Abstract
Fasting increases neuropeptide Y (NPY) expression, peptide levels, and the excitability of NPY-expressing neurons in the hypothalamic arcuate (ARC) nucleus. A subpopulation of ARC-NPY neurons (∼40%) are glucose-inhibited (GI)-type glucose-sensing neurons. Hence, they depolarize in response to decreased glucose. Because fasting enhances NPY neurotransmission, we propose that during fasting, GI neurons depolarize in response to smaller decreases in glucose. This increased excitation in response to glucose decreases would increase NPY-GI neuronal excitability and enhance NPY neurotransmission. Using an in vitro hypothalamic explant system, we show that fasting enhances NPY release in response to decreased glucose concentration. By measuring relative changes in membrane potential using a membrane potential-sensitive dye, we demonstrate that during fasting, a smaller decrease in glucose depolarizes NPY-GI neurons. Furthermore, incubation in low (0.7 mM) glucose enhanced while leptin (10 nM) blocked depolarization of GI neurons in response to decreased glucose. Fasting, leptin, and glucose-induced changes in NPY-GI neuron glucose sensing were mediated by 5′-AMP-activated protein kinase (AMPK). We conclude that during energy sufficiency, leptin reduces the ability of NPY-GI neurons to sense decreased glucose. However, after a fast, decreased leptin and glucose activate AMPK in NPY-GI neurons. As a result, NPY-GI neurons become depolarized in response to smaller glucose fluctuations. Increased excitation of NPY-GI neurons enhances NPY release. NPY, in turn, shifts energy homeostasis toward increased food intake and decreased energy expenditure to restore energy balance.
Keywords: arcuate nucleus, glucose-inhibited neuron, adenosine 5′-monophosphate kinase, leptin, fasting
energy homeostasis is regulated by a neural network that responds to nutrients, hormones, and neuronal signals (28). The neuropeptide Y (NPY) neurons within the arcuate nucleus of the hypothalamus (ARC) stand out as prime candidates for sensing and responding to signals of energy homeostasis. Fasting increases NPY gene expression and peptide content and enhances NPY neuronal excitability (5, 36, 40). Stimulation of NPY neurotransmission activates neuronal circuitry that increases feeding and promotes energy storage to restore energy homeostasis (27).
Nutrients and hormones inform ARC-NPY neurons about peripheral energy status. For example, ∼40% of ARC-NPY neurons belong to the subtype of glucose-sensing neurons that are inhibited by glucose (GI neurons) (22). GI and NPY-GI neurons also respond to hormonal signals of energy balance. For instance, leptin inhibits both NPY and GI neurons (2, 6, 32). Both glucose and leptin concentrations are proportional to peripheral energy status (1, 9). Therefore, during positive energy balance, elevated glucose and leptin would inhibit both NPY and GI neurons. In contrast, during fasting, when energy stores are low, reduced glucose and leptin would increase the response of NPY-GI neurons to decreased glucose. Under these conditions, small decreases in glucose would activate NPY-GI neurons and enhance NPY neurotransmission.
The identity of the cellular protein(s) conferring glucose sensitivity to NPY-GI neurons is unclear. However, our data and that of others suggest that the cellular energy sensor, 5′-AMP-activated protein kinase (AMPK), plays a key role in mediating glucose sensing (6, 7, 22). Hypothalamic AMPK activity varies with nutritional status. During conditions of energy deficit, like fasting, AMPK activation leads to increased food intake and decreased energy expenditure to restore energy homeostasis (3, 16, 17, 21). Glucose and leptin inhibit hypothalamic AMPK (3, 13, 19, 21). Moreover, AMPK activation mimics the excitatory effect of low glucose in GI neurons while mice lacking the AMPKα2-subunit have no detectable GI neurons (6, 7). These data suggest that reduced leptin and glucose during fasting activates AMPK in NPY-GI neurons. AMPK activation would, in turn, increase the response of NPY-GI neurons to reduced glucose.
The aim of this study was to determine whether fasting enhances the response of NPY-GI neurons to decreased glucose through the AMPK pathway. We used two approaches to test this hypothesis. First, we compared hypothalamic NPY release in response to decreased extracellular glucose from fed and fasted rats using an in vitro hypothalamic explant system. Second, we determined whether fasting-induced AMPK activation altered the glucose sensitivity of NPY-GI neurons using membrane potential-sensitive dye. Our results suggest that alterations in the glucose sensitivity of NPY-GI neurons may underlie fasting-induced changes in hypothalamic NPY release.
MATERIALS AND METHODS
In Vitro Hypothalamic Peptide Release
Animals and feeding regimen.
Male Sprague-Dawley rats (Charles River, 8–12 wk old) were housed in a temperature and humidity-controlled facility with a 12:12-h light-dark cycle (lights on 0700–1900). They were fed standard rodent chow (Teklad no. 4012) and were randomly assigned to fed or fasted feeding regimens. For fasted rats, food was removed at ∼3 PM, 2 days before the experiment. Water was supplied to all rats ad libitum. Body weight was recorded on each of the 2 days before and on the day of the experiment. The cumulative body weight change was calculated for each feeding group. Trunk blood was collected for glucose and leptin determinations. Leptin was measured by ELISA using commercially available kits according to the manufacturer's instructions (Linco, Indianapolis IN). Glucose levels were measured using a glucose analyzer (AccuCheck, Roche Diagnostics, Indianapolis, IN). The effect of food restriction was determined using a one-way ANOVA. The difference between individual feeding regimens was determined using Bonferroni's post hoc test. All procedures using laboratory animals were approved by the Institutional Animal Care and Use Committee of Merck Research Laboratories and the University of Medicine and Dentistry of New Jersey.
Tissue preparation.
On the morning of the experiment (between 1000 and 1130), rats were euthanized by decapitation. The brains were rapidly removed and put into ice-cold oxygenated perfusion solution (composition in mM: 2.5 KCl, 1.25 NaH2PO4, 7 MgCl2, 0.5 CaCl2, 28 NaHCO3, 7 glucose, 1 ascorbate, 3 pyruvate, and 233 sucrose, pH 7.4). The hypothalami were dissected from the brain by blocking the tissue using the following anatomical landmarks: 1) the optic chiasm rostrally, 2) the hypothalamic fissures laterally, 3) the mamillary bodies caudally, and 4) the ventral surface of the thalamus dorsally. The isolated block of tissue was cross-chopped into 300-μm3 pieces using a McIlwain tissue chopper (Mickle Laboratory Engineering, Guilford, UK) and loaded into individual chambers of the Brandel Suprafusion System (Brandel, Gaithersburg MD).
Measurement of hypothalamic NPY release.
The tissue was perfused with fresh, oxygenated Krebs buffer solution (composition: 120 mM NaCl, 5 mM KCl, 2.6 mM CaCl2, 0.7 mM MgSO4, 1.2 mM KH2PO4, 28 mM NaHCO3, 10 mM glucose, 0.1 mg/ml aprotinin, and 0.1% BSA, pH 7.4, 37°C) for approximately 30–60 min. Incubation in a supraphysiological glucose concentration (7–10 mM) protects neuronal tissue during dissection and sectioning. This recovery period does not affect the ability to detect glucose-sensing neurons using electrophysiological techniques (23, 24, 30, 33, 38, 39). Furthermore, incubation and testing hypothalamic tissue in 10 mM glucose do not impair the ability to detect changes in NPY neurotransmission caused by fasting (20, 36). Tissue was then acclimated in the reagent chamber for a minimum of 1 h before the start of the experiment in Krebs buffer containing 1.4, 2.5, 5, or 10 mM glucose as described for individual experiments. Perfusate was collected over a total of seven 15-min intervals. The first three intervals confirmed stable basal NPY release (fractions 1–3). Experimental manipulations (e.g., electrical stimulation or acute glucose concentration shift) occurred between fractions 3 and 4. Electrical stimulation parameters were determined experimentally. The threshold electrical stimulus was the minimal electrical stimulus that significantly increased NPY release. Maximal electrical stimulus yielded NPY release that was five times that released in response to threshold stimulation. Perfusate was collected for three 15-min intervals after experimental manipulation (fractions 4–6). Tissue viability was confirmed after a 20-min washout period using a 20-min exposure to 100 mM KCl. KCl-stimulated NPY release of viable tissue samples (95% of those tested) was at least two times the levels seen during the baseline period. Collected perfusate was stored at 4°C and assayed for NPY content using a commercially available radioimmunoassay kit (Phoenix Pharmaceuticals, Belmont, CA).
The average NPY released from viable tissue samples was calculated by measuring the NPY content for each time interval. Since the entire hypothalamus was used for these assays, the tissue contained all hypothalamic NPY neuronal populations. Normalizing NPY release to either tissue weight or total peptide content had no effect on relative differences in NPY release due to feeding state or experimental manipulations (i.e., electrical stimulation or glucose concentration change). The average baseline release was calculated by averaging NPY released during each of the three prestimulation intervals. The percent change in NPY release for each posttreatment fraction was calculated relative to the average baseline release. The effect of electrical and glucose-stimulated NPY release was compared to baseline release using a paired Student's t-test.
The effect of fasting, electrical, and glucose-stimulated NPY release was determined using a one-way ANOVA or unpaired Student's t-test where appropriate.
Double c-Fos and Green Fluorescent Protein Immunohistochemistry
As previously described (11), hypothalamic sections containing the ARC of overnight-fasted and ad libitum-fed NPY-green fluorescent protein (GFP) mice were first incubated in rabbit antiserum against Fos protein (0.1%; Ab-5 antibody, Oncogene, Cambridge, MA) overnight at 4°C. After washing, sections were incubated in biotinylated goat anti-rabbit IgG (0.2%; Jackson ImmunoResearch, West Grove, PA) and finally in streptavidin-peroxidase conjugate (Jackson ImmunoResearch). Following diaminonezidine-nickel revelation, sections were incubated in rabbit polyclonal antibody against GFP (0.1%; Molecular Probes, Carlsbad, CA). The second antigen-antibody complex was revealed by diaminobenzidine. Consequently, Fos-positive cells presented with black nuclei while GFP-expressing cells presented with brown cytoplasm.
Fos+, GFP+, and Fos+/GFP+ cells were counted using a computerized image analysis (Visilog 6.2 software, Noesis). Total numbers of single (GFP) or double-labeled (Fos-GFP) cells were counted bilaterally on six sections per ARC. Data are expressed as means ± SE. The operator was blinded to the feeding group. The effect of fasting was determined using an unpaired Student's t-test, and P < 0.05 was taken as significant.
In Vitro Determination of Glucose Sensitivity in GI Neurons Using Fluorescence Imaging Plate Reader Membrane Potential Dye
Animals and feeding regimen.
Male, 4- to 6-wk-old C57BL/6 mice (Taconic Laboratories, Germantown, NY) were housed in a temperature and humidity-controlled facility with a 12:h light-dark cycle (lights on 0700–1900). NPY-GFP mice (stock no. 006417, Jackson Laboratories, Bar Harbor, ME) were obtained at 4–5 wk of age. All mice were weaned at 21 days of age and tested at 4–6 wk of age. They were fed standard rodent chow (Purina 5001). Mice were randomly assigned to one of two feeding regimens: 1) fed ad libitum (Fed) or 2) food removal between 0900–1200 on the day before the experiment (Fasted). All mice were weighed, and body weight recorded on the day before and the day of the experiment. The cumulative body weight change was calculated for each feeding group. Water was supplied ad libitum. Mice were decapitated, and trunk blood was collected for glucose and leptin determination. Glucose levels were determined using a glucose meter (AccuCheck, Roche Diagnostics, Indianapolis, IN). Leptin levels were determined by ELISA using a commercially available kit (CrystalChem, Downers Grove, IL). The effect of fasting was determined using a one-way ANOVA.
Measurement of membrane potential using fluorescence imaging plate reader membrane potential dye.
Isolated ventromedial hypothalamic (VMH) neurons were obtained as described previously (6, 38). Briefly, wild-type (C57BL/6) or NPY-GFP mice were anesthetized with pentobarbital sodium (100 mg/kg ip) and transcardially perfused with ice-cold oxygenated (95% O2-5% CO2) perfusion solution containing (in mmol/l) 2.5 KCl, 7 MgCl2, 1.25 NaH2PO4, 28 NaHCO3, 0.5 CaCl2, 7 glucose, 1 ascorbate, and 3 pyruvate (osmolarity adjusted to ∼300 mosM with sucrose, pH 7.4). Brains were quickly removed and placed in an ice-cold (slushy) oxygenated perfusion solution. Sections (350 μm) were made through the hypothalamus using a vibratome (Vibroslice; Camden Instruments) as described previously (6, 38). Incubation in a supraphysiological glucose concentration (7 mM) protects neuronal tissue during dissection and sectioning. This recovery period does not affect the ability to detect glucose-sensing neurons using electrophysiological techniques (23, 24, 30, 33, 38, 39). Furthermore, incubation and testing hypothalamic tissue in 10 mM glucose does not impair the ability to detect changes in NPY neurotransmission caused by fasting (20, 36).
Brain slices were then placed in glucose-free Hibernate A/B27 (Brain Bits, Carlsbad, CA) to which 2.5 mmol/l glucose, 3 mmol/l pyruvate, 1 mmol/l lactic acid, 0.5 mmol/l-glutamine, and B27 supplement (1:50 vol:vol; Invitrogen, Carlsbad, CA) were added and held at 30°C. The VMH (ARC + ventromedial hypothalamic nucleus) was dissected and digested in Hibernate A (same composition as Hibernate A/B27 excluding the B27) with Papain (20 U/ml). The tissue was incubated for 30 min in a 30°C water bath with a platform rotating at 100 rpm and was then rinsed with Hibernate A/B27 and subjected to gentle trituration. After triturating, the cell suspension was centrifuged and the pellet was resuspended in glucose-free growth medium (Neurobasal; Invitrogen, Springfield, IL) to which 2.5 mM glucose, 3 mmol/l pyruvate, 1 mmol/l lactic acid, 0.5 mmol/l-glutamine, and B27 supplement (1:50 vol:vol; Invitrogen, Carlsbad, CA) were added. Neurons were plated in growth medium with fluoresbrite beads (Polysciences, Warrington PA) (Neurobasal; Invitrogen, Springfield, IL) and used within 1 day. Fluoresbrite beads were used for data normalization.
VMH neurons were incubated in 1.75% fluorescence imaging plate reader membrane potential dye (FLIPR-MPD) at 37°C in extracellular solution (ECF; composition in mM: 135 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, and 2.5 glucose, pH 7.4) beginning 30 min before and throughout the duration of all experiments. Neurons were visualized on an Olympus BX61 WI microscope with a ×10 objective equipped with a red filter (excitation 545 nm, emission 563–647 nm) for visualization of FLIPR-MPD dye and a green filter (excitation 470 nm, emission 500–530 nm) for visualization of NPY-GFP neurons. Images were captured at 1-min intervals (150-ms exposure time) over the course of each experiment using a charge-coupled device camera (Cool Snap, Photometrics, Tucson, AZ). Images were acquired and analyzed using MetaMorph software (Molecular Devices, Sunnyvale CA). The fluorescence intensity of each image (expressed as gray scale units per pixel) was normalized to that of the coincubated fluorescent beads. Images were captured both before and after changing the extracellular glucose concentrations. The extracellular solution bathing isolated VMH neurons initially contained 2.5 mM glucose. Five minutes after initiation of image acquisition, this solution was exchanged for an identical solution containing one of the following glucose concentrations: 2.5 (control), 1.4, 1.0, 0.7, 0.5, or 0.3 mM. Images were acquired every minute for 20 min after glucose concentration change. Percent change in fluorescence intensity was calculated relative to the fluorescence intensity before solution change.
Definition of depolarization using FLIPR-MPD dye.
We previously showed, in rats, that the FLIPR-MPD fluorescence intensity of GI neurons increased within 5–10 min and reached a stable plateau between 10 and 20 min following extracellular glucose reduction (6). This is analogous to our electrophysiological and Ca2+ imaging studies that show that glucose responses are observed by 5 min and are maximal by 10 min after glucose change (15, 30). Thus the average percent change in FLIPR-MPD fluorescence intensity between 10 and 20 min after glucose change [%Δ FLIPR-MPD (10–20)] was used to evaluate neuronal depolarization. The criterion to define membrane depolarization was determined experimentally by evaluating the distribution of %Δ FLIPR-MPD (10–20) in response to a solution exchange in which glucose remained constant at 2.5 mM. These studies showed that 97% of VMH neurons obtained from both fed and fasted mice exhibited a %Δ FLIPR-MPD (10–20) of <11%. Therefore, neurons were considered to be depolarized in response to decreased glucose if their %Δ FLIPR-MPD (10–20) was greater than 11%. Cell viability was confirmed using a 5-min exposure to 200 μM glutamate at the conclusion of the imaging session for each dish. Neurons were considered to be viable if glutamate exposure increased FLIPR-MPD fluorescence intensity by at least 20%. Only glutamate responsive neurons were used.
Glucose response curves were constructed, and the half-maximal glucose concentration change to detect neuronal depolarization was calculated using nonlinear regression analysis (four-parameter logistic fit) (Graphpad PRISM for Windows version 4.00, Graphpad Software, San Diego, CA.). The half-maximal glucose concentration change to detect neuronal depolarization from both feeding regimens was compared using F-test with P < 0.05 indicating significant difference.
Western Blot Analysis
Western blot analysis was performed using VMH sections from fed or fasted 4- to 5-wk-old male C57BL/6 mice. Sections were treated as described in results. Sections were homogenized in lysis buffer (50 mM Tris, 150 mM NaCl, 0.02% sodium azide, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, 0.5% deoxycholic acid, 2 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml aprotinin, and 2 μg/ml pepstatin A) at 4°C. Lysate supernatant was collected (10 min at 14 000 g at 4°C) and frozen at −80°C. Protein (30 μg; determined by modified Bradford assay) was loaded on a 10% Tris·HCl gel (Bio-Rad, Hercules, CA) and electrophoresed for 1.5 h at 120 V. Proteins were transferred to nitrocellulose membranes (Bio-Rad) for 2 h at 350 mA. Immunodetection was performed overnight at 4°C using rabbit polyclonal antibodies against each protein of interest: anti-phospho-AMPKα (Thr172; 1:1,000; Cell Signaling, Danvers, MA), anti-AMPKα2 (1:1,000; Abcam, Cambridge, MA), or anti-glial fibrillary acidic protein (GFAP; 1:50,000; Abcam). Membranes were then incubated with a horseradish peroxidase-conjugated secondary antibody (anti-rabbit 1:10 000; Jackson Immunoresearch) for 1 h at room temperature before signals were visualized using the SuperSignal West Pico ECL kit (Thermo Scientific, Rockford, IL). Quantification was performed using Scion Image software (Fredrick, MD). Results are presented as percentage of control after normalization to GFAP. The effect of experimental manipulations on AMPK phosphorylation was determined using an unpaired Student's t-test or one-way ANOVA.
Chemicals
The AMPK activator, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR; Toronto Research Chemicals, Toronto, ON, Canada), was prepared in ECF. The AMPK inhibitor, Compound C (Calbiochem, Gibbstown, NJ) was prepared as a 1,000× stock solution in DMSO and diluted in ECF so that the final concentration of DMSO did not exceed 0.1%. The concentrations of Compound C and AICAR were chosen on the basis of published sources. Compound C is a small molecule-selective inhibitor of AMPK. It inhibits AMPK activity by a reversible and competitive mechanism with respect to ATP (Ki ≈ 100 nM). Compound C at concentrations >10 μM inhibits AICAR (0.5 mM)-induced activation of acetyl CoA carboxylase in hepatocytes (41). Leptin (Preprotech, Rocky Hill, NJ) was prepared as 1000× stock in sterile water and then diluted to the final test concentration in ECF. Test compound concentrations are described in results. Test compounds remained in the extracellular fluid throughout the imaging procedure unless otherwise noted.
RESULTS
Hypothalamic NPY Release is Dependent on Extracellular Glucose Concentration
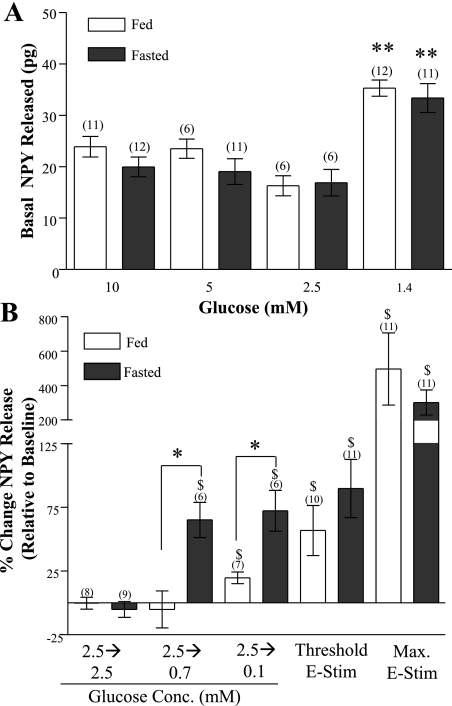
Hypothalamic NPY release in fed and fasted rats was significantly greater in 1.4 mM glucose compared with that in 2.5, 5, or 10 mM (Fig. 1A). Decreasing the extracellular glucose concentration from 2.5 to 0.1 mM significantly increased hypothalamic NPY release in fed rats (Fig. 1B; P < 0.05). However, decreasing the glucose concentration from 5 or 10 mM to 0.1 mM did not stimulate hypothalamic NPY release (data not shown; P > 0.05).
Fig. 1.
A: effect of glucose concentration on basal (nonstimulated) hypothalamic neuropeptide Y (NPY) release in fed and fasted male rats. Collections from three consecutive 15-min periods were averaged. Basal NPY release was the same in fed and fasted rats. NPY release in 1.4 mM glucose was significantly increased compared with higher glucose concentrations. Data are presented as means ± SE. **P < 0.001. B: effect of decreasing glucose and electrical stimulation (E-Stim) on hypothalamic NPY release in fed and fasted rats. NPY release was measured 15 min after glucose concentration change or electrical stimulation. Decreased glucose increased hypothalamic NPY release to a greater extent in fasted vs. fed rats. In contrast, electrical stimulation evoked the same amount of NPY from fed and fasted rats. The amount of NPY released in response to decreased glucose and electrical stimulation was within the same range. The amount of NPY release in response to decreased glucose was within the dynamic range of detection for our assay. Data are expressed relative to baseline levels and are presented as means ± SE. $P < 0.05 vs. baseline (i.e., in 2.5 mM glucose). N values are in parentheses above bars. *P < 0.05 fed vs. fasted.
Fasting Enhances NPY Release in Response to Decreased Glucose
Fasting significantly reduced body weight and plasma leptin and glucose levels in rats (Table 1). These fasting-induced changes were associated with changes in NPY release in response to decreased glucose. Reducing glucose from 2.5 to either 0.7 or 0.1 mM significantly increased NPY release from hypothalami of fasted rats (P < 0.01; Fig. 1B). Unlike fasted rats, hypothalamic NPY release from fed rats was not elevated in response to a glucose decrease from 2.5 to 0.7 mM. (Fig. 1B). Furthermore, although a glucose decrease from 2.5 to 0.1 mM increased hypothalamic NPY release in fed rats, it was significantly less than that observed in fasted rats (P < 0.05; Fig. 1B).
Table 1.
Effect of fasting on body weight and glucose and leptin levels in rats and mice
| Species/Feeding Regimen | n | Total Body Weight Change, g | Plasma Glucose, mM | Plasma Leptin, ng/ml |
|---|---|---|---|---|
| Rat | ||||
| ad libitum | 22 | +22.3±1.28 | 6.9±0.22 | 3.6±0.55 |
| Fasted | 28 | −20.5±1.01* | 4.5±0.29* | 0.7±0.29* |
| Mouse | ||||
| ad libitum | 10 | +0.7±0.23 | 8.5±0.38 | 6.3±0.45 |
| Fasted | 17 | −3.0±0.17 * | 4.3±0.10* | 1.1±0.2* |
Values are means ± SE. Rats and mice were weighed and body weight was recorded daily. Rats were randomly assigned to one of two feeding regimens: 1) ad libitum: free access to food; or 2) fasted: food removed at 3 PM 2 days before blood collection. Mice were randomly assigned to one of two feeding regimens: 1) ad libitum: food available; or 2) fasted: food withheld at 12 PM the day before blood collection. Cumulative body weight change was calculated for each feeding group. Glucose levels were determined on the final day of fasting. Fasting significantly reduced body weight, glucose levels, and leptin levels in both rats and mice.
P < 0.01 compared with ad libitum-fed group.
In contrast to glucose-induced changes in hypothalamic NPY release, electrical and KCl stimulation increased NPY release to the same extent in fed and fasted rats. Both intermediate (50 mA, 0.5 Hz, 60 s) and maximal electrical stimulation (50 mA, 25 Hz, 150 s) stimulated NPY release to the same extent in hypothalami from fed and fasted rats (Fig. 1B). Similarly, the amount of NPY released in response to intermediate (50 mM for 5 min) and maximal (100 mM for 20 min) KCl stimulation was the same in fed vs. fasted rats (intermediate KCl: fed: 38 ± 18 pg; fasted: 39 ± 4 pg; P > 0.05; maximum KCl: fed: 254 ± 90 pg;, fasted: 304 ± 75 pg; P > 0.05, data not shown). Baseline NPY release was the same in all experimental test groups. The levels of NPY released (pg/15 min) were approximately fivefold greater in response to maximal vs. threshold/intermediate levels of both stimuli. Furthermore, hypothalamic NPY release in response to an acute glucose reduction from 2.5 to 0.1 mM and that from a moderate electrical or KCl stimulus was within the same range (∼20–150 pg) and did not approach the maximal levels of NPY release (Fig. 1B; ∼300 pg).
Fasting Enhances the Response of VMH-GI and NPY-GI Neurons to Decreased Glucose
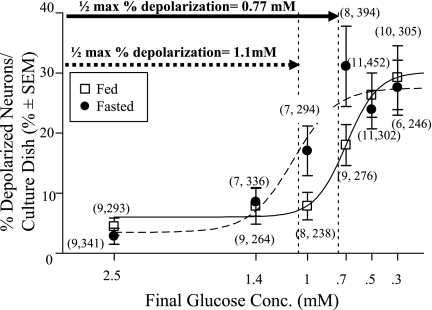
Fasting also significantly reduced body weight and plasma leptin and glucose levels in mice (Table 1). The relationship between an incremental decrease in glucose concentration and the percentage of detectable depolarized GI neurons [those that increased %Δ FLIPR-MPD (10–20) >11%] was sigmoidal for both the fed (r2 = 0.90; P = 0.01) and fasted (r2 = 0.82, P = 0.06) mice (Fig. 2). For VMH neurons from fed mice, a significant increase in the percentage of depolarized neurons was first observed when glucose was decreased from 2.5 to 0.7 mM (P < 0.05; Fig. 2). The maximum number of detectable VMH-GI neurons (∼25%) was observed when the glucose concentration was decreased from 2.5 to concentrations <0.5 mM. The half-maximal percentage of depolarized VMH-GI neurons was calculated to occur with a decrease in glucose from 2.5 to 0.77 mM. In contrast, in VMH neurons from fasted mice, a significant increase in the percentage of depolarized neurons was first observed with a glucose decrease from 2.5 to 1.0 mM (P < 0.05). The maximum number of detectable VMH-GI neurons (∼25%) was observed with decreases in glucose concentration from 2.5 to less than 0.7 mM. In VMH neurons from fasted mice, the half-maximal percentage of depolarized VMH-GI neurons was calculated to occur with a decrease in glucose from 2.5 to 1.1 mM. The calculated glucose concentration at which the half-maximal percentage of depolarized VMH neurons (i.e., GI neurons) would occur in response to a decrease from 2.5 mM was significantly greater for VMH neurons from fasted mice [1.1 mM (95% confidence interval = 0.94 to 1.31 mM)] compared with that for VMH neurons from fed mice [0.77 mM (95% confidence interval = 0.59 to 0.87 mM)] [F = 4.96 (2, 82); P < 0.05]. There was no significant difference between the total percentage of detectable VMH-GI neurons when glucose was decreased from 2.5 mM to concentrations lower than 0.5 mM in VMH neurons from fed or fasted mice (Fig. 2; P > 0.05). Therefore, during fasting, a smaller decrease in glucose concentration is sufficient to activate the GI neuron population.
Fig. 2.
The percentage of depolarized ventromedial hypothalamic (VMH) neurons from fed (□) and fasted (•) mice observed in response to decreased glucose using fluorescence imaging plate reader membrane potential dye (FLIPR-MPD) fluorescence intensity. Glucose was reduced from 2.5 to 1.4, 1.1, 0.7, 0.5, or 0.3 mM. Glucose-inhibited (GI) neurons were defined as those neurons whose average percent change in FLIPR fluorescence between 10 and 20 min post glucose change [%Δ FLIPR (10–20)] was >11%. Isolated VMH neurons from fasted mice depolarized in response to a significantly smaller glucose decrease compared with those from fed mice (calculated glucose concentration at which half of GI neurons depolarized to detectable levels: fasted, 1.1 mM; fed, 0.77 mM; P < 0.05). Data points (means ± SE) show the percentage of GI neurons per culture dish. Number of dishes used and number of cells analyzed are shown above each data point (no. dishes, no. total cells). Each plate was composed of cells pooled from at least 3 mice.
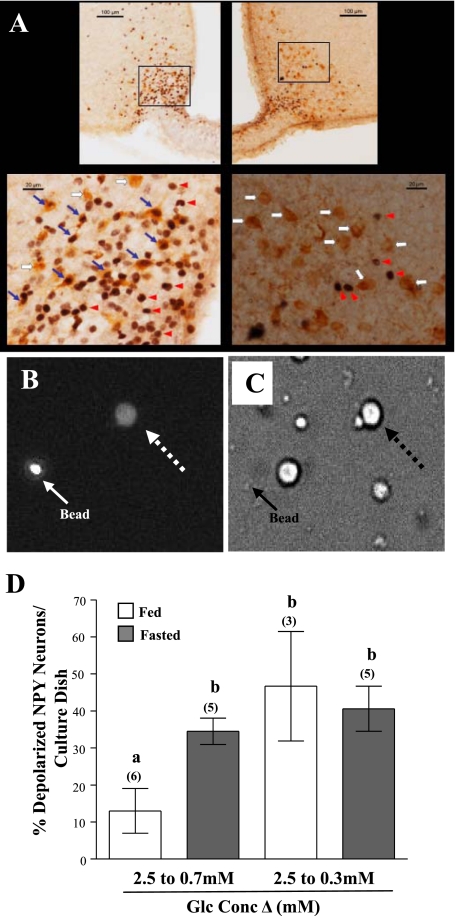
Next, we evaluated the effect of fasting specifically on NPY neurons. First, we confirmed that arcuate NPY neurons were activated by fasting using immunodetection of the protein product of the immediate-early gene c-fos, as a marker of neuronal activation, in NPY-GFP mice. Overnight fasting significantly increased the number of double-labeled c-Fos-GFP (NPY)-positive ARC cells [Fig. 3; fed: 3 ± 1 (n = 3); fasted: 181 ± 25 (n = 3); P < 0.05]. Fasting did not affect the total number of GFP-positive cells [fed: 256 ± 28 (n = 3); fasted: 280 ± 28 (n = 3); P > 0.05].
Fig. 3.
Effect of fasting and reduced glucose on hypothalamic arcuate (ARC)-NPY neurons. A: photomicrographs of the ARC nucleus from fed (right) and fasted (left) NPY-green fluorescent protein-expressing (GFP) mice. A higher magnification of each square is illustrated at bottom. Red arrowhead indicates c-Fos-immunopositive nuclei, white arrow indicates GFP-immunopositive neuron, and blue arrow indicates double-labeled neurons. B and C: fluorescence (B) and bright field (C) images of an NPY-GFP neuron stained with FLIPR-MPD (dashed arrow) visualized with a ×40 objective. The solid arrow points to a fluorescent bead. D: percentage of depolarized NPY neurons from fed and fasted NPY GFP mice in response to decreased glucose (2.5 to 0.7 mM or 0.3 mM) using changes in FLIPR-MPD fluorescence intensity. Significantly more NPY neurons from fasted mice depolarized when glucose was reduced from 2.5 to 0.7 mM. The percentage of NPY neurons that depolarized when glucose decreased from 2.5 to 0.3 mM was the same in fed and fasted NPY-GFP mice. Glc, glucose. Data points (means ± SE) show the percentage of NPY-GI neurons per culture dish. Number of dishes analyzed is shown above each bar. Bars with different letters are statistically different (P < 0.05).
We then determined whether fasting specifically altered the glucose sensitivity of NPY-GI neurons using NPY-GFP mice (Fig. 3, B–D). When glucose was reduced from 2.5 to 0.7 mM, significantly more NPY neurons from fasted [6 out of 17 NPY-GFP neurons (35%)] vs. fed [3 out of 19 NPY-GFP neurons (16%) mice depolarized; t(8) = 2.661; P = 0.03; Fig. 3D]. The same percentage of NPY neurons were depolarized in both the fed [6 of 13 NPY neurons (47%)] and the fasted [5 of 14 NPY neurons (41%)] groups in response to a glucose decrease from 2.5 to 0.3 mM (P > 0.05). Thus, fasting did not alter the total percentage of NPY-GI neurons. In addition, the total percentage of visible GFP neurons per culture dish was the same in fed and fasted mice (P > 0.05).
AMPK Mediates the Effects of Fasting
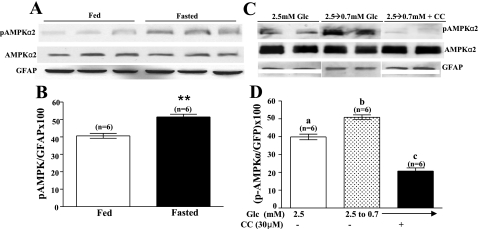
We next determined whether the increased response of VMH-GI neurons to decreased glucose during fasting was mediated by increased AMPK activity. Immunoblots of VMH tissue using a specific mouse phospho-AMPKα2 antibody showed that fasting significantly increased the level of VMH AMPKα2-subunit phosphorylation [Fig. 4, A and B; t(10) = 5.165, P < 0.001]. Similarly, lowering glucose from 2.5 to 0.7 mM for 30 min also increased VMH AMPKα2-subunit phosphorylation in fed mice; this effect was blocked by Compound C [Fig. 4, C and D; F(2,15) = 94.93, P < 0.0001]. There were no differences in total AMPKα2 or GFAP between any treatment groups.
Fig. 4.
A: a representative immunoblot of VMH phospho-AMPKα2 (pAMPKα2) and total AMPKα2 from fed and fasted mice. Fasting increased phosphorylation of AMPKα2 without changing total AMPK. Each lane contains the VMH from an individual mouse. B: immunoblots of pAMPK were measured using densitometry and quantitated relative to glial fibrillary acidic protein (GFAP) levels. **P < 0.001. C: a representative immunoblot of phospho-AMPKα2 performed on hypothalamic slices containing the VMH from fed mice. The extracellular glucose concentration bathing a subset of slices was decreased from 2.5 to 0.7 mM in the presence or absence of Compound C (CC; 30 μM). Control tissue was held in 2.5 mM extracellular glucose. Reducing the glucose concentration increased AMPKα2 phosphorylation; this was blocked by Compound C (30 μM). Neither decreasing glucose nor adding Compound C altered total AMPK levels. Each lane contains VMH from an individual mouse. D: immunoblots of pAMPK and total AMPK were measured using densitometry and quantitated relative to GFAP levels. Bars with different letters are statistically different (P < 0.05).
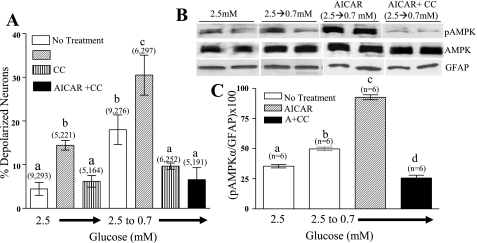
In fed mice, the addition of 0.5 mM AICAR to 2.5 mM glucose increased the percentage of detectable VMH-GI neurons measured using FLIPR-MPD fluorescence imaging. Moreover, the percentage of VMH-GI neurons observed when glucose was reduced in the presence of AICAR was significantly greater than with AICAR or decreased glucose alone. While Compound C (in 2.5 mM glucose) did not alter the percentage of VMH-GI neurons, it did completely block the effect of decreasing glucose in the presence or absence of AICAR (Fig. 5A).
Fig. 5.
A: the percentage of depolarized VMH neurons from fed mice measured using changes in FLIPR-MPD fluorescence intensity. The addition of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR; 0.5 mM) to 2.5 mM glucose or decreasing glucose from 2.5 to 0.7 mM increased the percentage of depolarized VMH neurons. The percentage of depolarized VMH neurons was significantly greater when glucose was decreased in the presence of AICAR compared with either treatment singly. Compound C (30 μM) in 2.5 mM glucose alone was without effect; however, it completely blocked the effects of AICAR and decreased glucose singly or in combination. Data are presented as means ± SE and show the percentage of GI neurons per culture dish. Number of dishes used and number of cells analyzed are shown above each data point (no. dishes, no. cells). Each plate was composed of cells pooled from at least 3 mice. Bars with different letters are statistically different (P < 0.05). B: a representative immunoblot of phospho-AMPKα2 and total AMPKα2 performed on hypothalamic slices containing the VMH from fed mice. Reducing the glucose concentration increased AMPKα2 phosphorylation. The addition of AICAR (0.5 mM) while reducing glucose potentiated the increased AMPKα2 phosphorylation. Compound C (30 μM) blocked the effect of decreasing glucose in the presence of AICAR. There was no difference in total AMPK levels between treatment groups. Each lane contains the VMH from an individual mouse. C: immunoblots of pAMPK were measured using densitometry and quantitated relative to GFAP levels. Bars with different letters are statistically different (P < 0.01).
Decreasing glucose from 2.5 to 0.7 mM significantly increased VMH AMPKα2-subunit phosphorylation in fed mice. This increase in VMH AMPKα2-subunit phosphorylation was significantly enhanced when glucose was lowered in the presence of AICAR (Fig. 5, B and C; P < 0.05). Compound C completely blocked AMPKα2-subunit phosphorylation observed when glucose was lowered in the presence of AICAR (Fig. 5, B and C). There were no differences in total AMPKα2 or GFAP between any treatment groups.
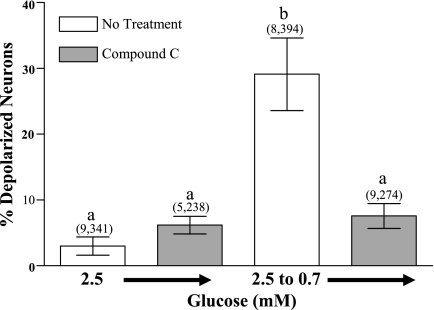
Similar results were obtained in fasted mice. That is, the addition of Compound C in 2.5 mM glucose did not change the percentage of VMH-GI neurons. However, Compound C completely blocked the increase in the percentage of GI neurons observed in response to a 2.5 to 0.7 mM glucose concentration reduction (Fig. 6). Finally, vehicle (0.1% DMSO) did not change FLIPR-MPD fluorescence (6.1 ± 1%) nor did it alter the number of depolarized VMH neurons observed when the glucose concentration was reduced from 2.5 to 0.7 mM (21 ± 2%).
Fig. 6.
The percentage of depolarized VMH neurons from fasted mice measured using changes in FLIPR-MPD fluorescence intensity. Compound C (30 μM) decreased the percentage of depolarized neurons observed as glucose was reduced from 2.5 to 0.7 mM. Data are presented as means ± SE and show the percentage of GI neurons per culture dish. Number of dishes used and number of cells analyzed are shown above each data point (no. dishes, no. cells). Each plate was composed of cells pooled from at least 3 mice. Bars with different letters are statistically different (P < 0.01).
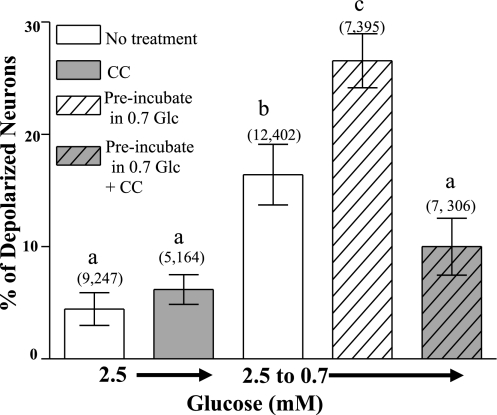
To determine whether decreased glucose per se increased the number of depolarized GI neurons in response to reduced glucose, VMH neurons from fed mice were preincubated in 0.7 mM glucose for 1 h. A glucose concentration of 0.7 mM corresponds to VMH glucose levels measured in rats after an overnight fast (9). Glucose (2.5 mM) was reapplied for 30 min. When the glucose concentration was subsequently decreased to 0.7 mM, the percentage of detectable VMH-GI neurons in dishes preincubated in 0.7 mM glucose was significantly greater than those not preincubated in this lower glucose level (P < 0.001; Fig. 7). Compound C prevented the effect of preincubation in 0.7 mM glucose. When glucose was reduced from 2.5 to 0.3 mM, the same percentage of VMH-GI neurons were detected in dishes that were preincubated in 0.7 mM and those that were not (0.7 mM glucose preincubation: 27 ± 5%; 2.5 mM glucose: 22 ± 2%; P > 0.05). Finally, cells preincubated in 0.7 mM glucose were stained with Trypan blue to confirm cell viability. The percentage of Trypan blue-labeled cells was identical in cultures incubated in 0.7 mM glucose for 4 h to those maintained in 2.5 mM glucose (0.7 mM glucose: 14 ± 2%; 83 of 536 from 6 plates; 2.5 mM glucose: 11 ± 2%; 108 of 979 from 6 plates, respectively; P > 0.05).
Fig. 7.
The percentage of depolarized VMH neurons from fed mice measured using changes in FLIPR-MPD fluorescence intensity. Preincubation in 0.7 mM glucose for 1 h increased the percentage of depolarized VMH neurons observed when glucose decreased from 2.5 to 0.7 mM. Pretreatment with the AMPK inhibitor Compound C (30 μM) blocked this effect. Data are presented as means ± SE and show the percentage of GI neurons per culture dish. Number of dishes used and number of cells analyzed are shown above each data point (no. dishes, no. cells). Each plate was composed of cells pooled from at least 3 mice. Bars with different letters are statistically different (P < 0.01).
Leptin Alters Glucose Sensitivity of GI Neurons via AMPK Inhibition
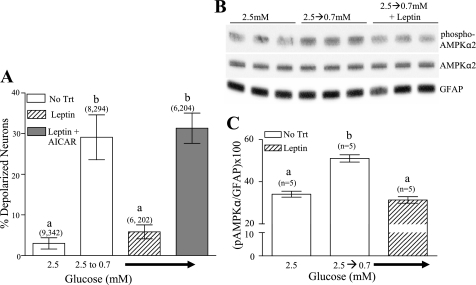
To determine whether decreased leptin contributes to the fasting-induced changes in glucose sensitivity of GI neurons, cultures from fasted mice were incubated with leptin (10 nM) for 1 h. Glucose levels were then decreased from 2.5 to 0.7 mM in the presence of leptin. Leptin significantly reduced the number of GI neurons detected as glucose was reduced from 2.5 to 0.7 mM (Fig. 8A; P < 0.05). To determine whether the leptin-associated suppression of GI neuronal response to decreased glucose persisted, VMH neurons were exposed to leptin for 1 h. Leptin was then removed, and 6 h later, the glucose concentration was reduced from 2.5 to 0.7 mM. The percentage of depolarized VMH neurons was 5 ± 2.5% in dishes exposed to leptin compared with 26 ± 4% in those without leptin (P < 0.01; data not shown). Coapplication of leptin with AICAR blocked the inhibitory effect of leptin on the depolarization of GI neurons in response to decreased glucose (Fig. 8A). Moreover, decreasing glucose from 2.5 to 0.7 mM in the presence of leptin completely blocked the increase in phosphorylation of α2AMPK-subunit seen when glucose was decreased in the absence of leptin (Fig. 8, A–C).
Fig. 8.
The percentage of depolarized VMH neurons from fasted mice measured using changes in FLIPR-MPD fluorescence intensity. Leptin (10 nM) significantly decreased the percentage of VMH neurons that depolarized in response to decreased glucose from 2.5 to 0.7 mM. AICAR (0.5 mM) reversed the effect of leptin. Data are presented as means ± SE and show the percentage of GI neurons per culture dish. Number of dishes used and number of cells analyzed are shown above each data point (no. plates, no. cells). Each plate was composed of cells pooled from at least 3 mice. No Trt, no treatment. Bars with different letters are statistically different (P < 0.05). B: a representative immunoblot of phospho-AMPKα2 and total AMPKα2 performed on hypothalamic slices containing the VMH from fed mice. Reducing the glucose concentration increased AMPKα2 phosphorylation. Leptin (10 nM) blocked the effect of decreasing glucose. There was no difference in total AMPK levels between treatment groups. Each lane contains VMH from an individual mouse. C: immunoblots of pAMPK were measured using densitometry and quantitated relative to GFAP levels. Bars with different letters are statistically different (P < 0.01).
DISCUSSION
This study shows that fasting enhances NPY release and the number of depolarized NPY-GI neurons in response to decreased glucose. Thus, fasting shifts the glucose concentration range to one where NPY-GI neurons are activated in higher glucose levels. This change in the glucose sensitivity of NPY-GI neurons may be due to decreased glucose and/or leptin concentration during fasting. This conclusion derives from our observations that leptin decreases, whereas low glucose increases, the response of NPY-GI neurons to decreased glucose. Moreover, our data strongly suggest that AMPK is the cellular target by which fasting-induced changes in glucose and leptin regulate the glucose sensitivity of NPY-GI neurons. These results are consistent with the established role of NPY in maintaining energy homeostasis (8, 34, 35). Increased responsiveness of NPY-GI neurons to decreased glucose would enhance NPY neurotransmission because NPY-GI neurons would become activated in response to smaller decreases in extracellular glucose. Activation of NPY neurons causes increased food intake and decreased energy expenditure. This positive shift in energy balance would restore energy homeostasis.
Our data show that reduced glucose, per se, stimulates hypothalamic NPY release. This is expected given that ∼40% of NPY neurons are also GI neurons (11). Gozali et al. (12) have previously reported that reducing glucose did not change hypothalamic NPY release. However, these investigators used glucose concentrations that exceed those found in the brain during peripheral euglycemia (e.g., a glucose decrease from 8 to 1.5 mM) (9, 29). We show here that hypothalamic NPY release is lower in glucose concentrations >1.4 mM. In fact, decreased glucose from either 10 or 5 mM to 0.1 mM did not stimulate hypothalamic NPY release in fed rats. Thus, the inability of Gozali et al. to detect increases in NPY release was most likely due to the inhibitory effect of high glucose on NPY-GI neurons. Our data and those of Gozali et al. emphasize the importance of studying NPY neurotransmission in physiological glucose concentrations.
Our studies show that fasting enhances hypothalamic NPY release in response to decreased glucose. This observation is consistent with our result showing that fasting also increases c-fos expression in ARC-NPY neurons. In addition, the fasting-induced increase in NPY release is correlated with increased numbers of depolarized NPY-GI neurons in response to decreased glucose seen in fasting. Although increased NPY mRNA and peptide levels are observed in fasted animals (25, 40), this cannot explain increased NPY release in fasted vs. fed rats in response to decreased glucose. If enhanced hypothalamic NPY release were simply a function of increased NPY mRNA or peptide, then any stimulus should cause differential release. However, both KCl and electrically stimulated hypothalamic NPY release were the same in fed and fasted rats. Moreover, the absolute amounts of NPY release (pg) in response to decreased glucose and to intermediate electrical or KCl stimulation were of similar magnitude. Therefore, the amount of NPY release in response to decreased glucose was within the dynamic range of detection for our assay. Furthermore, the lack of differential NPY release in the fed vs. fasted groups in response to intermediate KCl or electrical stimulation was not due to a plateau in NPY release. These data support our conclusion that the fasting-induced increase in NPY release in response to decreased glucose, which we observed in vitro, is not due to increased NPY mRNA expression and/or peptide levels. Rather, it is more likely due to increased numbers of depolarized NPY-GI neurons in decreased glucose. On the other hand, increased NPY mRNA and peptide levels seen during fasting would replenish the peptide pool in the face of increased NPY release.
In our study, we used FLIPR-MPD fluorescence intensity changes to demonstrate that GI neurons from fasted mice depolarize in response to smaller decreases in extracellular glucose compared with those from fed mice. Our results show that using the FLIPR-MPD fluorescence intensity change is a viable technique for screening glucose sensitivity in GI and NPY-GI neurons. Importantly, both patch-clamp recordings and FLIPR-MPD imaging indicate that GI neurons make up approximately 25–30% of neurons in the VMH (31). Similarly, both techniques reveal that ∼40% of NPY neurons are also GI neurons (11). Moreover, we have observed similar glucose sensitivity of GI neurons from rats and mice using patch-clamp recording, FLIPR-MPD, and calcium imaging (11, 14, 30). Finally, while the entire VMH was used in the majority of experiments herein to have a sufficient population of GI neurons per culture dish for statistical significance, a subset of studies specifically evaluated NPY-GI neurons using NPY-GFP mice. Both FLIPR-MPD imaging and patch-clamp recordings indicate that NPY-GI neurons are similar to the overall population of VMH-GI neurons (11).
Fasting decreases leptin and glucose concentrations (9, 10). Therefore, we hypothesize that alterations in leptin and glucose regulate the glucose sensitivity of GI neurons. As part of its overall function, leptin inhibits both GI and NPY neurons (6, 22). The studies herein agree with literature showing that leptin blocks the ability of GI neurons to sense decreased glucose (22). They also concur with those showing that exogenous leptin blocks fasting-associated increases in the neuronal activity ARC-NPY neurons (36). We show that incubation of hypothalamic neurons for an hour in leptin blocks the response of GI neurons to decreased glucose. These data suggest that under normal energy balance, leptin decreases the activation of GI neurons in response to decreased glucose. Thus, in the presence of leptin, glucose levels must fall further before GI neurons depolarize. Our data also show that leptin's effect on glucose sensitivity persists for 6 h after washout. This prolonged effect of leptin prevents oscillations of metabolic neurocircuitry in response to small glucose fluctuations. However, during fasting, reduced leptin enables NPY-GI neurons to depolarize in response to smaller glucose decreases. Finally, leptin inhibits NPY gene expression (18). Thus, decreased leptin during fasting also contributes to elevated NPY expression. This would serve to replenish the NPY pool in the face of increased neuronal activity as discussed above.
To determine whether decrease in glucose levels per se plays a role in fasting-induced changes in the glucose sensitivity of GI neurons, VMH neurons were incubated in 0.7 mM glucose before FLIPR-MPD fluorescence measurements. An extracellular glucose concentration of 0.7 mM is consistent with that measured in the VMH of a fasted rat (9). We show here that 1-h exposure of VMH-GI neurons to 0.7 mM glucose increases the response of GI neurons to decreased glucose. Thus, there may be a synergistic effect of leptin and glucose on the fasting-induced changes in glucose sensitivity of GI and NPY-GI neurons.
Alterations in AMPK activity mediate the effects of fasting, leptin, and glucose on GI neurons' glucose sensing (6). Previously, we found that AMPK activation with AICAR mimics the effect of decreased glucose on GI neuronal activity (6). Our present data and that of others (21) show that fasting increases VMH AMPKα2-subunit phosphorylation. Moreover, both leptin and glucose inhibit fasting-induced increases in AMPK phosphorylation (3, 19). We show here that leptin also prevented increased AMPK phosphorylation in response to decreased glucose. Moreover, AMPK activation with AICAR enhanced the response of GI neurons to decreased glucose and enhanced VMH AMPKα2-subunit phosphorylation. In contrast, inhibition of AMPK with Compound C reduced the response of GI neurons to decreased glucose and blocked the effects of AICAR on VMH AMPKα2 phosphorylation. Finally, AICAR reversed the inhibitory effect of leptin on GI neuron glucose sensing. These observations are consistent with Mountjoy et al. (22). We conclude that low leptin and glucose levels during fasting enhance AMPK activity and the ability of GI neurons to respond to decreases in glucose. Increased numbers of depolarized GI neurons in response to decreased glucose increases the excitability of the NPY-GI neuron population. This leads to increased NPY release in response to decreased glucose during fasting.
Finally, although our data clearly show that both leptin and glucose may mediate, in part, fasting's effects on GI neurons, we cannot rule out a role of other nutrients and hormones. For example, fasting also decreases insulin levels and increases tissue and circulating free fatty acids (4). Similar to leptin, insulin decreases NPY expression (26). Moreover, both leptin and insulin activate phosphatidylinositol 3-kinase (PI3K) and inhibit AMPK in hypothalamic neurons (6, 21). Surprisingly, we have shown that while GI neurons possess both PI3K and AMPK signaling pathways, leptin's effects do not appear to be mediated by PI3K nor insulin's effects mediated by AMPK in these neurons (6). That is, insulin increases while leptin decreases nitric oxide production in GI neurons via the PI3K and AMPK signaling pathways, respectively. Thus, it is difficult to predict the effects of insulin on the glucose sensitivity of GI neurons without detailed experimentation. Clearly, however, fasting-induced changes in extracellular insulin concentration may affect NPY-GI neurons. Similarly, while we find no overlap between glucose and oleic acid-sensing neurons in the arcuate nucleus (37), we cannot rule out a role of free fatty acids in the fasting-induced changes in NPY-GI neurons.
In conclusion, our data suggest that during normal energy balance, leptin decreases the response of NPY-GI neurons to decreased glucose. Thus, when energy stores are sufficient, a greater reduction in glucose is required to activate NPY-GI neurons. However, fasting-induced decreases in leptin and glucose cause NPY-GI neurons to be activated in response to smaller changes in glucose concentration. Because intermeal glucose levels fluctuate, it is not efficient for potent metabolic neurocircuitry, such as the NPY system, to be activated in response to subtle reductions in glucose. This would preclude maintenance of stable long-term energy balance. However, during energy deficit (e.g., fasting), decreased glucose is a greater threat due to depletion of alternate energy stores. Thus, increased responsiveness of NPY-GI neurons to small fluctuations in glucose would more readily activate the systems needed to restore energy balance. Moreover, enhanced responses of NPY-GI neurons to decreased glucose would contribute to the overall increase in NPY neurotransmission during fasting.
GRANTS
This work was funded by National Institutes of Health Grant DK-55619.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahima RS, Flier JS. Leptin. Annu Rev Physiol 62: 413–437, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Andersson U, Flippson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279: 12005–12008, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Boden G Gluconeogenesis and glycogenolysis in health and diabetes. J Investig Med 52: 375–378, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Brady LS, Smith M, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology 52: 441–447, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Canabal DD, Song Z, Potian JG, Beuve A, McArdle J, Routh VH. Glucose, insulin and leptin signalling pathways modualte nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 292: R1418–R1428, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Claret M, Smith M, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117: 2325–2336, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark JT, Kalra P, Kalra SP. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology 117: 2435–2442, 1985. [DOI] [PubMed] [Google Scholar]
- 9.De Vries MG, Arseneau L, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes 52: 2767–2773, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Faggioni R, Moser A, Feingold KR, Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am J Pathol 156: 1781–1787, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fioramonti X, Contie S, Song Z, Routh VH, Lorsignol A, Penicaud L. Characterization of glucosensing neuron subpopulations in the Arcuate nucleus. Diabetes 56: 1219–1227, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Gozali M, Pavia J, Morris MJ. Involvement of neuropeptide Y in glucose sensing in the dorsal hypothalamus of streptozotocin diabetic rats - in vitro and in vivo studies of transmitter release. Diabetologia 45: 1332–1339, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Hardie D The AMP-activated protein kinase pathway–new players upstream and downstream. J Cell Sci 117: 5479–5487, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Kang L, Dunn-Meynell A, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang B, Levin BE. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55: 412–420, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53: 549–559, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem 279: 19970–19976, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Kim MS, Lee KU. Role of hypothalamic 5′-AMP-activated protein kinase in the regulation of food intake and energy homeostasis. J Mol Med 83: 514–520, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Korner J, Savontaus E, Chua SC Jr, Leibel RL, Wardlaw SL. Leptin regulation of Agrp and NPY mRNA in the rat hypothalamus. J Neuroendocrinol 13: 959–966, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Lee K, LiB, Xi X, Suh Y, Martin RJ. Role of neuronal energy status in the regulation of adenosine 5′-monophosphate-activated protein kinase, orexigenic neuropeptides expression, and feeding behavior. Endocrinology 146: 3–10, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Li JY, Finniss S, Yang YK, Zeng Q, Qu SY, Barsh G, Dickinson C, Gantz I. Agouti-related protein-like immunoreactivity: characterization of release from hypothalamic tissue and presence in serum. Endocrinology 141: 1942–1950, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569–574, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Mountjoy PD, Bailey S, Rutter GA. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase. Diabetologia 50: 168–177, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Ono T, Nishino H, Fukuda M, Sasaki K, Muramoto K, Oomura Y. Glucoresponsive neurons in rat ventromedial hypothalamic tissue slices in vitro. Brain Res 232: 494–499, 1981. [DOI] [PubMed] [Google Scholar]
- 24.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurons of the rat hypothalamus. Nature 222: 282–284, 1969. [DOI] [PubMed] [Google Scholar]
- 25.Pritchard LE, Oliver R, McLoughlin JD, Birtles S, Lawrence CB, Turnbull AV, White A. Proopiomelanocortin-derived peptides in rat cerebrospinal fluid and hypothalamic extracts: evidence that secretion is regulated with respect to energy balance. Endocrinology 144: 760–766, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz MW, Sipols A, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP, Daniel Porte Jr. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology 130: 3608–3616, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes 52: 232–238, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci 14: 5068–5076, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 50: 2673–2681, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Song Z, Routh VH. Recurrent hypoglycemia reduces the glucose sensitivity of glucose-inhibited neurons in the ventromedial hypothalamus nucleus. Am J Physiol Regul Integr Comp Physiol 291: R1283–R1287, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390: 521–525, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3: 757–758, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Stanley BG, Kyrkouli S, Lampert S, Leibowitz SF. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides 7: 1189–1192, 1986. [DOI] [PubMed] [Google Scholar]
- 35.Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci USA 82: 3940–3943, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi KA, Cone R. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146: 1043–1047, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Wang R, Cruciani-Guglielmacci C, Migrenne S, Magnan C, Cotero VE, Routh VH. Effects of oleic acid on distinct populations of neurons in the hypothalamic arcuate nucleus are dependent on extracellular glucose levels. J Neurophysiol 95: 1491–1498, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes 53: 1959–1965, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Yang XJ, Kow LM, Funabashi T, Mobbs CV. Hypothalamic glucose sensor: similarities to and differences from pancreatic beta-cell mechanisms. Diabetes 48: 1763–1772, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Yoshihara Honma S T, Katsuno Y, Honma K. Dissociation of paraventricular NPY release and plasma corticosterone levels in rats under food deprivation. Am J Physiol Endocrinol Metab 271: E239–E245, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]