Abstract
Dietary phytosterols inhibit intestinal cholesterol absorption and regulate whole body cholesterol excretion and balance. However, they are biochemically heterogeneous and a portion is glycosylated in some foods with unknown effects on biological activity. We tested the hypothesis that phytosterol glycosides reduce cholesterol absorption in humans. Phytosterol glycosides were extracted and purified from soy lecithin in a novel two-step process. Cholesterol absorption was measured in a series of three single-meal tests given at intervals of 2 wk to each of 11 healthy subjects. In a randomized crossover design, participants received ∼300 mg of added phytosterols in the form of phytosterol glycosides or phytosterol esters, or placebo in a test breakfast also containing 30 mg cholesterol-d7. Cholesterol absorption was estimated by mass spectrometry of plasma cholesterol-d7 enrichment 4–5 days after each test. Compared with the placebo test, phytosterol glycosides reduced cholesterol absorption by 37.6 ± 4.8% (P < 0.0001) and phytosterol esters 30.6 ± 3.9% (P = 0.0001). These results suggest that natural phytosterol glycosides purified from lecithin are bioactive in humans and should be included in methods of phytosterol analysis and tables of food phytosterol content.
Keywords: diet, oils, mass spectrometry, deuterium
phytosterols are cholesterol-like compounds that occur naturally in plant foods and reduce cholesterol absorption (11, 17–19) and plasma LDL cholesterol (7, 12). Thus the U.S. National Cholesterol Education Program Adult Treatment Panel III has recommended adding 2.0 g/day of phytosterols to the diet of adults to reduce LDL cholesterol and coronary heart disease risk (2). Phytosterols at levels present in natural foods have also been shown to reduce cholesterol absorption (17, 19).
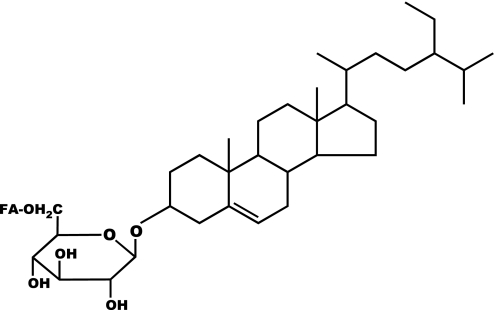
Phytosterols occur in diverse forms in food matrixes and not all have been tested for bioactivity (10). The form of the phytosterols is critical for bioactivity because crystalline phytosterols are not readily soluble in bile and do not reduce cholesterol absorption (20). Properly formulated with lecithin or other emulsifiers, free phytosterols do reduce cholesterol absorption and LDL cholesterol (3). Phytosterol esters solubilized in the triglyceride phase of margarines are also bioactive (6, 22). However, the amphipathic structure of phytosterol glycosides (Fig. 1) raises questions about the degree of solubility in intestinal bile salt micelles and reactivity with pancreatic enzymes. Although literature on the physiology of phytosterol glycosides is sparse, previous workers have shown that fatty acids are cleaved from glycosylated phytosterols in vitro by pancreatin, but the sugar moiety itself is not removed (9). This was confirmed by another study in which [14C]sitostanol glucoside was not cleaved in the stomach or small bowel after intragastric administration (23). If cleavage of the sugar from glycosylated phytosterols is needed for bioactivity, or if poor solubility limits physiological interactions, then phytosterol glycosides might not necessarily be biologically active.
Fig. 1.
Example of glycosylated phytosterol structure. Sitosterol on the right is bonded to glucose, which is then attached to a fatty acid (FA). Potential structural variability is present in the phytosterol moiety, the orientation of the bond from phytosterol to the sugar, the structure of the sugar, and the presence and type of fatty acid. When a fatty acid is present the material is referred to as acylated phytosterol glycoside.
Because phytosterol glycosides comprise a significant proportion of naturally occurring phytosterols in some foods (5, 13, 14), we tested the effect of phytosterol glycosides in a clinical trial in which cholesterol absorption was measured in repeated single-meal tests. In a randomized crossover study design, we compared phytosterol glycosides to placebo using deuterated cholesterol as a tracer with detection by mass spectrometry. Phytosterol esters were included as an experimental positive control, based on reduction in cholesterol absorption observed with phytosterol esters (15). Because large amounts of phytosterol glycosides are not commercially available, we first developed a method for the purification of gram quantities from soy lecithin.
MATERIALS AND METHODS
Subjects.
Twelve subjects were recruited by the Volunteer for Health Program at Washington University School of Medicine. Inclusion criteria were healthy individuals aged 18–80 yr with plasma LDL cholesterol below 190 mg/dl, triglycerides lower than 250 mg/dl, and no active medical or surgical illnesses. Subjects also were required to have stable body weight within 5 pounds during the preceding 2 mo and be willing to maintain their body weight throughout the study. Exclusion criteria included lipid abnormalities requiring treatment under current guidelines, history of clinical lactose intolerance, breastfeeding, and anticipated or current pregnancy. Four of the participants took birth control pills but no other medications potentially affecting lipid metabolism. The protocol was approved by the Washington University Human Research Protection Office. Informed consent was obtained in writing. One subject dropped out before receiving any treatments and 11 completed the entire protocol.
Materials.
[25,26,26,26,27,27,27-2H7]Cholesterol was purchased from CDN Isotopes (Pointe-Claire, QC, Canada). Soybean oil (Bakers & Chefs, Sam's Club) was purified as described to reduce the content of phytosterols (19). Hunt's Snack Pack fat-free pudding was purchased from a local supermarket in vanilla or chocolate flavor. Lecithin was purchased from The Vitamin Shoppe (North Bergen, NJ). Magnesol (a food-grade magnesium trisilicate) was purchased from Dallas Group of America (Whitehouse, NJ). CoroWise phytosterol esters containing 60.6% phytosterol moiety in phytosterol esters were a gift from Cargill (Minneapolis, MN).
Purification of phytosterol glycosides from lecithin.
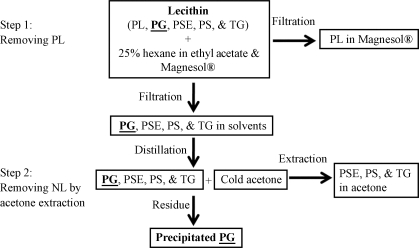
Soybean-derived commercial lecithin containing 3% glycosylated phytosterols was used as a starting material. Glycosides are of intermediate polarity compared with high-polarity phospholipids and low-polarity neutral lipids (triglycerides, phytosterol esters, unesterified phytosterols). The unique polarity of phytosterol glycosides was used to isolate them by using hexane, ethyl acetate, solid-phase magnesium trisilicate and acetone (Fig. 2).
Fig. 2.
Flow chart for purifying phytosterol glycosides from lecithin. Lecithin [containing phospholipids (PL), phytosterol glycosides (PG), phytosterol esters (PSE), unesterified phytosterols (PS), and triglycerides (TG)] was dissolved in 25% hexane in ethyl acetate followed by mixing with Magnesol (187.5 g/l). PL bound to Magnesol and were removed by vacuum filtration. The rest of the components remained in the solvents. After distillation to remove the solvents, the neutral lipids (NL: PSE, PS, and TG) were removed by cold acetone extraction. The precipitated PG was dried and analyzed.
Two steps were employed to purify glycosylated phytosterols from lecithin. Solvent-resistant plasticware was used and the procedure was performed in a clean room. The first step was removal of phospholipids. Fifty grams of lecithin (which is slow to dissolve in ethyl acetate but readily soluble in hexane) was dissolved in 313 ml of hexane and diluted to 25% hexane with 938 ml of ethyl acetate. Next, Magnesol was added at 187.5 g/l and the suspension was mixed for 10 min and then filtered under vacuum. Under these conditions, phospholipids bound to Magnesol whereas phytosterol glycosides and neutral lipids did not. Residual phospholipids were removed from the filtrate by repeating the treatment with 120 g/l Magnesol (not shown in Fig. 2). The filtrate was then dried.
The second step was removal of neutral lipids. Since these contaminants are much more soluble than phytosterol glycosides in acetone, they were removed by extraction. Ice-cold acetone equal in weight to the dried filtrate was added with intermittent swirling on ice for 30 min, followed by decanting the acetone. This process was repeated five times. The solid glycosylated phytosterols were lyophilized and analyzed.
Preparation of test meals.
Cholesterol-d7 was first disinfected by dissolving in ethanol followed by filtration through a 0.22-μm solvent-resistant FG filter and evaporation of the solvent. Lecithin was dissolved by heating at 100°C for 1 h in purified soybean oil, which was then cooled to room temperature. The lecithin/oil mixture was centrifuged at 1,600 g for 30 min at room temperature. The supernatant was added to the dried cholesterol-d7. Phytosterol esters or glycosides were added to 15% lecithin in purified soybean oil containing cholesterol-d7, warmed at 65°C until completely dissolved and then cooled to room temperature. Lecithin was added to the oil to increase the solubility of phytosterol glycosides. Each test meal consisted of 240 g of pudding and 12 g of 15% lecithin in purified soybean oil with or without phytosterol additions. The oil was heated again in a 65°C water bath for 10 min before being weighed into a bowl and mixed with room temperature pudding just prior to serving of the test meal. The meal was consumed within 10 min, and the subjects had nothing else to eat for 4 h. All test meals contained 303 kcal, 44 g carbohydrate, 2.4 g protein, 12.5 g fat, 82 mg of background phytosterols derived from pudding, oil, and lecithin, and 30 mg cholesterol-d7. To the meals were added one of the following: 1) 300 mg phytosterols from the phytosterol glycoside preparation (experimental treatment), 2) 325 mg phytosterols added in the form of phytosterol esters (positive control treatment), or 3) no addition (placebo treatment). Since phytosterols given in single-meal tests at a dose of 300 mg have a nearly maximum effect on cholesterol absorption in humans (20), we chose an experimental dose of 300 mg provided either by phytosterol esters or phytosterol glycosides.
Clinical protocol.
Eleven subjects received three cholesterol absorption tests each, which were performed 2 wk apart in a randomized crossover design. For each test they reported to the Clinical Trials Unit after an overnight fast, and a baseline plasma sample was drawn. The test meal was consumed and fasting blood samples were drawn 4 and 5 days later for analysis.
Analyses.
Plasma samples were saponified, extracted, converted to pentafluorobenzoyl esters, and analyzed for natural cholesterol and cholesterol-d7 by negative ion methane chemical ionization gas chromatography-mass spectrometry (NCI GC-MS) with an Agilent Technologies 5,973 positive/negative ion mass spectrometer in the Washington University Mass Spectrometry Resource as described (19). The cholesterol area ratio of mass-to-charge ratio (m/z) 587 (M) for tracer to m/z 581 (M+1) for natural cholesterol was calculated and converted to mole ratio of cholesterol-d7 to cholesterol by reference to a standard curve. The mole ratio in the plasma before each test meal was subtracted from that of day 4 and day 5. The net mole ratios of the 2 days were averaged and reported. Reduction in the mole ratio for a given treatment compared with the value during the placebo test was used as a measurement of reduction in percent cholesterol absorption as described previously (20). The sensitivity of this method was calculated from the m/z 587/581 area ratios of the standard curve point containing no added cholesterol-d7 run on different days. The between-assay standard deviation of these measurements was converted to a mole ratio of cholesterol-d7/natural cholesterol (0.0000055), multiplied by 2, and then divided by the mean change in mole ratio observed in plasma cholesterol during the placebo test (0.00038), yielding a value of 0.029. This represents an estimate of the fraction of average cholesterol absorption or change in cholesterol absorption that can be detected by the mass spectrometric method with ∼95% confidence.
The phytosterol glycoside fraction was analyzed in two stages. First, total phytosterols were determined after acid hydrolysis followed by alkaline hydrolysis (double hydrolysis), a procedure that liberates free sterols from both phytosterol esters and phytosterol glycosides (5). Then, in a separate analysis, the sum of free and esterified phytosterols was determined after alkaline hydrolysis only (single hydrolysis). Phytosterols contained in phytosterol glycosides were calculated as the difference between total phytosterols obtained from double hydrolysis and free plus esterified phytosterols from single hydrolysis. The hydrolysates were derivatized to pentafluorobenzoyl esters and analyzed by NCI GC-MS using pentadeuterated soy sterols as an internal standard (16).
Statistical analyses.
Statistical analyses were conducted with SAS (version 9.2, SAS Institute, Cary, NC). The independent effects of sequence of treatment, period, and treatment on the net enrichment of cholesterol-d7 tracer were analyzed by using the Proc Mixed of SAS with patient within sequence as the random effect. The significance of multiple comparisons among the treatments was adjusted with the Tukey procedure. The Proc Mixed procedure also was used to analyze possible carryover effects from one period to the next, with the previous treatment encoded as a separate class variable. Values represent means ± SE.
RESULTS
Purification of glycosylated phytosterols from lecithin.
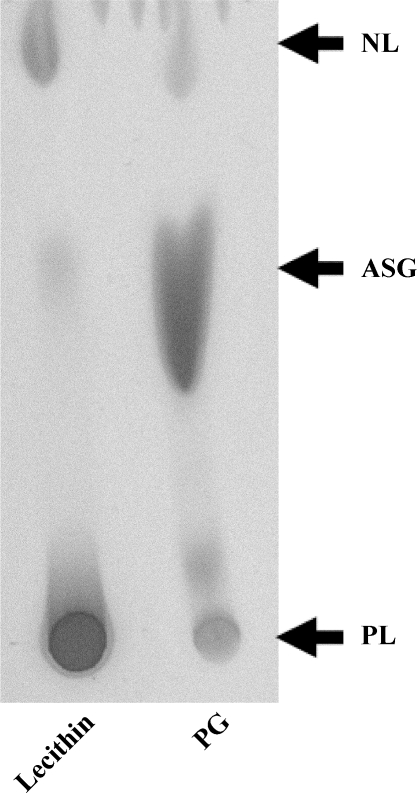
We have developed a protocol to purify glycosylated phytosterols from lecithin in gram quantities. The final product contained principally acylated phytosterol glycoside as shown by thin layer chromatography (Fig. 3). Most neutral lipids and phospholipids had been removed. The total phytosterol content was determined by acid hydrolysis (to break glycosidic bonds) followed by alkaline hydrolysis and yielded a value of 41.6% phytosterols by weight. Residual free and esterified phytosterols determined after alkaline hydrolysis were only 1.1% by weight, demonstrating that phytosterol glycosides contributed 97.3% of the total phytosterols present. Assuming a molecular weight of 852 for sitosterol glucoside linoleate as a principal form, the preparation had a phytosterol glycoside content of 73% by weight.
Fig. 3.
Analysis of lecithin and the purified PG by TLC. Lecithin or the purified PG [120 μg each (3 μl of 40 μg/μl in hexane)] was spotted on a TLC slide, run in pure ethyl acetate, and developed in iodine. Lecithin contained mostly PL and NL (PSE, PS, and TG), whereas purified PG was enriched with acylated sterol glycosides (ASG).
Effects of phytosterol esters and glycosides on cholesterol-d7 enrichment in the plasma.
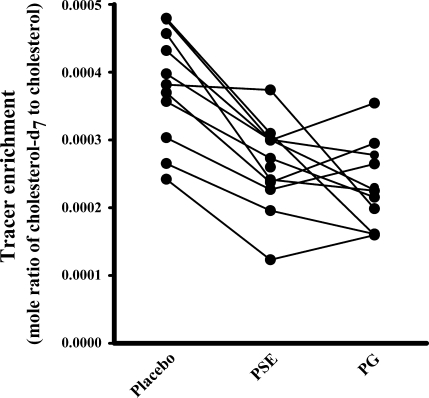
The baseline measurements for the 11 subjects are listed in Table 1. Phytosterol glycosides were solubilized in purified soybean oil containing 15% lecithin and cholesterol-d7 tracer and then mixed with low-phytosterol commercial pudding and consumed as a test breakfast. The resulting enrichment of plasma cholesterol-d7 was determined 4 and 5 days later during the time of peak plasma concentration by GC-MS. Each subject received three cholesterol absorption tests in random order that included phytosterol glycosides, phytosterol esters, or no addition (placebo). Plasma cholesterol-d7 enrichment was highest after the placebo test, and it was reduced by 37.6 ± 4.8% by phytosterol glycosides (P < 0.0001) and 30.6 ± 3.9% by phytosterol esters (P = 0.0001) (Fig. 4). There was no significant difference between phytosterol glycosides and phytosterol esters (P = 0.39). No carryover effects were found from the different supplementations on the net cholesterol-d7 tracer enrichment.
Table 1.
Participant characteristics at baseline
| N or Mean ± SE | |
|---|---|
| Women/Men | 10/1 |
| Age, yr | 37.7±3.2 |
| Weight, kg | 84.8±7.2 |
| Body mass index, kg/m2 | 30.7±2.4 |
| Lipids | |
| Total cholesterol, mg/dl | 192±5.7 |
| LDL cholesterol, mg/dl | 120±4.8 |
| HDL cholesterol, mg/dl | 53±3.5 |
| Triglycerides, mg/dl | 95±10.3 |
Fig. 4.
Effects of PG and PSE on cholesterol absorption. Eleven patients were randomly assigned in a crossover design to receive either placebo, PG, or PSE in 3 single-meal cholesterol absorption tests given 2 wk between treatments. The net increase in plasma cholesterol tracer enrichment was determined 4 and 5 days after the test and is reported as a mole ratio of tracer to natural cholesterol.
DISCUSSION
Plants have a tendency to glycosylate many cell components, and this modification needs to be taken into account in dietary studies for which bioavailability is a concern. In particular, phytosterols are glycosylated in many plants (10, 13, 14) and may even be the predominant form encountered in some foods such as potatoes, in which phytosterol glycosides have been reported to comprise 82% of phytosterols (5). However, only a few studies have addressed the potential biological activity of these compounds, owing in part to the lack of commercial availability in the amounts needed.
Clinical studies usually require several grams of phytosterols. Although it is possible to prepare analytically pure material at a milligram scale by techniques such as high-performance liquid chromatography, the low throughput and high solvent consumption make these methods impractical or expensive at gram scale. To obviate these difficulties, we used simple adsorptive methods beginning with soy lecithin in which phytosterol glycosides constitute ∼3% of the total mass (Fig. 2). Phospholipids, the principal contaminant, were removed by selective adsorption to magnesium trisilicate. Then neutral lipids, including most free and esterified phytosterols, were removed by extraction into cold acetone. Contaminating free phytosterols and phytosterol esters comprised only 2.7% of phytosterols in the final phytosterol glycoside fraction, which was suitable for use in human studies.
To approximate the natural delivery of phytosterol glycosides, we dissolved them in soybean oil containing 15% lecithin, a condition associated with improved solubility at room temperature. Phytosterol glycosides were compared with placebo in a series of three single-meal cholesterol absorption tests performed in each of 11 normal subjects. The subjects consumed 30 mg cholesterol-d7 tracer, and plasma enrichment was measured several days later at the peak of appearance in the plasma by negative ion mass spectrometry. The results show that phytosterol glycosides reduced cholesterol absorption by 37.6%, a typical reduction observed during other studies of conventional forms of phytosterols (22), and comparable to the 30.6% reduction observed simultaneously with phytosterol esters. Thus phytosterol glycosides are biologically active in humans.
We are not aware of previous studies of the effect of phytosterol glycosides on cholesterol absorption. However, glycosides of related nonphytosterol compounds have been shown to reduce cholesterol absorption and provide a framework in which to consider the present results. Synthetic glycosides of spirostane derivatives (saponins) reduced cholesterol absorption in monkeys (8). Similarly, spirostane diglycosides reduced cholesterol absorption in hamsters (1) and humans (4). Thus, there is precedent suggesting that glycosylated steroids might also be expected to reduce cholesterol absorption. Explanations of the mechanism by which such glycosides are active would need to take account of the reports that phytosterol glycosidic bonds are not cleaved by mammalian pancreatic enzymes in vitro (9). Furthermore, a study in rats showed that [4-14C]sitosteryl β-d-glucoside remains unhydrolyzed and unabsorbed in rat stomach and small intestine (23). This suggests that phytosterol glycosides may be active with the glycosidic bond intact and not necessarily hydrolyzed to yield free phytosterols. If this is the case, then phytosterol glycosides would necessarily need to enter intestinal micelles and exert actions there. Further work is needed to test this hypothesis.
The biological activity of soy phytosterol glycosides has implications both for analytical methods and for nutrition databases. Traditional protocols for extracting and analyzing phytosterols from foods employ only alkaline hydrolysis and do not measure glycosylated forms (24). To include phytosterol glycosides in the results, an acidic hydrolytic step must be included to cleave the phytosterol-glycosidic bond so that the free phytosterol is liberated and measured (5, 10). Alternatively, phytosterol glycosides have been measured directly by gas chromatography of the intact material (21). Further work is needed to validate these methods to be sure that phytosterols are adequately extracted, not destroyed by the hydrolytic conditions, and are included in the final analytical method.
In the present work, phytosterol glycosides were solubilized in lecithin and triglyceride before study. Since our material was derived from soy lecithin and ultimately from crude soybean oil, this appears to be a reasonable formulation. However, phytosterol glycosides in other foods may not necessarily be associated with lecithin, with unpredictable effects on cholesterol absorption. Bioavailability might also be adversely affected by different food matrixes, and this needs further experimental work. Although the measured reduction of cholesterol absorption by phytosterol glycosides and phytosterol esters was similar, only a single dose was analyzed; a much larger study would be needed to establish bioequivalence.
Currently, our nutritional databases include relatively little information about phytosterols despite their demonstrated ability to lower cholesterol absorption and LDL cholesterol. Our work suggests that food tables and databases that report phytosterol amounts may have underestimated the true values by failing to measure and report glycosylated phytosterols. Additional studies on bioactivity of glycosylated phytosterols are needed to understand the actions of this important class of nutrients.
GRANTS
This work was supported by National Institutes of Health Grant R01 50420, the Washington University Mass Spectrometry Resource (P41 RR00954), the Washington University Diabetes Center (P60 DK20579), Washington University Institute of Clinical and Translational Sciences, Center for Applied Research Sciences (UL1 RR024992), the Washington University Digestive Disease Center (P30 DK52574), and the Washington University Clinical Nutrition Research Center (P30 DK056341).
DISCLOSURES
Dr. Ostlund and Washington University have a financial interest in Lifeline Technologies, a biotechnology company developing bioactive phytosterols. Lifeline products are not used in this study, and Lifeline has no financial interest in phytosterol glycosides.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.DeNinno MP, McCarthy PA, Duplantier KC, Eller C, Etienne JB, Zawistoski MP, Bangerter FW, Chandler CE, Morehouse LA, Sugarman ED, Wilkins RW, Woody HA, Zaccaro LM. Steroidal glycoside cholesterol absorption inhibitors. J Med Chem 40: 2547–2554, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Gremaud G, Dalan E, Piguet C, Baumgartner M, Ballabeni P, Decarli B, Leser ME, Berger A, Fay LB. Effects of non-esterified stanols in a liquid emulsion on cholesterol absorption and synthesis in hypercholesterolemic men. Eur J Nutr 41: 54–60, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Harris WS, Dujovne CA, Windsor SL, Gerrond LLC, Newton FA. Inhibiting cholesterol absorption with CP-88,818 (b-tigogenin cellobioside; Tiqueside): studies in normal and hyperlipidemic subjects. J Cardiovasc Pharmacol 30: 55–60, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Jonker D, van der Hoek GD, Glatz JFC, Homan C, Posthumus MA, Katan MB. Combined determination of free, esterified and glycosilated plant sterols in foods. Nutr Rep Int 32: 943–951, 1985. [Google Scholar]
- 6.Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc 78: 965–978, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Law MR Plant sterol and stanol margarines and health. West J Med 173: 43–47, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malinow MR Effects of synthetic glycosides on cholesterol absorption. Ann NY Acad Sci 454: 23–27, 1985. [DOI] [PubMed] [Google Scholar]
- 9.Moreau RA, Hicks KB. The in vitro hydrolysis of phytosterol conjugates in food matrices by mammalian digestive enzymes. Lipids 39: 769–776, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Moreau RA, Whitaker BD, Hicks KB. Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog Lipid Res 41: 457–500, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Nestel P, Cehun M, Pomeroy S, Abbey M, Weldon G. Cholesterol-lowering effects of plant sterol esters and non-esterified stanols in margarine, butter and low-fat foods. Eur J Clin Nutr 55: 1084–1090, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen TT The cholesterol-lowering action of plant stanol esters. J Nutr 129: 2109–2112, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Normen L, Bryngelsson S, Johnsson M, Evheden P, Ellegard L, Brants H, Andersson H, Dutta P. The phytosterol content of some cereal foods commonly consumed in Sweden and in the Netherlands. J Food Comp Anal 15: 693–704, 2002. [Google Scholar]
- 14.Normen L, Johnsson M, Andersson H, van Gameren Y, Dutta P. Plant sterols in vegetables and fruits commonly consumed in Sweden. Eur J Clin Nutr 38: 84–89, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Ostlund RE, Lin X. Regulation of cholesterol absorption by phytosterols. Curr Atheroscler Rep 8: 487–491, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Ostlund RE, McGill JB, Zeng CM, Covey DF, Stearns J, Stenson WF, Spilburg CA. Gastrointestinal absorption and plasma kinetics of soy Δ5-phytosterols and phytostanols in humans. Am J Physiol Endocrinol Metab 282: E911–E916, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Ostlund RE, Racette SB, Okeke A, Stenson WF. Phytosterols that are naturally present in commercial corn oil significantly reduce cholesterol absorption in humans. Am J Clin Nutr 75: 1000–1004, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Ostlund RE, Racette SB, Stenson WF. Effects of trace components of dietary fat on cholesterol metabolism: phytosterols, oxysterols, and squalene. Nutr Rev 60: 349–359, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Ostlund RE, Racette SB, Stenson WF. Inhibition of cholesterol absorption by phytosterol-replete wheat germ compared with phytosterol-depleted wheat germ. Am J Clin Nutr 77: 1385–1389, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Ostlund RE, Spilburg CA, Stenson WF. Sitostanol administered in lecithin micelles potently reduces cholesterol absorption in humans. Am J Clin Nutr 70: 826–831, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Phillips KM, Ruggio DM, Ashraf-Khorassani M. Analysis of steryl glucosides in foods and dietary supplements by solid-phase extraction and gas chromatography. J Food Lipids 12: 124–140, 2005. [Google Scholar]
- 22.Spilburg CA, Goldberg AC, McGill JB, Stenson WF, Racette SB, Bateman J, McPherson TB, Ostlund RE Jr. Fat-free foods supplemented with soy stanol-lecithin powder reduce cholesterol absorption and LDL cholesterol. J Am Diet Assoc 103: 577–581, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Weber N Metabolism of sitosteryl beta-d-glucoside and its nutritional effects in rats. Lipids 23: 42–47, 1988. [DOI] [PubMed] [Google Scholar]
- 24.Weihrauch JL, Gardner JM. Sterol content of foods of plant origin. J Am Diet Assoc 73: 39–47, 1978. [PubMed] [Google Scholar]