Abstract
Expression of spermine/spermidine-N1-acetyltransferase (SSAT), the rate-limiting enzyme of polyamine backconversion cascade, increases after ischemia-reperfusion injuries (IRI). We hypothesized that SSAT plays an important role in the mediation of IRI. To test our hypothesis, wild-type (SSAT-wt) and SSAT-deficient (SSAT-ko) mice were subjected to liver or kidney IRI by ligation of hepatic or renal arteries. The liver and kidney content of putrescine (Put), a downstream by-product of SSAT activity, increased in SSAT-wt animals but not in SSAT-ko animals after IRI, indicating that polyamine backconversion is not functional in SSAT-deficient mice. When subjected to hepatic IRI, SSAT-ko mice were significantly protected against liver damage compared with SSAT-wt mice. Similarly, SSAT-ko animals subjected to renal IRI showed significantly greater protection against damage to kidney tubules than SSAT-wt mice. These studies indicate that SSAT-deficient animals are protected against IRI and suggest that SSAT is an important mediator of the tissue damage in IRI.
Keywords: polyamine catabolism, polyamine backconversion, acute liver failure, acute kidney injury
ischemia-reperfusion injury (IRI) is a complication of a number of clinical conditions, including stroke, myocardial infarction, acute kidney injury and acute liver failure and is a major cause of morbidity and mortality. The ischemic phase of the injury (i.e., disruption of blood supply to the affected organ) leads to ATP depletion and accumulation of metabolites leading to cellular acidosis as a result of switching from aerobic to anaerobic metabolism, whereas reperfusion (i.e., reestablishment of blood flow to the affected organ) causes the production of reactive oxygen intermediates (6, 44). The latter molecules contribute to oxidative tissue damage in IRI (6, 44).
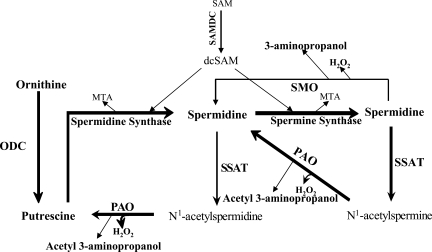
We have identified an apparent linkage between IRI and polyamine backconversion (4, 38). Our previous studies indicate that the expression of SSAT and to a lesser extent spermine oxidase (SMO) increase in the kidney and liver after IRI (4, 38). Polyamines are aliphatic cations that are important in interactions between nucleic acids and between nucleic acids and proteins, as well as between proteins (12, 16, 19, 27). These molecules are critical in the regulation of DNA and chromatin structure, gene transcription, and signal transduction (12, 16, 19, 27). Because of their physiological significance the metabolism of polyamines is tightly regulated. The synthetic arm of polyamine pathway consists of decarboxylation of ornithine by ornithine decarboxylase (ODC) to produce putrescine (Put) followed by sequential addition of aminopropyl groups to Put and spermidine (Spd) to form Spd and spermine (Spm), respectively (Fig. 1). Polyamine catabolism, on the other hand, is mediated by the backconversion pathway in which Spm or Spd are first acetylated by SSAT and then oxidized by polyamine oxidase (PAO) to yield Spd or Put, respectively, along with stoichiometric amounts of hydrogen peroxide (H2O2) and acetyl-3-aminopropanal. Alternatively, Spm can be directly converted to Spd via the spermine oxidase (SMO) reaction, which also generates H2O2 and 3-aminopropanal.
Fig. 1.
Schematic diagram depicting the polyamine metabolic pathway. Spermidine (Spd) and spermine (Spm) are synthesized by the sequential addition of aminopropyl groups derived from decarboxylated S-adenosylmethionine (dcSAM) onto putrescine (Put) and Spd to form Spd and Spm, respectively. Catabolism of polyamines proceeds via the backconversion pathway or via the 1-step Spm oxidation by spermine oxidase (SMO). In the spermidine/spermine N-1-acetyltransferase (SSAT)/polyamine oxidase (PAO)-mediated polyamine backconversion pathway, Spm and Spd are first acetylated by SSAT, the rate-limiting enzyme of the pathway, and then oxidized by PAO, leading to Put and cytotoxic by-products (i.e., H2O2, acetyl-3-aminopropionaldehyde). SAM, S-adenosylmethionine; SAMDC, S-adenosylmethionine decarboxylase; MTA, 5-methylthioadenosine; ODC, ornithine decarboxylase.
As a result of changes in polyamine metabolism, tissue polyamine levels are altered after cerebral, myocardial, kidney, and liver IRI (5, 11, 13, 15, 18, 31). During liver IRI, hepatic expression of SSAT and SMO increases causing the Spm and Spd levels to decrease and the backconversion product, Put, to increase dramatically (4). Similarly, the expression of SSAT and SMO also increases in the kidneys subjected to IRI (38). This leads to a significant buildup of Put in the injured kidneys (38).
SSAT is encoded by a single gene on the X chromosome and is the rate-limiting enzyme in the polyamine backconversion pathway (Fig. 1). The consequences of increased SSAT activity have been studied in cultured cells and in transgenic animals (1–3, 28–30, 34, 36, 37). Increased expression of SSAT in HEK293 cells leads to the depletion of Spd and Spm and increased Put content (36, 37) and is associated with altered morphology, reduced adhesion as well as decreased cell proliferation (36, 37). These cells have been found to have high levels of DNA damage and are G2 arrested (37). These events are presumed to be recapitulated during SSAT induction in IRI (36). Transgenic animals that express high levels of SSAT have depleted tissue Spm and Spd pools and increased tissue levels of Put and N1-acetylspermidine (1–3, 28, 29, 34). Mice overexpressing SSAT develop follicular cysts in the dermis, permanent hair loss and excessive wrinkling of the skin, all of which are partially attributable to increased polyamine backconversion (30). A detailed analysis of polyamine metabolism reveals that in addition to having elevated polyamine backconversion, SSAT transgenic animals have elevated levels of polyamine synthesis (22). This effect derives from lowering of Spd and Spm levels by SSAT and a subsequent upregulation of the key polyamine biosynthetic enzymes, ODC and S-adenosylmethionine decarboxylase (SAMDC). This simultaneous ratcheting up of the anabolic and catabolic arms of the polyamine pathway gives rise to “polyamine flux” whereby Spd and Spm are backconverted by SSAT and PAO to Put, which is then cycled back to Spd and Spm by ODC and SAMDC (22, 35). The end result of polyamine flux is an ongoing futile cycle, which depletes pathway precursors such as the SSAT coenzyme acetyl-CoA or overproduces pathway by-products such as Put, H2O2, and aldehydes (22, 35).
On the basis of in vitro effects of enhanced SSAT expression and the fact that we have observed induction of SSAT expression and activity following IRI, we hypothesize that metabolic events associated with increased SSAT activity and altered polyamine backconversion play an integral role in mediating tissue damage in IRI. To test this hypothesis, we compared the severity of IRI-induced tissue damage in liver and kidney in SSAT-deficient (SSAT-ko) and wild-type (SSAT-wt) mice.
MATERIALS AND METHODS
Animals and Animal Models
Genotyping.
Generation of SSAT-ko animals used in these studies was previously described (20). These animals do not exhibit any overt phenotypic abnormalities. However, they have increased white adipose acetyl- and malonyl-CoA pools, lowered adipose palmitate levels and decreased glucose oxidation. When placed on high-fat diet the SSAT-deficient animals have a distinct tendency to accumulate white adipose tissue (20). For these studies SSAT-ko mice and their SSAT-wt littermates were derived from mating of male SSAT deficient and female SSAT heterozygote mice. Genotypes of the litters were determined by PCR analysis of genomic DNA obtained from their tail clippings. Briefly, genomic DNA from tail clippings was PCR amplified (94°C for 3 min-94°C 30 s, 50°C 30 s, 72°C 90 s for 35 cycles-72°C for 10 min) using 5′SSAT (CTCCTCCTGCTGTTCAAGTA), 3′SSAT (CAGTTCCTGGGGACGAC) and NEO 5′SSAT (GCGCCCGGTTCTTTTTGTCA) primers. The amplified DNA was size fractionated on a 1% agarose-Tris/acetate/EDTA gel and analyzed for the presence of 408-bp and/or 680-bp products representing the SSAT-wt and disrupted SSAT (SSAT-ko) alleles, respectively (Fig. 2).
Fig. 2.
Genotyping of wild-type (SSAT-wt) and SSAT-deficient (SSAT-ko) mice. Genomic DNA was isolated and subjected to PCR amplification by using specific primers to identify the SSAT-wt and SSAT-ko mice. All animals depicted except the heterozygous animal were males. The amplified DNA was size fractionated on a 1% agarose-Tris/acetate/EDTA gel and analyzed for the presence of 408 bp (SSAT-wt) and 680 bp (SSAT-ko) PCR amplification products. Lane marked as Std contains DNA size standard.
Hepatic IRI.
Partial hepatic IRI was induced in male SSAT-wt and their male SSAT-ko littermates (28–32 g, 20–26 wk old). Briefly, mice were anesthetized, a midline laparotomy was performed, and an atraumatic clip was used to interrupt the blood supply to the left lateral and median lobes of the liver. Animals were well hydrated and their body temperature was controlled around 94°F by use of an adjustable heating pad. After 90 min the clip was removed to initiate reperfusion. Sham-operated SSAT-wt and -ko animals were used as controls. At 3 and 12 h postsurgery, animals were killed and their blood and livers were harvested. Blood samples were processed to obtain serum. Liver samples were fixed in paraformaldehyde and preserved in 70% ethanol or snap frozen in liquid nitrogen.
Renal IRI.
Renal IRI was induced in male SSAT-wt mice and their male SSAT-ko littermates (28–32 g, 20–26 wk old) by occluding the renal pedicles with nontraumatic microvascular clamps for 30 min. The blood flow to the kidneys was reestablished by removal of the clamps. Animals subjected to sham operation (identical treatment except the renal pedicles were not clamped) were used as controls. During the procedure, animals were well hydrated and their body temperature was controlled around 94°F with an adjustable heating pad. At timed intervals (24 to 96 h) postsurgery, animals were killed and their blood and kidneys were harvested. Blood samples were processed to obtain serum and kidneys were fixed in paraformaldehyde and preserved in 70% ethanol or snap frozen in liquid nitrogen.
Measurement of Tissue Polyamine Levels
Livers and kidneys were harvested at timed intervals after the onset of reperfusion and snap frozen. The samples were extracted and analyzed for polyamines as described previously by reversed-phase HPLC (21, 22).
Assessment of Liver and Kidney Function
The extent of liver injury was assessed by measuring the serum levels of alanine aminotransferase (ALT) as described elsewhere (4). Renal function was assessed by determining the serum creatinine levels with a colorimetric assay (Quanti Chrome Creatinine Assay kit, BioAssay systems). In this assay the change in absorbance over 30 min was measured. The change in absorbance was used to calculate the serum creatinine levels in the context of absorbance change over a 30-min period in standard samples.
Histopathology of Liver and Kidney
The histology of uninjured and injured livers and kidneys from SSAT-wt and SSAT-ko mice were compared by light microscopic examination of hematoxylin and eosin (H&E)-stained sections. Briefly, tissue samples fixed in paraformaldehyde and preserved in ethanol were paraffin embedded. Tissue sections (5 μm) were cut, stained with H&E, and examined (×20 magnification) for signs of tissue damage. Tissue damage in the liver sections was determined by mean percentage of necrotic areas in three high-power fields per slide (percentage of necrotic areas were determined in the affected lobes). The extent of renal injury was assessed by examining the cortical and corticomedullary regions of the kidney for lymphocyte infiltration, tubular dilatation, cast formation, and edema. Kidneys were assigned an injury score of 0–3 (0, no injury; 1, mild injury; 2, moderate injury; 3, severe injury) to compare the severity of tissue damage in the SSAT-wt and SSAT-ko animals.
Northern Hybridization
Total RNA was extracted from kidneys (30 μg/lane), size fractionated on a 1.2% agarose-formaldehyde denaturing gel, and transferred to nylon membranes by capillary transfer using 10× SSPE buffer. Membranes were cross-linked by UV light and baked. Hybridization was performed according to established methods using 32P-labeled cDNA fragments for SMO (corresponding to nucleotides 100 to 716 of a mouse SMO cDNA; GenBank accession no. NM_145533) or ODC (corresponding to nucleotides 477 to 1046 of a mouse ODC cDNA; GenBank accession no. M10624). Membranes were washed, blotted dry, and exposed to phosphorescent screens at room temperature for 24–72 h. The amount of radioactivity hybridized to specific mRNA was estimated by scanning densitometry of the membranes using a PhosphoImager SI (Molecular Dynamics, Amersham).
Statistical Analyses
Values are expressed as means ± SE. The significance of difference between mean values was examined using ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Assessment of Tissue Polyamine Levels in the Livers of SSAT-wt and SSAT-ko Mice After Partial Hepatic IRI
To confirm the genotype and assess the effect of SSAT ablation on the polyamine backconversion pathway, we compared the liver polyamine contents of sham-operated animals and animals subjected to hepatic IRI of both genotypes. Our results indicate that Put levels in the livers of sham-operated SSAT-wt (82 ± 12 pmol/mg protein) and SSAT-ko (84 ± 17 pmol/mg protein) animals were identical (Fig. 3A). The tissue Put levels increased after IRI in the livers of SSAT-wt mice by greater than sixfold (550 ± 74 pmol/mg protein). In contrast Put levels of SSAT-ko mouse livers (151 ± 47 pmol/mg protein) only increased marginally (less than twofold) (Fig. 3A). These results demonstrate that inactivation of SSAT precludes the polyamine backconversion pathway and thus reduces the buildup of Put in the livers of SSAT-ko animals after IRI.
Fig. 3.
Comparison of polyamine levels in SSAT-wt and SSAT ko mice after liver ischemia-reperfusion injury (IRI). A: liver Put levels. Liver Put levels were measured in injured and sham-operated (Cont) SSAT-wt and SSAT-ko animals. The Put content in the livers of both SSAT-wt and -ko mice were similar in sham-operated animals. However, in IRI animals, Put levels increased by ∼6-fold in the livers of wild-type animals compared with sham-operated animals of the same genotype. The hepatic Put levels in the IRI SSAT-ko animals were similar to those of the SSAT-ko sham-operated mice. B: liver Spd and Spm levels. The concentration of Spm and Spd in the livers of uninjured SSAT-wt and SSAT-ko animals were similar. However, the concentrations of Spm and Spd in the livers of SSAT-wt mice were significantly lower than those of sham-operated and SSAT-ko mice.
We also examined the tissue content of Spd and Spm in the injured lobes of the livers of SSAT-wt and -ko animals. The concentration of Spm and Spd in the livers of sham-operated SSAT-wt and SSAT-ko animals were similar (8,440 ± 880 and 6,150 ± 780 vs. 9,100 ± 1,100 and 6,930 ± 920 pmol/mg protein, respectively) (Fig. 3B). The concentrations of Spm and Spd in the livers of SSAT-wt mice (2,448 ± 320 and 3,225 ± 660 pmol/mg protein, respectively) were significantly lower than those of sham-operated mice of both genotypes and the SSAT-ko mice subjected to partial hepatic IRI (8,015 ± 678 and 5,212 ± 570 pmol/mg protein) (Fig. 3B).
Comparison of ODC and SMO Expression in the Livers of SSAT-wt and SSAT-ko Mice
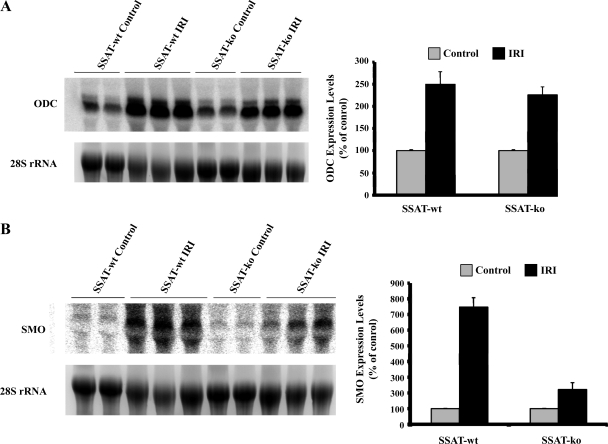
We next examined the expression of ODC and SMO transcripts in livers subjected to IRI in SSAT-wt and SSAT-ko animals by Northern blot analysis. Fig. 4A (left) demonstrates that the expression of ODC increases in IRI in both SSAT-wt and -ko animals. Densitometric analysis of the results indicates that the expression levels of ODC increased by ∼2.5 ± 0.28 and ∼2.3 ± 0.22 fold in SSAT-wt and SSAT-ko mice, respectively, with IRI relative to sham-operated animals (P < 0.05) (Fig. 4B, right). Fig. 4A (left) demonstrates that the SMO mRNA levels increase in the injured SSAT-wt and -ko animals compared with sham-operated animals of either genotype. Densitometric analysis of the Northern blot results revealed that the expression of SMO mRNA increased by ∼7.5 ± 0.6- and 2.2 ± 0.4-fold in the livers of SSAT wt and SSAT-ko mice after IRI compared with their genotype matched sham-operated animals (Fig. 4B, right). Our results indicate that the increases in ODC and SMO levels are of higher magnitude in the SSAT-wt animals. It should be noted that the signal strength for SMO mRNA was significantly weaker than that of ODC mRNA when blots were exposed for the same duration.
Fig. 4.
Northern blot analysis of ODC (A) and SMO (B) mRNA levels in livers of SSAT-wt and SSAT-ko animals after IRI. The expression of ODC increased (A, left) whereas the expression of SMO was only weakly induced (B, left) after IRI in both SSAT-wt and SSAT-ko animals. Densitometric analysis of the Northern blot results and normalization of the signal values against 28s rRNA were used to evaluate the induction profiles of each transcript. The semiquantitative values of the induction profiles of ODC and SMO are presented on the right of A and B, respectively.
Liver Function in SSAT-wt and SSAT-ko Mice Subjected to Hepatic IRI
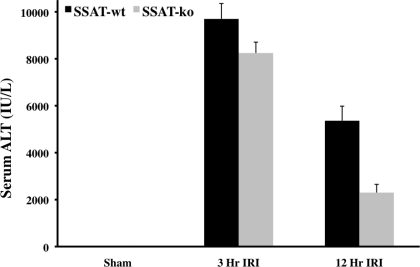
We next examined the effect of SSAT deficiency and disruption of polyamine backconversion on the severity of IRI by comparing the liver function of SSAT-ko and -wt animals. Liver function was assessed by measurement of serum marker enzyme, ALT, levels in SSAT-wt and SSAT-ko animals after sham operation and liver IRI. Our studies indicate that the serum ALT levels of sham-operated SSAT-ko and -wt animals were virtually identical (Fig. 5). Comparison of the SSAT-wt and -ko animals at 3 and 12 h after hepatic IRI indicate that SSAT-ko animals had significantly lower (∼64%, P < 0.05) serum ALT levels at 12 but not at 3 h after the onset of reperfusion (Fig. 5). The results presented in this section indicate that the disruption of polyamine backconversion protects the liver against damage caused by IRI.
Fig. 5.
Assessment of liver damage in SSAT-wt and SSAT-ko mice after IRI. The loss of liver function associated with liver IRI was compared in SSAT-wt and SSAT-ko mice (n = 4 per group) by examining serum alanine aminotransferase (ALT) levels. The serum ALT levels of sham-operated SSAT-wt and -ko mice were similar. The liver ALT of both genotypes were similarly elevated as early as 3 h after the onset of reperfusion; however, at 12 h after onset of reperfusion the SSAT-ko animals had significantly (P < 0.05) lower serum ALT levels than their SSAT-wt counterparts.
Histological Analysis of the Livers of SSAT-wt and SSAT-ko Mice after IRI
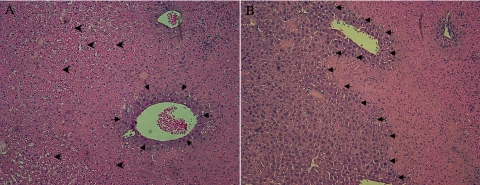
To confirm the functional studies in the previous section, we examined the histology of liver in SSAT-wt and SSAT-ko mice after sham operation or partial hepatic IRI. Comparison of sham-operated SSAT-wt and SSAT-ko livers did not reveal any histological differences (results not shown). The livers from SSAT-wt and SSAT-ko mice subjected to IRI showed the typical hemorrhagic necrosis and inflammatory cell infiltration. Comparison of the livers of SSAT-wt to SSAT-ko animals (Figs. 6, A and B, respectively) revealed the latter to be less congested without any discernable differences in the inflammatory cell infiltration (this was further confirmed by the lack of difference in the MPO activity in the injured SSAT-wt and SSAT-ko livers: 532 ± 184 vs. 567 ± 164, respectively). The analysis of percent necrosis in the affected liver sections revealed extensive hepatic necrosis in both SSAT-wt (77.5 ± 7.5%) and SSAT-ko (57.9 ± 7.4%) mice subjected to IRI (Table 1). The aforementioned studies combined with the ALT assay data indicate that ablation of SSAT reduces the severity of liver damage after IRI. These results suggest that SSAT and, by extension, polyamine backconversion, play a significant role in the mediation of liver damage in IRI.
Fig. 6.
Comparison of the histopathology of liver after partial hepatic IRI in SSAT-wt and SSAT-ko mice. The histology of livers of SSAT-wt (A) and -ko (B) animals subjected to 90 min of ischemia and 12 h of reperfusion were examined (n = 4/group). Histological comparison of uninjured SSAT-wt and -ko liver did not reveal any structural differences (data not shown). Histological assessment of livers from SSAT-wt and SSAT-ko (A and B, respectively) mice after induction of liver injury showed the typical extensive hemorrhagic necrosis and inflammatory infiltrates seen after IRI. However, in livers from SSAT-ko mice (B), there was far less congestion and the extent of necrosis was substantially reduced (small arrows delineate the injured vs. uninjured areas; large arrows indicate the areas of congestion).
Table 1.
Assessment of liver necrosis in SSAT-wt and SSAT-ko mice subjected to hepatic IRI
|
Quantification of Liver Necrosis, % necrotic area/field | ||
|---|---|---|
| SSAT-wt | SSAT-ko | |
| Sham | 0 | 0 |
| 12 h IRI | 77.5±7.5 | 57.9±7.9 |
Hematoxylin and eosin-stained liver sections (n = 4/group) were examined and the % necrotic area/field was determined by evaluating 3 random fields per slide at ×40 magnification. SSAT-ko, spermidine/spermine-N1-acetyltransferase deficient; SSAT-wt, wild-type; IRI, ischemia-reperfusion injury.
Determination of Issue Polyamine Levels in the Kidneys of SSAT-wt and SSAT-ko Mice After Bilateral Renal IRI
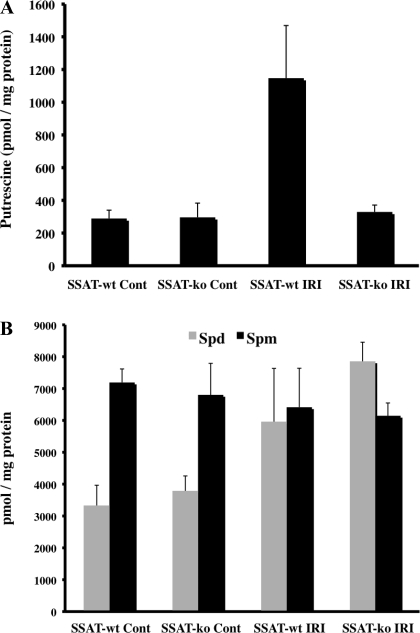
The kidney polyamine contents of SSAT-wt and SSAT-ko animals subjected to sham-operation or kidney IRI were compared. Our results indicate that Put levels in the kidneys of sham-operated SSAT-wt (289 ± 51 pmol/mg protein) and SSAT-ko (296 ± 87 pmol/mg protein) animals were identical (Fig. 7A). The tissue Put levels increased by nearly fourfold after IRI in the kidneys (1,147 ± 422 pmol/mg protein) of SSAT-wt mice. In contrast, Put levels of SSAT-ko mouse kidneys (329 ± 42 pmol/mg protein) remained at near-normal levels (Fig. 7A). These results demonstrate that inactivation of SSAT gene disrupts the polyamine backconversion pathway and thus reduces the buildup of Put in the kidneys of SSAT-ko animals after IRI.
Fig. 7.
Comparison of polyamine levels in SSAT-wt and SSAT ko mice after kidney IRI. A: kidney Put levels. Kidney Put levels were measured in injured and sham-operated SSAT-wt and -ko animals. The Put content in the kidneys of SSAT-wt were similar in sham-operated animals. After IRI, Put levels increased by ∼4-fold in the kidneys of SSAT-wt animals compared with sham-operated animals of the same genotype. The kidney Put levels in the injured SSAT-ko animals were not different from those of the SSAT-ko and SSAT-wt sham-operated mice. B: kidney Spd and Spm levels. The kidney Spd levels increased in SSAT-wt and SSAT-ko mice by ∼2-fold after IRI whereas the Spm levels were only marginally reduced in the injured kidneys of either SSAT-wt or SSAT-ko animals.
We also examined the tissue content of Spd and Spm in kidneys of SSAT-wt and SSAT-ko animals subjected to sham operation or renal IRI. The kidney Spd levels increased in SSAT-wt and SSAT-ko mice by nearly twofold after IRI (3,315 ± 651 vs. 5,954 ± 1,679 and 3,780 ± 477 vs. 7,854 ± 601 pmol/mg protein, respectively) whereas the kidney Spm levels were only marginally reduced in the injured kidneys of either SSAT-wt or SSAT-ko animals (7,191 ± 425 vs. 6,414 ± 1,223 and 6,802 ± 989 vs. 6,146 ± 402 pmol/mg protein, respectively) (Fig. 7B).
Comparison of ODC and SMO Expression in the Kidneys of SSAT-wt and SSAT-ko mice
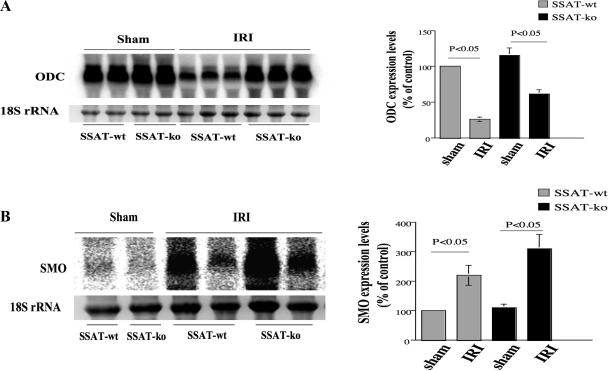
We next examined the expression of ODC and SMO in kidney IRI in SSAT-wt and SSAT-ko animals by Northern hybridization. Figure 8 A demonstrates that the expression of ODC decreased in IRI in both SSAT-wt and SSAT-ko animals (left). Densitometric analysis of the results indicates that the expression levels of ODC decreased by ∼74 and ∼46% in the kidneys of SSAT-wt and SSAT-ko mice subjected to renal IRI relative to sham-operated animals (P < 0.05, n = 3 mice for each injured group and n = 2 mice for each sham group) (right). Figure 8B demonstrates that the expression of SMO increased in IRI in both SSAT-wt and SSAT-ko animals (left). Densitometric analysis of the Northern blot indicates that the expression levels of SMO increased by ∼220 and ∼300% in SSAT wt and SSAT-ko mice in kidney IRI (right). Although the expression of SMO mRNA in both SSAT-wt and SSAT-ko animals subjected to IRI was significantly higher than their sham-operated counterparts, the differences between the genotypes was not statistically significant.
Fig. 8.
Northern blot analysis of ODC (A) and SMO (B) mRNA expression in kidneys of SSAT-wt and SSAT-ko animals after sham operation or IRI. The expression of ODC decreased (A, left) whereas the expression of SMO increased (B, left) after IRI in both SSAT-wt and SSAT-ko animals. Densitometric scanning of the gels and normalization of the values against 28s rRNA was used to evaluate the signals. The semi-quantitative values of the induction profiles of ODC and SMO are presented on the right of A and B, respectively.
Comparison of the Kidney Functions of SSAT-wt and SSAT-ko Mice After Renal IRI
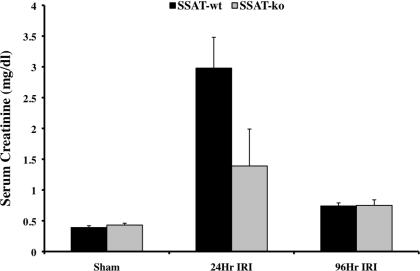
We next examined the effect of SSAT deficiency and disruption of polyamine backconversion on the severity of IRI. The severity of kidney damage in SSAT-wt and SSAT-ko animals subjected to renal IRI was examined by comparing their serum creatinine levels (Fig. 9). Our results indicate that the serum creatinine levels of sham-operated SSAT-wt and -ko animals were similar. Serum creatinine levels of SSAT-wt and SSAT-ko animals 24 h after IRI indicate that SSAT-ko animals have significantly lower (∼55%, P < 0.05) serum creatinine levels. However, serum creatinine levels of mice from both genotypes were not significantly different when samples from 96-h reperfusion were compared. These results indicate that although the disruption of polyamine backconversion does not affect kidney function under normal conditions, it partially protects the kidney against IRI.
Fig. 9.
Assessment of kidney damage in SSAT-wt and SSAT ko mice after IRI. The loss of renal function caused by bilateral IRI was compared in SSAT-wt and -ko mice (n = 6 per group) by comparing serum creatinine levels. Whereas serum creatinine levels of sham-operated SSAT-ko and -wt mice were similar, the SSAT-ko animals subjected to renal IRI had significantly (P < 0.05) lower serum creatinine levels than SSAT-wt animals subjected to renal IRI. The serum creatinine levels in both genotypes were significantly reduced and near normal levels by 96 h postreperfusion.
Histological Analysis of the Kidneys of SSAT-wt and SSAT-ko Mice After IRI
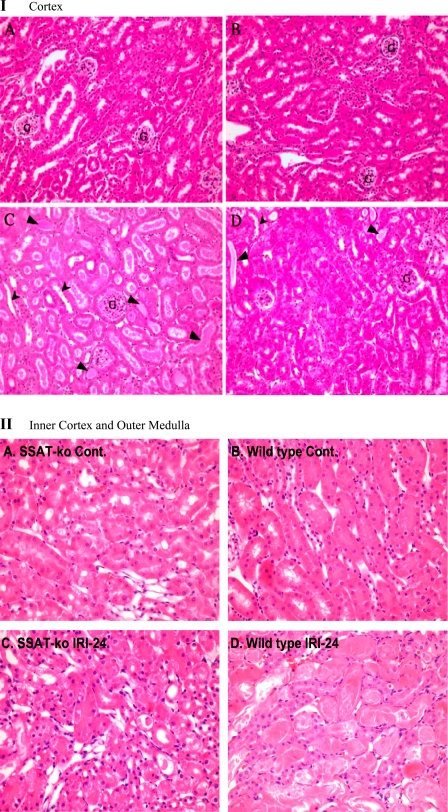
To confirm the results of the functional studies described in the previous section, we examined the histology of kidneys in SSAT-wt and SSAT-ko mice after sham operation or renal IRI. Examination of the cortical region of sham-operated (uninjured) animals at high (×20) magnification did not reveal any histological differences in the SSAT-wt (Fig. 10IA) and SSAT-ko mice (Fig. 10IB). Comparison of the cortical histopathology of injured animals under high magnification revealed that whereas SSAT-wt mice (Figs. 10IC) showed mild injuries including tubular dilatation, loss of brush border, edema, and cast formation, the SSAT-ko mice were protected against these changes (Fig. 10ID). Comparison of the corticomedullary region of sham-operated (uninjured) animals also did not reveal any histological differences in the SSAT-ko and -wt mice (Fig. 10II, A and B, respectively). Examination of the histology of corticomedullary region of animals subjected to IRI revealed that the SSAT-ko mice (Fig. 10IIC) display significant protection against structural damage relative to SSAT-wt animals (Fig. 10IID). In general the SSAT-deficient animals exhibited less severe necrotic injuries and epithelial loss. We did not observe a significant inflammatory response in the injured kidneys of either population (i.e., there were not any differences in the number of leukocytes in the interstitium or tubular lumen of injured and control animals of either genotype) at 24 h of reperfusion; however, the SSAT-wt animals had marginally higher number of nucleated cells in their peritubular capillaries at this time point. For semiquantitative analysis of renal injury the severity of tubular damage was scored as outlined in materials and methods. The injury scores are shown in Table 2. Our results suggest that the severity of parenchymal damage is reduced in SSAT-ko animals. The reduction of the severity of kidney damage in SSAT-ko animals together with the data indicating that these animals have better preserved kidney function indicates that ablation of SSAT partially protects the kidneys against IRI. By extension, these studies suggest that polyamine backconversion plays an important role in the mediation of kidney IRI.
Fig. 10.
Comparison of the histopathology of kidneys after renal IRI in SSAT-wt and SSAT-ko mice. I: cortex. The histology of the kidney cortical regions of SSAT-ko and SSAT-wt sham-operated animals (A and B, respectively) and animals subjected to 30 min of ischemia and 24 h of reperfusion (C and D, respectively) were examined microscopically. Comparison of the kidneys of sham-operated animals did not reveal any differences between the SSAT-wt and SSAT-ko mice. Our results indicated that after IRI, the kidneys of SSAT-ko had reduced tubular dilatation (arrowheads) as well as edema, and cast formation (small arrows) relative to the kidneys of SSAT-wt animals. G, glomeruli. II: inner cortex and outer medulla. The histology of the corticomedullary region of kidneys of SSAT-ko and SSAT-wt sham-operated animals (A and B, respectively) and animals subjected to 30 min of ischemia and 24 h of reperfusion (C and D) were examined microscopically. Comparison of the kidneys of sham-operated animals from SSAT-wt and -ko genotypes (B vs. A) did not reveal any differences in the renal architecture. Kidneys from SSAT-ko animals (C) subjected to IRI display significant protection against structural damage in the corticomedullary (inner cortex/outer medulla) region relative to SSAT-wt animals (D).
Table 2.
Renal histopathology scores for SSAT-wt and SSAT-ko mice
|
Cortex |
Inner Cortex/Outer Medulla
|
|||
|---|---|---|---|---|
| Inflammation | Tubular Damage | Inflammation | Tubular Damage | |
| SSAT-wt Sham | 0±0 | 0±0 | 0±0 | 0±0 |
| SSAT-wt IRI | 0±0 | 1.25±0.5 | 0±0 | 3±0 |
| SSAT-ko Sham | 0±0 | 0±0 | 0±0 | 0±0 |
| SSAT-ko IRI | 0±0 | 0.5±0.5 | 0±0 | 2.5±0.3 |
Each kidney section (n = 4/group) examined received a score of 0 to 3 depending on the severity of tissue injury. Inflammation reflects the presence of interstitial and luminal leukocytes. Tubular damage reflects the composite core of the severity of tubular dilatation, edema, and cast formation.
DISCUSSION
Previous studies from our laboratory demonstrate that the expression of SSAT increases dramatically in kidneys and livers after IRI (4, 38). The early induction of SSAT (12–24 h postreperfusion) and its enhanced expression in models of kidney injury implicate this molecule as a mediator of renal injury (4, 38). Our studies showing that enhanced expression of SSAT in cultured cells leads to DNA damage, growth arrest, and alterations in cell adhesion suggest that increased expression of this enzyme and the attendant increase in polyamine backconversion are critically involved in the mediation of tissue damage in IRI (36, 37). This hypothesis was specifically tested in the present studies by the assessment of severity of tissue damage in models of hepatic and renal IRI by comparison studies in SSAT-wt and SSAT-ko mice. Comparing liver function and structure after hepatic IRI indicates that the SSAT-ko animals have a reduced buildup of Put in their livers (Fig. 3A), better liver function as indicated by lower serum ALT levels (Fig. 5), and less severe liver injury (Fig. 6). In parallel studies we examined the effect of SSAT deficiency on the severity of kidney IRI. Again, SSAT-ko animals had lower levels of Put in their kidneys (Fig. 7A), better renal function as indicated by lower serum creatinine levels (Fig. 9), and less severe renal injuries (Fig. 10). These results indicate that SSAT-deficient animals display significant but not complete protection against IRI. We propose that the partial protection against hepatic and renal damage is due to the multifactorial nature of IRI and that it would be unlikely that alterations in any one factor would ameliorate the injury entirely. However, the extent to which SSAT ablation reduces injury is substantial and suggests that SSAT and by extension polyamine backconversion cascade is important in the etiology of IRI in both liver and the kidney.
Our studies indicate that the ablation of SSAT significantly reduces the organ damage after IRI; therefore, we propose that this enzyme is a mediator of tissue damage. The role of SSAT in this regard is supported by in vivo and in vitro evidence showing that its expression leads to oxidative stress and that it is detrimental to the integrity of tissues and organs (1–3, 28–30, 34, 36, 37). Studies examining the effect of transgenic expression of SSAT in mice indicate that it adversely effects the integrity and function of various tissues (28, 30, 34). These results are complemented by in vitro observations indicating that SSAT expression leads to alterations in cell physiology, cell cycle arrest, and apoptosis (36, 37). For example, induced expression of SSAT by the Spm analog N1,N11-diethylnorspermine in L56Br-C1 cells resulted in the depletion of the cellular polyamine pools, inhibition of cell proliferation, and onset of a distinct apoptotic response (14). Similarly, in SK-MEL-28 human melanoma cells, depletion of polyamines secondary to induction of SSAT resulted in decreased cell growth and induction of apoptosis (7). Our studies indicate that conditional overexpression of SSAT leads to oxidative stress, DNA damage, activation of DNA damage checkpoint and G2 to M cell cycle arrest (36, 37). Although SSAT overexpression leads to a variety of adverse effects its ablation is not detrimental (20). To date, other than what is described in this manuscript, the only phenotype associate with SSAT deficiency is the increase in white adipose acetyl- and malonyl-CoA pools, a decrease in adipose palmitate, enhanced glucose oxidation, and an accumulation of body fat that is exaggerated when SSAT-deficient animals are placed on a high-fat diet (20).
The nature of the polyamine pathway response and its role in the mediation of IRI seem to vary from organ to organ. Spm and Spd levels decrease in the liver and brain after IRI (4, 5). Examination of the content of the latter polyamines in the livers indicated that the tissue content of these molecules decrease in the livers of the SSAT-wt animals subjected to hepatic IRI but remains stable in similarly treated SSAT-ko mice (Fig. 3B). In line with the above observation, whereas the Put levels increase in injured SSAT-wt animals they only increase marginally in the livers of injured SSAT-ko animals (Fig. 3A). The aforementioned changes in tissue polyamine content suggest that polyamine backconversion is upregulated in the liver of injured SSAT-wt but not SSAT-ko mice. The changes in the polyamine content of the kidney after IRI follow a different dynamic. After renal IRI, the increase in kidney Put levels are not associated with a significant reduction in Spm levels, which remain fairly stable, but they are accompanied by a more than twofold increase in the Spd content.
Examination of ODC and SMO in the livers and kidneys of SSAT-wt and SSAT-ko animals revealed that the expression of ODC is also differentially regulated in liver and kidney after IRI. ODC expression decreased in the kidney whereas it increased in liver after IRI (Figs. 4 and 8). The magnitude of alteration in the expression of ODC in kidney or liver was significantly more profound in SSAT-wt relative to SSAT-ko mice subjected to IRI (Figs. 4 and 8). The decrease in ODC expression levels suggests that enhanced polyamine synthesis is not responsible for the maintenance of tissue Spm levels or elevated levels of Put in kidneys subjected to IRI. Our results further indicate that the expression of SMO is increased in kidney IRI in both SSAT-wt and SSAT-ko mice (Figs. 4 and 8). The increase in tissue Spd levels in kidney IRI could be explained by the increase in tissue SMO levels. Taken together, our results suggest that although polyamine flux (i.e., concomitant increase in polyamine backconversion and polyamine biosynthesis) does not play a major role in the etiology of renal IRI it may contribute to the tissue damage caused by hepatic IRI. Furthermore, our results also suggest that increased SMO levels may play a significant role in IRI in the kidney (evidenced by increased SMO mRNA expression and increased Spd level) but not in the liver (low abundance and weak induction of SMO).
The studies presented here suggest that polyamine depletion per se may not be a contributing factor to renal IRI. However, since tissue damage caused by IRI in the kidney is not uniform (i.e., the S3 portion of proximal tubule is damaged whereas the glomerulus, S1, and S2 portions of proximal tubule and distal tubule are resistant), our data may not completely reflect the localized effect of enhanced polyamine catabolism in susceptible vs. resistant regions. Our results also indicate that the dynamics of polyamine metabolism after IRI differs considerably in the liver and kidney. However, despite the differences in the polyamine response after IRI, our results indicate that SSAT and polyamine backconversion pathway play a significant role in the mediation of tissue damage in both liver and kidney IRI.
In hepatic IRI, the reduction in the levels of Spm, an antioxidant and a modulator of the inflammatory response, may lead to increased severity of tissue damage (8–10, 25, 26, 32, 33, 39–41, 43). The potential role of Spm in the modulation of oxidative tissue damage is supported by previous studies indicating that its administration reduces the severity of cardiac and cerebral IRI (8, 9, 43). There is also a significant buildup of Put in the liver after IRI (4, 38). Increased levels of Put were shown to decrease cell viability in cultured hepatocytes (4). Enhanced polyamine backconversion may also contribute to tissue damage through the polyamine pool changes and/or through the generation of toxic by-products (i.e., acetyl 3-aminopropanal and H2O2). The PAO by-product H2O2 is known to lead to tissue injury through production of hydroxyl radicals as the result of Fenton reaction (23, 24, 42). Several studies have shown that aminoaldehydes such as 3-aminopropanol play a significant role in mediating tissue injury and that neutralization of these molecules protects the brain against damage in cerebral IRI (17, 18). In kidney IRI, the maintenance of Spm and Spd levels at near normal or elevated levels, respectively (Fig. 6B), suggests that the depletion of these molecules may not play a significant role in the mediation of kidney injury. On the other hand, the by-products of polyamine backconversion, H2O2 and acetyl 3-aminopropanal, may be of great significance in renal IRI. This is in part supported by in vitro studies showing that catalase reduces the expression of oxidative marker, heme oxygenase 1, in SSAT-expressing renal epithelial cells (36). Additional studies are needed to evaluate in detail the role of molecules generated via polyamine catabolism in mediation of tissue damage in IRI.
In conclusion, the data presented here indicate that ablation of SSAT provides significant protection against tissue damage and organ failure in kidney and liver following IRI. Given the specificity of SSAT upregulation in injured organs in major models of IRI (heart, brain, liver, and kidney) and the role of this enzyme in the mediation of tissue damage, we propose that SSAT suppression or inhibition represents a possible strategy for therapeutic interventions aimed at preventing tissue damage and organ failure in IRI.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases grant DK-66589 (to K. Zahedi and M. Soleimani), National Institutes of Health Grants DK-56029 and HL-72552 (to A. B. Lentsch), National Cancer Institutes Grants CA-22153 and CA-76428 (to C. W. Porter), a Merit Review Award, and grants from Dialysis Clinic (to M. Soleimani).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alhonen L, Karppinen A, Uusi-Oukari M, Vujcic S, Korhonen VP, Halmekyto M, Kramer DL, Hines R, Janne J, Porter CW. Correlation of polyamine and growth responses to N1,N11-diethylnorspermine in primary fetal fibroblasts derived from transgenic mice overexpressing spermidine/spermine N1-acetyltransferase. J Biol Chem 273: 1964–1969, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Alhonen L, Parkkinen JJ, Keinanen T, Sinervirta R, Herzig KH, Janne J. Activation of polyamine catabolism in transgenic rats induces acute pancreatitis. Proc Natl Acad Sci USA 97: 8290–8295, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhonen L, Rasanen TL, Sinervirta R, Parkkinen JJ, Korhonen VP, Pietila M, Janne J. Polyamines are required for the initiation of rat liver regeneration. Biochem J 362: 149–153, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barone S, Okaya T, Rudich S, Petrovic S, Tehrani K, Wang Z, Zahedi K, Casero RA, Lentsch AB, Soleimani M. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia/reperfusion injury. Am J Physiol Cell Physiol 289: C826–C835, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Baskaya MK, Rao AM, Dogan A, Donaldson D, Gellin G, Dempsey RJ. Regional brain polyamine levels in permanent focal cerebral ischemia. Brain Res 744: 302–308, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Bonventre JV Mechanisms of ischemic acute renal failure. Kidney Int 43: 1160–1178, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Kramer DL, Diegelman P, Vujcic S, Porter CW. Apoptotic signaling in polyamine analogue-treated SK-MEL-28 human melanoma cells. Cancer Res 61: 6437–6444, 2001. [PubMed] [Google Scholar]
- 8.Clarkson AN, Liu H, Pearson L, Kapoor M, Harrison JC, Sammut IA, Jackson DM, Appleton I. Neuroprotective effects of spermine following hypoxic-ischemic-induced brain damage: a mechanistic study. FASEB J 18: 1114–1116, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Coert BA, Anderson RE, Meyer FB. Exogenous spermine reduces ischemic damage in a model of focal cerebral ischemia in the rat. Neurosci Lett 282: 5–8, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Das KC, Misra HP. Hydroxyl radical scavenging and singlet oxygen quenching properties of polyamines. Mol Cell Biochem 262: 127–133, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Dogan A, Rao AM, Hatcher J, Rao VL, Baskaya MK, Dempsey RJ. Effects of MDL 72527, a specific inhibitor of polyamine oxidase, on brain edema, ischemic injury volume, and tissue polyamine levels in rats after temporary middle cerebral artery occlusion. J Neurochem 72: 765–770, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Hasan R, Alam MK, Ali R. Polyamine induced Z-conformation of native calf thymus DNA. FEBS Lett 368: 27–30, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa S, Nakano M, Hamana K, Taniguchi Y, Iwasaki T, Kanda T, Suzuki T, Nagai R. Decrease in myocardial polyamine concentration in rats with myocardial infarction. Life Sci 60: 1643–1650, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Hegardt C, Johannsson OT, Oredsson SM. Rapid caspase-dependent cell death in cultured human breast cancer cells induced by the polyamine analogue N(1),N(11)-diethylnorspermine. Eur J Biochem 269: 1033–1039, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Higaki I, Matsui-Yuasa I, Tanaka H, Terakura M, Kinoshita H, Otani S. The effect of hepatic ischemia-reperfusion on polyamine metabolism in some organs of the rat. Transplantation 55: 268–273, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi K, Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun 271: 559–564, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Ivanova S, Batliwalla F, Mocco J, Kiss S, Huang J, Mack W, Coon A, Eaton JW, Al-Abed Y, Gregersen PK, Shohami E, Connolly ES Jr, Tracey KJ. Neuroprotection in cerebral ischemia by neutralization of 3-aminopropanal. Proc Natl Acad Sci USA 99: 5579–5584, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanova S, Botchkina GI, Al-Abed Y, Meistrell M 3rd, Batliwalla F, Dubinsky JM, Iadecola C, Wang H, Gregersen PK, Eaton JW, Tracey KJ. Cerebral ischemia enhances polyamine oxidation: identification of enzymatically formed 3-aminopropanal as an endogenous mediator of neuronal and glial cell death. J Exp Med 188: 327–340, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janne J, Alhonen L, Leinonen P. Polyamines: from molecular biology to clinical applications. Ann Med 23: 241–259, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Jell J, Merali S, Hensen ML, Mazurchuk R, Spernyak JA, Diegelman P, Kisiel ND, Barrero C, Deeb KK, Alhonen L, Patel MS, Porter CW. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA. J Biol Chem 282: 8404–8413, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Kramer D, Mett H, Evans A, Regenass U, Diegelman P, Porter CW. Stable amplification of the S-adenosylmethionine decarboxylase gene in Chinese hamster ovary cells. J Biol Chem 270: 2124–2132, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Kramer DL, Diegelman P, Jell J, Vujcic S, Merali S, Porter CW. Polyamine acetylation modulates polyamine metabolic flux, a prelude to broader metabolic consequences. J Biol Chem 283: 4241–4251, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Kunduzova OR, Escourrou G, De La Farge F, Salvayre R, Seguelas MH, Leducq N, Bono F, Herbert JM, Parini A. Involvement of peripheral benzodiazepine receptor in the oxidative stress, death-signaling pathways, and renal injury induced by ischemia-reperfusion. J Am Soc Nephrol 15: 2152–2160, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Linas SL, Whittenburg D, Parsons PE, Repine JE. Ischemia increases neutrophil retention and worsens acute renal failure: role of oxygen metabolites and ICAM 1. Kidney Int 48: 1584–1591, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Lovaas E Antioxidative and metal-chelating effects of polyamines. Adv Pharmacol 38: 119–149, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Lovaas E, Carlin G. Spermine: an anti-oxidant and anti-inflammatory agent. Free Radic Biol Med 11: 455–461, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol 35: 55–91, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Min SH, Simmen RCM, Alhonen L, Halmekyto M, Porter CW, Janne J, Simmen FA. Altered levels of growth-related and novel gene transcripts in reproductive and other tissues of female mice overexpressing spermidine/spermine N1-acetyltransferase (SSAT). J Biol Chem 277: 3647–3657, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Niiranen K, Halmekyto M, Pietila M, Diegelman P, Parkkinen JJ, Eloranta T, Porter CW, Alhonen L, Janne J. Relation of skin polyamines to the hairless phenotype in transgenic mice overexpressing spermidine/spermine N-acetyltransferase. Mol Pharmacol 59: 231–238, 2001.11160858 [Google Scholar]
- 30.Pietila M, Alhonen L, Halmekyto M, Kanter P, Janne J, Porter CW. Activation of polyamine catabolism profoundly alters tissue polyamine pools and affects hair growth and female fertility in transgenic mice overexpressing spermidine/spermine N1-acetyltransferase. J Biol Chem 272: 18746–18751, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Rao AM, Donaldson D, Prasad MR, Dempsey RJ, Baskaya MK. Regional brain polyamine levels in permanent focal cerebral ischemia. Neurosurgery 40: 364–370, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Sava IG, Battaglia V, Rossi CA, Salvi M, Toninello A. Free radical scavenging action of the natural polyamine spermine in rat liver mitochondria. Free Radic Biol Med 41: 1272–1281, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Soda K, Kano Y, Nakamura T, Kasono K, Kawakami M, Konishi F. Spermine, a natural polyamine, suppresses LFA-1 expression on human lymphocyte. J Immunol 175: 237–245, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Suppola S, Heikkinen S, Parkkinen JJ, Uusi-Oukari M, Korhonen VP, Keinanen T, Alhonen L, Janne J. Concurrent overexpression of ornithine decarboxylase and spermidine/spermine N(1)-acetyltransferase further accelerates the catabolism of hepatic polyamines in transgenic mice. Biochem J 358: 343–348, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tucker JM, Murphy JT, Kisiel N, Diegelman P, Barbour KW, Davis C, Medda M, Alhonen L, Janne J, Kramer DL, Porter CW, Berger FG. Potent modulation of intestinal tumorigenesis in Apcmin/+ mice by the polyamine catabolic enzyme spermidine/spermine N1-acetyltransferase. Cancer Res 65: 5390–5398, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Zahedi K, Barone S, Tehrani K, Rabb H, Matlin K, Casero RA, Soleimani M. Overexpression of SSAT in kidney cells recapitulates various phenotypic aspects of kidney ischemia-reperfusion injury. J Am Soc Nephrol 15: 1844–1852, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Zahedi K, Bissler JJ, Wang Z, Josyula A, Lu L, Diegelman P, Kisiel N, Porter CW, Soleimani M. Spermidine/spermine N1-acetyltransferase overexpression in kidney epithelial cells disrupts polyamine homeostasis, leads to DNA damage, and causes G2 arrest. Am J Physiol Cell Physiol 292: C1204–C1215, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Zahedi K, Wang Z, Barone S, Prada AE, Kelly CN, Casero RA, Yokota N, Porter CW, Rabb H, Soleimani M. Expression of SSAT, a novel biomarker of tubular cell damage, increases in kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol 284: F1046–F1055, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Borovikova LV, Wang H, Metz C, Tracey KJ. Spermine inhibition of monocyte activation and inflammation. Mol Med 5: 595–605, 1999. [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M, Caragine T, Wang H, Cohen PS, Botchkina G, Soda K, Bianchi M, Ulrich P, Cerami A, Sherry B, Tracey KJ. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med 185: 1759–1768, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang M, Wang H, Tracey KJ. Regulation of macrophage activation and inflammation by spermine: a new chapter in an old story. Crit Care Med 28: N60–N66, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Wang M, Xie HY, Zhou L, Meng XQ, Shi J, Zheng S. Role of reactive oxygen species in mediating hepatic ischemia-reperfusion injury and its therapeutic applications in liver transplantation. Transplant Proc 39: 1332–1337, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Zhao YJ, Xu CQ, Zhang WH, Zhang L, Bian SL, Huang Q, Sun HL, Li QF, Zhang YQ, Tian Y, Wang R, Yang BF, Li WM. Role of polyamines in myocardial ischemia/reperfusion injury and their interactions with nitric oxide. Eur J Pharmacol 562: 236–246, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M. A short burst of oxygen radicals at reflow induces sustained release of oxidized glutathione from postischemic hearts. J Biol Chem 268: 18532–18541, 1993.8395507 [Google Scholar]