Abstract
Inflammatory bowel diseases (IBD) can involve widespread gastrointestinal dysfunction, even in cases in which inflammation is localized to a single site. The underlying pathophysiology of dysfunction in noninflamed regions is unclear. We examined whether colitis is associated with altered electrogenic ion transport in the ileal mucosa and/or changes in the properties of ileal submucosal neurons. Colitis was induced by administration of trinitrobenzene sulfonic acid (TNBS), and the uninflamed ileum from animals was examined 3, 7, and 28 days later. Electrogenic ion transport was assessed in Ussing chambers. Intracellular microelectrode recordings were used to examine the neurophysiology of the submucosal plexus of the ileum in animals with colitis. Noncholinergic secretion was reduced by 33% in the ileum from animals 7 days after the induction of colitis. The epithelial response to vasoactive intestinal peptide (VIP) was unaltered in animals with colitis, but the response to carbachol was enhanced. Slow excitatory synaptic transmission was dramatically reduced in VIP-expressing, noncholinergic secretomotor neurons. This change was detected as early as 3 days following TNBS treatment. No changes to fast synaptic transmission or the number of VIP neurons were observed. In addition, cholinergic secretomotor neurons fired more action potentials during a given stimulus, and intrinsic primary afferent neurons had broader action potentials in animals with colitis. These findings implicate changes to enteric neural circuits as contributing factors in inflammation-induced secretory dysfunction at sites proximal to a localized inflammatory insult.
Keywords: submucosal plexus, electrogenic secretion, ion transport, synaptic transmission, vasoactive intestinal peptide
the integrative nerve circuitry of the enteric nervous system (ENS) provides regulatory control of the gastrointestinal (GI) tract (16). The output of the ENS is modulated by extrinsic autonomic influences from the sympathetic and parasympathetic nervous systems. When the gut is diseased or damaged, the nervous system attempts to adapt to maintain the essential digestive and defensive functions of the GI tract. However, in chronic disease, gut function is frequently compromised. Inflammatory bowel diseases (IBD), which include Crohn's disease and ulcerative colitis, are relapsing and remitting chronic intestinal inflammatory conditions. They are the source of considerable morbidity, arising from disturbances of motility and secretion (10, 20, 54). These functional alterations develop throughout the GI tract, even at sites distant from a localized region of active inflammation (2, 28, 47, 51). Abnormalities in function also occur during periods of remission from IBD, when there is resolution of inflammation (14, 27, 55). To understand how inflammation in one region of the gut gives rise to functional disturbances at distant sites, we have examined the enteric neural circuitry in noninflamed regions of the gut.
In animals with ileitis we observed changes to the neural circuitry of the submucosal plexus of the colon that were qualitatively and quantitatively similar to those observed at the site of inflammation (45). Specifically, neurons that display a long afterhyperpolarization (AHP) (AH neurons), which are regarded as intrinsic primary afferent neurons (19), become hyperexcitable (33, 34, 38, 39). Fast synaptic input to synaptic (S) neurons is facilitated owing to the recruitment of additional presynaptic neurotransmitters (38, 39). These changes are reflected in functional alterations in the control of electrogenic secretion in the colon. To date, the enteric neural circuitry of the submucosal plexus of the ileum in animals with colitis has not been examined. However, it has been demonstrated that secretory responses to direct stimulation of the epithelium and to capsaicin are blunted over the first 7 days of ileitis, but these responses returned to normal by 30 days (41).
In the present study we tested the hypothesis that submucosal neurons of the ileum are affected by inflammation of the colon. The potential impacts of changes to submucosal neurons include alterations to secretion and absorption (15, 57, 58), as well as maintenance of the integrity of the epithelial barrier (43). We first examined whether there were secretory abnormalities in the ileum of animals with colitis. We also assessed the proportion of vasoactive intestinal peptide (VIP) neurons in the submucosal plexus and VIP release. We then went on to study the enteric neurophysiology in these animals. Our results indicate that there are marked changes to the noncholinergic secretomotor neurons and that these are accompanied by a reduction in noncholinergic secretion. Moreover, the ileal submucosal plexus is altered in response to colitis in a fundamentally different manner from than seen previously in the colon.
METHODS
Animals.
The methods used in this study were approved by the University of Calgary Animal Care Committee and conform to the guidelines of the Canadian Council on Animal Care. Male albino guinea pigs (250–350 g, Charles River, Montreal, QC, Canada) were administered intracolonic trinitrobenzene sulfonic acid (TNBS, 0.3–0.5 ml, 20–25 mg/ml in 25% ethanol, Sigma-Aldrich, St. Louis, MO) or saline under halothane anesthetic as described elsewhere (34). Briefly, TNBS or saline were given via enema through a flexible polyethylene catheter inserted rectally 7 cm proximal to the anus. At 3, 7, or 28 days later animals were exsanguinated under deep halothane anesthesia and the distal ileum (10 cm proximal to the ileocecal junction) and distal colon were removed. Body weight change and macroscopic damage score of the colon and ileum were used to assess the severity of colitis as previously described (40).
Measurement of electrogenic ion transport.
Segments of ileum 10 cm proximal to the ileocecal junctions were maintained in oxygenated Krebs solution (in mM: 117 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, and 11 d-glucose; aerated with 95% O2-5% CO2). Mucosal preparations with an intact submucosal plexus were obtained by opening the ileum along the mesenteric border and removing the external muscle layers (comprising the circular and longitudinal smooth muscle layers plus the myenteric plexus) by blunt dissection. Preparations were subsequently mounted in Ussing chambers (exposed mucosal area of 0.6 cm2) containing 6 ml of oxygenated (95% O2-5% CO2) Krebs solution maintained at 37°C. The serosal bathing solution contained 11 mM d-glucose, whereas in the mucosal solution an equimolar concentration of mannitol was substituted for glucose.
Tissues were voltage clamped at 0 mV with an automatic voltage clamp (DVC 4000, World Precision Instruments, Sarasota, FL), and changes in the short-circuit current (ΔISC) required to maintain the 0 mV potential were continuously monitored by use of DataTrax software (World Precision Instruments). All pharmacological agents were added to the serosal (submucosal) reservoir.
To evaluate the total neurogenic secretion from the epithelium, veratridine (10 μM, Sigma-Aldrich), which activates voltage-gated Na+ channels, was used to stimulate secretomotor neurons. The effects of veratridine were blocked by tetrodotoxin (TTX, 300 nM, Sigma-Aldrich). Preliminary experiments were performed using various concentrations of veratridine and 10 μM was found to be approximately the ED50 value for secretion. Values for veratridine-stimulated secretion were taken from the peak ΔISC. Agonist-induced Cl− secretion in GI epithelia occurs via Ca2+-dependent or cAMP-dependent signaling pathways (3). Thus, to determine tissue viability, carbachol (10 μM, Sigma-Aldrich), a cholinergic receptor agonist that evokes Ca2+-dependent secretion, was utilized as a positive control. To determine noncholinergic secretion, veratridine-stimulated secretion was measured in the presence of atropine (1 μM, Sigma-Aldrich). In these experiments forskolin (10 μM, Sigma-Aldrich), which stimulates cAMP-dependent secretion, was used as positive control. In additional experiments, VIP (30 nM, Sigma-Aldrich) was added to the serosal chamber in the presence of TTX to stimulate secretion directly from the epithelium.
Quantification of VIP release from the ileum of animals with colitis.
To address the possibility of reduced VIP release from enteric neurons of the submucosal plexus, medium was collected following incubation of ileal mucosa-submucosal preparations for radioimmunoassay (RIA) of VIP release (21, 22). Briefly, tissues from control animals and animals treated with TNBS 7 days previously were prepared as described above. Following isolation and for all subsequent incubations, the tissue was maintained in Krebs containing 1% bovine serum albumin, 10 μM amastatin, and 1 μM phosphoramidon (Sigma-Aldrich). Following washing (3 × 10 min Krebs), a 10-min baseline sample was collected, followed by a 10-min vehicle (ethanol) sample. The same tissue was then washed again (3 × 10 min Krebs), and a second baseline sample was collected followed by a veratridine (10 μM) 10-min induced release sample.
VIP was measured in duplicate by RIA as previously described (21, 22) (Bachem, Bubendorf, Switzerland). The limit of detection of the assay was 0.01 ng/ml and the 50% inhibitory concentration of the assay was 0.28 ng/ml. The concentration of VIP measured in this study ranged from 11.8 to 82.7 pg/ml. Unless otherwise stated, results are expressed as percent increase in VIP release per gram of tissue wet weight per minute compared with the baseline sample collected immediately prior to treatment.
Tissue preparation for neurophysiology.
The oral end of the ileum was marked and the tissue was maintained in oxygenated Krebs solution (in mM: 117 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, and 11 d-glucose; aerated with 95% O2-5% CO2) containing nicardipine (3 μM, Sigma-Aldrich) and scopolamine (1 μM, Sigma-Aldrich). The submucosa was dissected from a segment of ileum as previously described (35, 38). Submucosal preparations were transferred to Silastic elastomer-lined recording chamber and superfused with oxygenated Krebs preheated to 33–34°C.
Neurophysiological characterization of submucosal neurons.
Neurons were impaled with microelectrodes fabricated from 1-mm-outer-diameter borosilicate glass (World Precision Instruments) and filled with 1% biocytin in 1 M KCl. Electrode resistances were 90–150 mΩ. Recordings were made with a Multiclamp 700A amplifier in current-clamp mode (Axon Instruments, Molecular Devices, Sunnyvale, CA). Recordings were digitized at 5–50 kHz and stored and analyzed by using PC-based data acquisition and analysis software (pClamp 9.2 suite, Axon Instruments). Following a 10-min stabilization period after impalement, passive and active electrical properties were evaluated, as well as synaptic events evoked by focal stimulation of adjacent ganglia. Only neurons that fired action potentials of greater than 40 mV in amplitude, which overshoot 0 mV and had a resting membrane potential more negative than −40 mV, were included in the electrophysiological analyses.
Excitability was measured by injecting 500-ms depolarizing and hyperpolarizing current pulses to reveal input resistance and the number of action potentials at threshold (rheobase), twice rheobase, and the maximum number of action potentials each neuron fired during a 500-ms depolarizing step. Synaptic inputs were stimulated by using a concentric bipolar stimulating electrode positioned on interganglionic nerve bundles. Stimulus pulses of 0.5-ms duration and 5- to 15-V intensity were delivered via a Grass SD9 (Grass Medical Instruments, Quincy, MA) stimulator. For the measurement of fast excitatory postsynaptic potentials (EPSPs), cells were hyperpolarized to −80 mV to inhibit action potential firing. The amplitude of three fast EPSPs were averaged for each neuron under control conditions and in inflamed preparations, and after superfusion with hexamethonium (100 μM, Sigma-Aldrich) to examine noncholinergic neurotransmission. To elicit slow synaptic input, 1-s trains of 20-Hz 5- to 15-V stimulation were applied to interganglionic nerve bundles. Slow EPSPs were evoked from the resting membrane potential.
To examine neurotransmitter release properties, two pairs of fiber tract stimuli were applied 50 ms apart to generate two fast EPSPs. Fast EPSPs were elicited at a −80 mV membrane potential to inhibit action potentials. The paired-pulse ratio (PPR) was determined as the ratio of the maximum amplitude of the second fast EPSP to the maximum amplitude of the first. Paired-pulsed facilitation was determined to occur if the PPR was greater than one. Paired-pulse depression occurred if the PPR was less than one.
Visualization and classification of recorded neurons.
At the end of each recording, depolarizing pulses from the intracellular electrode were delivered to the neuron to ensure complete filling of the neuron with biocytin. For post hoc identification of neurons the shape of the ganglion was drawn and the location in the preparation recorded. At the end of each experiment the tissue was fixed in Zamboni's fixative (0.1 M PBS containing 2% formaldehyde plus 0.2% picric acid) overnight at 4°C. Following fixation the tissue was washed in PBS. For biocytin visualization, the tissue was washed three times in PBS containing 0.1% Triton X-100 (3 × 10 min) followed by incubation with FITC-conjugated avidin for 2 h at room temperature (1:100, Jackson ImmunoResearch, West Grove, PA) (35). Neurons were classified as AH or S according to well-established criteria (59). AH neurons must possess the characteristic action potential shape, as well as a prolonged AHP. In addition, AH neurons possess a multiaxonal Dogiel type II morphology. S neurons have narrow action potential shapes, receive fast EPSPs, and have a uniaxonal Dogiel type I morphology. S neurons can further be divided into choline acetyltransferase (ChAT)-expressing S neurons (S-ChAT) or VIP-expressing S neurons (S-VIP) by electrophysiological criteria. S-VIP neurons receive slow inhibitory postsynaptic potentials (IPSPs), whereas S-ChAT neurons receive no inhibitory input (8). However, to confirm this we also performed immunohistochemistry on filled neurons.
Immunohistochemistry.
To determine the proportion of VIP-immunoreactive neurons in the ileal submucosal plexus and to examine biocytin-filled preparations, we performed immunohistochemistry with a mouse anti-VIP antibody (1:500, UBC Regulatory Peptide Group, Vancouver, BC, Canada). Submucosal preparations were also double labeled with a neuronal nuclear marker (1:1,000, Fos4, Santa Cruz Biotechnology, Santa Cruz, CA) (46). Segments of ileum, 10 cm from the ileocecal junction, from control and TNBS-treated animals (7 days) were placed in a Sylgard-lined dissecting dish, opened along the mesenteric border, and pinned tautly with the mucosa facing up. Tissue was fixed overnight in Zamboni's fixative at 4°C. Fixative was removed by three 10-min washes in PBS. Ileal segments were dissected to yield preparations of submucosal plexus. Preparations were incubated in the primary antisera for 48 h at 4°C. Following incubation in primary antiserum, immunoreactivity was detected following incubation of preparations for 2 h at room temperature in donkey anti-mouse IgG-CY3 (1:100; Biocan Scientific, Mississauga, ON, Canada) and anti-rabbit IgG-FITC (1:50; Biocan Scientific). Following washes in PBS, the preparations were mounted in bicarbonate-buffered glycerol at pH 8.6. The whole-mount preparations or preparations containing filled neurons were visualized with a Zeiss Axioplan fluorescence microscope (Zeiss Instruments, Toronto, ON, Canada) and photographed with a Sensys digital camera (Sensys, Photometrics, Tucson, AZ).
Statistics.
Statistical analyses and data plotting were carried out by use of Prism 3 (Graphpad Software, San Diego, CA). Differences between neurons from normal and inflamed animals were compared by using unpaired two-tailed Student's t-tests, a one-way ANOVA with a Dunnett post hoc test, or a two-way ANOVA with the Bonferroni posttest to compare values to controls. Linear regression analysis and two-way ANOVA were utilized to compare current-frequency relationships. A P value of <0.05 was considered statistically significant.
RESULTS
Macroscopic characterization of the ileum and colon after the induction of colitis.
As previously reported (39), guinea pigs treated with TNBS lost weight over the first 3–4 days, whereas control guinea pigs maintained or increased body weight (data not shown). At 3, 7, and 28 days following induction of inflammation significant macroscopic damage was observed in the colon compared with saline-treated controls. The macroscopic damage scores of the colon were as follows: control, 0.33 ± 0.03, n = 45; 3-day colitis, 7.0 ± 0.54, n = 34; 7-day colitis, 6.12 ± 0.43, n = 49; 28-day colitis, 2.37 ± 0.29, n = 20. At all time points following TNBS administration to the colon, the appearance and macroscopic damage score of the ileum from treated animals was indistinguishable from that of normal ileal tissue. Damage scores were as follows: control, 0.23 ± 0.1, n = 25; 3-day colitis, 0.23 ± 0.1, n = 17; 7-day colitis, 0.24 ± 0.1, n = 30; 28-day colitis, 0.23 ± 0.1, n = 12.
Short-circuit current measurements from the ileum of animals with colitis.
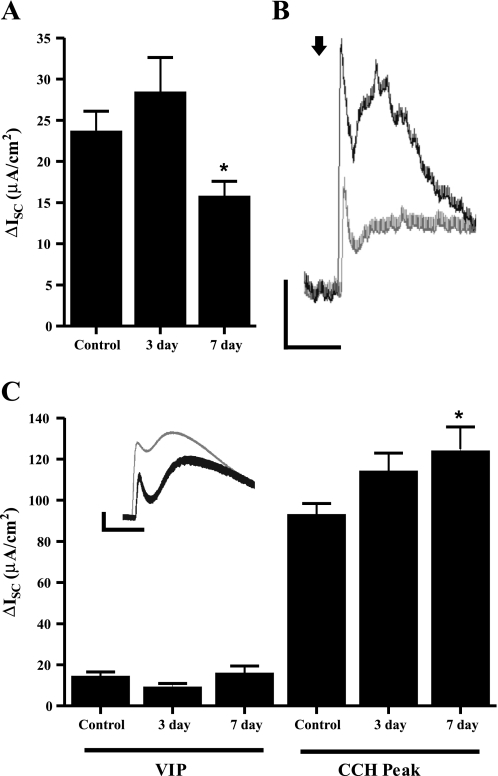
We investigated whether there were secretory abnormalities in the ileum of animals with colitis in the absence of any morphological alterations in the tissue. Preparations of mucosa-submucosa containing the submucosal plexus from the terminal ileum of inflamed animals were mounted in Ussing chambers to examine electrogenic ion transport. Baseline ISC and conductance values in the ileum of animals treated 7 days previously to induce colitis were similar to those of controls (Table 1). Baseline secretion was significantly reduced 3 days following TNBS treatment, without a change in conductance (Table 1). The maintenance of tissue resistance suggests that epithelial integrity was unaltered in the ileum of animals with colitis. The Na+ channel activator veratridine (10 μM) was applied to the serosal side of the tissue to elicit neurogenic secretion, measured as an increase in ISC, as previously described (26). Veratridine caused a rapid biphasic increase in the ISC that was abolished in the presence of TTX (data not shown). The total magnitude of the veratridine-stimulated response was unchanged in animals 7 days after TNBS treatment (control, 64.9 ± 11.3 μA/cm2, n = 9; 7-day TNBS, 60.0 ± 8.0 μA/cm2, n = 9, P > 0.05). To confirm that the relative contributions of cholinergic and noncholinergic components of neurally mediated responses were unchanged, veratridine stimulation was repeated in the presence of atropine (1 μM) to block the cholinergic component of the ISC. Atropine had no effect on baseline secretion from the ileum in any group of animals, but it significantly reduced the veratridine-stimulated response, confirming the inhibition of the cholinergic component of ISC evoked by veratridine. In the ileum of animals treated with TNBS 7 days previously, the magnitude of this reduction in secretion was significantly greater than that observed in controls (Fig. 1). This effect was not observed 3 days following TNBS treatment (Fig. 1).
Table 1.
Characteristics of ileal mucosa-submucosal preparations from control and TNBS-treated animals
| Baseline, μA/cm2 | Conductance, mS | |
|---|---|---|
| Control (n = 9) | 219.4±26.4 | 15.6±0.1 |
| 3-day TNBS (n = 6) | 135.7±26.2* | 13.8±1.2 |
| 7-day TNBS (n = 9) | 209.6±15.4 | 17.0±1.1 |
Data are presented as means ± SE. TNBS, trinitrobenzene sulfonic acid.
Significantly reduced vs. control, P < 0.05.
Fig. 1.
Effect on inflammation on neurally evoked secretion and changes to the epithelial responsiveness to secretagogues. A: noncholinergic secretion elicited by veratridine (10 μM) in the presence of atropine (1 μM) in tissue from control animals, and animals treated 3 and 7 days previously with trinitrobenzene sulfonic acid (TNBS). B: representative traces of veratridine response in control (solid) and 7-day inflamed animals (shaded) in the presence of atropine. Scale bars: 10 μA, 50 s. C: response to vasoactive intestinal peptide (VIP, 30 nM) and the fast peak response to carbachol (CCH, 1 μM) in the presence of tetrodotoxin (TTX, 300 nM). Inset: representative trace of change in short-circuit current (ΔISC) following CCH stimulation. Scale bars: 20 μA, 50 s. *P < 0.05.
The major noncholinergic neurotransmitter for mucosal secretion is VIP (31, 48). We hypothesized that the reduced atropine-resistant component of secretion in TNBS-treated animals was due to blunting of VIP signaling. To test this, we added VIP (30 nM) to the serosal side of our preparations in the presence of TTX (300 nM) to block neuronal transmission. VIP responses of the epithelium were virtually identical between TNBS-treated and untreated animals (Fig. 1), indicating that we were not observing a blunted epithelial responsiveness to VIP. Furthermore, the response to the adenylate cyclase activator forskolin (10 μM) was also unchanged between groups (control, ΔISC 32.6 ± 3.8 μA/cm2, n = 7; 3 days, ΔISC 40.7 ± 4.9 μA/cm2, n = 9; 7 days, ΔISC 31.3 ± 6.9 μA/cm2, n = 7, P > 0.05).
Carbachol elicits a biphasic response when added to the serosal chamber, a rapid transient peak, followed by a slowly developing sustained increase in ISC (48). The fast peak of carbachol-evoked secretion was significantly increased 7 days following TNBS treatment (Fig. 1). There was a trend for the slow component of carbachol secretion to be increased (control, ΔISC 120.8 ± 13.2 μA/cm2, n = 7; 3 days, ΔISC 166.3 ± 15.5 μA/cm2, n = 6; 7 days, ΔISC 150.8 ± 13.2 μA/cm2, n = 7, P > 0.05), but this did not reach statistical significance. The area under the curve was also not significantly changed following inflammation.
Quantification of VIP-immunoreactive neurons in the submucosal plexus.
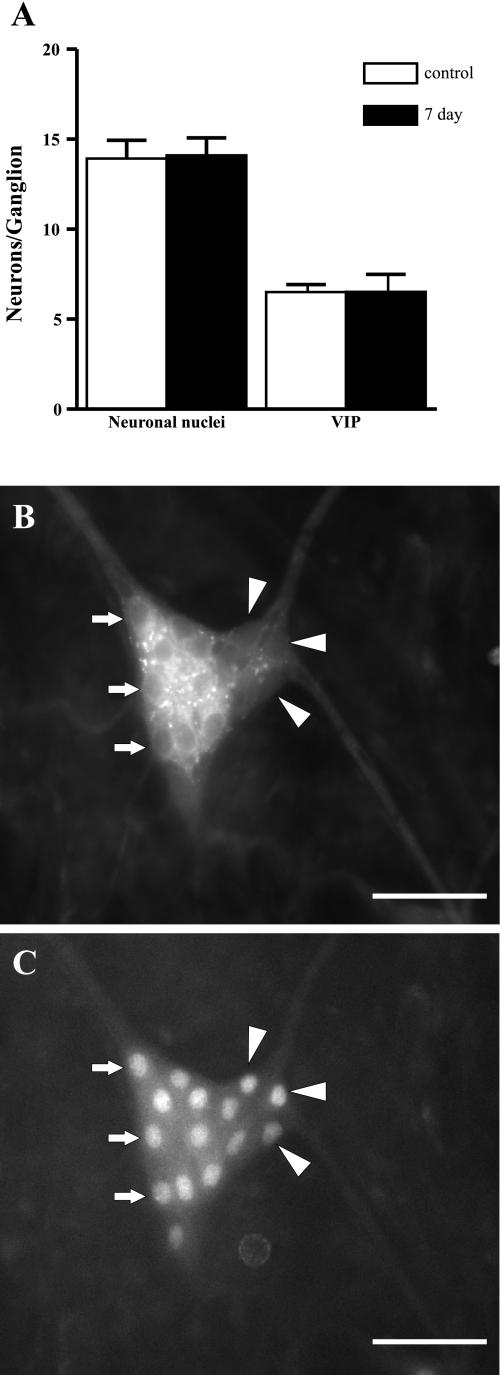
Another possible explanation for the reduced noncholinergic secretory response is that the number of VIP neurons in the submucosal plexus was reduced. Therefore, we counted the number of VIP-expressing neurons in the submucosal plexus of the ileum to address this possibility. At 7 days following TNBS treatment, the number of VIP-expressing neurons was unchanged (Fig. 2). Furthermore, the average number of neuronal nuclei was also unchanged in animals with colitis, indicating no generalized loss of ileal submucosal neurons.
Fig. 2.
Quantification of the proportion of VIP-expressing neurons in the submucosal plexus of the ileum. A: absolute numbers of neuronal nuclei per ganglion and proportion of VIP positive neurons are unchanged 7 days following TNBS administration. B: representative submucosal VIP staining from the ileum of an inflamed animal. C: neuronal nuclei in the same ganglion. Arrows indicate VIP-expressing neurons, and arrowheads indicate VIP-negative neurons. Scale bars: 50 μm.
Quantification of VIP release from the ileum of animals with colitis.
The RIA employed in this study was able to reliably detect VIP peptide released from the ileal mucosa-submucosa preparations of the guinea pig. However, VIP release between individual preparations was highly variable. There were no significant differences in baseline VIP release between control and TNBS-treated animals (control, 59.3 ± 4.9 pg·g wet wt−1·min−1, n = 5; TNBS, 49.6 ± 2.1 pg·g wet wt−1·min−1, n = 5, P > 0.05). Ethanol treatment (0.1% in Krebs) had an inhibitory effect on the rate of VIP release from the tissue that was similar in control and TNBS-treated animals (control, 23.8 ± 8.7% reduction; TNBS, 13.3 ± 9.5% reduction); in contrast, veratridine caused an increase in the rate of VIP release of 38.6 ± 17.4% for controls and 15.3 ± 15.4 for TNBS-treated animals. This trend of reduced VIP secretion from inflamed animals did not reach statistical significance (P > 0.05).
Neurophysiological characteristics of submucosal S neurons.
Having identified an abnormality involving the neural control of secretion, in the absence of a corresponding defect in epithelial responsiveness, we investigated the neurophysiological properties of the submucosal plexus. We focused primarily on enteric S neurons, since these have been established as the secretomotor neurons of the guinea pig ileum (18). In S neurons, input resistance, resting membrane potential, rheobase, and the number and width of action potentials were largely unchanged in TNBS-treated animals (Table 2). There was a trend (P = 0.06) toward a reduced rheobase for action potential generation in S neurons 3 days following TNBS treatment (Table 2). The maximum duration a neuron could fire action potentials was significantly prolonged 3 days after the induction of colitis, but it returned to the normal level by 7 days. The duration of firing was 215.7 ± 25.3 ms (n = 43) in controls, which increased to 334.9 ± 48.5 ms (n = 14) at 3 days following TNBS (P < 0.05) and fell to 216.4 ± 38.5 ms (n = 17) at 7 days and 309.2 ± 63.4 ms (n = 9) at 28 days after TNBS treatment.
Table 2.
Summary of basal and stimulated electrophysiological characteristics of S neurons
| Rin, mΩ | RMP, mV | Rheobase, pA | APs at Rheobase | APs at 2× Rheobase | AP50, ms | |
|---|---|---|---|---|---|---|
| Control (n = 42–47) | 240±17 | −50.3±1.5 | 91.9±11.7 | 1.9±0.4 | 5.5±0.9 | 1.2±0.1 |
| 3-day TNBS (n = 14) | 255±28 | −47.6±1.1 | 50.0±5.9 | 1.6±0.4 | 7.6±1.7 | 1.3±0.1 |
| 7-day TNBS (n = 18) | 336±58 | −48.6±1.7 | 73.6±12.0 | 2.7±0.8 | 5.3±1.0 | 1.3±0.1 |
| 28-day TNBS (n = 9) | 261±61 | −54.6±2.7 | 97.2±32.9 | 2.0±0.4 | 5.9±0.9 | 1.3±0.04 |
Data are presented as means ± SE. Data from S neurons of saline control animals and animals 3, 7, and 28 days following TNBS treatment. Rin, input resistance; RMP, resting membrane potential; rheobase, minimum current injection required to elicit an action potential; APs, action potentials; AP50, duration (width) of the AP at 50% amplitude.
When we examined the two populations of S-ChAT and S-VIP neurons separately, the basal electrical properties were comparable between the two groups with one exception. In S-VIP neurons, the number of actions potentials fired at rheobase was increased over that observed in controls compared with 7 days following TNBS treatment (control, 1.3 ± 0.2 action potentials, n = 23 vs. TNBS, 2.5 ± 0.6, n = 13; P < 0.05). This effect was not observed at earlier (3-day) and or later (28-day) time points (data not shown).
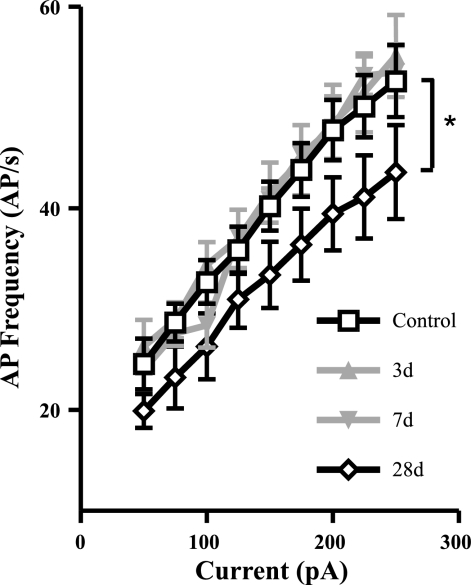
As noted above, colitis caused changes in the S neurons in the ileum. For the most part none of these persisted as colitis resolved (28 days). However, at the 28-day time point, a reduction in excitability, as determined by the reduction in the relationship between current injection and action potential generation, was detected (Fig. 3).
Fig. 3.
Changes to the action potential (AP) firing characteristics in S neurons following inflammation. A: current frequency (I-F) relationship of S neurons 3, 7, and 28 days (3d, 7d, and 28d, respectively) following TNBS administration compared with control values. The slope of the I-F relationship was unchanged from controls at 3 and 7 days following TNBS, but it was significantly reduced at 28 days. *P < 0.05 linear regression analysis.
Changes in synaptic inputs to S neurons of the ileum in animals with colitis.
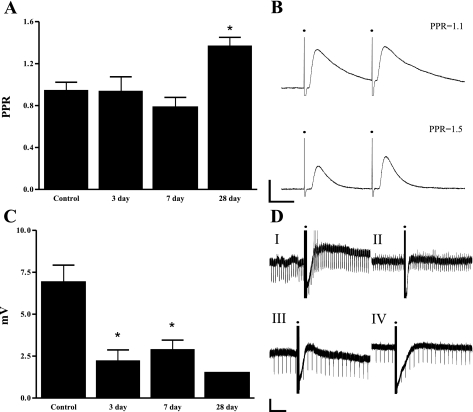
S neurons receive both fast and slow synaptic inputs (18). All S neurons retained the capacity to receive fast EPSPs after inflammation. The EPSP amplitudes of the pooled population were 19.1 ± 2.8 mV, n = 8 at 3 days, 18.7 ± 2.7 mV, n = 21 at 7 days, and 16.8 ± 4.8 mV, n = 5 at 28 days following TNBS. These were not different from controls (21.4 ± 1.7 mV, n = 34). After administration of hexamethonium, responses were typically abolished in preparations from control and inflamed animals. A subpopulation (control, 41.2%; 3 days, 37.5%; 7 days, 66.7%, 28 days, 40%) of neurons remained which received small hexamethonium-resistant EPSPs. Neither the size of this subpopulation nor the size of the hexamethonium-resistant EPSP differed significantly between tissues from control and TNBS-treated animals (amplitude of hexamethonium resistant EPSP: control, 9.3 ± 1.6 mV, n = 14; 3 days, 7.7 ± 2.9 mV, n = 3; 7 days, 5.6 ± 1.1 mV, n = 13; 28 days, 14.9 ± 11.2 mV, n = 2; P > 0.05). Thus the changes in the neurochemical properties of fast synaptic transmission seen the colon in colitis and in ileitis were not observed in the present study (38, 39, 45). We utilized the paired-pulse protocol described above, to examine vesicular handling in presynaptic neurons of the submucosal plexus. No change in the PPR was detected 3 or 7 days following TNBS treatment (Fig. 4). However, when we examined the dynamics of neurotransmission at the later time point (28 days following the induction of colitis), the PPR was significantly increased compared with control animals (Fig. 4). The amplitude of the two EPSPs are nearly equal at control synapses (PPR = 0.93 ± 0.1, n = 5). At 28 days, a paired-pulse facilitation was detected (1.37 ± 0.08, n = 3; P < 0.05).
Fig. 4.
Changes to synaptic transmission received by S neurons of the submucosal plexus. A: change in the paired-pulse ratio (PPR) in controls and inflamed animals. B: representative PPR recording from a control neuron (top), and a neuron from a 28-day TNBS-treated animal (bottom). Scale bars: 5 mV, 20 ms. C: magnitude of the slow excitatory postsynaptic action potential (EPSP) received by VIP-expressing S neurons (S-VIP neurons) from control and inflamed animals. D: representative traces of reduced slow EPSP magnitude from S-VIP neurons. The recording demonstrates the prominent inhibitory postsynaptic action potentials received by these neurons. Large long-lasting slow EPSPs are received by control neurons (I), which occasionally reach AP threshold (spikes on trace). Downward deflections on traces reflect current injections used to monitor input resistance during the EPSP. Slow EPSP magnitude is reduced at 3 (II), 7 (III), and 28 (IV) days. Scale bars: 10 mV, 5 s. Solid circles in B and D indicate the stimulus artifact. *P < 0.05.
In contrast to the modest changes observed in fast synaptic transmission, slow synaptic transmission to S neurons was altered as early as 3 days after the induction of inflammation, and this persisted to 28 days after the induction of colitis. Data from all S neurons indicated a reduction in the evoked slow EPSP. When data from S-VIP neurons were analyzed separately this reduction was significant at the 7-day time point (Fig. 4). In contrast, there were no changes in the magnitude of the slow EPSP in S-ChAT neurons (data not shown). In the S-VIP cells, the slow EPSP was reduced from a control amplitude of 6.6 ± 1.2 mV, n = 14, to 2.4 ± 0.9 mV, n = 3 at 3 days and 3.05 ± 0.5 mV, n = 4, at 7 days following TNBS (P < 0.05 for both groups). Interestingly, there was a trend for a long-term reduction of slow EPSP amplitudes (28 days, 1.5 mV ± 0, n = 2). The magnitude of the slow IPSP, which is a characteristic property of these neurons, was unchanged at all time points (control, −23.8 ± 1.8 mV, n = 23; 3 days, −28.1 ± 0.8 mV, n = 5; 7 days, −27.2 ± 2.5 mV, n = 11; 28 days, −31.6 ± 1.4 mV, n = 2).
Changes to AH neurons of the ileum in animals with colitis.
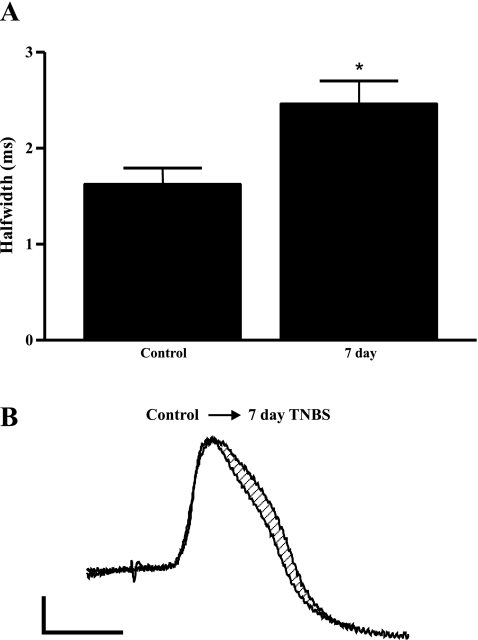
AH neurons in the submucosal plexus respond to luminal stimuli and in turn initiate secretory and vasodilatory responses from submucosal S neurons. They provide a major source of synaptic inputs to S neurons, which are the effector neurons in this pathway (19). We studied the electrical properties of AH neurons to test the hypothesis that alterations to AH neurons underlie changes to synaptic transmission. Similar to S neurons, basal characteristics of AH neurons were unchanged following TNBS colitis (Table 3). This is in contrast to studies of AH neurons within the TNBS-inflamed colon, which consistently demonstrate increased excitability of AH neurons during and following inflammation (30, 33, 34, 37–39). Examination of the shape of the action potential in AH neurons demonstrated an increased half-width in the 7-day TNBS treatment group (control, 1.63 ± 0.16 ms, n = 7; 7-day TNBS, 2.47 ± 0.23 ms, n = 5; Fig. 5). The area under the curve of the AHP, which follows an intracellular generated action potential in these neurons, was unchanged from −11,850 ± 2,644 mV·ms, n = 5 in controls and −12,320 ± 4,012 mV·ms, n = 6, P > 0.05, 7 days following TNBS treatment.
Table 3.
Summary of basal characteristics of AH neurons
| RMP, mV | Rheobase, pA | APs at Rheobase | |
|---|---|---|---|
| Control (n = 5) | −49.1±6.0 | 141.7±51.4 | 1.6±0.4 |
| 7-day TNBS (n = 6) | −50.9±3.0 | 198.7±52.5 | 1±0 |
Data from AH neurons from control animals and animals 7 days following TNBS treatment (TNBS). RMP, resting membrane potential; rheobase, minimum current injection required to elicit an action potential; APs at rheobase, number of action potentials generated at rheobase.
Fig. 5.
Changes to the action potential half-width in neurons that display a long afterhyperpolarization (AH neurons) following inflammation. A: duration of half-width in control neurons and neurons from animals at 7 following administration of TNBS. B: representative traces of AH action potentials from control and from 7-day TNBS-inflamed animals. Scale bars: 20 mV, 2 ms. *P < 0.05.
DISCUSSION
This study examined the function of the ileum of animals with colitis to determine whether the adaptations observed in the secretomotor neural circuitry at the site of inflammation, and at sites distal to inflammation, were also observed at proximal sites. We identified secretory abnormalities in the ileum of guinea pigs with colitis that appear to involve alterations in the physiology of synaptic transmission in the submucosal plexus. We also detected changes to the electrical properties of enteric neurons that may account for the changes in synaptic behavior in the ENS. The changes we observed in the ileum in animals with colitis differ considerably from those previously observed in the submucosal plexus of the colon in animals with colitis and ileitis (36–38, 45).
Neural control of secretion is altered in the ileum of animals with colitis.
Neurogenic secretion is regulated by cholinergic and noncholinergic nerves (12). In the guinea pig ileum, ∼50% of the neurons of the submucosal plexus are cholinergic, comprising two populations: secretomotor neurons (∼45% of total neurons) that project to the epithelium and a smaller population of AH (intrinsic primary afferent) neurons (∼10%) that activate secretion by local release of their transmitters (13, 17, 32). Noncholinergic secretomotor neurons express VIP and form a major population (∼45%) of the total submucosal neurons. In the guinea pig ileum, noncholinergic secretion accounts for 45–50% of the total secretion elicited by electrical field stimulation (11).
The alkaloid veratridine was utilized to depolarize enteric submucosal neurons and elicit neurogenic secretion. Veratridine has been shown to stimulate the release of VIP (5) and acetylcholine (60) and has been used previously for this purpose (39). The magnitude of the total secretory response recorded in the present study was similar to that observed by Cooke (11). Seven days following TNBS colitis, total neurogenic secretion evoked by veratridine was unchanged from controls. This was surprising in the light of previous observations in which secretion was consistently reduced in animals with colitis (1, 6, 45, 61). Furthermore, in a study of the jejunum of animals with ileitis, secretion evoked by capsaicin and carbachol were also attenuated (41). Baseline secretion was reduced 3 days following TNBS treatment, but all other evoked responses were the same in tissues from these animals. We infer from these observations that when inflammation is distal and sufficiently distant, the induction of inflammatory mediators that are thought to be responsible for these distant effects (41, 45, 61) are either not produced or insufficient to give rise to significant changes.
Despite not seeing changes in the total evoked response, we studied the relative contribution of cholinergic and noncholinergic components of secretion. Under control conditions we observed that ∼60% of the total response was cholinergic, which is similar to that observed by Cooke (11). In contrast, in animals 7 days following colitis, the cholinergic fraction was increased by ∼10%. There are a number of possible explanations for these data. We examined the effect of carbachol and VIP, as well as forskolin, which directly activates epithelial cells. In the presence of TTX to block neurogenic secretion, exogenous VIP as well as forskolin generated similar increases in ISC between control and tissue from inflamed animals. These findings suggest that the epithelium itself is intact and that the colitis was not altering epithelial responsiveness. Interestingly, carbachol added to the serosal chamber elicited a greater increase in ISC in the presence of TTX, 7 days following inflammation. These data suggest that there is either some degree of upregulation of the secretory capacity of the epithelium to a stimulus that activates intracellular calcium (12, 23) or that there is enhanced expression of cholinergic receptors on the epithelium. Muscarinic M3 receptors are the major subtype of epithelial cell cholinergic receptor mediating secretion (25). Currently it is not clear whether there is altered muscarinic receptor expression in the epithelium in colitis, but cholinergic signaling is altered at the site of inflammation (53). This effect requires a certain duration of inflammation to develop since it was not present at 3 days following TNBS treatment. Importantly, the total secretion was not changed for carbachol, indicating a specific effect of inflammation on the fast component of the phasic carbachol response. Further studies are required to elucidate the mechanism behind the potentiated response of the epithelium to cholinergic stimulation.
Our observation of unchanged responsiveness of the ileal epithelium to exogenous VIP suggested that the reduced noncholinergic secretion following TNBS colitis does not originate from an epithelial defect. Thus it remains most likely that this arises from a defect of VIP release or a reduced population of VIP neurons. We examined the latter and found no reduction in the proportion of VIP neurons in the submucosal plexus after TNBS treatment. The VIP release paradigm employed here demonstrated a trend toward a reduced VIP release in the presence of veratridine. However, this did not reach significance, probably reflecting the fact that we are only indirectly measuring VIP release; what we measure is the spillover into the medium bathing the tissue.
Neurophysiological properties of the submucosal plexus are altered in the ileum of animals with colitis.
We found that the basal characteristics of S neurons from the submucosal plexus of the ileum are largely unchanged following colitis and are in agreement with the basal properties demonstrated by others in the submucosal plexus of the ileum and colon (38, 45). S neurons from the submucosal plexus of the ileum demonstrated a subtly altered electrophysiology during colitis. When data from all S neurons were pooled, we found that neurons fired action potentials for a greater maximum duration 3 days after TNBS treatment. One possible mechanism for this is a change to the inactivation kinetics of the voltage-gated Na+ channels (56), since acutely following inflammation the neurons must be depolarized for a longer time to enter a refractory state. The impact of a longer train of action potentials in secretomotor S neurons would be a greater influx of Ca2+ into the presynaptic bouton and potentiated neurotransmitter release. Enteric S neurons of the ileal submucosal plexus express Nav1.2 and Nav1.3 α-subunits of voltage gated sodium channels (4). TNBS colitis (7) and inflammatory mediators such has prostaglandin E2 (52) can modulate voltage-gated sodium currents in the dorsal root ganglion. The possibility that TTX-sensitive voltage-gated sodium channels expression and kinetics may be altered in S neurons of the submucosal plexus following colitis warrants further investigation.
Excitatory slow synaptic transmission is reduced in the submucosal plexus of the ileum following colitis.
S neurons of the submucosal plexus receive fast and slow synaptic inputs from AH neurons and extrinsic nerve endings in the plexus (9, 35, 49). Alterations to the synapses on S neurons are expected to adversely affect gut function by either leading to excessive or reduced activation of these cells. When we examined synaptic transmission to S neurons we saw no change in fast EPSPs received by the neurons following TNBS treatment, which were comparable in amplitude to control preparations in other studies (38). This is in contrast to previous work, which demonstrated an increased amplitude and neurochemical composition of fast EPSPs following TNBS colitis (38, 39). The proportion of cholinergic and noncholinergic fast EPSPs were also unchanged following colitis. We observed a slightly higher percentage of hexamethonium-resistant EPSPs than has been seen by others in the submucosal plexus of the ileum (∼27%; Ref. 42). We conclude that fast EPSPs are not affected by inflammation in the submucosal plexus of the ileum following colitis.
Recordings made at the site of colitis revealed that slow EPSPs were unchanged in submucosal S neurons (38). In the ileum of animals with colitis, slow EPSPs in S-VIP neurons were reduced in magnitude, whereas those in S-ChAT neurons remained unchanged. We have not yet determined whether this effect involves pre- and/or postsynaptic mechanisms. However, from previous studies in the submucosal plexus of the colon, this suppressed excitability is not expected to originate from a desensitization of the postsynaptic neuron (38). In addition, it is not expected that changes in synaptic transmission arise in alterations to the connectivity between neurons in the plexus. Using electron microscopy, Krauter et al. (29) did not detect changes in synaptic connections within the myenteric plexus at the site of inflammation. However, the results of Nurgali et al. (44) could be interpreted to indicate that AH neurons lose their Dogiel type II morphology and become uniaxonal. This would suggest a loss of some synaptic connections from AH neurons occurring in the myenteric plexus of the ileum following ileitis. However, we did not observe Dogiel type I neurons with the characteristics of AH neurons in the submucosal plexus of the ileum following colitis. Further supporting a lack of alteration to the network of the submucosal plexus of the ileum was the observation that there the number of VIP neurons in the submucosal plexus remained unchanged following colitis, nor was the absolute number of neurons per submucosal ganglion reduced. We therefore consider that presynaptic alterations may be underlying the changes we have observed. Interestingly, S-VIP neurons, in the face of reduced slow excitation, fired more action potentials when depolarized to rheobase. This is consistent with an adaptation to the loss of synaptic excitation. Taken together, these findings suggest that there are alterations in the presynaptic transmitter expression or release in the ileal submucosal plexus and we intend to investigate this further.
Inflammation induced alterations to the currents underlying the action potential in ileal submucosal AH neurons following colitis.
Inflammation profoundly affects the AH neurons of the ENS at the site of inflammation in the case of colitis and at sites distal to inflammation such as the colon following ileitis (33, 34, 38, 39). The basal characteristics of AH neurons from the submucosal plexus of the ileum were largely unchanged following colitis and are in agreement with the basal electrophysiological properties demonstrated by others (38). AH neurons from the submucosal plexus of the ileum did not display the hyperexcitability seen in the colon (30, 34, 38, 39, 45) or of the myenteric plexus of the ileum following ileitis (44). Action potential firing threshold and frequency were unchanged, and size and duration of the AHP were comparable in saline-treated and TNBS-treated animals. However, AH neurons manifested a significant increase in the half-width of the action potential. This indicates a perturbation to the underlying currents that generate the action potential. A lack of change to the magnitude of the fast AHP indicates that the K+ currents were largely unchanged, whereas the maintenance of the AHP suggests that Ca2+ currents and mobilization of Ca2+ from intracellular stores were also similar. A greater accumulation of Ca2+ would generate a larger AHP and vice versa (19). Hence it is likely that Na+ currents were altered in these cells either in terms of absolute channel number or altered kinetics or subunit expression. Indeed, changes to dorsal root ganglion Na+ channel subunit composition and currents have been reported in response to TNBS colitis (7), suggesting that a similar phenomenon may occur in sensory neurons of the submucosal plexus. Regardless of the cause, the broadened action potential may have important physiological consequences. In the central nervous system, action potential broadening is an important form of short-term plasticity (24). If this were to occur in the GI tract, this may lead to increased local releases of transmitters, including acetylcholine. Another possible consequence is an amplification of signaling in the networks of AH neurons, leading to heightened signaling between these neurons.
Persistence of changes in the submucosal plexus of the ileum following colitis.
Inflammation-induced alterations to the ENS persist following the resolution of inflammation (30, 39). To examine the possible persistence of changes in the ileal submucosal plexus we examined enteric neurons 28 days following TNBS treatment. At this time point macroscopic damage to the colon has substantially resolved. The secretory abnormalities seen in the jejunum of animals with ileitis were resolved at this time point (41). However, others have shown that secretion and changes to enteric neurons remained altered at 56 days after TNBS (30, 39). At 28 days, basal electrophysiological characteristics were still largely unchanged from control and from earlier time points of inflammation. These are similar to results found in the submucosal plexus of the colon 56 days following colitis (39). However, at 28 days there was a subtle alteration from controls. The current-frequency relationship showed a depression in the firing frequency to a given depolarizing stimulus. The consequences of this change in the submucosal plexus would be expected to be a decrease in synaptic release to a prolonged depolarization. Depression of neuronal excitability in the ENS following inflammation has not been demonstrated previously. All previous findings indicate that the only alteration to S neurons is facilitation and/or a change in neurochemical coding of synaptic transmission (29, 30, 38, 39, 45).
Studying the PPR revealed that long-term changes also occur to the presynaptic neurons within the submucosal plexus. Submucosal S neurons from control animals and in animals treated with TNBS 3 and 7 previously demonstrated a slight PPR depression. PPR depression is also the normal situation in myenteric neurons from the ileum (50) and colon (29). However, 28 days following TNBS treatment, PPR facilitation in S neurons was observed. Similar results have been shown in the myenteric plexus of the colon 7 days following TNBS colitis (29). This is an intriguing finding because it suggests that vesicle handling and release have been altered in presynaptic neurons, most likely the AH neurons of the ileal submucosal plexus. The argument has been made that this could reflect an increase in vesicle recycling (29), and indeed that may be the case in the submucosal plexus of the ileum following colitis; however, a change in the Ca2+ sensitivity of the vesicular release machinery cannot be excluded.
Summary and perspective.
We demonstrated the impact of colitis on the submucosal plexus of the ileum. The effects that we observed are clearly different from the local inflammation-induced changes that occur in the ENS of the colon during colitis (29, 30, 34, 36, 38, 39), as well as during ileitis (45). Inflammation of the colon or of the ileum leads to characteristic increases in excitability of neurons, as well as alterations to synaptic transmission in both the submucosal and myenteric plexuses. However, in the submucosal plexus of the ileum following colitis, hyperexcitability is largely absent, whereas slow synaptic transmission is profoundly altered.
The pathophysiological consequences of alterations to the submucosal plexus of the ileum may be a reduction of nerve-mediated ileal secretion. Secretion plays an important role in the maintenance of epithelial barrier function. A compromised epithelial barrier will potentially permit the migration of pathogenic bacteria across the epithelium, and this in turn could lead to further inflammation. A reduced epithelial barrier may be one mechanism behind the relapsing nature of Crohn's disease and ulcerative colitis. Our data indicates that inflammation in a distal region of the gut will have wide-ranging consequences on the ENS and gut function. Future studies will address the mechanisms that propagate inflammatory changes throughout the large and small bowel.
GRANTS
This work was supported by grants from the Crohn's and Colitis Foundation of Canada (K. A. Sharkey, G. M. Mawe) and National Institutes of Health (DK-62267, G. M. Mawe). K. A. Sharkey is an Alberta Heritage Foundation for Medical Research Medical Scientist and the Crohn's and Colitis Foundation of Canada Chair in IBD Research at the University of Calgary.
Acknowledgments
We thank Aparna Ramchandran for assistance with some of the Ussing chamber studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Asfaha S, Bell CJ, Wallace JL, MacNaughton WK. Prolonged colonic epithelial hyporesponsiveness after colitis: role of inducible nitric oxide synthase. Am J Physiol Gastrointest Liver Physiol 276: G703–G710, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Aube AC, Cherbut C, Barbier M, Xing JH, Roze C, Galmiche JP. Altered myoelectrical activity in noninflamed ileum of rats with colitis induced by trinitrobenzene sulphonic acid. Neurogastroenterol Motil 11: 55–62, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62: 535–572, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bartoo AC, Sprunger LK, Schneider DA. Expression and distribution of TTX-sensitive sodium channel alpha subunits in the enteric nervous system. J Comp Neurol 486: 117–131, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Belai A, Burnstock G. Release of calcitonin gene-related peptide from rat enteric nerves is Ca2+-dependent but is not induced by K+ depolarization. Regul Pept 23: 227–235, 1988. [DOI] [PubMed] [Google Scholar]
- 6.Bell CJ, Gall DG, Wallace JL. Disruption of colonic electrolyte transport in experimental colitis. Am J Physiol Gastrointest Liver Physiol 268: G622–G630, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol 287: G845–G855, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Bornstein JC, Costa M, Furness JB. Synaptic inputs to immunohistochemically identified neurones in the submucous plexus of the guinea-pig small intestine. J Physiol 381: 465–482, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornstein JC, Costa M, Furness JB. Intrinsic and extrinsic inhibitory synaptic inputs to submucous neurones of the guinea-pig small intestine. J Physiol 398: 371–390, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins SM The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology 111: 1683–1699, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Cooke HJ Influence of enteric cholinergic neurons on mucosal transport in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 246: G263–G267, 1984. [DOI] [PubMed] [Google Scholar]
- 12.Cooke HJ Role of the “little brain” in the gut in water and electrolyte homeostasis. FASEB J 3: 127–138, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Cooke HJ, Sidhu M, Fox P, Wang YZ, Zimmermann EM. Substance P as a mediator of colonic secretory reflexes. Am J Physiol Gastrointest Liver Physiol 272: G238–G245, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol 98: 1578–1583, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Frieling T, Wood JD, Cooke HJ. Submucosal reflexes: distension-evoked ion transport in the guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 263: G91–G96, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Furness JB, Clerc N, Stebbing MJ, Vogalis F. The enteric nervous system and its extrinsic connections. In: Textbook of Gastroenterology, edited by Yamada T. Philadelphia: Lippincott Williams & Wilkins, 2003, p. 12–34.
- 17.Furness JB Types of neurons in the enteric nervous system. J Auton Nerv Syst 81: 87–96, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Furness JB, Alex G, Clark MJ, Lal VV. Morphologies and projections of defined classes of neurons in the submucosa of the guinea-pig small intestine. Anat Rec A Discov Mol Cell Evol Biol 272: 475–483, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol 72: 143–164, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Giaroni C, De Ponti F, Cosentino M, Lecchini S, Frigo G. Plasticity in the enteric nervous system. Gastroenterology 117: 1438–1458, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Grider JR, Jin JG. Vasoactive intestinal peptide release and l-citrulline production from isolated ganglia of the myenteric plexus: evidence for regulation of vasoactive intestinal peptide release by nitric oxide. Neuroscience 54: 521–526, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Grider JR, Makhlouf GM. Prejunctional inhibition of vasoactive intestinal peptide release. Am J Physiol Gastrointest Liver Physiol 253: G7–G12, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Hardcastle J, Hardcastle PT, Noble JM. The involvement of calcium in the intestinal response to secretagogues in the rat. J Physiol 355: 465–478, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature 371: 513–515, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Hirota CL, McKay DM. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol 149: 463–479, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyland NP, Cox HM. The regulation of veratridine-stimulated electrogenic ion transport in mouse colon by neuropeptide Y (NPY), Y1 and Y2 receptors. Br J Pharmacol 146: 712–722, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isgar B, Harman M, Kaye MD, Whorwell PJ. Symptoms of irritable bowel syndrome in ulcerative colitis in remission. Gut 24: 190–192, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson K, McHugh K, Collins SM. Experimental colitis alters myenteric nerve function at inflamed and noninflamed sites in the rat. Gastroenterology 109: 718–722, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Krauter EM, Linden DR, Sharkey KA, Mawe GM. Synaptic plasticity in myenteric neurons of the guinea-pig distal colon: presynaptic mechanisms of inflammation-induced synaptic facilitation. J Physiol 581: 787–800, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krauter EM, Strong DS, Brooks EM, Linden DR, Sharkey KA, Mawe GM. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil 19: 990–1000, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Kuwahara A, Radowicz-Cooke HJ. Epithelial transport in guinea-pig proximal colon: influence of enteric neurones. J Physiol 395: 271–284, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li ZS, Furness JB. Immunohistochemical localisation of cholinergic markers in putative intrinsic primary afferent neurons of the guinea-pig small intestine. Cell Tissue Res 294: 35–43, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Linden DR, Sharkey KA, Ho W, Mawe GM. Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J Physiol 557: 191–205, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol 547: 589–601, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lomax AE, Bertrand PP, Furness JB. Electrophysiological characteristics distinguish three classes of neuron in submucosal ganglia of the guinea-pig distal colon. Neuroscience 103: 245–255, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Lomax AE, Fernandez E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil 17: 4–15, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Lomax AE, Linden DR, Mawe GM, Sharkey KA. Effects of gastrointestinal inflammation on enteroendocrine cells and enteric neural reflex circuits. Auton Neurosci 126–127: 250–257, 2006. [DOI] [PubMed]
- 38.Lomax AE, Mawe GM, Sharkey KA. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J Physiol 564: 863–875, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lomax AE, O'Hara JR, Hyland NP, Mawe GM, Sharkey KA. Persistent alterations to enteric neural signaling in the guinea pig colon following the resolution of colitis. Am J Physiol Gastrointest Liver Physiol 292: G482–G491, 2007. [DOI] [PubMed] [Google Scholar]
- 40.McCafferty DM, Wallace JL, Sharkey KA. Effects of chemical sympathectomy and sensory nerve ablation on experimental colitis in the rat. Am J Physiol Gastrointest Liver Physiol 272: G272–G280, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Miceli P, Morris GP, MacNaughton WK, Vanner S. Alterations in capsaicin-evoked electrolyte transport during the evolution of guinea pig TNBS ileitis. Am J Physiol Gastrointest Liver Physiol 282: G972–G980, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Monro RL, Bertrand PP, Bornstein JC. ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J Physiol 556: 571–584, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol 285: G1028–G1036, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Nurgali K, Nguyen TV, Matsuyama H, Thacker M, Robbins HL, Furness JB. Phenotypic changes of morphologically identified guinea-pig myenteric neurons following intestinal inflammation. J Physiol 583: 593–609, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Hara JR, Lomax AE, Mawe GM, Sharkey KA. Ileitis alters neuronal and enteroendocrine signalling in guinea pig distal colon. Gut 56: 186–194, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parr EJ, Sharkey KA. The use of constitutive nuclear oncoproteins to count neurons in the enteric nervous system of the guinea pig. Cell Tissue Res 277: 325–331, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Rao SS, Read NW, Brown C, Bruce C, Holdsworth CD. Studies on the mechanism of bowel disturbance in ulcerative colitis. Gastroenterology 93: 934–940, 1987. [DOI] [PubMed] [Google Scholar]
- 48.Reddix R, Kuhawara A, Wallace L, Cooke HJ. Vasoactive intestinal polypeptide: a transmitter in submucous neurons mediating secretion in guinea pig distal colon. J Pharmacol Exp Ther 269: 1124–1129, 1994. [PubMed] [Google Scholar]
- 49.Reed DE, Vanner SJ. Converging and diverging cholinergic inputs from submucosal neurons amplify activity of secretomotor neurons in guinea-pig ileal submucosa. Neuroscience 107: 685–696, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Ren J, Galligan JJ. Dynamics of fast synaptic excitation during trains of stimulation in myenteric neurons of guinea-pig ileum. Auton Neurosci 117: 67–78, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritchie JA, Salem SN. Upper intestinal motility in ulcerative colitis, idiopathic steatorrhoea, and the irritable colon syndrome. Gut 6: 325–327, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rush AM, Waxman SG. PGE2 increases the tetrodotoxin-resistant Nav1.9 sodium current in mouse DRG neurons via G-proteins. Brain Res 1023: 264–271, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Sayer B, Lu J, Green C, Soderholm JD, Akhtar M, McKay DM. Dextran sodium sulphate-induced colitis perturbs muscarinic cholinergic control of colonic epithelial ion transport. Br J Pharmacol 135: 1794–1800, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharkey KA, Kroese AB. Consequences of intestinal inflammation on the enteric nervous system: neuronal activation induced by inflammatory mediators. Anat Rec 262: 79–90, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47: 804–811, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulbricht W Sodium channel inactivation: molecular determinants and modulation. Physiol Rev 85: 1271–1301, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Vanner S, Surprenant A. Cholinergic and noncholinergic submucosal neurons dilate arterioles in guinea pig colon. Am J Physiol Gastrointest Liver Physiol 261: G136–G144, 1991. [DOI] [PubMed] [Google Scholar]
- 58.Weber E, Neunlist M, Schemann M, Frieling T. Neural components of distension-evoked secretory responses in the guinea-pig distal colon. J Physiol 536: 741–751, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood JD Application of classification schemes to the enteric nervous system. J Auton Nerv Syst 48: 17–29, 1994. [DOI] [PubMed] [Google Scholar]
- 60.Yau WM, Dorsett JA, Youther ML. Calcium-dependent stimulation of acetylcholine release by substance P and vasoactive intestinal polypeptide. Eur J Pharmacol 120: 241–243, 1986. [DOI] [PubMed] [Google Scholar]
- 61.Zamuner SR, Warrier N, Buret AG, MacNaughton WK, Wallace JL. Cyclooxygenase 2 mediates post-inflammatory colonic secretory and barrier dysfunction. Gut 52: 1714–1720, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]