Abstract
Previously, we showed that ACh-induced proliferation of human colon cancer cells is mediated by transactivation of epidermal growth factor (EGF) receptors (EGFRs). In the present study, we elucidate the molecular mechanism underlying this action. ACh-induced proliferation of H508 colon cancer cells, which express exclusively M3 muscarinic receptors (M3Rs), was attenuated by anti-EGFR ligand binding domain antibody, a broad-spectrum matrix metalloproteinase (MMP) inhibitor, anti-MMP7 antibody, a diphtheria toxin analog that blocks release of an EGFR ligand [heparin-binding EGF-like growth factor (HBEGF)], and anti-HBEGF antibody. Conditioned media from ACh-treated H508 cells induced proliferation of SNU-C4 colon cancer cells that express EGFR but not M3R. These actions were attenuated by an EGFR inhibitor and by anti-EGFR and anti-HBEGF antibodies. In H508, but not SNU-C4, colon cancer cells, ACh caused a striking dose- and time-dependent increase in levels of MMP7 mRNA and MMP7 protein. Similarly, ACh induced robust MMP1 and MMP10 gene transcription. ACh-induced MMP1, MMP7, and MMP10 gene transcription was attenuated by atropine, anti-EGFR antibody, and chemical inhibitors of EGFR and ERK activation. In contrast, inhibitors of phosphatidylinositol 3-kinase and NF-κB activation did not alter MMP gene transcription. Collectively, these findings indicate that MMP7-catalyzed release of HBEGF mediates ACh-induced transactivation of EGFR and consequent proliferation of colon cancer cells. ACh-induced activation of EGFR and downstream ERK signaling also regulates transcriptional activation of MMP7, thereby identifying a novel feed-forward mechanism for neoplastic cell proliferation.
Keywords: epidermal growth factor receptor, gene transcription, heparin-binding epidermal growth factor-like growth factor
colon cancer, a common and frequently lethal disease, progresses in a multistep process that involves tumor initiation and promotion. Therapeutic strategies directed at interfering with tumor promotion are of particular interest, because this is generally a reversible, long-term process (13). Hence, there is great interest in identifying key colon cancer growth factors and their receptors. Increasing evidence indicates that muscarinic receptors and ligands play key roles in intestinal neoplasia (8, 9, 14, 15, 17, 27). The muscarinic cholinergic family of G protein-coupled receptors (GPCRs) consists of five muscarinic receptor subtypes designated M1R, M2R, M3R, M4R, and M5R (for reviews see Refs. 34 and 35). Using RT-PCR with primers specific for muscarinic receptor subtypes, Frucht and colleagues (14, 15) reported that 60% of colon cancer cell lines tested express M3R. Follow-up studies revealed that normal colon epithelial cells uniformly express M3R, and in 62% of colon cancers, M3R expression was increased up to eightfold compared with adjacent normal colon epithelium (37).
Work from our laboratory extended these observations by showing that cholinergic ligand-induced proliferation of H508 human colon cancer cells, which express high levels of M3R (∼2,500 binding sites per cell) (15), is mediated by cross talk between M3R and epidermal growth factor (EGF) receptors (EGFRs) (10) and that genetic ablation of M3R attenuates murine colon epithelial cell proliferation and neoplasia (27). EGFRs are commonly overexpressed in epithelial malignancies, and this feature frequently indicates a more aggressive phenotype (29). In particular, as observed with M3R, EGFR is frequently overexpressed in colon cancer: 25–77% of tumors overexpress EGFR compared with adjacent normal mucosa (22, 29). Coexpression of M3R and EGFR in many colon cancer cell lines and overexpression of these receptors in the majority of colon cancers suggest that the functional interaction we identified between M3R and EGFR is important for regulating colon cancer cell proliferation (10).
Interaction of cells with extracellular matrix components is essential for many physiological processes, including development, growth, and tissue repair. Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that play a key role in modulating cell-matrix interactions. Human MMPs, a family including ≥25 members, are expressed as secreted or membrane-bound proenzymes that are activated by proteolytic cleavage (4, 40). Besides degrading extracellular matrix substrates, MMPs may regulate “shedding” of cell surface growth factors, a process whereby MMP-catalyzed proteolysis releases extracellular domains of transmembrane signaling molecules. For example, by catalyzing release of EGFR ligands, MMP7 activation may be a critical component of cross talk between GPCRs and EGFR (25). On the basis of these observations, we hypothesized that MMP activation in H508 colon cancer cells mediates ACh-induced activation of EGFR and, in particular, that MMP7 plays a key role in this process.
The primary objective of the present study was to elucidate molecular mechanisms underlying ACh-induced activation of EGFR. We sought to determine whether MMPs, specifically MMP7, play a role in releasing an EGFR ligand. We used human H508 colon cancer cells, which coexpress M3R and EGFR (10). SNU-C4 human colon cancer cells, which express EGFR but not muscarinic receptors (15), were used as control. Our findings demonstrate that ACh-induced cell proliferation requires activation of MMP7 and release of heparin-binding EGF-like growth factor (HBEGF), an EGFR ligand. Moreover, ACh stimulated a striking induction of MMP7 gene transcription that was also mediated by activation of M3R and EGFR and by post-EGFR ERK signaling. In a systematic analysis of MMP gene transcription, we identified additional robust ACh-induced, EGFR/ERK-dependent transcriptional activation of MMP1 and MMP10.
MATERIALS AND METHODS
Materials.
ACh, atropine, and CRM197 were purchased from Sigma; PD168393, AG1478, PD98059, U0126, LY294002, SN50, and GM6001 from Calbiochem; anti-HBEGF antibody from Santa Cruz Biotechnology; anti-EGFR antibody from UBI; recombinant human MMP7, MMP10, amphiregulin (AR), anti-MMP7 neutralizing antibody, anti-AR neutralizing antibody, and Quantikine MMP7 immunoassay kit from R & D Systems; CellTiter 96 AQueous One solution cell proliferation assay [3-(4,5-dimethylthiazol)-5-(3-carboxymethoxyphenyl)-2(4-sulfophenyl)-2H-tetrazolium (MTS)] kit from Promega; and RPMI growth medium from GIBCO BRL. All other chemicals were obtained from Sigma or Fisher.
Cell culture.
H508 human colon cancer cells (American Type Culture Collection) were grown in RPMI 1640 growth medium supplemented with 10% fetal bovine serum (Biowhittaker). SNU-C4 human colon cancer cells are maintained in our laboratory in RPMI 1640 with 10% FBS (10). Adherent cells were passaged weekly at subconfluence after trypsinization. Cultures were maintained in incubators at 37°C in an atmosphere of 5% CO2-95% air.
Cell proliferation.
Cells were seeded in 96-well plates (Corning Glass Works, Corning, NY) at ∼10% confluence and allowed to attach for 24 h. After 24 h of serum starvation, 200 μl of fresh serum-free growth medium containing test agents were added. When chemical inhibitors and antibodies were used, they were added 30 min and 2 h, respectively, before test agents. Cells were incubated for 5 days at 37°C in an atmosphere of 5% CO2-95% air without further additions of test agents. After the 5-day incubation, cell proliferation was determined by addition of 20 μl of CellTiter 96 AQueous One solution (Promega) to each well. After 1–2 h of incubation at 37°C, absorbance was measured at 490 nm using a 96-well microtiter plate reader (SpectraMax384).
Soft agar colony assay.
Soft agar assays were performed using the CytoSelect 96-well Cell Transformation Assay kit (Cell Biolabs) according to the manufacturer's instruction. Briefly, 2.5 × 104 H508 cells were grown in RPMI 1640 with 10% FBS in each well of a 96-well plate. When chemical inhibitors and antibodies were used, they were added 1 h before test agents. After 7-day incubations, colonies were solubilized, transferred, and stained with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution. Absorbance was measured at 570 nm using a 96-well microtiter plate reader (SpectraMax384).
Conditioned media experiments.
H508 cells were seeded in T75 flasks at 50% confluence (∼2 × 106 cells) in RPMI 1640 medium containing 10% FBS and allowed to attach for 24 h. After 24 h of serum starvation, cells were washed once with serum-free medium. Cells were incubated with 10 ml of fresh RPMI 1640 medium for 10 min. Cell media at this point were used as untreated H508 media. ACh was added to cells for an additional 10 min. Supernatants were centrifuged at 500 rpm for 5 min and used as “conditioned media.” SNU-C4 cells were seeded in 96-well plates at ∼10% confluence in 200 μl of RPMI 1640 medium containing 10% FBS and allowed to attach for 24 h. After 24 h of serum starvation, 200 μl of fresh serum-free growth medium containing test agents was added. Cells were incubated for 5 days at 37°C in an atmosphere of 5% CO2-95% air without further addition of test agents. After the 5-day incubation, SNU-C4 cell proliferation was determined using the assay described above.
Quantitative real-time PCR.
Cells were subcultured in six-well plates at 106 cells per well. After 24 h of incubation at 37°C, cells were serum starved overnight in RPMI 1640 medium and washed once with RPMI 1640 medium before addition of test agents. Total cellular RNA was isolated from cells using TRIzol reagent (Invitrogen). First-strand cDNAs were synthesized using the Superscript III First Strand Synthesis System for RT-PCR (Invitrogen). Primers for RT-PCR were designed using the National Center for Biotechnology Information nucleotide database, SIM-4 gene alignment program, and online software (www.genscript.com/ssl-bin/app/primer). Quantitative real-time PCR (Q-PCR) was performed using the 7900HT Fast System (ABI) with Power SYBR Green Master Mix (ABI) and 20 ng of primer, and cDNA was synthesized from 50 ng of total RNA. PCR conditions included 5 min at 95°C followed by 35 cycles of 95°C for 15 s, 60°C for 20 s, and 72°C for 40 s and a final cycle at 95°C for 15 s, 60°C for 15 s, and 95°C for 15 s. PCR data were analyzed using ABI instrument software SDS 2.1. Specificity of amplifications was confirmed by melting-curve analysis. Relative levels of mRNA expression were calculated according to the standard cycle threshold (ΔΔCt) method. Individual expression values were normalized by comparison with GAPDH. Table 1 lists primers used for MMPs, GAPDH, and EGFR ligands.
Table 1.
Primers and product length for Q-PCR
| Genes | Forward Primer Sequence, 5′–3′ | Reverse Primer Sequence, 5′–3′ | Length, bp |
|---|---|---|---|
| MMP1 | AACTGCCAAATGGGCTTG | CCGTGTAGCACATTCTGTCC | 101 |
| MMP2 | CTGCAGGGCGCTACTTCT | TCACGCTCTTCAGACTTTGG | 69 |
| MMP3 | CCAAGCAAATAGCTGAAGACTTT | TTTCTTTGCATTTGGGTCAA | 125 |
| MMP7 | GGAGATGCTCACTTCGATGA | ATACCCAAAGAATGGCCAAG | 104 |
| MMP8 | AGCAATTGACGCAGCTGTT | GGAAAGGCACCTGATATGCT | 129 |
| MMP9 | GAGGTGGACCGGATGTTC | CCTGGCAGAAATAGGCTTTC | 82 |
| MMP10 | TGAGCCTAAGGTTGATGCTG | GTCACCATCCTGGCATTG | 99 |
| MMP12 | TTGGAGGTATGATGAAAGGAGA | TTTAGGCCCGATTCCTTG | 85 |
| MMP13 | GAAGACTTCCCAGGAATTGG | AAATGGAATTTGCTGGCAT | 142 |
| MMP14 | ACATCAAAGTCTGGGAAGGG | CCGGTTCTACCTTCAGCTTC | 132 |
| MMP19 | TTAAGCTGCACCCAGATGAT | AGTGGGCAGCTCTGTCTCTT | 101 |
| MMP21 | CTGGAATCCCAACACACAAC | ATCTTCTGTGAGGGCCTGAT | 131 |
| MMP26 | GGTCAGCTTCAGACACTGGA | AGCTCTGATTCCCAGAGTGC | 93 |
| MMP28 | CACTGCAGGAAAGATGGGT | GGAACCTCCAGCATCGAC | 108 |
| GAPDH | CCCCATGGTGTCTGAGCG | CGACAGTCAGCCGCATCTT | 67 |
| EGF | ACCAAGACCTCAAGAATGGG | TCCATGAAGTTGGTTGCATT | 84 |
| HBEGF | GGAGAGGAGGTTATGATGTGG | CGATTCCTTGAGCACAAGTCT | 89 |
| TGFα | CCCTCCTGAAGGGAAGAAC | CTCCTCTGGGCTCTTCAGAC | 64 |
| Amphiregulin | AGGAGAAGCTGAGGAACGAA | CACTGGAAAGAGGACCGACT | 146 |
| βcellulin | AGAAGAAATGGAAACTCTGGGT | GAGCAAGGCACTTTGCAG | 125 |
| Epiregulin | TATGTGGCTTTGACCGTGAT | TTTCGATTTCTGTACCATCTGC | 92 |
| Epigen | TTACTGCATCAACGGTGCTT | AGTGCTCACACCTTTCTCCA | 95 |
Q-PCR, quantitative real-time PCR; MMP, matrix metalloproteinase; EGF, epidermal growth factor; HBEGF, heparin-binding EGF-like growth factor; TGFα, transforming growth factor-α.
mRNA stability.
H508 cells were pretreated with actinomycin D (1 μg/ml final concentration) for 1 h before addition of ACh (100 μM). Total RNA was then extracted at 0–24 h, and MMP7 mRNA levels were measured by Q-PCR. Data are presented as values from cells treated with ACh relative to cells treated with water after normalization to a GAPDH control.
MMP7 ELISA.
H508 cells were seeded at 106 cells per 2 ml of medium per well in six-well plates. After serum starvation for 24 h, 100 μM ACh was added. At desired times, supernatants were collected and centrifuged at 500 rpm for 5 min. MMP7 was measured using the Quantikine System (R & D Systems) according to the manufacturer's instructions. Briefly, cell extracts and supernatants were diluted twofold in Calibrator Diluent RD6-28, and 50 μl were added directly to coated ELISA plates in duplicate. After 2 h of incubation at room temperature, wells were washed three times, MMP7 antibody conjugates were added for 2 h, wells were washed again three times, MMP substrate was added, and color was developed. Optical density was measured at 450 nm, with wavelength correction set at 540 nm.
Statistical analysis.
Values are means ± SE of at least three independent experiments. Statistical calculations were performed using Student's unpaired t-test (SigmaPlot, Systat Software, San Jose, CA). P < 0.05 was considered significant.
RESULTS
ACh induces dose-dependent proliferation of human colon cancer cells.
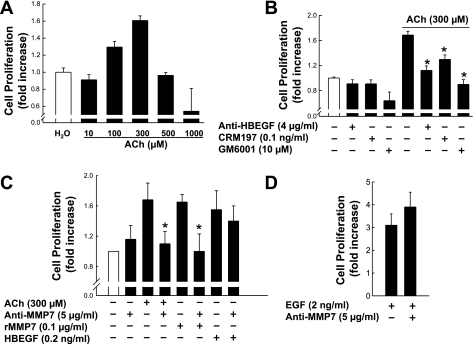
Previously, we showed that ACh induces proliferation of H508 human cancer cells that coexpress M3R and EGFR (10). To select appropriate ACh concentrations for the experiments that follow, we examined the dose-response curve for ACh-induced H508 cell proliferation. As shown in Fig. 1A, ACh-induced proliferation of H508 cells was detectable with 100 μM ACh and maximal with 300 μM ACh; a 1.7-fold increase in proliferation was observed after 5 days of incubation, a duration selected on the basis of previous work (10). At the concentrations shown, cholinergic agonists, including ACh, do not induce apoptosis (5, 10). From the dose-response data in Fig. 1A, to measure changes in cell proliferation, we selected 300 μM ACh as our test dose.
Fig. 1.
Effects of various agents on proliferation of H508 human colon cancer cells. A: dose-response of ACh-induced proliferation of H508 cells. H508 cells were incubated for 5 days at 37°C with 10–1,000 μM ACh. B: effect of heparin-binding epidermal growth factor-like growth factor (HBEGF) antibody, CRM197, and matrix metalloproteinase (MMP) inhibitor (GM6001) on cell proliferation. H508 cells were incubated with the indicated reagents alone or in combination for 5 days at 37°C. C and D: neutralizing antibody to MMP7 blocks ACh- and recombinant MMP7 (rMMP7)-stimulated cell proliferation. H508 cells were incubated with ACh alone, rMMP7 alone, or the indicated combined reagents for 5 days at 37°C. Cell proliferation was determined using the CellTiter 96 AQueous One solution assay. Values are means ± SE of ≥3 separate experiments. *P < 0.05 vs. ACh-stimulated cells (Student's t-test).
ACh-induced proliferation of H508 cells is mediated by MMP7-catalyzed release of HBEGF.
Conjugated secondary bile acids induce proliferation of colon cancer cells by a mechanism involving MMP7-catalyzed release of an EGFR ligand, HBEGF (9). Because bile acids interact functionally with muscarinic receptors and induce transactivation of EGFR (7, 9, 26), we hypothesized that the actions of ACh were mediated by a similar mechanism. To determine the role of HBEGF and MMPs in mediating ACh-induced proliferation of H508 cells, we examined the actions of HBEGF release inhibitors, neutralizing antibodies to HBEGF, and GM6001, a nonselective MMP inhibitor. As shown in Fig. 1B, at concentrations that did not significantly alter basal cell proliferation, CRM197, a nontoxic mutant form of diphtheria toxin that prevents HBEGF release, an anti-HBEGF antibody, and GM6001 attenuated the proliferative actions of ACh to basal or near-basal levels (P < 0.05). An inert control chemical for GM6001, designated NC GM6001, did not alter ACh-induced cell proliferation (not shown). These data were consistent with the conclusion that MMP-catalyzed HBEGF release and binding to EGFR mediated ACh-induced transactivation of EGFR and downstream stimulation of H508 cell proliferation.
To confirm the role of MMP7 in ACh-induced cell proliferation, we examined the actions of recombinant MMP7 (rMMP7) and neutralizing anti-MMP7 antibody (Fig. 1C). The efficacy for stimulating H508 cell proliferation was the same for the maximal concentration of rMMP7 (0.1 μg/ml) and 300 μM ACh. Anti-MMP7 antibody effectively blocked ACh- and rMMP7-induced cell proliferation (P < 0.05). As anticipated, control experiments showed that anti-MMP7 antibody did not alter proliferation induced by HBEGF (Fig. 1C) or EGF (Fig. 1D); both EGFR ligands activate EGFR directly, independent of MMP7 activation. These results provide compelling evidence that, as observed with bile acids (9), ACh-induced transactivation of EGFR and stimulation of H508 cell proliferation are mediated by MMP7 activation and HBEGF release.
Effect of conditioned media from H508 cells on proliferation of SNU-C4 colon cancer cells.
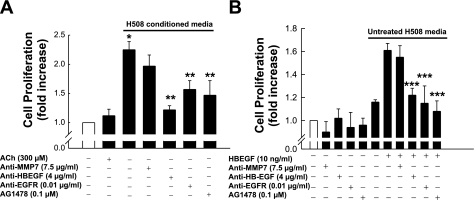
SNU-C4 human colon cancer cells express EGFR but not muscarinic receptors (15). As shown in Fig. 2A, 300 μM ACh did not stimulate SNU-C4 cell proliferation. We hypothesized that, after treatment of H508 cells with ACh, HBEGF released into the cell medium would stimulate SNU-C4 cell proliferation; that is, HBEGF in supernatant from H508 cells should activate EGFR on SNU-C4 cells. As predicted, 5 days of incubation with “conditioned media” from H508 cells stimulated a 2.3-fold increase in SNU-C4 cell proliferation (P < 0.05; Fig. 2A). This proliferative effect was attenuated by neutralizing antibodies against HBEGF and EGFR and by the EGFR inhibitor AG1478, but not by anti-MMP7 antibody (Fig. 2A). To mitigate the effects of any other possible growth factors that may be present in H508 medium, we used media from untreated H508 cells as a negative control and as a vehicle for HBEGF administration. As shown in Fig. 2B, positive control experiments showed that addition of HBEGF in media from untreated H508 cells stimulated proliferation of SNU-C4 cells, an effect that was attenuated by anti-HBEGF and anti-EGFR antibody, by the EGFR inhibitor AG1478, but not by anti-MMP7 antibody. Thus addition of HBEGF in untreated H508 media mimicked the results of addition of conditioned media from ACh-treated H508 cells. Collectively, these findings provide strong evidence that conditioned media from ACh-treated H508 cells contain released HBEGF, thereby inducing proliferation of SNU-C4 cells.
Fig. 2.
Effects of conditioned media from H508 cells (A) and addition of HBEGF to untreated H508 media (B) on proliferation of SNU-C4 human colon cancer cells. A: SNU-C4 cells were incubated with ACh or conditioned media from H508 cells and inhibitors for 5 days at 37°C. B: SNU-C4 cells were incubated with reagents alone in serum-free media or in combination in untreated H508 media. Values are means ± SE of ≥3 separate experiments. *P < 0.05 vs. ACh-stimulated cells. **P < 0.05 vs. cells stimulated by H508 supernatant alone. ***P < 0.05 vs. cells stimulated by HBEGF alone in untreated H508 media.
H508 cells express multiple EGFR ligands.
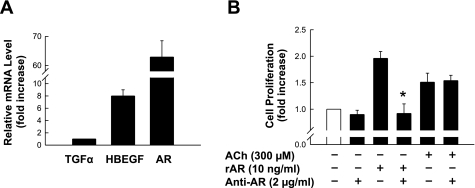
To determine whether H508 cells express EGFR ligands other than HBEGF, we performed Q-PCR using primers shown in Table 1. Of seven known EGFR ligands, we detected abundant mRNA for transforming growth factor-α, HBEGF, and AR (Fig. 3A). The other EGFR ligands, EGF, β-cellulin, epiregulin, and epigen, were not detected. We demonstrated previously that transforming growth factor-α does not mediate cholinergic agonist-induced H508 cell proliferation (9). To determine whether AR plays a role in ACh-induced H508 cell proliferation, we examined the actions of recombinant human AR (rAR) and neutralizing anti-human AR antibody on H508 cell proliferation (see materials and methods). As shown in Fig. 3B, 10 ng/ml rAR stimulated a twofold increase in H508 cell proliferation in 5 days, an action that was completely abolished by neutralizing anti-AR antibody. In contrast to the effects of anti-HBEGF antibody (Fig. 1B), ACh-induced H508 cell proliferation was not altered by neutralizing anti-AR antibody. Collectively, these results indicate that although several EGFR ligands are expressed, ACh-induced H508 cell proliferation is mediated exclusively by HBEGF. The function, if any, of AR in H508 cells remains to be determined.
Fig. 3.
H508 cells express multiple epidermal growth factor (EGF) receptor (EGFR) ligands. A: relative mRNA levels of 3 EGFR ligands were measured by quantitative real-time PCR (Q-PCR). B: ACh-induced H508 cell proliferation is not affected by blocking amphiregulin (AR) with a neutralizing antibody. H508 cells were incubated with ACh alone, recombinant AR (rAR) alone, AR antibody alone, or combined reagents for 5 days at 37°C. Cell proliferation was determined using the CellTiter 96 AQueous One solution assay. TGFα, transforming growth factor-α. Values are means ± SE of 3 separate experiments. *P < 0.05 vs. AR-stimulated cell proliferation.
ACh enhances anchorage-independent growth of colon cancer cells.
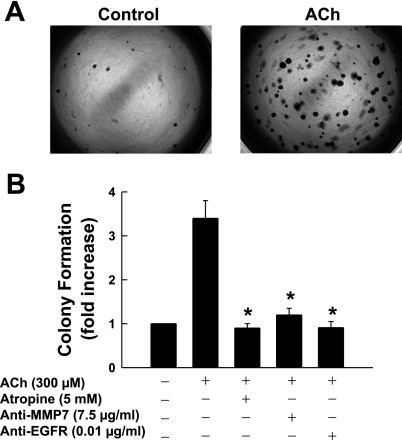
Anchorage-independent growth is a hallmark of malignant cell transformation (30). It requires fewer extracellular growth factors and is independent of cell-cell interaction (30). Hence, measurement of anchorage-independent growth is considered an accurate and stringent test for uncontrolled cell proliferation (18, 20, 39). To determine whether ACh promotes anchorage-independent growth of H508 colon cancer cells, we used a soft agar assay (see materials and methods). After 7 days of incubation, ACh (300 μM) stimulated a >3.5-fold increase in the number of H508 cell colonies formed in agarose (Fig. 4, A and B). ACh-enhanced colony formation was blocked by atropine and by neutralizing anti-MMP7 and anti-EGFR antibodies, thereby confirming that, similar to ACh-induced cell proliferation, anchorage-independent H508 cell growth is mediated by activation of muscarinic receptors, MMP7, and EGFR (Fig. 4B). These findings indicate that, as predicted from the many actions of EGFR activation that result in tumor progression (12, 21), downstream effects of muscarinic receptor activation also result in multiple proneoplastic actions.
Fig. 4.
ACh enhances H508 cell colony formation. A: representative photographs of colonies formed in soft agar assay in the presence and absence of ACh (300 μM). Dark spots represent H508 cell colonies. B: ACh-induced colony formation is attenuated by atropine and by anti-MMP7 and anti-EGFR antibodies. Values are means ± SE of 3 separate experiments. *P < 0.05 vs. ACh-stimulated colonies.
Effect of ACh on MMP7 gene transcription and MMP7 protein expression.
Having shown that M3R-induced transactivation of EGFR and cell proliferation are mediated by MMP7 activation, we considered the possibility that cholinergic ligand-induced M3R activation alters MMP7 gene expression. The MMP7 gene promoter region contains transcriptional regulation sites that are potential targets of transcription factors activated downstream of ERK (e.g., activator protein-1). To determine whether MMP7 gene expression is altered by M3R-mediated transactivation of EGFR, we performed Q-PCR (primers shown in Table 1). As depicted in Fig. 5, A and B, ACh induced a striking time- and dose-dependent increase in MMP7 mRNA levels. Induction of MMP7 mRNA was robust (∼50-fold) but transient, peaking at 4–6 h and returning to baseline levels by 24 h (Fig. 5A). As shown in Fig. 5B, 1 μM ACh caused a 15-fold increase in MMP7 mRNA levels, whereas 100–300 μM ACh caused a maximal, ∼50-fold, increase in MMP7 gene transcription. The dose response for ACh-stimulated MMP7 gene induction was similar to that for stimulation of H508 cell proliferation (cf. Figs. 1A and 5B). Nonetheless, because the increase in MMP7 gene transcription was maximal with 100 μM ACh, we used that concentration in the experiments described below.
Fig. 5.
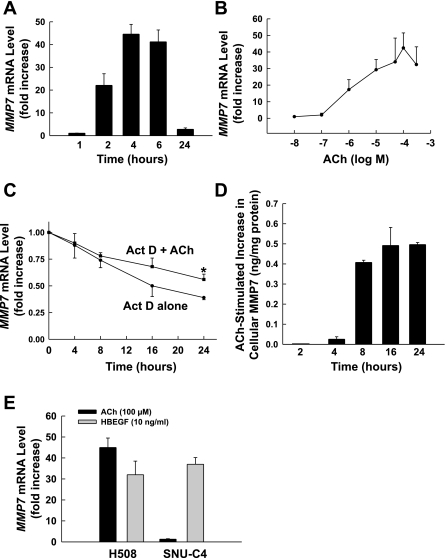
ACh stimulates time- and dose-dependent MMP7 mRNA and protein expression. A: ACh induces time-dependent upregulation of MMP7 transcription. Levels of MMP7 mRNA were determined by Q-PCR. B: ACh causes dose-dependent increases in MMP7 gene transcription. Q-PCR was performed using cDNAs that were synthesized from total RNAs prepared from H508 cells that were stimulated by different concentrations of ACh for 4 h. Fold increase was determined by comparison with controls. C: MMP7 mRNA decay was measured by Q-PCR after addition of actinomycin D (1 μg/ml) in the presence or absence of 100 μM ACh. Values are expressed as percentage of mRNA remaining compared with value at time 0 (i.e., before addition of ACh). *P < 0.05 vs. ACh-stimulated cells. D: ACh induces MMP7 protein expression determined by ELISA. In H508 cell extracts, at the indicated times after addition of 100 μM ACh, increases in MMP7 protein above basal levels were determined by ELISA. E: ACh (100 μM) induces upregulation of MMP7 transcription in H508 cells but not in SNU-C4 cells. HBEGF (10 ng/ml) upregulates MMP7 gene transcription in H508 and SNU-C4 colon cancer cells. Q-PCR was performed using cDNAs that were synthesized from total RNAs prepared from cells that were stimulated by test agents for 4 h. Values are means ± SE of ≥3 separate experiments.
To confirm that ACh-stimulated increases in MMP mRNA were primarily a consequence of increased transcription, and not reduced degradation of the transcript, we used actinomycin D (1 μg/ml)-induced inhibition of mRNA synthesis to determine whether ACh altered the half-life of MMP7 mRNA (see materials and methods). As shown in Fig. 5C, in H508 cells the half-life of MMP7 mRNA was ∼16 h in the absence of ACh and increased to ∼26 h with ACh. Although ACh increases the half-life of MMP7 mRNA by ∼10 h, it is apparent from these data that the 50-fold induction of MMP7 mRNA levels by ACh at 4–6 h occurs predominantly as a consequence of increased gene transcription and cannot be explained by the relatively minor change in MMP7 mRNA degradation.
To confirm that MMP7 transcriptional induction results in increased translation, we used ELISA to measure MMP7 protein levels in H508 cell extracts and culture media. In unstimulated H508 cells, the basal level of cellular MMP7 was 0.37 ± 0.07 ng/mg protein. The data shown in Fig. 5D illustrate increases in MMP7 protein above basal levels after addition of 100 μM ACh. Increased MMP7 protein expression was first detected at 4 h (0.03 ± 0.01 ng/mg protein above basal), peaked at 16 h (0.49 ± 0.04 ng/mg protein above basal), and remained elevated at 24 h (0.49 ± 0.01 ng/mg protein above basal). We also measured MMP7 protein levels in cell culture media. In media from unstimulated H508 cells, MMP7 was below the limits of detection (0.02 ng/ml). After addition of 100 μM ACh, increased MMP7 protein released into the cell culture media was first detected at 2 h (1.54 ± 0.06 ng/ml), peaked at 8 h (2.78 ± 0.09 ng/ml), and remained elevated at 24 h (2.23 ± 0.25 ng/ml). This temporal pattern for the increase in MMP7 protein production and release into the media is consistent with a delay between mRNA synthesis and translation.
ACh-induced MMP7 gene transcription requires expression of muscarinic receptors.
We compared effects of ACh on H508 colon cancer cells that express both M3R and EGFR with effects on SNU-C4 cells that express EGFR but not muscarinic receptors (15). As shown in Fig. 5E, whereas ACh stimulated a robust, ∼50-fold, increase in the level of MMP7 mRNA in H508 cells, there was a negligible response in SNU-C4 cells. In contrast, HBEGF stimulated a robust increase in MMP7 mRNA levels in both cell lines (Fig. 5E). These findings confirm that muscarinic receptor expression is required for ACh-induced MMP7 gene transcription. Moreover, as observed for cell proliferation (Fig. 1C), HBEGF mimicked the actions of ACh on MMP7 gene induction (Fig. 5E).
ACh-induced MMP7 gene transcription is mediated by transactivation of EGFR and post-EGFR ERK signaling.
We used chemical inhibitors and neutralizing antibodies to determine whether the mechanism underlying ACh-induced MMP7 gene induction was similar to that for ACh-induced cell proliferation (i.e., mediated by activation of M3R and EGFR and by post-EGFR signaling). As shown in Fig. 6A, ACh-induced MMP7 transcriptional upregulation was abolished by atropine, anti-EGFR antibody, and two EGFR activation inhibitors, PD168393 and AG1478. These findings confirm that, as observed for ACh-induced cell proliferation, ACh-induced transcriptional activation of MMP7 requires transactivation of EGFR.
Fig. 6.
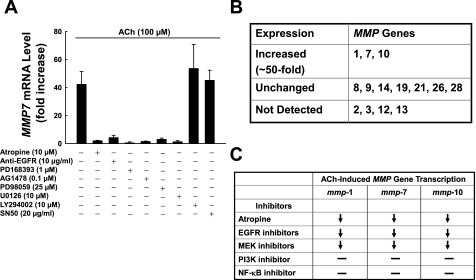
Spectrum and mechanism of ACh-induced MMP gene transcription. A: actions of inhibitors of key signaling molecules on ACh-induced upregulation of MMP7 gene transcription. H508 cells were incubated with 100 μM ACh for 4 h in the absence or presence of the indicated concentrations of atropine, EGFR antibody, EGFR inhibitors (PD168393 and AG1478), MEK inhibitors (PD98059 and U0126), phosphatidylinositol 3′-kinase (PI3K) inhibitor (LY294002), and NF-κB inhibitor (SN50). MMP7 mRNA was measured by Q-PCR. Values are means ± SE of ≥3 separate experiments. B: actions of ACh on expression of MMP genes in H508 colon cancer cells. MMP mRNA was measured by Q-PCR using primers shown in Table 1. C: actions of inhibitors of key signaling molecules on ACh-induced upregulation of MMP1, MMP7, and MMP10 gene transcription. Inhibitors and concentrations are the same as those used in Fig. 5A. ↓, Attenuation; −, no effect.
Post-EGFR signaling occurs primarily by activation of two post-receptor signaling cascades, ERK and phosphatidylinositol 3′-kinase (PI3K/AKT). To determine which of these pathways is required for ACh-induced MMP7 transcriptional induction, we examined the actions of ERK and PI3K inhibitors. As shown in Fig. 6A, inhibitors of MEK (PD98059 and U0126), the kinase immediately upstream of ERK activation, completely abolished MMP7 gene induction, whereas neither a PI3K inhibitor (LY294002) nor an NF-κB inhibitor (SN50) altered MMP7 gene induction. These results indicate that ACh-induced MMP7 transcriptional upregulation is mediated exclusively by post-EGFR ERK signaling.
ACh induces transcriptional upregulation of MMP1, MMP7, and MMP10.
Motivated by these intriguing results, we performed a systematic analysis of MMP gene expression in H508 cells (see Table 1 for primers). As shown in Fig. 6B, MMP2, MMP3, MMP12, and MMP13 are not expressed in H508 cells. MMP8, MMP9, MMP14, MMP19, MMP21, MMP26, and MMP28 are expressed, but levels are not altered by treatment with ACh. Nonetheless, in addition to MMP7, ACh caused robust time- and dose-dependent transcriptional upregulation of MMP1 and MMP10 (∼50-fold induction at 4–6 h; not shown). Compared with induction of MMP7, ACh-induced expression of MMP1 and MMP10 had similar dose responses and kinetics (i.e., peak induction of mRNA levels at 4–6 h with a return to baseline levels by 24 h; not shown). Moreover, in experiments using actinomycin D (1 μg/ml) to inhibit mRNA synthesis in H508 cells (see materials and methods), half-lives of MMP1 and MMP10 mRNA, ∼24 and 18 h, respectively, were not altered by ACh (data not shown). The half-lives of MMP genes in H508 cells were comparable to those reported by others using different cell lines (3, 24, 33). These findings indicate that, as observed with MMP7, induction of MMP1 and MMP10 mRNA levels by ACh at 4–6 h is primarily a consequence of increased transcription, not reduced degradation of mRNA transcripts.
Finally, as observed with MMP7, ACh-induced transcriptional upregulation of MMP1 and MMP10 was abolished by atropine, anti-EGFR antibody, and two EGFR inhibitors, PD168393 and AG1478 (Fig. 6C; data not shown). These results indicate that, as observed with ACh-induced MMP7 gene transcription, ACh-induced transcriptional activation of MMP1 and MMP10 requires activation of M3R and EGFR/ERK signaling. Moreover, as observed with MMP7, MEK inhibitors completely abolished MMP1 and MMP10 gene induction, whereas PI3K and NF-κB inhibitors had no effect (Fig. 6C; data not shown). These results indicate that, as observed with MMP7, ACh-induced MMP1 and MMP10 transcriptional upregulation is mediated by post-EGFR ERK signaling. However, unlike MMP7 (Fig. 1C), recombinant MMP1 and MMP10 do not stimulate colon cancer cell proliferation (5) (data for MMP10 not shown).
DISCUSSION
Proliferation of well-differentiated cancer cells is regulated by growth factors and their receptors. Hence, at early stages of neoplasia, it remains possible to attenuate tumor progression by treating cancer cells with inhibitors of growth factor receptor activation or post-receptor signaling. Our previous findings that muscarinic receptor expression and activation are critical for murine intestinal cell proliferation and neoplasia (27) and that production and release of ACh by human colon cancer cells result in autocrine stimulation of cell proliferation (8) highlight the importance of muscarinic ligands and receptors for colon neoplasia. Nonetheless, the mechanisms whereby muscarinic receptor activation stimulates colon cancer cell proliferation were largely undefined. Although activation of EGFR was clearly a necessary component of this mechanism (10), the mechanism underlying the M3R-EGFR interaction remained to be demonstrated. This mechanistic information is a prerequisite for developing targeted treatment strategies to arrest or reverse muscarinic receptor agonist-regulated intestinal neoplasia.
Collectively, the findings described in the present study provide strong evidence favoring a mechanism for ACh-induced colon cancer cell proliferation that involves MMP7-catalyzed release of HBEGF. Proliferative actions of ACh were blocked by inhibitors of MMP7 activation and HBEGF release. Addition of MMP7 or HBEGF to H508 cells mimicked the proliferative actions of ACh (Fig. 1) (9). In addition to stimulating cell proliferation (Fig. 1), activation of muscarinic signaling increases the ability of H508 to form colonies in agarose (Fig. 4). The ability of cells to proliferate independent of anchoring substrate is a requirement for successful tumor implantation (metastasis). As observed with ACh-induced cell proliferation, ACh-induced colony formation is dependent on activation of MMP7 and EGFR (Fig. 4B).
Experiments with conditioned media from H508 cells treated with ACh provided additional evidence for the crucial role of released HBEGF. Whereas conditioned media stimulated robust proliferation of SNU-C4 colon cancer cells that do not express M3R, this action was blocked by addition of anti-HBEGF antibody, anti-EGFR antibody, and an EGFR activation inhibitor (Fig. 2A), and the effects of conditioned media were mimicked by treatment of SNU-C4 cells with HBEGF in untreated H508 media (Fig. 2B). Our previous demonstration that pro-MMP7 and pro-HBEGF are colocalized on H508 cell plasma membranes (9), thereby facilitating MMP7-catalyzed shedding of HBEGF, lends further support to our conclusions. It is noteworthy that although HBEGF is pleiotropic with respect to receptors, including other members of the human EGFR (HER) family, our data using EGFR-neutralizing antibodies (Fig. 2B) strongly suggest that, in H508 cells, EGFR is the primary HER for HBEGF.
MMP7 has long been known to play a key role in colon neoplasia. MMP7 plays a critical role in the communication between GPCRs and EGFR (38). In human colon cancer cells, MMP7 mediates transactivation of EGFR by releasing HBEGF, one of seven known EGFR ligands (9). MMP7 is overexpressed in the majority of colon cancers (1, 2). Genetic ablation of MMP7 in the ApcMin mouse model of intestinal neoplasia attenuates tumor formation (36). Our findings provide a novel mechanism whereby MMP7 exerts proneoplastic effects by mediating cross talk between muscarinic and EGF receptors.
Besides identifying the mechanism whereby exposure of human colon cancer cells to cholinergic ligands results in activation of EGFR and post-receptor proliferative signaling (10), unanticipated findings demonstrate that activation of the signaling mechanism underlying ACh-induced cell proliferation also stimulates a striking induction of MMP gene transcription. Treatment of H508 cells with ACh stimulated dose- and time-dependent ∼50-fold increases in MMP1, MMP7, and MMP10 mRNA levels. These actions were attenuated by pretreatment with atropine and by inhibitors of EGFR activation and ERK signaling (Fig. 6A). ACh has little effect on degradation of MMP mRNA (Fig. 5C), indicating that these effects result primarily from upregulated MMP gene transcription.
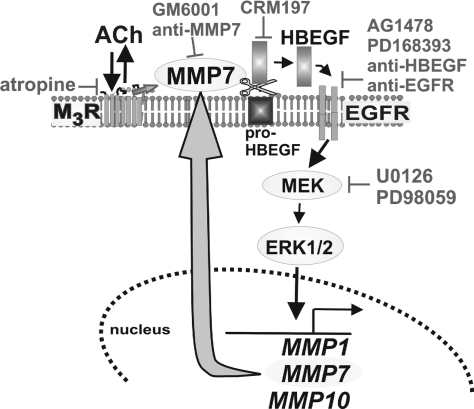
On the basis of the present findings, in Fig. 7 we propose a model delineating key steps leading from ACh interaction with M3R to MMP gene induction. Reversible interaction of ACh with M3R results in activation of MMP7. Activated MMP7 catalyzes cleavage of pro-HBEGF and release of the EGFR ligand HBEGF. As observed by others, post-EGFR signaling by the ERK pathway induces transcriptional activation of many genes that are associated with progression of colon neoplasia (c-MYC, COX2, cyclin D1, and many others) (11, 23, 28). We discovered that this pathway results in robust transcriptional activation of MMP7 (Fig. 5). It is proposed that this model of ACh-induced activation of post-M3R/EGFR ERK signaling constitutes a feed-forward mechanism whereby activated MMP7 can be replenished by the robust increase in MMP7 mRNA gene transcription and subsequent increase in MMP7 protein (Fig. 5). Although the magnitude of ACh-induced upregulation of MMP1 and MMP10 gene transcription is similar to that of MMP7, we do not know what functional roles, if any, MMP1 or MMP10 plays in H508 colon cancer cells. MMP1 and MMP10 do not stimulate H508 cell proliferation (9; present study). Future studies, beyond the scope of the present work, will address the role of ACh-induced MMP1 and MMP10 gene induction in colon cancer.
Fig. 7.
Model depicting mechanisms underlying ACh-induced activation of MMP7, EGFR, ERK signaling, and upregulation of MMP1, MMP7, and MMP10 gene transcription. Interaction of ACh with M3 muscarinic receptor (M3R) results in activation of MMP7. MMP7 catalyzes release of HBEGF from pro-HBEGF. HBEGF is an EGFR ligand. Activation of EGFR results in post-receptor ERK signaling, which stimulates MMP1, MMP7, and MMP10 gene transcription. Agents that block this cascade include a muscarinic receptor inverse agonist (atropine), inhibitors of MMP7 activation (GM6001 and anti-MMP7), an inhibitor of pro-HBEGF cleavage (CRM197), inhibitors of EGFR activation (AG1478, PD168393, anti-HBEGF, and anti-EGFR antibodies), and inhibitors of MEK (U0126 and PD98059). As indicated by the large curved arrow, our findings provide evidence that we have identified a novel feed-forward mechanism regulated by muscarinic signaling whereby increased MMP7 gene transcription can replenish activated MMP7.
Regarding in vivo activation of muscarinic receptor signaling in colon cancer, several potential sources of muscarinic agonists exist in the colon. ACh release by enteric neurons stimulates activation of intestinal ion channels, increases motility, and regulates other physiological processes (16, 31). In a similar fashion, neuronal release of ACh may stimulate progression of neoplastic intestinal cells via activation of muscarinic receptors (10). Recent provocative data from our laboratory indicate that nonneuronal ACh production by colon cancer cells provides an autocrine and/or paracrine growth factor (8). Using high-performance liquid chromatography-electrochemical detection, we demonstrated that several colon cancer cell lines, including H508, release 3–7 μM ACh into cell culture media; in this concentration range, ACh stimulates robust MMP7 gene induction (Fig. 5B). In addition, choline acetyltransferase, a critical enzyme for ACh synthesis, was detected in human colon cancer cell lines and colon cancer surgical specimens. Such nonneuronal production of ACh, which may be common in neoplastic cells (32), provides another source of this efficacious growth factor. In addition to ACh, bile acids, long reported to increase colon cancer risk, provide an additional pool of muscarinic receptor ligands. We previously demonstrated that bile acids are muscarinic receptor agonists that stimulate colon epithelial cell proliferation (5–7, 9). We reported that “physiological” concentrations of conjugated secondary bile acids interact functionally with muscarinic receptors, particularly M3R (5, 7, 9, 19). Hence, by the mechanisms delineated here, neuronal and nonneuronal ACh, acting in autocrine, paracrine, or neurocrine modes, and luminal bile acids can promote colon cancer cell proliferation and invasiveness. Our findings in a murine colon cancer model indicate that, regardless of the responsible muscarinic ligand(s), genetic ablation of muscarinic receptor expression attenuates intestinal neoplasia (27).
Finally, our results may have promising clinical implications. ACh induction of MMP7 expression by EGFR-dependent ERK signaling can be blocked at several steps (Fig. 7). As observed with other cancers, safe and successful treatment strategies are generally based on the specificity of growth factor receptor expression in colon tumors. Hence, in the majority of cancers that overexpress muscarinic receptors (37), it is likely that blocking M3R activation or cross-communication with EGFR will attenuate neoplastic cell proliferation by blocking EGFR-dependent signaling and MMP7 gene induction. In contrast, in the minority of cancers that do not overexpress M3R, as shown with SNU-C4 cells (Figs. 2 and 5E), this approach is unlikely to be effective. Instead, as shown in Fig. 2 with SNU-C4 cells, proliferation of cells that express EGFR, but not muscarinic receptors, can be attenuated by inhibiting EGFR or signaling downstream of EGFR (e.g., MEK and ERK; Fig. 7). We anticipate that further unraveling the mechanisms underlying this novel proproliferative interaction between M3R activation and MMP7 gene induction will identify additional therapeutic targets.
GRANTS
This work was supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs and by National Cancer Institute Grants CA-107345 and CA-120407 (J-P. Raufman). G. Xie was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants T32 DK-067872 (J.-P. Raufman) and K08 DK-080843 (G. Xie).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adachi Y, Yamamoto H, Itoh F, Arimura Y, Nishi M, Endo T, Imai K. Clinicopathologic and prognostic significance of matrilysin expression at the invasive front in human colorectal cancers. Int J Cancer 95: 290–294, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Adachi Y, Yamamoto H, Itoh F, Hinoda Y, Okada Y, Imai K. Contribution of matrilysin (MMP7) to the metastatic pathway of human colorectal cancers. Gut 45: 252–258, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinckerhoff CE, Plucinska IM, Sheldon LA, O'Connor GT. Half-life of synovial cell collagenase mRNA is modulated by phorbol myristate acetate but not by all-trans-retinoic acid or dexamethasone. Biochemistry 25: 6378–6384, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253: 269–285, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Cheng K, Chen Y, Zimniak P, Raufman J, Xiao Y, Frucht H. Functional interaction of lithocholic acid conjugates with M3 muscarinic receptors on a human colon cancer cell line. Biochim Biophys Acta 1588: 48–55, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Cheng K, Khurana S, Chen Y, Kennedy RH, Zimniak P, Raufman JP. Lithocholylcholine, a bile acid/acetylcholine hybrid, is a muscarinic receptor antagonist. J Pharmacol Exp Ther 303: 29–35, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Cheng K, Raufman JP. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol 70: 1035–1047, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Cheng K, Samimi R, Xie G, Shant J, Drachenberg C, Wade M, Davis RJ, Nomikos G, Raufman JP. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am J Physiol Gastrointest Liver Physiol 295: G591–G597, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng K, Xie G, Raufman JP. Matrix metalloproteinase-7-catalyzed release of HBEGF mediates deoxycholyltaurine-induced proliferation of a human colon cancer cell line. Biochem Pharmacol 73: 1001–1012, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng K, Zimniak P, Raufman JP. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res 63: 6744–6750, 2003. [PubMed] [Google Scholar]
- 11.Cohen G, Mustafi R, Chumsangsri A, Little N, Nathanson J, Cerda S, Jagadeeswaran S, Dougherty U, Joseph L, Hart J, Yerian L, Tretiakova M, Yuan W, Obara P, Khare S, Sinicrope FA, Fichera A, Boss GR, Carroll R, Bissonnette M. Epidermal growth factor receptor signaling is up-regulated in human colonic aberrant crypt foci. Cancer Res 66: 5656–5664, 2006. [DOI] [PubMed] [Google Scholar]
- 12.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, Pinto A, Normanno N. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol 214: 559–567, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc Natl Acad Sci USA 91: 609–613, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frucht H, Gazdar AF, Park JA, Oie H, Jensen RT. Characterization of functional receptors for gastrointestinal hormones on human colon cancer cells. Cancer Res 52: 1114–1122, 1992. [PubMed] [Google Scholar]
- 15.Frucht H, Jensen RT, Dexter D, Yang WL, Xiao Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin Cancer Res 5: 2532–2539, 1999. [PubMed] [Google Scholar]
- 16.Geibel JP Secretion and absorption by colonic crypts. Annu Rev Physiol 67: 471–490, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Gutkind JS, Novotny EA, Brann MR, Robbins KC. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc Natl Acad Sci USA 88: 4703–4707, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature 400: 464–468, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton JP, Xie G, Raufman JP, Hogan S, Griffin TL, Packard CA, Chatfield DA, Hagey LR, Steinbach JH, Hofmann AF. Human cecal bile acids: concentration and spectrum. Am J Physiol Gastrointest Liver Physiol 293: G256–G263, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu K, Buchanan FG, Katkuri S, Morrow JD, Inoue H, Otaka M, Watanabe S, DuBois RN. Oncogenic potential of MEK1 in rat intestinal epithelial cells is mediated via cyclooxygenase-2. Gastroenterology 129: 577–590, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366: 2–16, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Ozgul C, Karaoz E, Erdogan D, Dursun A. Expression of epidermal growth factor receptor in normal colonic mucosa and in adenocarcinomas of the colon. Acta Physiol Hung 85: 121–128, 1997. [PubMed] [Google Scholar]
- 23.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med 8: 289–293, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Poulalhon N, Farge D, Roos N, Tacheau C, Neuzillet C, Michel L, Mauviel A, Verrecchia F. Modulation of collagen and MMP1 gene expression in fibroblasts by the immunosuppressive drug rapamycin. A direct role as an antifibrotic agent? J Biol Chem 281: 33045–33052, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHBEGF. Nature 402: 884–888, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Raufman JP, Chen Y, Cheng K, Compadre C, Compadre L, Zimniak P. Selective interaction of bile acids with muscarinic receptors: a case of molecular mimicry. Eur J Pharmacol 457: 77–84, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Raufman JP, Samimi R, Shah N, Khurana S, Shant J, Drachenberg C, Xie G, Wess J, Cheng K. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res 68: 3573–3578, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26: 3291–3310, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19: 183–232, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Shin SI, Freedman VH, Risser R, Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci USA 72: 4435–4439, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh SK, Binder HJ, Boron WF, Geibel JP. Fluid absorption in isolated perfused colonic crypts. J Clin Invest 96: 2373–2379, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song P, Sekhon HS, Jia Y, Keller JA, Blusztajn JK, Mark GP, Spindel ER. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res 63: 214–221, 2003. [PubMed] [Google Scholar]
- 33.Sundareshan P, Nagle RB, Bowden GT. EGF induces the expression of matrilysin in the human prostate adenocarcinoma cell line, LNCaP. Prostate 40: 159–166, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Wess J Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol 10: 69–99, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Wess J Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol 44: 423–450, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA 94: 1402–1407, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang WL, Frucht H. Cholinergic receptor up-regulates COX-2 expression and prostaglandin E2 production in colon cancer cells. Carcinogenesis 21: 1789–1793, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Yu WH, Woessner JF Jr, McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev 16: 307–323, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu S, Bjorge JD, Cheng HC, Fujita DJ. Decreased CHK protein levels are associated with Src activation in colon cancer cells. Oncogene 27: 2027–2034, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev 23: 101–117, 2004. [DOI] [PubMed] [Google Scholar]