucleotidases that generate extracellular PPi as an end-product and also MeATP-insensitive NTDPase-family ecto-ATPases that produce Pi rather than PPi. Direct analysis of Pi production with 500 μM ATP as the substrate indicated an ecto-ATPase rate of 3.23 ± 0.12 nmol·h−1·μg protein−1 in the absence of MeATP versus an ecto-ATPase of 3.38 ± 0.26 nmol·h−1·μg protein−1 in the presence of 300 μM MeATP.
Fig. 1.
Expression and activities of ectonucleotidases and inorganic pyrophosphate (PPi) homeostatic proteins in rat aortic vascular smooth muscle cells (VSMC). A: RT-PCR analysis of mRNAs for ectonucleotidases and PPi homeostatic proteins in VSMC. NPP, nucleotide pyrophosphatase/phosphodiesterase; ANK, progressive ankylosis gene; TNAP, tissue nonselective alkaline phosphatase; NTPD, nucleoside 5′-triphosphate diphosphohydrolase. B: suspensions of intact VSMC [106 cells in 200 μl basal salt solution (BSS)] at 23°C were pulsed with 100 nM exogenous ATP in the absence (▪) or presence (□) of 300 μM β,γ-methylene ATP (MeATP); •, cell-free control. ATP was assayed by luciferase-based luminescence. Data points represent the average ± range of duplicates from a representative experiment repeated three times using different VSMC preparations. C and D: metabolism of exogenous (10 μM) ATP coupled to extracellular PPi generation by adherent VSMC monolayers in the absence (▪) or presence (□) of 300 μM MeATP. Data represent the average ± range of duplicates from a representative experiment repeated three times using adherent VSMC monolayers bathed in 1 ml BSS at 37°C.
ATP released from VSMC is efficiently coupled to extracellular PPi accumulation.
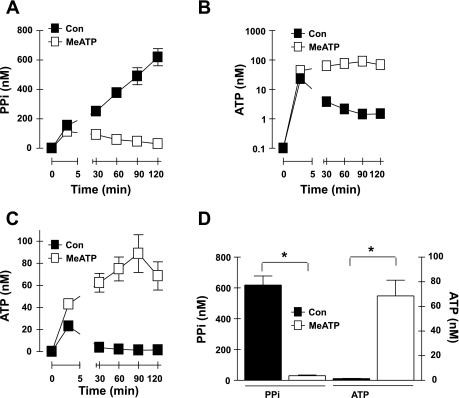
Most mammalian cell types constitutively release ATP at low but significant rates thereby conditioning extracellular compartments to contain nanomolar concentrations of ATP and ATP metabolites at steady state (24, 29). Perturbation of the steady-state relationship between the ATP release rate and the ATP clearance rate, by either increasing the rate of ATP release (e.g., due to mechanical stimulation) or repressing activity of the opposing ecto-ATPases, leads to higher levels of extracellular ATP. We assessed the ability of adherent VSMC to condition their extracellular medium with endogenous ATP and PPi during 2-h test incubations in serum-free BSS. It is important to note that transfer of the VSMC monolayers from the regular tissue culture media to this experimental test media (including two intermediate washes with PBS) constitutes a transient mechanical stimulus that triggers rapid release of endogenous ATP and other low-molecular-mass metabolites into the extracellular compartment. Thus, Fig. 2, B (log scale) and C (linear scale), shows that 10–20 nM extracellular ATP was present at the earliest time point (3 min) at which the assay medium was sampled. Notably, ∼150 nM extracellular PPi was also rapidly accumulated at this early time point (Fig. 1A) followed by a sustained increase in extracellular [PPi] to >600 nM as the VSMC progressively conditioned the assay medium over the 2-h experimental time course (Fig. 2, A and D). In contrast, the initial burst of accumulated extracellular ATP progressively decreased at the later time points to a steady-state level of 1 nM ATP within 30 min. Notably, when VSMC were transferred to MeATP-containing assay medium, the accumulation of both extracellular ATP (Fig. 2, B and C) and PPi (Fig. 2A) was markedly altered. Although the initial burst of PPi accumulation was not affected, the VSMC did not accumulate additional extracellular PPi but slowly cleared the initially accumulated PPi. Conversely, inclusion of MeATP reversed the clearance of the initially released ATP and facilitated accumulation of extracellular [ATP] in the 20–60 nM range during the 2 h of subsequent incubation (Fig. 2, B and C). These data indicate that VSMC continue to release ATP after the initial mechanical stimulus at a rate that is matched by an opposing MeATP-sensitive ecto-ATPase (and MeATP-insensitive ecto-ATPases) to yield a steady-state level of 1 nM extracellular ATP in the absence of MeATP. The sustained increase in extracellular PPi levels during maintenance of this low steady-state ATP level indicates that ATP release from the VSMC is very efficiently coupled to PPi generation via an eNPP-type ecto-ATPase. Suppression of this eNPP-mediated ATP metabolism in the presence of MeATP coordinately blocks accumulation of extracellular PPi and facilitates increased levels of ATP.
Fig. 2.
Autocrine ATP release and coupled extracellular PPi accumulation in rat aortic VSMC. A–C: extracellular levels of PPi (A, linear scale) and ATP (B, log scale; C, linear scale) following transfer of cultured VSMC to basal saline assay medium in the absence [▪,control (Con)] or presence (□) of 300 μM MeATP. Data points in A–C represent means ± SE from 11–16 separate experiments (each performed in duplicate) with different VSMC preparations at passages 3-4; for some data points, the error bars are smaller than the symbols. D: statistical analysis at the 120-min time points. *P < 0.05, two-way ANOVA with the Bonferroni post hoc test.
Tissue nonselective alkaline phosphatase (TNAP) acts as a potent PPi hydrolyzing ectoenzyme (40, 42, 43). We used 5 mM levamisole, a well-characterized TNAP inhibitor, to test whether basally expressed TNAP significantly limited the magnitude of extracellular PPi accumulation by the cultured VSMC (43). If TNAP activity is high, then the presence of levamisole should potentiate PPi accumulation. However, this was not observed. In the presence of levamisole, extracellular PPi accumulation (measured at 2 h as described in Fig. 2D) by intact VSMC was 71 ± 29% (n = 3 experiments) of the normalized values measured in the absence of levamisole. This concentration of levamisole strongly inhibited alkaline phosphatase activity (supplemental Fig. 2), as measured by standard p-nitrophenyl phosphate hydrolysis assays, in lysates of VSMC or lysates of cAMP-differentiated L-cells that express high levels of TNAP (5). Notably, the levamisole-sensitive alkaline phosphatase-specific activity in VSMC lysates was ∼16-fold lower than in L-cell lysates. Thus, basally expressed TNAP is not a major regulator of extracellular PPi homeostasis in cultured VSMC. Direct measurements indicated that exogenous PPi was only slowly cleared (20% of a 1 μM pulse was metabolized within 2 h) by these cultured VSMC (data not shown).
Attenuation of extracellular PPi accumulation by methylene diphosphonate, a product of eNPP1-mediated metabolism of MeATP.
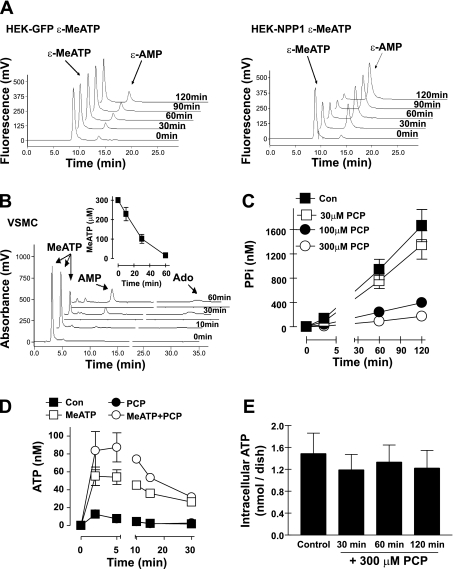
We previously reported that MeATP inhibits ATP hydrolysis by eNPP-type ecto-ATPases by acting as a competing substrate (25) and have verified this rapid catabolism of extracellular MeATP in both HEK-293 cells stably transfected with human eNPP1 (Fig. 3A) and in rat VSMC (Fig. 3B). Thus, eNPP1 will catalyze the reaction (MeATP → PCP + AMP) to generate PCP, a bisphosphonate analog, as a by-product. In turn, the PCP that accumulates during incubation of VSMC with MeATP may affect the autocrine mechanisms that drive accumulation of extracellular PPi. This was supported by our observation that PPi accumulation was reduced in a dose-dependent manner by exogenously added PCP, with 300 μM producing an eightfold decrease (Fig. 3C). Notably, we verified that these concentrations of PCP had no inhibitory actions on either the luciferase or ATP-sulfurylase reactions used to quantitate the PPi levels in conditioned medium. PCP (300 μM) by itself did not affect the transient accumulation of extracellular ATP induced by the medium exchange stimulus. Rather, the presence of both PCP and MeATP in the exchange medium further potentiated the marked ATP accumulation facilitated by MeATP alone (Fig. 3D). This suggests that PCP may act as a product inhibitor of eNPP1 and thereby attenuate both PPi accumulation and ATP clearance.
Fig. 3.
MeATP is rapidly metabolized by eNPP1 in VSMC to generate methylene diphosphonate (PCP) that acts as an inhibitor of autocrine PPi accumulation. A: HPLC chromatograms of extracellular media from intact HEK-green fluorescent protein (GFP) cells (left) or HEK-NPP1 cells (right) pulsed with 20 μM exogenous etheno-β,γ-methylene ATP (ɛ-MeATP) and incubated for the indicated times. B: HPLC chromatograms of extracellular media from rat VSMC pulsed with 300 μM exogenous MeATP and incubated for the indicated times. Inset: time course of MeATP (300 μM) hydrolysis based on calibrated absorbance from the HPLC chromatogram in B. C: extracellular PPi generation by VSMC incubated in the absence (▪) or presence of 30 μM (□), 100 μM (•), or 300 μM (○) PCP. Data points represent the average ± range of duplicates from representative experiments (each repeated three times with similar results) using passage 4 VSMC. D: extracellular ATP release by VSMC incubated in the absence (▪) or presence of 300 μM MeATP (□), 300 μM PCP (•), or 300 μM MeATP + 300 μM PCP (○). PCP data points indicated by solid circles overlap with the control data points indicated by solid squares. E: total intracellular ATP was measured in control or PCP-treated VSMC by rapid permeabilization of the cell monolayers with digitonin (50 μg/ml) to release ATP for quantification by the luciferase-based assay. VSMC were incubated in the absence or presence of 300 μM PCP for the indicated times before digitonin permeabilization. Data points in D and E represent means ± SE from 3 separate experiments (each performed in triplicate) with VSMC preparations at passages 2-4 (in D, error bars are smaller than symbols for some data points).
PCP is similar to the non-nitrogen-containing bisphosphonates used in antiosteoporosis therapy. The therapeutic action of such bisphosphonates involves their accumulation within osteoclasts and intracellular incorporation into cytotoxic ATP analogs via a back reaction with AMP-aminoacyl tRNA synthetase conjugates (48). We tested whether the observed ability of PCP to suppress accumulation of extracellular PPi by VSMC might involve toxic effects that result in decreased intracellular ATP content. However, exposure of VSMC to 300 μM PCP for up to 2 h (the usual duration of the test incubations for measuring PPi accumulation rates) did not result in significant reduction in intracellular ATP levels (Fig. 3E).
Attenuation of extracellular PPi generation by probenecid.
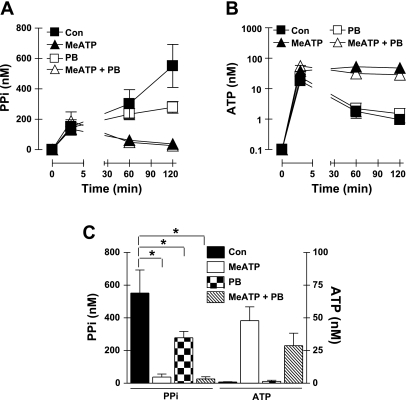
Given the high expression of ANK mRNA in VSMC (Fig. 1A), we tested the possible contribution of ANK-regulated PPi efflux by comparing PPi accumulation and ATP release in the absence or presence of PB, MeATP, or combined PB plus MeATP. PB acts as an inhibitor of multiple anion transporters and has been shown to reduce PPi efflux mediated or regulated by ANK (17). Fig. 4, A and C, shows that extracellular PPi accumulation was reduced twofold in the presence of 2.5 mM PB, which contrasted with the near-complete suppression of PPi generation by MeATP. These experiments indicated that a PB-sensitive mechanism accounts for ∼50% of the PPi that accumulates in extracellular medium acutely conditioned by VSMC. PB alone had no obvious effect on the initial mechanically induced ATP release or the clearance of this released ATP (Fig. 4B). However, exposure of VSMC to both PB and MeATP resulted in lower (albeit not statistically significant) accumulation of extracellular ATP (at the later times following medium transfer) compared with cultures incubated with only MeATP (Fig. 4, B and C). This suggested that the inhibitory effects of PB on PPi levels might additionally involve reduction in the ATP release that is correlated with the MeATP-sensitive PPi accumulation.
Fig. 4.
Attenuation of extracellular PPi accumulation by probenecid (PB). A and B: extracellular levels of PPi (A, linear scale) and ATP (B, log scale) following transfer of cultured VSMC to basal saline assay medium in the absence (▪, control) or presence of inhibitors; ▴, 300 μM MeATP; □, 2.5 mM PB; ▵, MeATP + PB. Data points in A and B represent means ± SE from 3 separate experiments (each performed in duplicate) with different VSMC preparations at passages 3-4; for some data points, the error bars are smaller than the symbols. C: statistical analysis at the 120-min time points. *P < 0.05, two-way ANOVA with the Bonferroni post hoc test.
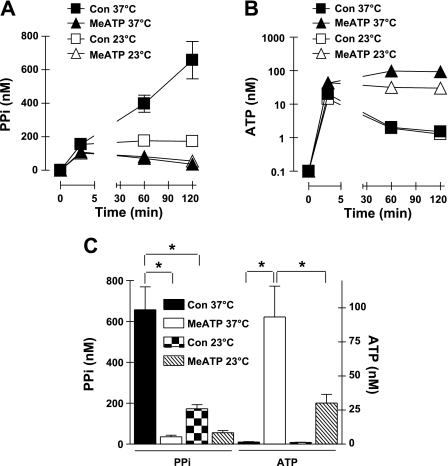
Accumulation of extracellular PPi and ATP by VSMC is attenuated by reduced temperature but not brefeldin A.
Basal nucleotide release can reflect export of nucleotides contained within the Golgi-derived secretory vesicles that continuously bring new protein and lipid cargo to the plasma membrane (26). Because secretion of Golgi-derived vesicles is a highly temperature-sensitive process, we tested the effect of reduced incubation temperature (23°C relative to the control 37°C) on PPi accumulation (Fig. 5, A and C) and ATP release (Fig. 5, B and C) by VSMC. These experiments showed that the sustained rate, but not the initial burst, of extracellular PPi accumulation was greatly reduced at the lower assay temperature. This could reflect reduced rates of 1) eNPP1-catalyzed conversion of released ATP to PPi, 2) ANK-dependent PPi efflux, or 3) an ATP release process per se. The latter possibility was supported by reduced ATP accumulation observed at 23°C in the presence of MeATP. Reduced temperature did not decrease the initial burst of ATP released after medium transfer, but no additional accumulation of ATP was observed in the presence of MeATP at 23°C. BFA inhibits the GTP/GDP exchange cycle of ARF-family small GTPases and thereby attenuates anterograde membrane traffic both from the endoplasmic reticulum to the Golgi and from the Golgi to the plasma membrane to suppress constitutive secretion (4, 41) in most cells, including VSMC (39). Given the marked temperature sensitivity of the PPi and ATP accumulation, we tested whether the effects of reduced temperature would be mimicked by BFA. VSMC were preincubated with 3 μg/ml BFA for 2 h before the transfer to serum-free test medium and were also incubated with this concentration of BFA throughout the media conditioning period. The BFA-treated cells were characterized by extracellular PPi accumulation (measured at 2 h as described in Fig. 5C) that was 86 ± 12% (n = 4 experiments) of the normalized values measured in the absence of BFA. Likewise, BFA treatment did not affect the release or metabolism of extracellular ATP.
Fig. 5.
Accumulation of extracellular PPi and ATP by VSMC is attenuated by reduced temperature. A and B: extracellular levels of PPi (A, linear scale) and ATP (B, log scale) following transfer of cultured VSMC to basal saline assay medium in the absence (▪, □: control) or presence (▴, ▵) of 300 μM MeATP at 37°C (filled symbols) and 23°C (open symbols). Data points in A and B represent means ± SE from 7 separate experiments (each performed in duplicate) with different VSMC preparations at passages 2-4; for some data points, the error bars are smaller than the symbols. C: statistical analysis at the 120-min time points. *P < 0.05, three-way ANOVA with the Bonferroni post hoc test.
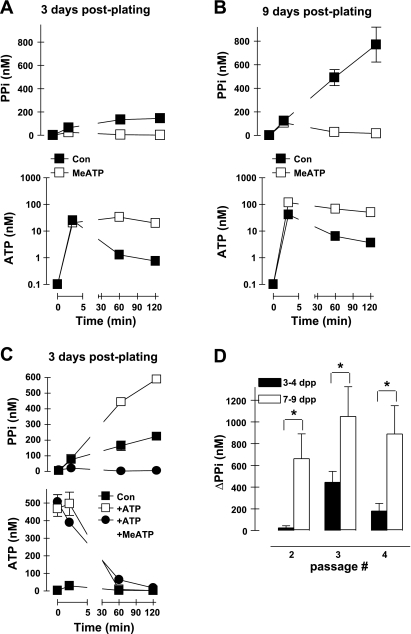
PPi accumulation in rat aortic VSMC cultures is modulated by time postplating.
The ATP release and PPi accumulation experiments illustrated in Figs. 2–5 used rat aortic VSMC cultures at passages 3-4, and most were performed at ∼4 days postplating of 4 × 105 cells per well of the six-well assay dishes. In vitro calcification experiments routinely involve more prolonged in vitro culture (7–14 days postplating) in the presence of various promineralizing stimuli (e.g., elevated phosphate, osteogenic cytokines) (22); the controls for such experiments use VSMC cultured for similarly prolonged times but in the absence of promineralizing factors (13). We observed increases in the rate and extent of PPi accumulation when control VSMC from the same aortic isolate and passage were assayed at 9 days postplating (Fig. 6, B and D) versus 3 days postplating (Fig. 6, A and D). Control experiments with cells plated at increasing densities indicated that this effect of time postplating could not be ascribed simply to increased cell numbers per well (data not shown). Differences in PPi accumulation between short-term (3–4 days postplating) versus longer-term (7–9 days) cultures were observed at all passages (Fig. 6D). This may reflect differences in 1) ATP release, 2) hydrolysis of ATP to PPi by eNPP1, 3) competing hydrolysis of released ATP by ecto-ATPases other than eENPP1, or 4) efflux of intracellular PPi via anion transporters. Figure 6C shows that reduced expression or activity of eNPP1 per se is an unlikely explanation for the low rates of PPi generation observed in shorter-term VSMC cultures because these cells readily generated extracellular PPi via a MeATP-sensitive mechanism when challenged with a 500 nM pulse of exogenous ATP to supplement the ATP released from endogenous stores.
Fig. 6.
Extracellular PPi accumulation in VSMC cultures is modulated by time postplating. A: extracellular levels of PPi (top, linear scale) and ATP (bottom, log scale) following transfer of cultured VSMC to basal saline assay medium in the absence (▪, control) and presence (□) of 300 μM MeATP. Data points represent means ± SE from the triplicates of a representative experiment repeated three times using passage 2 VSMC at 3 days postplating (dpp). B: VSMC from the same preparation as in A, but cultured 6 additional days before the analysis of PPi and ATP accumulation. Data points represent means ± SE from the triplicates of a representative experiment repeated three times. C: VSMC from the same preparation as in A and assayed for PPi and ATP levels following transfer of basal saline assay medium alone (▪, control; −ATP) or assay medium supplemented with 500 nM exogenous ATP (□) or with 500 nM ATP plus 300 μM MeATP (•). Data represent means ± SE from the triplicates of a representative experiment repeated three times. D: statistical analysis of net PPi generation (ΔPPi) by VSMC as a function of passage number and days postplating. Data points represent means ± SE from 3 separate experiments (each performed in triplicate) with different VSMC preparations. *P < 0.05, two-way ANOVA with the Bonferroni post hoc test. ΔPPi = [PPi]120 min − [PPi]3 min.
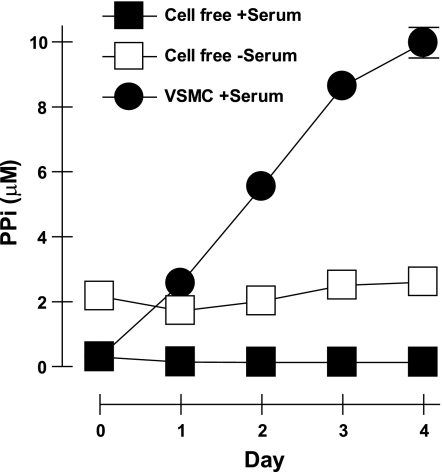
Steady-state PPi accumulation by rat aortic VSMC in tissue culture.
The fetal bovine serum used for tissue culture of VSMC contains substantial alkaline phosphatase activity, which efficiently hydrolyzes PPi to Pi. In the experiments shown thus far, VSMC were transferred to serum-free assay medium to analyze extracellular PPi and ATP accumulation during short-term (≤2 h) incubations under defined in vitro conditions. We also tested how VSMC accumulate extracellular PPi when maintained in standard serum-supplemented DMEM-type tissue culture medium (Fig. 7). Our stocks of DMEM, in the absence of serum or conditioning by cells, contained ∼2 μM PPi as presumed contaminant from added antibiotics or other cofactors. However, the addition of serum, which contains alkaline phosphatase, rapidly reduces this to 100 nM PPi. Rat aortic VSMC maintained in serum-supplemented DMEM steadily conditioned this medium to produce an extracellular level of 10 μM PPi within 4 days. The VSMC-conditioned DMEM contained very low (<1 nM) extracellular ATP due to the combined ecto-ATPase activities of both cells and the serum (data not shown). Thus, VSMC constitutively generate extracellular PPi at a rate sufficient to counteract serum-associated pyrophosphatase activity and thereby elevate PPi to the supramicromolar levels required for suppression of vascular calcification.
Fig. 7.
Extracellular PPi accumulation by VSMC maintained under tissue culture conditions. VSMC were cultured in serum-supplemented medium as described in experimental procedures. Media samples were assayed for PPi levels (•) at the indicated days following transfer of the VSMC to fresh culture medium. Data points represent means ± SE of triplicates from a representative experiment repeated 3 times using passage 2 VSMC. Day 0 represents addition of fresh growth media to VSMC that were seeded 3 days prior. ▪, PPi levels in serum-containing tissue culture media; □, PPi levels in serum-free tissue culture media.
DISCUSSION
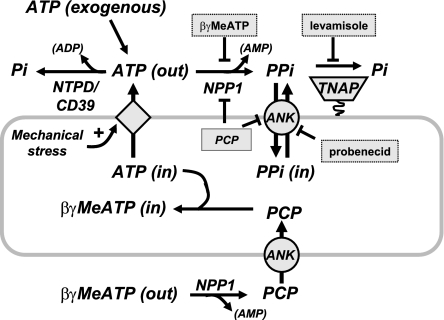
This study provides the first direct characterization of an autocrine pathway wherein the release of endogenous ATP stores is tightly coupled to extracellular ATP hydrolysis and the sustained accumulation of extracellular pyrophosphate in cultured VSMC. Our experiments indicate that this coupled pathway of ATP release and extracellular hydrolysis operates in synchrony with another autocrine mechanism wherein intracellular PPi, generated as a by-product of multiple intracellular nucleotide-consuming metabolic reactions (60), is directly released into the extracellular compartment via a probenecid-sensitive transport process. This probenecid-sensitive PPi accumulation may be mediated or regulated by ANK, which is highly expressed in these VSMC. Other results suggest that the expression and/or activities of the signaling proteins that underlie these autocrine pathways of PPi accumulation may be modulated as the growth state of VSMC and their secretion of extracellular matrix elements changes during sustained tissue culture. Taken together, these observations support significant roles for local release of both ATP and PPi in the steady-state suppression of pathological calcification in blood vessels as in the model illustrated in Fig. 8. Perturbation of either autocrine pathway may contribute to the complex etiology of cardiovascular calcification that is associated with dysregulation of multiple endocrine, paracrine, and metabolic factors (23, 56).
Fig. 8.
Model of extracellular ATP metabolism and PPi generation in VSMC.
These data extend previous findings using cultured aortic VSMC isolated from enpp1−/− and ank/ank murine models, which indicated that eNPP1- and ANK-dependent mechanisms provided equal and additive contributions to the accumulation of extracellular PPi (22). That study used VSMC cultured under promineralizing conditions (elevated extracellular phosphate and reduced serum) that favor upregulation of PPi-degrading TNAP, whereas the present experiments used standard synthetic phenotype VSMC that express much lower levels of TNAP. Thus, our finding that a PB-sensitive pathway comprised ∼50% of the acute PPi-generating capacity in these latter VSMC further suggests that the autocrine ANK and eNPP1 pathways are coordinately utilized for PPi accumulation under both basal conditions and during induction of proosteogenic gene expression. This reinforces in vivo observations that absence of either eNPP1 or ANK predisposes mice to spontaneous aortic calcification and markedly accelerates the aortic mineralization induced by hyperphosphatemia (42). Although our experiments focused on VSMC, the coordinate roles of eNPP1- and ANK-based pathways in the suppression of mineralization have been most extensively characterized in osteoblasts and chrondrocytes (15, 16). These latter cells share a mesenchymal stem cell lineage with VSMC, fibroblasts, and adipocytes (21). ANK expression in murine fibroblasts is increased during growth stimulation in response to multiple mitogens (14), while downregulation of eNPP1 is required for optimal adipogenic differentiation of murine fibroblasts (34). Whether similar dynamic regulation of coordinate ATP release, eNPP1, or ANK activity occurs as VSMC switch between contractile and synthetic phenotypes is an important question for future studies.
Our use of MeATP as an experimental tool presented both advantages and limitations. Ecto-ATPases encompass two families of structurally and functionally distinct cell surface proteins: 1) the ectonucleoside 5′-triphosphate diphosphohydrolases (NTPDases), which include CD39 (NTPDase1) and four related members (47); and 2) the eNPPs, which include eNPP2 and eNPP3 in addition to eNPP1 (57). Although both NTPDases and eNPPs can use ATP as a preferred substrate, their catalytic properties are distinct in that NTPDases serially hydrolyze ATP to produce AMP plus two phosphates (Pi) while eNPPs act as pyrophosphatases hydrolyzing ATP to generate AMP plus PPi. RT-PCR analysis and direct measurement of ecto-ATPase activity confirmed that the cultured VSMC express multiple ecto-ATPases (NTPDase1, NTPDase3, and eNPP3) in addition to eNPP1 (Fig. 1). At present, there are no selective inhibitors of either ecto-ATPase family (32). We previously described (and confirmed in this study) pharmacological experiments indicating that MeATP competitively inhibits ATP utilization by eNPPs but not NTPDases (25). Significantly, recent analysis of the crystal structure of NTPDase2 showed that MeATP/AMPPCP, in contrast to AMPPNP (5′-adenylyl-β,γ-imidodiphosphate), cannot fit into the ATP-binding site of that ecto-ATPase (63). This explains our findings that even submillimolar MeATP does not affect the hydrolysis of micromolar ATP by cells expressing only NTDPases and no eNPPs (25) (supplemental Fig. 1). This extraordinary selectivity also underlies our observation that MeATP completely suppresses the generation of extracellular PPi by VSMC challenged with a 10 μM pulse of exogenous ATP while only modestly slowing clearance of this added ATP by the combined ecto-ATPase activities of eNPP1, eNPP3, NTPDase1/CD39, and NTPDase3. It is important to note that MeATP also acts as a substrate for eNPP1-mediated hydrolysis in VSMC (Fig. 3B) with its by-products being extracellular AMP and PCP. Notably, we observed that PCP, which is a nonhydrolyzable PPi analog, potently inhibited the autocrine accumulation of extracellular PPi by VSMC (Fig. 3C). Under our experimental conditions, PCP did not suppress ATP accumulation (Fig. 3D) nor did it induce a decrease in intracellular ATP content (Fig. 3E). These findings indicate that PCP may suppress accumulation of extracellular PPi in two ways: 1) by blocking PPi efflux via ANK; and 2) by acting as a product-inhibitor of eNPP1-mediated hydrolysis of released ATP into PPi and AMP (Fig. 8). Although short-term PCP exposure did not induce obvious cytotoxic or proapoptotic responses, we have observed that more prolonged incubation (>6 h) in the presence of PCP induces a significant rounding up and detachment of cultured VSMC. This suggests that PCP may eventually induce proapoptotic effects similar to those triggered by non-nitrogen-containing bisphosphonates.
Extracellular PPi homeostasis and mineralization reactions are further regulated by the expression and functional activity of TNAP, a glycosylphosphatidylinositol-anchored ectoenzyme that degrades locally accumulated extracellular PPi into Pi (40). However, we observed that the cultured rat aortic VSMC express only low levels of TNAP mRNA (Fig. 1A) and levamisole-sensitive alkaline phosphatase activity (supplemental Fig. 2). Thus, TNAP does not significantly limit the rate or extent of PPi accumulation during acute medium conditioning in this cultured VSMC model. However, TNAP is markedly upregulated in several VSMC models during culture in the presence of promineralizing stimuli, such as elevated phosphate and various proosteogenic cytokines (33, 43, 46, 58). A robust levamisole-sensitive pyrophosphatase activity is also observed in freshly isolated rat aortic rings, and this activity is increased in aortic segments derived from uremic rats (38, 43). Thus, the relative expression of TNAP appears to vary with the well-known phenotype plasticity of VSMC (55): modest expression in the differentiated, contractile phenotype cells that predominate in intact blood vessels or aortic rings, low expression in the proliferating, synthetic phenotype cells that predominate in tissue culture, and high expression in the calcifying phenotype cells that accumulate in response to osteogenic stimuli. However, it remains possible that levamisole-insensitive phosphatase(s) also described in explanted rat aortic rings may act as predominant pyrophosphatases in the synthetic phenotype VSMC (43).
A major unresolved question is the mechanism(s) by which ATP is released from VSMC to drive the eNPP1-mediated accumulation of extracellular PPi. A significant limitation in addressing this issue is the very efficient coupling between release of ATP into the cell surface microenvironment and its rapid metabolism by eNPP1 and other ecto-ATPases (28). We have previously characterized the functional colocalization of ATP release pathways and eNPP activity during receptor-mediated stimulation of ATP release from human 1321N1 astrocytes (3, 24). Significant accumulation of ATP in the bulk extracellular compartment was observed only when eNPP activity was inhibited by MeATP. In contrast, accumulation of submicromolar ATP in the cell surface microenvironment was measured in the absence of eNPP inhibition using a surface-localized luciferase sensor that effectively competed with eNPP for the released ATP. In the present study, we observed that extracellular ATP in the conditioned bulk medium compartment of VSMC was steadily maintained in the 1–2 nM range in the absence of ecto-ATPase inhibitors but at higher levels, ranging from 10–80 nM, in the presence of MeATP. These results suggest that eNPP1 is in close proximity to ATP release sites in VSMC. This model of continuous ATP release and eNPP1-dependent hydrolysis is further supported by the sustained and MeATP-sensitive increase in extracellular PPi by VSMC.
The ability of reduced temperature to attenuate this continuous ATP release from nominally unstimulated VSMC (Fig. 6) initially suggested that constitutive exocytosis of Golgi-derived secretory vesicles might provide the pathway for ATP export. Recent studies by Lazarowski and colleagues have demonstrated that a BFA-sensitive pathway underlies the constitutive release of UDP-glucose from several cell types (extracellular UDP-glucose functions as an agonist for Gi-coupled P2Y14 receptors) (26, 30). However, using similar BFA treatment protocols, we failed to observe any significant attenuation of ATP and PPi accumulation in the conditioned medium of VSMC. In addition to exocytosis, cells can release ATP by mechanisms that involve facilitated efflux of the cytosolic ATP pool via channels or transporters (see review in Ref. 29). Given the very favorable electrochemical driving force for ATP efflux, even low-frequency opening of such channels can deliver significant amounts of ATP to extracellular compartments.
Multiple transport proteins or conductances have been suggested as “ATP channels,” including some ATP-binding cassette-family transporters, volume-regulated anion channels, maxi-anion channels, and plasma membrane variants of the mitochondrial voltage-dependent anion channel porins (29). However, there is growing support for the involvement of so-called hemichannels, composed of protein subunits from the well-characterized connexin (Cx) family or the recently described pannexin (Px) family (1, 9, 19, 20, 35, 36, 54). Although Cx-based channels are generally associated with the transcellular movement of molecules through gap junction channels, Cx (and Px) hemichannels at nonjunctional membrane sites can also be gated to the open state to act as possible conduits for ATP and other small organic metabolites. VSMC express multiple connexins, including Cx43. In preliminary studies, we have observed that extracellular accumulation of ATP and PPi is significantly attenuated in VSMC treated with 100 μM carbenoxolone, a nonselective inhibitor of connexin and pannexin channels. This suggests that low-frequency opening of such hemichannels may comprise a major ATP release pathway in these cells. Previous studies have indicated that the gating of connexin gap junction channels is sensitive to temperature (6, 7) and that this might underlie the attenuated accumulation of ATP and PPi observed at 23°C versus 37°C. Alternatively, the lower temperature may act via indirect effects on the homeostasis of ions (e.g., H+ or Ca2+) that allosterically modulate hemichannel gating.
Many recent studies have shown increased activity of Cx- or Px-based hemichannels in response to various types of mechanical stimulation, such as fluid shear, cell swelling, or direct non-lytic deformation of the plasma membrane (2, 12, 49, 53, 62). In this regard, unavoidable mechanical stimulation accompanies routine manipulations used in our experimental assays including 1) the initial transfer of VSMC from growth medium to assay medium and 2) periodic removal of extracellular medium samples for analysis of ATP and PPi content. These seemingly innocuous manipulations may generate mechanical stimuli sufficient to gate nonjunctional hemichannels or other mechanosensitive channels with appreciable permeability to ATP. The possible role of mechanical stimulation as a regulator of the ATP/eNPP1-mediated pathway for PPi accumulation is mechanistically appealing given the basal physiological state of VSMC within the aorta and other calcification-prone elastic arteries. These cells undergo constant cyclic mechanical perturbation due to the repetitive systolic stretch and diastolic recoil entrained by the cardiac cycle. Thus, future experiments should test the effects of graded cyclic mechanical strain on both the ATP/eNPP1 and the ANK pathways used by VSMC for the autocrine regulation of extracellular PPi levels.
GRANTS
This work was supported in part by National Institutes of Health (NIH) Grants P01-HL-18708 (to G. R. Dubyak and A. M. Romani), RO1-GM-36387 (to G. R. Dubyak), and RO1-DK-69681 (to W. C. O'Neill) and by a grant from the Genzyme Renal Innovations Program (to W. C. O'Neill). D. A. Prosdocimo was supported by NIH Grant T32-HL-07887.
Supplementary Material
Acknowledgments
We thank Dr. Robert Terkeltaub (University of California, San Diego, School of Medicine, La Jolla, CA) for providing the human eNPP1 plasmid; Dr. Steve Fisher and lab members for technical assistance in the isolation of rat aortic VSMC; Gregg DiNuoscio for technical assistance; and Andrew E. Blum for useful discussions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA 99: 9840–9845, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CA. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol 285: F423–F429, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Blum AE, Joseph SM, Przybylski RJ, Dubyak GR. Rho-family GTPases modulate Ca2+-dependent ATP release from astrocytes. Am J Physiol Cell Physiol 295: C231–C241, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JK, Knight PA, Wright SH, Thornton EM, Miller HR. Constitutive secretion of the granule chymase mouse mast cell protease-1 and the chemokine, CCL2, by mucosal mast cell homologues. Clin Exp Allergy 33: 132–146, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Brown NA, Stofko RE, Uhler MD. Induction of alkaline phosphatase in mouse L cells by overexpression of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem 265: 13181–13189, 1990. [PubMed] [Google Scholar]
- 6.Bukauskas FF, Elfgang C, Willecke K, Weingart R. Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophys J 68: 2289–2298, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukauskas FF, Weingart R. Temperature dependence of gap junction properties in neonatal rat heart cells. Pflügers Arch 423: 133–139, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol 22: 364–373, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci 18: 8794–8804, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation 117: 2938–2948, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diglio CA, Grammas P, Giacomelli F, Wiener J. Angiogenesis in rat aorta ring explant cultures. Lab Invest 60: 523–531, 1989. [PubMed] [Google Scholar]
- 12.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res 20: 41–49, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giachelli CM, Speer MY, Li X, Rajachar RM, Yang H. Regulation of vascular calcification: roles of phosphate and osteopontin. Circ Res 96: 717–722, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y, Hsu DK, Feng SL, Richards CM, Winkles JA. Polypeptide growth factors and phorbol ester induce progressive ankylosis (ank) gene expression in murine and human fibroblasts. J Cell Biochem 84: 27–38, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millan JL. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol 164: 1199–1209, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA 99: 9445–9449, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science 289: 265–270, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Huang MS, Sage AP, Lu J, Demer LL, Tintut Y. Phosphate and pyrophosphate mediate PKA-induced vascular cell calcification. Biochem Biophys Res Commun 374: 553–558, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 104: 6436–6441, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iovine MK, Gumpert AM, Falk MM, Mendelson TC. Cx23, a connexin with only four extracellular-loop cysteines, forms functional gap junction channels and hemichannels. FEBS Lett 582: 165–170, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iyemere VP, Proudfoot D, Weissberg PL, Shanahan CM. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J Intern Med 260: 192–210, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Johnson K, Polewski M, van Etten D, Terkeltaub R. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1-/- mice. Arterioscler Thromb Vasc Biol 25: 686–691, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 99: 1044–1059, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem 278: 23331–23342, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Joseph SM, Pifer MA, Przybylski RJ, Dubyak GR. Methylene ATP analogs as modulators of extracellular ATP metabolism and accumulation. Br J Pharmacol 142: 1002–1014, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreda SM, Seminario-Vidal L, Heusden C, Lazarowski ER. Thrombin-promoted release of UDP-glucose from human astrocytoma cells. Br J Pharmacol 153: 1528–1537, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahser FC, Malcolm BA. A continuous nonradioactive assay for RNA-dependent RNA polymerase activity. Anal Biochem 325: 247–254, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem 275: 31061–31068, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64: 785–795, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol Pharmacol 63: 1190–1197, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem 279: 36855–36864, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levesque SA, Lavoie EG, Lecka J, Bigonnesse F, Sevigny J. Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br J Pharmacol 152: 141–150, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res 98: 905–912, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Liang J, Fu M, Ciociola E, Chandalia M, Abate N. Role of ENPP1 on adipocyte maturation. PLoS ONE 2: e882, 2007. [DOI] [PMC free article] [PubMed]
- 35.Lin JH, Yang J, Liu S, Takano T, Wang X, Gao Q, Willecke K, Nedergaard M. Connexin mediates gap junction-independent resistance to cellular injury. J Neurosci 23: 430–441, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA 103: 7655–7659, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI, O'Neill WC. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol 15: 1392–1401, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Lomashvili KA, Garg P, Narisawa S, Millan JL, O'Neill WC. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int 73: 1024–1030, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendez AJ Monensin and brefeldin A inhibit high density lipoprotein-mediated cholesterol efflux from cholesterol-enriched cells. Implications for intracellular cholesterol transport. J Biol Chem 270: 5891–5900, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Millan JL Alkaline phosphatases: structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal 2: 335–341, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller SG, Carnell L, Moore HH. Post-Golgi membrane traffic: brefeldin A inhibits export from distal Golgi compartments to the cell surface but not recycling. J Cell Biol 118: 267–283, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev 19: 1093–1104, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narisawa S, Harmey D, Yadav MC, O'Neill WC, Hoylaerts MF, Millan JL. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res 22: 1700–1710, 2007. [DOI] [PubMed] [Google Scholar]
- 44.North RA Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002. [DOI] [PubMed] [Google Scholar]
- 45.O'Neill WC Vascular calcification: not so crystal clear. Kidney Int 71: 282–283, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Proudfoot D, Skepper JN, Shanahan CM, Weissberg PL. Calcification of human vascular cells in vitro is correlated with high levels of matrix Gla protein and low levels of osteopontin expression. Arterioscler Thromb Vasc Biol 18: 379–388, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2: 409–430, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers MJ New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des 9: 2643–2658, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Romanello M, Pani B, Bicego M, D'Andrea P. Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun 289: 1275–1281, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Ronaghi M, Karamohamed S, Pettersson B, Uhlen M, Nyren P. Real-time DNA sequencing using detection of pyrophosphate release. Anal Biochem 242: 84–89, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Hohne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nurnberg P. Mutations in ENPP1 are associated with 'idiopathic' infantile arterial calcification. Nat Genet 34: 379–381, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Rutsch F, Terkeltaub R. Parallels between arterial and cartilage calcification: what understanding artery calcification can teach us about chondrocalcinosis. Curr Opin Rheumatol 15: 302–310, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Sauer H, Hescheler J, Wartenberg M. Mechanical strain-induced Ca2+ waves are propagated via ATP release and purinergic receptor activation. Am J Physiol Cell Physiol 279: C295–C307, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Schock SC, Leblanc D, Hakim AM, Thompson CS. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem Biophys Res Commun 368: 138–144, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Shanahan CM Mechanisms of vascular calcification in renal disease. Clin Nephrol 63: 146–157, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol 26: 1423–1430, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci 30: 542–550, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res 89: 1147–1154, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Terkeltaub R Physiologic and pathologic functions of the NPP nucleotide pyrophosphatase/phosphodiesterase family focusing on NPP1 in calcification. Purinergic Signal 2: 371–377, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Terkeltaub RA Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol 281: C1–C11, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Volonte C, Amadio S, D'Ambrosi N, Colpi M, Burnstock G. P2 receptor web: complexity and fine-tuning. Pharmacol Ther 112: 264–280, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto K, Sokabe T, Ohura N, Nakatsuka H, Kamiya A, Ando J. Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol 285: H793–H803, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Zebisch M, Strater N. Structural insight into signal conversion and inactivation by NTPDase2 in purinergic signaling. Proc Natl Acad Sci USA 105: 6882–6887, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.