Abstract
The integrity of the intestinal epithelial barrier depends on intercellular junctions that are highly regulated by numerous extracellular and intracellular factors. E-cadherin is found primarily at the adherens junctions in the intestinal mucosa and mediates strong cell-cell contacts that have a functional role in forming and regulating the epithelial barrier. Polyamines are necessary for E-cadherin expression, but the exact mechanism underlying polyamines remains elusive. The current study was performed to determine whether polyamines induce E-cadherin expression through the transcription factor c-Myc and whether polyamine-regulated E-cadherin plays a role in maintenance of the epithelial barrier integrity. Decreasing cellular polyamines reduced c-Myc and repressed E-cadherin transcription as indicated by a decrease in levels of E-cadherin promoter activity and its mRNA. Forced expression of the c-myc gene by infection with adenoviral vector containing c-Myc cDNA stimulated E-cadherin promoter activity and increased E-cadherin mRNA and protein levels in polyamine-deficient cells. Experiments using different E-cadherin promoter mutants revealed that induction of E-cadherin transcription by c-Myc was mediated through the E-Pal box located at the proximal region of the E-cadherin promoter. Decreased levels of E-cadherin in polyamine-deficient cells marginally increased basal levels of paracellular permeability but, remarkably, potentiated H2O2-induced epithelial barrier dysfunction. E-cadherin silencing by transfection with its specific small interfering RNA also increased vulnerability of the epithelial barrier to H2O2. These results indicate that polyamines enhance E-cadherin transcription by activating c-Myc, thus promoting function of the epithelial barrier.
Keywords: paracellular permeability, adherens junctions, gene expression, ornithine decarboxylase, intestinal epithelial cells
epithelial cells line the gastrointestinal mucosa and form an important barrier to a wide array of noxious substances in the lumen. The effectiveness and stability of this epithelial barrier depend on specialized structures composing different intercellular junctions, including tight junctions and adherens junctions (14, 20, 45, 48). Under physiological conditions, these junctional proteins completely surround the subapical region of epithelial cells and maintain the structural integrity and normal functions of the intestinal epithelium (14, 45). The tight junction is the most apical of these junctional complexes, and it seals epithelial cells together in a way that prevents even small molecules from leaking between cells (34, 47, 48). Immediately below the tight junctions are the cadherin-rich adherens junctions that mediate strong cell-cell adhesion and tightly interconnect epithelial cells together (6, 17, 18, 35). An increasing body of evidence indicates that the prior formation of adherens junctions is essential for the assembly of tight junctions between epithelial cells and that alteration in levels of the cadherin-dependent adherens junctions regulates the stability of tight junction complex and affects intestinal epithelial paracellular permeability (2, 20, 30).
E-cadherin is found primarily at the adherens junctions and is the major cadherin expressed in epithelial cells, including those of the gastrointestinal tract (1, 49). As a classic member of the adhesion molecules, E-cadherin contains a single transmembrane domain, a cytoplasmic domain of ∼150 amino acid residues, and an ectodomain of ∼550 amino acid residues comprising five tandemly repeated domains (2, 49). The dimers of E-cadherin bind homotypically to similar dimers on neighboring cells in the intestinal epithelium, and its conserved cytoplasmic domain interacts with the cytoskeleton through actin-binding proteins, leading to strengthened cell-cell adhesion (49). These strong E-cadherin-mediated cell-cell adhesions are thought to be critical for epithelial barrier function, and signals that are transmitted through the adherens junctions also regulate tight junction function (17, 18, 20, 49). In support of this notion, inhibition of the cadherin-dependent adherens junctions by ectopic expression of an E-cadherin dominant negative mutant retards the assembly of tight junctions and disrupts epithelial barrier function (1, 13, 32, 50). Furthermore, adherens junctions are shown to be more vulnerable than tight junctions in some clinical conditions (15, 21) and are of significance in the pathogenesis of epithelial barrier failure (49).
The natural polyamines (putrescine, spermidine, and spermine) are ubiquitous polycationic molecules found in all eukaryotic cells and play distinct regulatory roles in intestinal epithelial cells (IECs) (7, 16, 46, 54). Polyamines regulate expression of various genes involved in epithelial cell division (28, 38, 52, 55), repair (41, 51), and apoptosis (27, 57, 59), whereas polyamine deficiency inhibits mucosal growth and delays healing after injury. We (19, 20) recently demonstrated that polyamines also modulate intercellular junction levels and that decreasing cellular polyamines by inhibiting ornithine decarboxylase (ODC; a key enzyme of polyamine biosynthesis) with α-difluoromethylornithine (DFMO) results in a reduction in levels of adherens junction proteins such as E-cadherin, β-catenin, and α-catenin. To define the mechanism underlying polyamines in adherens junction expression, our studies have further shown that polyamines stabilize E-cadherin mRNA and protein at least partially by altering Ca2+ signaling (19, 43). However, the role and mechanism of polyamines in the regulation of E-cadherin gene transcription remain elusive.
E-cadherin transcription is tightly controlled through the proximal region of the E-cadherin promoter that contains several cis-elements, including a CAAT-box, GC-rich region, and a 12-bp palindromic element named the E-Pal box (5, 12, 23, 30). The transcription factor AP-2 has been shown to stimulate E-cadherin transcription through its direct interaction with the GC-rich region (23), whereas the retinoblastoma protein (Rb) and the proto-oncogene product c-Myc specifically activate the E-cadherin promoter by acting as coactivators of AP-2 (4). The zinc-finger protein WT1 also interacts with the GC-rich region of the E-cadherin promoter and induces E-cadherin transcription (24). Another candidate transcription factor interacting with the E-cadherin promoter is the hepatocyte nuclear factor-3, which synergizes with AML-1 and p300 to stimulate E-cadherin transcription (30). In this study, we set out to study whether polyamines induce E-cadherin transcription through c-Myc. The data presented indicate that decreasing cellular polyamines represses E-cadherin transcription, which is prevented by ectopic c-Myc overexpression. Induction in E-cadherin transcription by c-Myc is mediated through the E-Pal box that is located at the proximal region of the E-cadherin promoter, thus providing new insight into the mechanism underlying polyamines in the regulation of E-cadherin transcription.
MATERIALS AND METHODS
Chemicals and supplies.
Disposable culture ware was purchased from Corning Glass Works (Corning, NY). Tissue culture media and dialyzed FBS were obtained from Invitrogen (Carlsbad, CA), and biochemicals were obtained from Sigma (St. Louis, MO). The monoclonal antibodies against E-cadherin and β-catenin were purchased from Transduction Laboratories (Lexington, KY); the anti-ODC antibody (0–1136) and the secondary antibody, anti-mouse IgG conjugated to horseradish peroxidase (A-8924), were purchased from Sigma. The antibody against c-Myc was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and DFMO was obtained from Genzyme (Cambridge, MA). 14C-labeled mannitol and 3H-labeled inulin were purchased from Amersham Pharmacia Biotech (Piscataway, NJ), and the 12-mm Transwell filters (0.4-μm pore size, clear polyester) were obtained from Costar (Cambridge, MA).
Recombinant viral construction and reporter plasmids.
Adenoviral vectors were constructed using the Adeno-X expression system (Clontech) according to the protocol provided by the manufacturer. Briefly, the full-length cDNA of human c-Myc was cloned into the pShuttle by digesting BamHI/HindIII and ligating the resultant fragments into the XbaI site of the pShuttle vector. pAdeno-c-Myc(AdMyc) was constructed by digesting pShuttle construct with PI-SceI/I-CeuI and ligating the resultant fragment into the PI-SceI/I-CeuI sites of the pAdeno-X adenoviral vector. Recombinant adenoviral plasmids were packaged into infectious adenoviral particles by transfecting human embryonic kidney (HEK)-293 cells using Lipofectamine Plus reagent (GIBCO Bethesda Research Laboratory, Gaithersburg, MD). The adenoviral particles were propagated in HEK-293 cells and purified with cesium chloride ultracentrifugation. Titers of the adenoviral stock were determined by standard plaque assay. Recombinant adenoviruses were screened for the expression of the introduced gene by Western blot analysis using anti-c-Myc antibody. pAdeno-X, which was the recombinant replication-incompetent adenovirus carrying no c-Myc cDNA insert (Adnull), was grown and purified as described above and served as a control adenovirus. IEC-6 cells were infected with AdMyc or Adnull, and expression of c-Myc was assayed at 24 or 48 h after the infection.
The full-length E-cadherin promoter was cloned from human genomic DNA, and the construct of full-length E-cadherin promoter luciferase (Luc) reporter, F-Luc (∼270-bp regulatory region upstream of the E-cadherin gene fused to the Luc reporter gene) was generated as described previously (28). The 5′-deletion E-cadherin promoter constructs, including 70-Luc and 60-Luc, and the E-Pal, CAAT, GC1, and GC2 point mutants of the E-cadherin promoter were generated using the QuikChange site-directed mutagenesis kit and performed according to the manufacturer's instructions (Stratagene, La Jolla, CA). Mutations of various binding sites within the E-cadherin promoter were verified by DNA sequencing. Transient transfection was performed using the Lipofectamine kit as recommended by the manufacturer (Invitrogen). Cells were collected 48 h after the transfection, and luciferase assay was performed using the Bright-Glo luciferase assay system as recommended by the manufacturer (Promega, Madison, WI). The activity from individual transfection was normalized by the β-galactosidase activity from cotransfected pRSV β-galactosidase plasmid. The experiments were done in triplicate and are reported as the means of relative light unit normalized to β-galactosidase.
Cell culture.
The IEC-6 cell line was purchased from the American Type Culture Collection at passage 13. The cell line was derived from normal rat intestine and was developed and characterized by Quaroni et al. (40). IEC-6 cells originated from intestinal crypt cells as judged by morphological and immunologic criteria. They are nontumorigenic and retain undifferentiated characteristics of intestinal crypt cells. Stock cells were maintained in T-150 flasks in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% heat-inactivated FBS, 10 μg/ml insulin, and 50 μg/ml gentamicin. Flasks were incubated at 37°C in a humidified atmosphere of 90% air-10% CO2, and passages 15–20 were used in experiments. IEC-6 cells at passages 15–20 exhibit a stable phenotype (9, 42).
RNA interference.
The small interfering RNA (siRNA) specifically targeting the coding region of E-cadherin mRNA (siE-cad) was purchased from Sequitur (Natick, MA). Scrambled control siRNA (C-siRNA), which had no sequence homology to any known genes, was used as the control. The siE-cad and C-siRNA were transfected into cells as described previously (41, 42). Briefly, for each 60-mm cell culture dish, 15 μl of the 20 μM stock siE-cad or C-siRNA were mixed with 300 μl of Opti-MEM medium (Invitrogen). This mixture was gently added to a solution containing 10 μl of Lipofectamine 2000 in 300 μl of Opti-MEM medium. The solution was incubated for 20 min at room temperature and gently overlaid onto the monolayer of cells in 3 ml of medium, and cells were harvested for various assays after 48-h incubation.
Real-time quantitative PCR analysis.
Total RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA). Equal amounts of total RNA (2 μg) were transcribed to synthesize single-stranded cDNA with an RT-PCR kit (Invitrogen). Real-time quantitative PCR (Q-PCR) was performed using Applied Biosystems Instruments (Foster City, CA) specific primers, probes, and software as described in our previous publications (56, 58). The levels of E-cadherin mRNA were quantified by Q-PCR analysis and normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels.
Western blot analysis.
Cell samples, placed in SDS sample buffer (250 mM Tris-HCl, pH 6.8, 2% SDS, 20% glycerol, and 5% mercaptoethanol), were sonicated and then centrifuged (10,000 g) at 4°C for 15 min. The supernatant from cell samples was boiled for 5 min and then subjected to electrophoresis on 7.5% acrylamide gels. After the transfer of protein onto nitrocellulose filters, the filters were incubated for 1 h in 5% nonfat dry milk in 1× phosphate-buffered saline-Tween 20 [PBS-T: 15 mM NaH2PO4, 80 mM Na2HPO4, 1.5 M NaCl, pH 7.5, and 0.5% (vol/vol) Tween 20]. Immunologic evaluation was then performed for 1 h in 1% BSA/PBS-T buffer containing 1 μg/ml of the specific antibody against c-Myc or E-cadherin proteins. The filters were subsequently washed with 1× PBS-T and incubated for 1 h with the second antibody conjugated to peroxidase by protein cross linking with 0.2% glutaraldehyde. After extensive washing with 1× PBS-T, the immunocomplexes on the filters were developed using the enhanced chemiluminescence method according to the manufacturer's instructions (Amersham Pharmacia Biotech, Arlington Heights, IL).
Chromatin immunoprecipitation.
IEC-6 cells were infected with the AdMyc or Adnull for 48 h and then fixed with 1% formaldehyde to cross link chromatin. Chromatin immunoprecipitation (ChIP) analysis was performed using the Active Motif ChIP-IT kit (Carlsbad, CA), following the manufacturer's recommendations with minor modification, as described previously (10, 55). Briefly, cells were suspended in lysis buffer and gently dounced on ice with 10 strokes to aid in nuclei release. After centrifugation, the nuclear pellet was suspended in digestion buffer and the chromatin was sheared with Enzymatic Shearing Cocktail. The sheared DNA samples were centrifuged, and the supernatants were collected and precleared with protein G beads. The precleared DNA samples were then incubated with the anti-c-Myc or control IgG antibodies overnight with constant rotation. The immunocomplexes were captured by addition of protein G beads, and the immunoprecipitated DNA was collected from the beads using ChIP elution buffer. DNA-protein cross links were reversed and deproteinized, and DNA was recovered and amplified by PCR. Primers to amplify the proximal region of the E-cadherin promoter containing an E-Pal box were 5′-TGCCACCAACTACAGACAGG-3′ and 5′-GGGCAGGAGTCTAGCAGAAG-3′. Primers to amplify the proximal region of the GAPDH promoter (a negative control) were 5′-TACTAGCGGTTTTACGGGCG-3′ and 5′-TCGAACAGGAGGAGCAGAGAGCGA-3′. The DNA isolated through IgG ChIP was used as a negative control. The input DNA, obtained from chromatin that was processed (cross linked and reversed) similarly to the samples, served as a positive control for PCR effectiveness.
Paracellular tracer flux assay.
Flux assays were performed on the 12-mm Transwell as described in our previous publications (19, 20). Briefly, cells were grown in control cultures or cultures containing 5 mM DFMO or DFMO plus 5 μM spermidine for 4 days, trypsinized and plated at confluent density of 4 × 104 cells/cm2 on the insert, and maintained at same culture conditions for additional 48 h to establish tight monolayers. Two different membrane-impermeable molecules, [14C]mannitol (mol wt 184) and [3H]inulin (mol wt 5,200), served as paracellular tracers in this experiment. At the beginning of the flux assay, both sides of the bathing wells of Transwell filters were replaced with fresh medium containing either 5 mM unlabeled mannitol or inulin. Each of the tracers was added to a final concentration of 3.6 nM to the apical bathing wells that contained 0.5 ml of medium. The basal bathing well had no added tracers and contained 1.5 ml of the same flux assay medium as in the apical compartment. All flux assays were performed at 37°C, and the basal medium was collected 2 h after addition of [14C]mannitol or [3H]inulin for a Beckman liquid scintillation counter. The results were expressed as percentage of total count values of tracer.
Statistics.
All data are means ± SE from three to six samples. Immunoblotting results were repeated three times. The significance of the difference between means was determined using analysis of variance (ANOVA). The level of significance was determined using Duncan's multiple-range test (22) or two-way ANOVA.
RESULTS
Decreased levels of endogenous c-Myc are associated with repression of E-cadherin transcription following polyamine depletion.
Our previous study showed that polyamines stabilize E-cadherin protein by increasing Ca2+ influx through control of Kv channel activity and membrane potential (20, 43). The focus of the current study was to further determine whether polyamines are implicated in the regulation of E-cadherin gene transcription via c-Myc. Exposure of IEC-6 cells to 5 mM DFMO for 6 days completely inhibited ODC enzyme activity and almost totally depleted cellular polyamines. Levels of putrescine and spermidine were undetectable on day 6 after treatment with DFMO, and spermine had decreased by ∼60% as reported in our previous publications (19, 28). Consistent with our previous findings (29, 38), polyamine depletion by DFMO also significantly decreased the steady-state levels of c-Myc protein, which was completely prevented by exogenous spermidine (5 μM) given together with DFMO (Fig. 1A). Putrescine (10 μM) had an effect equal to that of spermidine on levels of c-Myc protein when it was added to cultures that contained DFMO (data not shown).
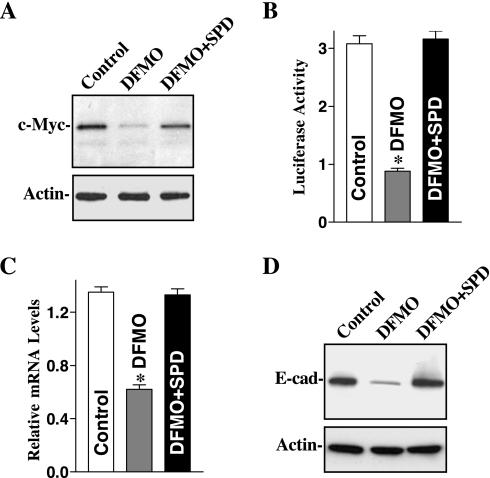
Fig. 1.
Effect of depletion of cellular polyamines by inhibiting ornithine decarboxylase (ODC) on c-Myc and E-cadherin expression in intestinal epithelial cell (IEC)-6 cells. Cells were grown in control cultures, cultures in which ODC activity was inhibited by 5 mM α-difluoromethylornithine (DFMO), and cultures inhibited with DFMO and supplemented with 5 μM spermidine (SPD) for 6 days. A: representative immunoblots. Whole cell lysates were harvested, applied to each lane (20 μg) equally, and subjected to electrophoresis on a 10% acrylamide gel. Levels of c-Myc protein were identified with a specific antibody that recognizes c-Myc. After the membrane was stripped, it was reprobed with actin that served as an internal control for equal loading. B: E-cadherin promoter activity in cells described in A. Cells were grown in medium containing either DFMO alone or DFMO + SPD for 4 days and were then transfected using the E-cadherin promoter luciferase reporter construct. After incubation for 48 h in the same culture conditions, transfected cells were harvested and assayed for luciferase activity. Data were normalized according to β-galactosidase activity from cotransfected plasmid pRSV β-galactosidase. Values are means ± SE of data from 3 separate experiments. *P < 0.05 compared with controls and cells exposed to DFMO + SPD. C: levels of E-cadherin mRNA in cells described in A. Total cellular RNA was isolated, and levels of E-cadherin mRNA were measured using real-time quantitative PCR analysis. Values are means ± SE of data from 3 separate experiments. *P < 0.05 compared with control and cells treated with DFMO + SPD. D: representative immunoblots of E-cadherin in cells described in A. E-cad, E-cadherin. Data are representative of 3 independent experiments showing similar results.
Decreased levels of endogenous c-Myc in the polyamine-deficient cells were associated with a significant inhibition of E-cadherin transcription as demonstrated by decreases in E-cadherin promoter activity (Fig. 1B) and E-cadherin mRNA (Fig. 1C). Levels of E-cadherin promoter activity and E-cadherin mRNA were decreased by ∼60% in cells exposed to DFMO for 6 days, which were associated with a significant decrease in the level of E-cadherin protein (Fig. 1D). Expression of E-cadherin protein was inhibited by ∼80% in DFMO-treated cells. Furthermore, this repressed E-cadherin expression following decreased c-Myc in polyamine-deficient cells was accompanied by dysfunction of the intestinal epithelial barrier as indicated by an increase in paracellular permeability as described in our previous publications (20). In the presence of DFMO, exogenous spermidine not only prevented the reduction in c-Myc but also returned E-cadherin expression to near normal level. These results suggest that repression of E-cadherin transcription following polyamine depletion results from an inhibition of c-Myc.
c-Myc ectopic expression stimulates E-cadherin transcription.
To further define the role of polyamine-modulated c-Myc in the regulation of E-cadherin transcription in IECs, we examined the effect of overexpression of the c-myc gene on E-cadherin expression in the presence or absence of cellular polyamines. The adenoviral vector containing the corresponding human c-Myc cDNA under the control of the pCMV promoter was constructed as described in our previous publication (28). Adenoviral vectors used in this study have been shown to infect intestinal epithelial cells with near 100% efficiency (28, 29). In fact, >95% of IEC-6 cells were positive when they were infected for 24 h with the adenoviral vector encoding green fluorescent protein (data not shown). As noted in Fig. 2A, the c-Myc protein was expressed in amounts increasing with the viral load and reached ∼15- and 25-fold higher than control levels when adenovirus at a concentration of 10 or 50 plaque-forming units (pfu)/cell was used. An adenovirus that lacked exogenous c-Myc cDNA (Adnull) was used as negative control in this experiment and did not induce c-Myc levels when it was infected at equal concentration.
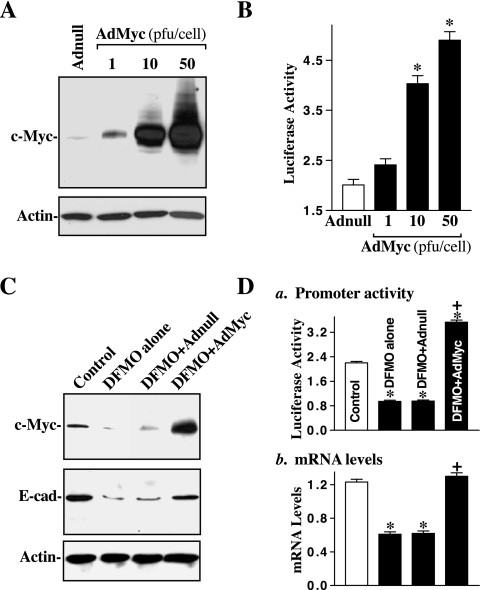
Fig. 2.
Effect of c-Myc overexpression on E-cadherin expression in normal and polyamine-deficient cells. A: representative immunoblots. The c-Myc adenoviral expression vector (AdMyc) was constructed in which the complete open reading frame of the human c-Myc cDNA was cloned to pShuttle of the Adeno-X expression system under the control of the human cytomegalovirus immediate-early promoter pCMV. Cells were infected with the AdMyc or adenoviral vector containing no c-Myc cDNA (Adnull) at a multiplicity of infection of 1–50 plaque-forming units (pfu)/cell, and c-Myc expression was analyzed 48 h after the infection. B: changes in E-cadherin promoter activity in cells described in A. The luciferase activity was measured 48 h after the transfection with the E-cadherin-promoter luciferase reporter construct, and data were normalized according to β-galactosidase activity from cotransfected plasmid pRSV β-galactosidase. Values are means ± SE of data from 3 separate experiments. *P < 0.05 compared with cells infected with the Adnull. C: effect of ectopic expression of c-Myc on E-cadherin expression in polyamine-deficient cells. Cells were grown in control cultures and cultures containing 5 mM DFMO for 4 days and then infected with the AdMyc or Adnull at a concentration of 10 pfu/cell. After 48 h in the presence of DFMO, whole cell lysates were harvested; levels of c-Myc and E-cadherin proteins were measured by Western blot analysis. Equal loading was monitored by actin immunoblotting. D: changes in levels of E-cadherin promoter activity (a) and its mRNA (b) in cells described in C. Values are means ± SE of data from 3 separate results. *P < 0.05 compared with controls. +P < 0.05 compared with DFMO-treated cells and cells treated with DFMO and then infected with the Adnull.
Transient infection with the AdMyc dose-dependently increased E-cadherin promoter activity in normal IEC-6 cells (Fig. 2B). The levels of E-cadherin promoter activity in cells infected with the AdMyc were increased by ∼2-fold at a dose of 10 pfu/cell and by ∼2.5-fold at doses of 50 pfu/cells compared with cells infected with the Adnull in the same concentrations. On the other hand, it is interesting and important that forced expression of c-Myc by infection with the AdMyc had similar stimulatory effect on E-cadherin transcription in polyamine-deficient cells. The level of c-Myc protein was increased by more than 12-fold when polyamine-deficient cells were infected with the AdMyc (10 pfu/cell) relative to DFMO-treated cells infected with Adnull at the same concentration (Fig. 2C). Consistently, induced c-Myc significantly prevented the decrease in levels of E-cadherin transcription (Fig. 2D) and its protein in polyamine-deficient cells. The level of E-cadherin protein in DFMO-treated cells infected with the AdMyc was increased by ∼60% relative to DFMO-treated cells infected with Adnull. These results indicate that induced c-Myc increases E-cadherin regardless of the presence or absence of cellular polyamines, suggesting that polyamines regulate E-cadherin transcription through a c-Myc-mediated mechanism in normal IECs.
c-Myc regulates E-cadherin transcription through the E-Pal box.
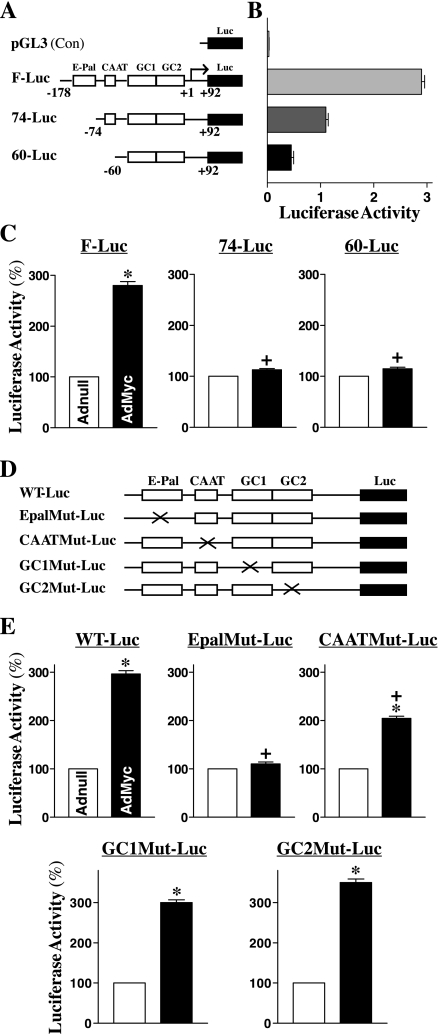
To define the mechanism by which c-Myc stimulates E-cadherin transcription, we cloned the E-cadherin promoter fragment from human genomic DNA, which contained the E-Pal box, CAAT box, and GC1 and GC2 region as illustrated in Fig. 3A. To map the c-Myc-responsive region of the E-cadherin promoter, we prepared different reporter constructs containing deletions in the E-cadherin promoter. As shown in Fig. 3B, the elements that contained a 270-bp region of the E-cadherin promoter were required for basal and regulatory E-cadherin expression in IEC-6 cells, because the 5′-deletions of the E-cadherin promoter gradually decreased to basal levels of reporter gene activity. The results presented in Fig. 3C further show that ectopic expression of the c-myc stimulated E-cadherin promoter activity and that this induction was mediated through an E-Pal box within its promoter region. The E-cadherin promoter activity increased significantly after c-Myc overexpression when cells were transfected with the F-Luc construct that contains the E-Pal box. However, this induction in E-cadherin promoter activity by c-Myc completely disappeared when cells were transfected with either the 74-Luc or 60-Luc (both promoter fragments containing no E-Pal-box). To further determine the exact function of this E-Pal box within the E-cadherin promoter region, we generated different E-Pal, CAAT, GC1, and GC2 point mutants of the E-cadherin promoter, as indicated in Fig. 3D. Consistent with observations from deletion study, the point mutation of the E-Pal box within the E-cadherin promoter region completely prevented induction in the E-cadherin promoter by c-Myc (Fig. 3E, top center). When cells were transfected with the EpalMut-Luc, there were no significant differences in levels of E-cadherin promoter activity between controls (Adnull) and cells overexpressing c-Myc. In addition, the CAAT point mutation of the E-cadherin promoter also marginally but significantly reduced the stimulation of the E-cadherin promoter by c-Myc (Fig. 3E, top right). On the other hand, GC1 or GC2 point mutation of the E-cadherin promoter failed to alter c-Myc-induced stimulation of E-cadherin promoter (Fig. 3E, bottom).
Fig. 3.
Effect of ectopic expression of the c-myc gene on E-cadherin promoter activity after mutation of different binding sites. A: schematic representation of deletion of E-cadherin promoter luciferase (Luc) reporter constructs. E-Pal, CAAT, GC1, and GC2 indicate the relative locations of specific binding sites within the E-cadherin-promoter. B: basal activity of various deletion mutants of the E-cadherin promoter in IEC-6 cells without c-Myc overexpression. Cells were transfected with different deletion mutants of E-cadherin-promoter, and levels of the luciferase reporter activity were detected 48 h after the transfection. Results were normalized relative to β-galactosidase activity from cotransfected plasmid pRSV β-galactosidase. Values are means ± SE of data from 3 separate experiments. C: changes in luciferase reporter activity of deletion constructs after c-Myc overexpression. Cells were infected with either the AdMyc or Adnull at s concentration of 10 pfu/cell for 24 h and then transfected using different E-cadherin promoter luciferase reporter deletion constructs. The levels of luciferase activity was assayed 48 h after transfection. Values are means ± SE of data from 3 separate experiments. *P < 0.05 compared with cells transfected with the Adnull. +P < 0.05 compared with cells infected with the AdMyc and then transfected with the F-Luc. D: schematic representation of various point mutants. Mutagenic oligonucleotides were designed to hybridize to the E-cadherin-promoter construct to create the mutant (Mut) where the E-Pal, CAAT, GC1, or GC2 consensus site was eliminated by making 2 base changes. E: levels of reporter gene activity. Values are means ± SE of data from 3 separate experiments. *P < 0.05 compared with cells infected with the Adnull. +P < 0.05 compared with cells infected with the AdMyc and then transfected with the WT-Luc.
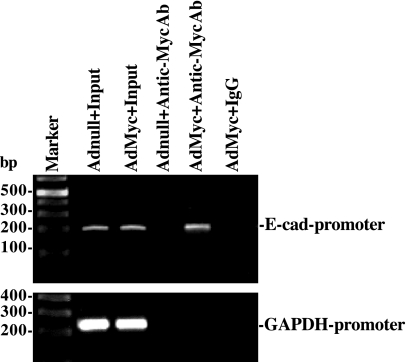
ChIP analysis also was used to examine the in vivo association of c-Myc with the E-cadherin promoter region. Nuclear fractions were immunoprecipitated using a specific anti-c-Myc antibody in cells infected with the c-Myc overexpression or control vectors, and the associated DNA was purified. Using specific PCR primers, we obtained a 205-bp PCR product that matched the sequence of a proximal region of E-cadherin promoter from −144 to 61 (containing the E-Pal box) relative to the transcriptional start site. c-Myc was bound to the E-cadherin promoter in vivo, as shown using an anti-c-Myc antibody in cells overexpressing c-Myc (Fig. 4, top). This association was specific for c-Myc, since no PCR product was detectable in AdMyc-infected cells when a nonspecific antibody (IgG) or primers to an unrelated promoter such as the GAPDH promoter were used (Fig. 4, bottom). These results indicate that c-Myc stimulates E-cadherin transcription by interacting with the E-Pal box within the proximal region of the E-cadherin promoter.
Fig. 4.
Activity of c-Myc binding to E-cadherin promoter as measured by chromatin immunoprecipitation (ChIP) analysis. The association of c-Myc with the E-cadherin promoter (between −144 and 61) was determined using an anti-c-Myc antibody (Ab); IgG was used as a negative control. Cross-linked chromatin isolated from cells infected with either the AdMyc or Adnull was immunoprecipitated using an anti-c-Myc Ab, and the associated chromosomal DNA fragments were amplified by PCR using E-cadherin promoter-specific primers and GAPDH promoter-specific primers as described in materials and methods. The expected size of the PCR product was ∼205 bp. Chromosomal DNA input was subject to the same procedures and served as a positive control. Three experiments were performed that showed similar results.
Decreased E-cadherin in polyamine-deficient cells is accompanied by dysfunction of the epithelial barrier.
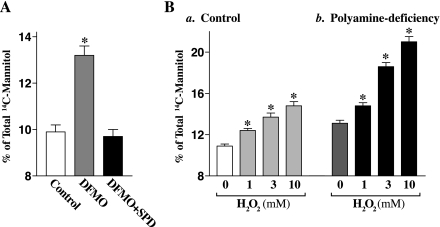
To determine the involvement of polyamine-modulated E-cadherin in maintaining the intestinal barrier integrity, we examined epithelial paracellular permeability by measuring the paracellular flux of membrane-impermeable tracer across the confluent monolayer in controls and polyamine-deficient cells with or without the challenge by H2O2. Cells were grown in control cultures or in cultures containing DFMO or DFMO plus putrescine for 4 days, plated at confluent density on the insert, and maintained for an additional 48 h to establish a tight monolayer in the same culture conditions. A widely accepted hydrophilic paracellular tracer molecule, [14C]mannitol, was used in this study. To verify the system used for paracellular permeability assays, we tested the effect of removal of extracellular Ca2+ on the paracellular flux of [14C]mannitol, which served as a positive control. As expected, exposure to the Ca2+-free medium for 2 h remarkably increased paracellular permeability (data not shown). As shown in Fig. 5A, decreased levels of E-cadherin expression following reduction of c-Myc in polyamine-deficient cells were associated with an increase in basal level of paracellular permeability, which was completely prevented by exogenous spermidine given together with DFMO. Results presented in Fig. 5B further show that the epithelial barrier in polyamine-deficient cells that exhibited decreased levels of E-cadherin was more vulnerable than that observed in control cells after challenge by H2O2. When H2O2 at different concentrations was added to the medium, levels of paracellular flux of [14C]mannitol in controls were increased by ∼25% at 3 mM and by ∼36% at 10 mM, whereas levels of the paracellular flux in polyamine-deficient cells were increased by ∼40% at 3 mM and by ∼61% at 10 mM, respectively. In addition, H2O2 did not affect cell viability as measured by Trypan blue staining (data not shown).
Fig. 5.
Changes in paracellular permeability in control and polyamine-deficient IEC-6 cells with or without challenge with H2O2. A: basal levels of paracellular permeability. After cells were grown in control cultures or cultures containing DFMO or DFMO + SPD for 4 days, they then were trypsinized, plated at confluent density on the insert, and maintained under the same culture conditions for an additional 48 h. Membrane-impermeable tracer molecule, [14C]mannitol, was added to the insert medium, and the entire basal medium was collected 2 h thereafter for paracellular tracer flux assays. Values are means ± SE of data from 6 samples. *P < 0.05 compared with control cells and cells treated with DFMO + SPD. B: paracellular permeability in controls (a) and polyamine-deficient cells (b) after challenge with different concentrations of H2O2 for 3 h. After cells were treated with H2O2 for 1 h, [14C]mannitol was added to the insert medium, and levels of [14C]mannitol were measured 2 h thereafter. Values are means ± SE of data from 6 samples. *P < 0.05 compared with control cells.
E-cadherin silencing disrupts function of the epithelial barrier.
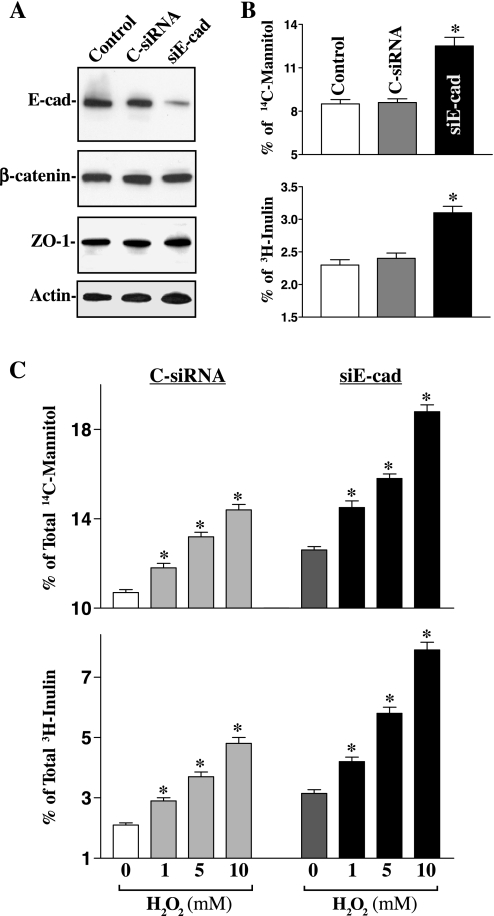
This study examined whether specific inhibition of E-cadherin expression by transfection with its specific siRNA (siE-cad) impairs function of the epithelial barrier with or without H2O2 challenge. These specific siE-cad nucleotides were designed to cleave rat E-cadherin mRNA by activating endogenous RNase H and to have a unique combination of specificity, efficiency, and reduced toxicity. Initially, we determined the transfection efficiency of the siRNA nucleotides in IEC-6 cells and demonstrated that >95% of cells were positive when they were transfected with a fluorescent FITC-conjugated siRNA for 24 h (data not shown). As shown in Fig. 6A, transfection with the siE-cad specifically decreased E-cadherin protein but had no effect on β-catenin and zonula occludens-1 (ZO-1) proteins. Level of E-cadherin protein was decreased by ∼80% when cells were exposed to siE-cad for 48 h. As expected, E-cadherin silencing also disrupted function of the epithelial barrier as indicated by an increase in levels of paracellular flux of [14C]mannitol and [3H]inulin (Fig. 6B). Levels of epithelial permeability were increased by ∼45% in [14C]mannitol and by ∼31% in [3H]inulin, respectively, compared with those observed in control cells and cells transfected with C-siRNA. Results presented in Fig. 6C further show that E-cadherin silencing by siE-cad also remarkably potentiated H2O2-induced epithelial barrier dysfunction. When cells were challenged by H2O2 at the concentration of 10 mM, levels of paracellular flux of [14C]mannitol were increased from 14.4 ± 0.25% (n = 6) in cells transfected with C-siRNA to 18.8 ± 0.3% (n = 6) in E-cadherin-silenced cells, whereas levels of the paracellular flux of [3H]inulin were increased from 4.8 ± 0.2% (n = 6) in control populations to 7.9 ± 0.3% (n = 6) in E-cadherin-silenced populations. In addition, neither siE-cad nor C-siRNA affected cell viability in the presence or absence of H2O2 challenge as measured by Trypan blue staining (data not shown). These findings strongly suggest that E-cadherin plays a critical role in maintenance of intestinal barrier integrity.
Fig. 6.
Effect of E-cadherin silencing on basal levels of paracellular permeability. A: representative immunoblots for E-cadherin, β-catenin, and zonula occludens-1 (ZO-1) proteins. Cells were transfected with specific small interfering RNA (siRNA) targeting the coding region of E-cadherin mRNA (siE-cad) or control siRNA (C-siRNA) for 48 h, and levels of E-cadherin, β-catenin, and ZO-1 proteins were measured by Western blot analysis. B: paracellular permeability in cells described in A. After cells were transfected with siE-cad or C-siRNA, they were plated at confluent density on the insert and maintained for an additional 48 h. Levels of paracellular permeability were measured 2 h after addition of [14C]mannitol or [3H]inulin. Values are means ± SE of data from 6 samples. *P < 0.05 compared with controls and cells transfected with C-siRNA. C: changes in levels of paracellular permeability after challenge with H2O2 in E-cadherin knockdown cells described in A. Cells were transfected with siE-cad or C-siRNA for 48 h, and levels of paracellular permeability were measured 2 h after addition of [14C]mannitol or [3H]inulin. Values are means ± SE of data from 6 samples. *P < 0.05 compared with cells transfected with C-siRNA.
DISCUSSION
Polyamines play a critical role in the maintenance of intestinal epithelial integrity (46, 54), but the exact mechanisms underlying polyamines at cellular and molecular levels remain unclear. We (10, 19, 20) recently demonstrated that polyamines regulate the intestinal epithelial barrier function and that polyamine depletion increases epithelial paracellular permeability at least partially by repressing expression of ZO-1 and E-cadherin. Our studies have further shown that polyamines modulate expression of various intercellular junction proteins through distinct cellular signaling pathways. In this regard, polyamines promote occludin mRNA translation and increase its protein stability (19), whereas polyamines modulate ZO-1 transcription by altering the interaction of the ZO-1 gene promoter with the transcription factor JunD (10). In this report, we provide new evidence showing that polyamines are necessary for E-cadherin transcription through its gene promoter, thus advancing our understanding of the functions of cellular polyamines in IECs. The most significant of new finding reported in this study is that polyamines promote E-cadherin transcription by activating c-Myc that interacts the E-Pal box in the proximal region of the E-cadherin promoter.
Results reported in the present study clearly show that decreasing cellular polyamine levels by inhibiting ODC with DFMO decreased levels of c-Myc protein, which was associated with an inhibition of E-cadherin transcription (Fig. 1). Because both decreased c-Myc and inactivation of E-cadherin transcription in DFMO-treated cells were completely prevented by the addition of exogenous spermidine, these observed changes in levels of c-Myc protein and E-cadherin transcription are more likely related to polyamine depletion rather than to the nonspecific effect of DFMO. Our previous studies (28, 29, 38, 53) and others (7, 8, 16) have show that polyamines are absolutely required for c-Myc expression and that polyamine depletion represses c-Myc by inhibiting its gene transcription and mRNA translation. Since the E-cadherin is a potential target of c-Myc (4), it is likely that decreased levels of E-cadherin transcription following polyamine depletion result from a reduction in c-Myc transcriptional activity. This possibility is strongly supported by results presented in Fig. 2 indicating that forced c-Myc expression in polyamine-deficient cells not only prevented the inhibition of E-cadherin transcription but also restored its protein levels to near normal.
The results reported presently also indicate that there is a functional E-Pal box in the E-cadherin-promoter and that increased c-Myc activates E-cadherin transcription through direct interaction with the E-Pal box. It has been recognized for many years that levels of the E-cadherin in various tissues are highly regulated and its expression is cell type dependent in manner (11, 12, 30, 31, 39). As shown in Fig. 3, the upstream fragment (positions −178 to +92) of the E-cadherin gene mediates strong expression of a luciferase reporter in IECs, although in some nonepithelial cells this promoter is either inactive or less active (23). There are several cis-acting elements in this fragment that have been identified to contribute to tissue-specific activity of the E-cadherin promoter (5, 49). These regulatory elements include the CAAT box, two AP-2 binding sites in a GC-rich region, and the E-Pal box, as shown in Fig. 3A. Although the GC-rich region and CAAT box always exhibit positive regulatory activity in most cells, the E-Pal box displays distinct regulatory effects, depending on the nature of stress, cell type, and other factors (49). For example, the activation of E-Pal box suppresses E-cadherin promoter activity in mesenchymal cells, but it mediates the activation of E-cadherin promoter in epithelial cells (23).
Several pieces of evidence from the current studies demonstrate that the E-Pal box is crucial for E-cadherin induction by c-Myc. First, 5′-deletion of this proximal E-Pal box from the E-cadherin promoter (74-Luc and 60-Luc) almost completely prevented c-Myc-induced activation of the E-cadherin promoter, although this remaining fragment still contains a CAAT box and GC1 and GC2 regions (Fig. 3C). Second, the stimulation of E-cadherin-promoter by c-Myc overexpression was also totally abolished when this E-Pal box was eliminated by point mutation (Fig. 3, D and E). Our results also show that point mutation of the CAAT box in the E-cadherin promoter partially blocked the induction in E-cadherin promoter by c-Myc (Fig. 3E, right), suggesting that the CAAT plays a marginal role in mediating c-Myc-induced activation of the E-cadherin transcription. Third, ChIP assay revealed that c-Myc bound to the proximal region of the E-cadherin promoter in vivo (Fig. 4). Together, these experiments establish that the sequence (CACCTGCAGGTGCACCTTTAGGTG) located at −94/−78 within the E-cadherin promoter is a functional E-Pal element in normal IECs and that induced c-Myc activates E-cadherin through this E-Pal box. These findings are consistent with results from others (4) who have demonstrated that c-Myc specifically activates transcription of the E-cadherin promoter in Madin Darby canine kidney (MDCK) epithelial cells but not in NIH-3T3 mesenchymal cells and that mutation of the E-Pal box prevents E-cadherin activation by c-Myc. In MDCK cells, c-Myc also functions as a coactivator of AP-2 to regulate E-cadherin transcription.
The data from the present study further show that polyamine-modulated E-cadherin expression plays an important role in the maintenance of normal function of the epithelial barrier. Results reported in Figs. 5 and 6 not only confirm our previous observations (20) showing that decreased levels of E-cadherin were associated with an increase in basal levels of epithelial paracellular permeability but also provide new evidence indicating that reduction in E-cadherin levels by polyamine depletion or E-cadherin silencing remarkably potentiated H2O2-induced epithelial barrier dysfunction. This finding is of physiological significance, because normal IECs contain high levels of polyamines and their cellular levels are changed rapidly in response to various physiological and pathological stimuli (16, 46, 54). On the other hand, E-cadherin mediates strong cell-cell adhesion and has functional role in forming and regulating the epithelial barrier. Disruption of the epithelial barrier function occurs commonly in various pathological conditions such as inflammatory bowel disease, intestinal infections, cancers, and critical surgical stresses (3, 15, 25). To date, many signaling pathways, including tyrosine kinases, Ca2+, protein kinase C, and phospholipase C-γ, have been implicated in the regulation of paracellular permeability in epithelial cells (26, 33, 37, 44). The current studies provide strong evidence for a role of polyamine-induced c-Myc in the control of E-cadherin transcription and are thus involved in the regulation of the intestinal epithelial barrier.
In summary, these results indicate that polyamines regulate E-cadherin transcription through c-Myc and are implicated in the control of intestinal epithelial barrier function. Our previous studies (28, 38, 53) have shown that increased polyamines stimulate c-Myc expression, leading to an increase in nuclear c-Myc in IECs. Results obtained in the present study further show that polyamine-induced c-Myc activates E-cadherin transcription, probably through its interaction with the E-Pal box within the proximal region of E-cadherin promoter, thus increasing levels of E-cadherin protein and enhancing the epithelial barrier function. In contrast, reduction in c-Myc following polyamine depletion inhibits E-cadherin transcription, resulting in the barrier dysfunction. These findings suggest that c-Myc is a biological regulator for adherens junction expression and that c-Myc-mediated E-cadherin transcription plays a critical role in the maintenance of intestinal epithelial barrier integrity under physiological conditions.
GRANTS
This work was supported by a Merit Review Grant from the Department of Veterans Affairs and by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-57819, DK-61972, and DK-68491. J.-Y. Wang is a Research Career Scientist, Medical Research Service, U.S. Department of Veterans Affairs.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Angst BD, Marcozzi C, Magee AI. The cadherin superfamily. J Cell Sci 114: 625–626, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci 114: 629–641, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 134: 1031–1049, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batsché E, Muchardt C, Behrens J, Hurst HC, Crémisi C. RB and c-Myc activate expression of the E-cadherin gene in epithelial cells through interaction with transcription factor AP-2. Mol Cell Biol 18: 3647–3658, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrens J, Löwrick O, Klein-Hitpass L, Birchmeier W. The E-cadherin promoter: functional analysis of a GC-rich region and an epithelial cell-specific palindromic regulatory element. Proc Natl Acad Sci USA 88: 11495–11499, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell 18: 189–200, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casero RA, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov 6: 373–390, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Celano P, Baylin SB, Casero RA Jr. Polyamines differentially modulate the transcription of growth-associated genes in human colon carcinoma cells. J Biol Chem 264: 8922–8927, 1989. [PubMed] [Google Scholar]
- 9.Chen J, Rao JN, Zou T, Liu L, Marasa BS, Xiao L, Zeng X, Turner DJ, Wang JY. Polyamines are required for expression of Toll-like receptor 2 modulating intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol 293: G568–G576, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Xiao L, Rao JN, Zou T, Liu L, Bellavance E, Gorospe M, Wang JY. JunD represses transcription and translation of the tight junction protein zona occludens-1 modulating intestinal epithelial barrier function. Mol Biol Cell 19: 3701–3712, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiFeo A, Narla G, Camacho-Vanegas O, Nishio H, Rose SL, Buller RE, Friedman SL, Walsh MJ, Martignetti JA. E-cadherin is a novel transcriptional target of the KLF6 tumor suppressor. Oncogene 25: 6026–6031, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 24: 2375–2385, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Fujimori T, Takeichi M. Disruption of epithelial cell-cell adhesion by exogenous expression of a mutated nonfunctional N-cadherin. Mol Biol Cell 4: 37–47, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem 74: 219–245, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermüller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol 281: G216–G228, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Gerner EW, Meyskens FL Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 4: 781–792, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol 107: 1575–1587, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumbiner BM Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84: 345–357, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Guo X, Rao JN, Liu L, Zou T, Keledjian KM, Boneva D, Marasa BS, Wang JY. Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 288: G1159–G1169, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Guo X, Rao JN, Liu L, Zou T, Turner DJ, Bass BL, Wang JY. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol 285: C1174–C1187, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Hanby AM, Chinery R, Poulsom R, Playford RJ, Pignatelli M. Downregulation of E-cadherin in the reparative epithelium of the human gastrointestinal tract. Am J Pathol 148: 723–729, 1996. [PMC free article] [PubMed] [Google Scholar]
- 22.Harter JL Critical values for Duncan's new multiple range test. Biometrics 16: 671–685, 1960. [Google Scholar]
- 23.Hennig G, Löwrick O, Birchmeier W, Behrens J. Mechanisms identified in the transcriptional control of epithelial gene expression. J Biol Chem 271: 595–602, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Hosono S, Gross I, English MA, Hajra KM, Fearon ER, Licht JD. E-cadherin is a WT1 target gene. J Biol Chem 275: 10943–10953, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Huber D, Balda MS, Matter K. Occludin modulates transepithelial migration of neutrophils. J Biol Chem 275: 5773–5778, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol 148: 791–800, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Rao JN, Bass BL, Wang JY. NF-κB activation and susceptibility to apoptosis after polyamine depletion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 280: G992–G1004, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Guo X, Rao JN, Zou T, Marasa BS, Chen J, Greenspon J, Casero RA Jr, Wang JY. Polyamine-modulated c-Myc expression in normal intestinal epithelial cells regulates p21Cip1 transcription through a proximal promoter region. Biochem J 398: 257–267, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Li L, Rao JN, Zou T, Zhang HM, Boneva D, Bernard MS, Wang JY. Polyamine-modulated expression of c-myc plays a critical role in stimulation of normal intestinal epithelial cell proliferation. Am J Physiol Cell Physiol 288: C89–C99, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Liu YN, Lee WW, Wang CY, Chao TH, Chen Y, Chen JH. Regulatory mechanisms controlling human E-cadherin gene expression. Oncogene 24: 8277–8290, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Liu YN, Liu Y, Lee HJ, Hsu YH, Chen JH. Activated androgen receptor downregulates E-cadherin gene expression and promotes tumor metastasis. Mol Cell Biol 28: 7096–7108, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man Y, Hart VJ, Ring CJ, Sanjar S, West MR. Loss of epithelial integrity resulting from E-cadherin dysfunction predisposes airway epithelial cells to adenoviral infection. Am J Respir Cell Mol Biol 23: 610–617, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci 113: 2085–2090, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol 17: 453–458, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Nejsum LN, Nelson WJ. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J Cell Biol 178: 323–335, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson WJ Meeting of cell-cell adhesion, communication and signalling at the junction. Trends Cell Biol 6: 325–327, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun 69: 1329–1336, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel AR, Wang JY. Polyamines modulate transcription but not posttranscription of c-myc and c-jun in IEC-6 cells. Am J Physiol Cell Physiol 273: C1020–C1029, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol 24: 306–319, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 80: 248–265, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao JN, Liu L, Zou T, Marasa BS, Boneva D, Wang SR, Malone DL, Turner DJ, Wang JY. Polyamines are required for phospholipase C-γ1 expression promoting intestinal epithelial restitution after wounding. Am J Physiol Gastrointest Liver Physiol 292: G335–G343, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Rao JN, Liu SV, Zou T, Liu L, Xiao L, Zhang X, Bellavance E, Yuan JX, Wang JY. Rac1 promotes intestinal epithelial restitution by increasing Ca2+ influx through interaction with phospholipase C-γ1 after wounding. Am J Physiol Cell Physiol 295: C1499–C1509, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao JN, Platoshyn O, Li L, Guo X, Golovina VA, Yuan JX, Wang JY. Activation of K+ channels and increased migration of differentiated intestinal epithelial cells after wounding. Am J Physiol Cell Physiol 282: C885–C898, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Rao RK, Basuroy S, Rao VU, Karnaky KJ Jr, Gupta A. Tyrosine phosphorylation and dissociation of occluding-ZO-1 and E-cadherin-β-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 368: 471–481, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286: C1213–C1228, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Seiler N, Raul F. Polyamines and the intestinal tract. Crit Rev Clin Lab Sci 44: 365–411, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 22: 207–235, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda) 19: 331–338, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci 65: 3756–3788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venkiteswaran K, Xiao K, Summers S, Calkins CC, Vincent PA, Pumiglia K, Kowalczyk AP. Regulation of endothelial barrier function and growth by VE-cadherin, plakoglobin, and β-catenin. Am J Physiol Cell Physiol 283: C811–C821, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Wang JY, Johnson LR. Expression of protooncogenes c-fos and c-myc in healing of gastric mucosal stress ulcers. Am J Physiol Gastrointest Liver Physiol 266: G878–G886, 1994. [DOI] [PubMed] [Google Scholar]
- 52.Wang JY, McCormack SA, Viar MJ, Johnson LR. Stimulation of proximal small intestinal mucosal growth by luminal polyamines. Am J Physiol Gastrointest Liver Physiol 261: G504–G511, 1991. [DOI] [PubMed] [Google Scholar]
- 53.Wang JY, McCormack SA, Viar MJ, Wang H, Tzen CY, Scott RE, Johnson LR. Decreased expression of protooncogenes c-fos, c-myc, and c-jun following polyamine depletion in IEC-6 cells. Am J Physiol Gastrointest Liver Physiol 265: G331–G338, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Wang JY Polyamines and mRNA stability in regulation of intestinal mucosal growth. Amino Acids 33: 241–252, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Xiao L, Rao JN, Zou T, Liu L, Marasa BS, Chen J, Turner DJ, Passaniti A, Wang JY. Induced JunD in intestinal epithelial cells represses CDK4 transcription through its proximal promoter region following polyamine depletion. Biochem J 403: 573–581, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao L, Rao JN, Zou T, Liu L, Marasa BS, Chen J, Turner DJ, Zhou H, Gorospe M, Wang JY. Polyamines regulate the stability of activating transcription factor-2 mRNA through RNA-binding protein HuR in intestinal epithelial cells. Mol Biol Cell 18: 4579–4590, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang HM, Rao JN, Guo X, Liu L, Zou T, Turner DJ, Wang JY. Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J Biol Chem 279: 22539–22547, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Zou T, Mazan-Mamczarz K, Rao JN, Liu L, Marasa BS, Zhang AH, Xiao L, Pullmann R, Gorospe M, Wang JY. Polyamine depletion increases cytoplasmic levels of RNA-binding protein HuR leading to stabilization of nucleophosmin and p53 mRNAs. J Biol Chem 281: 19387–19394, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Zou T, Rao JN, Guo X, Liu L, Zhang HM, Strauch ED, Bass BL, Wang JY. NF-κB-mediated IAP expression induces resistance of intestinal epithelial cells to apoptosis after polyamine depletion. Am J Physiol Cell Physiol 286: C1009–C1018, 2004. [DOI] [PubMed] [Google Scholar]