Abstract
In cystic fibrosis, impaired secretion resulting from loss of activity of the cystic fibrosis transmembrane conductance regulator (CFTR) causes dehydration of intestinal contents and life-threatening obstructions. Conversely, impaired absorption resulting from loss of the NHE3 Na+/H+ exchanger causes increased fluidity of the intestinal contents and diarrhea. To test the hypothesis that reduced NHE3-mediated absorption could increase survival and prevent some of the intestinal pathologies of cystic fibrosis, Cftr/Nhe3 double heterozygous mice were mated and their offspring analyzed. Cftr-null mice lacking one or both copies of the NHE3 gene exhibited increased fluidity of their intestinal contents, which prevented the formation of obstructions and increased survival. Goblet cell hyperplasia was eliminated, but not the accumulation of Paneth cell granules or increased cell proliferation in the crypts. Microarray analysis of small intestine RNA from Cftr-null, NHE3-null, and double-null mice all revealed downregulation of genes involved in xenobiotic metabolism, including a cohort of genes involved in glutathione metabolism. Expression of energy metabolism genes was altered, but there were no changes in genes involved in inflammation. Total intracellular glutathione was increased in the jejunum of all of the mutants and the ratio of reduced to oxidized glutathione was reduced in Cftr-null mutants, indicating that CFTR deficiency affects intestinal glutathione metabolism. The data establish a major role for NHE3 in regulating the fluidity of the intestinal contents and show that reduced NHE3-mediated absorption reverses some of the intestinal pathologies of cystic fibrosis, thus suggesting that it may serve as a potential therapeutic target.
Keywords: Slc9a3, distal intestinal obstructive syndrome, meconium ileus, cytochrome P-450, glutathione transferase
cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), an apical membrane Cl− channel that serves as the major mechanism for anion secretion in lung, intestine, pancreas, and other epithelial tissues. Although CF affects many organs, one of the major pathological consequences of CF is the occurrence of intestinal obstructions. In humans, meconium ileus (MI) is a frequent occurrence in CF newborns, and ∼15% of adults with CF develop distal intestinal obstructive syndrome (DIOS) (12, 39). A similar phenotype occurs in CF mice, which are subject to lethal obstructions of the ileum beginning a few days after birth and rarely survive beyond 6 wk of age unless treated with an osmotic laxative (6) or maintained on a liquid diet (14). CF mice develop goblet cell hyperplasia and inflammation of the intestine (42, 54), which contribute to the disease process. They also have impaired innate immunity due to the failure of Paneth cell granules to dissolve in the crypts of the small intestine (5), and the crypts show an abnormally high proliferative capacity (18).
Treatment with an osmotic laxative reduces the incidence of DIOS in human CF patients (39) and leads to a reduction in intestinal obstructions and enhanced survival in CF mice (6), thus indicating that a major factor in CF intestinal disease is the reduced fluidity of the intestinal contents. Homeostatic regulation of intestinal fluidity depends on an appropriate balance between the secretion and absorption of ions (21), with perturbations leading to either obstructions or diarrhea. A widely accepted paradigm is that fluidity is principally influenced by apical secretory mechanisms, particularly anion secretion through the CFTR. Toxigenic stimulation of CFTR, for example, leads to diarrhea and sharply increased intestinal fluidity (17, 56), whereas a reduction in CFTR-mediated secretion leads to poor hydration of intestinal contents (54). However, in a contrasting phenotype, mice lacking the NHE3 Na+/H+ exchanger exhibit severe diarrhea and have increased luminal fluid throughout the intestinal tract (51). Additionally, toxin B of Clostridium difficile, which induces diarrhea in humans, acts by inhibiting NHE3 activity (25), and there have been reports of congenital Na+-wasting diarrhea that appear to be due to impaired Na+/H+ exchange (41).
Loss of NHE3 has been shown to cause a severe reduction in Na+ absorption in the intestine (22), with partial compensation occurring in the colon via upregulation of both the epithelial Na+ channel and the colonic H+-K+-ATPase (51), which is needed for maximum Na+ channel activity (55). Because of their opposing roles in the maintenance of intestinal fluid homeostasis (21), it seemed possible that the loss or reduction of NHE3-mediated absorption might counteract some of the pathological consequences of CFTR deficiency. To test this hypothesis, we crossed mice that were heterozygous for both the Cftr- and Nhe3-null mutations and analyzed their offspring with respect to survival rates, intestinal fluidity, histopathology and cell proliferation in the crypts. We also performed microarray analyses using RNA samples from small intestine of Cftr-null (Cftr−/−Nhe3+/+), Nhe3-null (Cftr+/+Nhe3−/−), and Cftr/Nhe3-double null (Cftr−/−Nhe3−/−) mice and wild-type controls. Although we had anticipated that NHE3 deficiency might counter some of the gene expression changes occurring in response to CFTR deficiency, the observed patterns indicated similar alterations in xenobiotic and glutathione metabolism. The results show that partial or complete loss of NHE3 in CF mice increases luminal fluidity and prevents goblet cell hyperplasia, thus reducing the incidence of obstructions and increasing the survival rate. The data suggest that absorption can dominate over secretion in regulating luminal fluidity and suggest that inhibition of NHE3-mediated absorption might be a useful strategy for treating some of the intestinal pathologies of CF.
METHODS
Animals.
Mice with mutations in the Cftr and Nhe3 genes were described previously (51, 54) and were inbred onto the FVB/N background for at least 10 generations prior to being used in this study. The CF mice (Cftrtm1Unc) carried the S486X mutation with a stop codon after Ser489 (54). Mice heterozygous for null mutations in both the Cftr and Nhe3 genes were mated and produced offspring of all nine genotypes. Cftrtm1Unc genotypes were determined by PCR as described by Jackson Laboratory (http://jaxmice.jax.org/pub-cgi/protocols/protocols.sh?objtype=protocol&protocol_id=798). Nhe3 genotypes were determined by PCR as described previously (63). Mice were fed standard laboratory chow and had access to food and water ad libitum. Animals were euthanized by asphyxiation in 100% CO2 followed by bilateral thoracotomy to induce pneumothorax. All animal experiments were approved by the University of Cincinnati or the University of Missouri Institutional Animal Care and Use Committees.
Measurements of fluidity of intestinal contents.
Fluidity of the intestinal contents was measured by a modification of the enteropooling assay, which is used to assess the severity of induced diarrhea in rodents (46). The luminal contents were collected from freshly excised intestine, the wet weight was determined, and the sample was dried at 55°C overnight. The dried contents were weighed and the weight of the original water content (wet weight − dry weight) was calculated. A numerical value for fluidity of the luminal contents was calculated as the ratio of water weight to dry weight. Fluidity measurements were made only in adult mice, precluding such measurements in Cftr−/−Nhe3+/+ mice, which exhibit early mortality.
Histological analysis.
All mice analyzed were ∼25 days old. Freshly excised tissue was flushed with phosphate-buffered saline, coiled into a Swiss Roll, fixed in 10% neutral buffered formalin at room temperature for 24 h, and embedded in paraffin. For analysis of crypt morphology and measurements of the crypt-villus axis (CVA), 5-μm sections were stained with hematoxylin and eosin. The number of crypts containing undissolved Paneth cell granules was reported as the percent of granule-positive crypt lumens within at least 10 fields of view per mouse. For morphometric analysis of the CVA, a minimum of 10 ileal CVAs were measured per mouse, with the crypt and villus heights measured separately. Only complete CVAs, in which the entire villus and its adjacent crypt were full length, were measured. To identify goblet cells, sections were stained with Alcian blue and periodic acid-Schiff (AB/PAS). Briefly, rehydrated sections were incubated with Alcian blue (pH 2.5) for 30 min, followed by 5 min in 0.5% periodic acid. Slides were then incubated with Schiff's reagent (Sigma, St. Louis, MO) for 30 min, washed in water, and counterstained with aqueous hematoxylin. At pH 2.5 acidic mucins stain blue and neutral mucins stain magenta. The number of goblet cells along each villus was normalized to 100 μm. For all histological analyses, the sample number per genotype was equal to the number of mice. All images were taken on a Zeiss Axioskop with an Axiocam MRc5 camera, and measurements were made by use of Axiovision 4.5 software.
Cell proliferation.
To assay proliferation, tissues were fixed as described above. Slides were deparaffinized in CitriSolve (Fisher Scientific, Pittsburgh, PA) and rehydrated through successive ethanol washes. Proliferating cell nuclear antigen (PCNA) was assayed by using a PCNA kit (Zymed Laboratories, San Francisco, CA) according to the manufacturer's directions, with an additional epitope retrieval step in boiling 10 mM sodium citrate, pH 8.6. The nuclei in at least 10 whole crypts per mouse were counted. Statistics were based on the number of PCNA-positive nuclei divided by the total number of nuclei in the crypts. The sample number used for statistics reflects the number of mice used; four to five mice per genotype were analyzed.
Microarray analyses.
Whole small intestine was homogenized in Tri-Reagent (Molecular Research Center, Cincinnati, OH) and total RNA was extracted according to the manufacturer's directions. Because all analyses were performed using RNA from adult mouse intestine, samples for the CFTR arrays (C57BL/6J Cftr−/−Nhe3+/+ mice and wild-type controls) were obtained from a colony at the University of Missouri that had been maintained on an osmotic laxative (Colyte; Schwarz Pharma, Seymore, IN) from birth. All other mice were from colonies at the University of Cincinnati and were on the FVB/N background. Microarray analyses were performed by the University of Cincinnati Genomics and Microarray Laboratory core facility by using spotted 70-mer oligonucleotide libraries. cDNA probes were generated from total RNA and were labeled with either the Cy3 or Cy5 fluorophores. Within each experiment, the Cy3 and Cy5 labeling was alternated between wild-type and mutant samples (dye-flip controls) to eliminate any influence of the fluorophore on probe hybridization. Values are presented as fold changes (ratio of fluorescence intensity values), with values greater than +1 indicating upregulation in mutant relative to wild-type samples and values less than −1 indicating downregulation in mutant relative to wild-type samples. Three separate sets of microarray experiments were performed: 1) Cftr-null, n = 4 pairs of Cftr−/−Nhe3+/+ and Cftr+/+Nhe3+/+ mice on the C57BL/6J background); 2) Nhe3-null, n = 6 pairs of Cftr+/+Nhe3−/− and Cftr+/+Nhe3+/+ mice on the FVB/N background; 3) Cftr/Nhe3 double-null, n = 6 pairs of Cftr−/−Nhe3−/− and Cftr+/+Nhe3+/+ mice on the FVB/N background. The Cftr (set 1) and Nhe3 (set 2) samples were analyzed by using a modified Operon v.3 platform (GEO: GPL5738), containing 28,878 array elements (Qiagen-Operon, Alameda, CA). The Cftr/Nhe3 (set 3) samples were analyzed by using the Illumina Mouse Exonic Evidence Based Oligonucleotide platform (GEO: GPL5137), which contains 38,467 elements (Invitrogen). The complete sets of microarray data, as well as lists of the significantly changed genes and more detailed descriptions of the methods, have been deposited in National Center for Biotechnology Information's Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO accession numbers GSM227447 (set 2, Cftr+/+Nhe3−/− vs. wild-type), GSM217788 (set 1, Cftr−/−Nhe3+/+ vs. wild-type), and GSM228723 (set 3, Cftr−/−Nhe3−/− vs. wild-type). Analysis was performed using R statistical software and the limma Bioconductor package (53), and significance was determined using a empirical Bayes procedure, Intensity-Based Moderated T-statistic, as previously described (48).
Northern blots.
Total RNA was prepared as described for microarrays. Membranes were blotted with 10 μg total RNA per lane and probed with 32P-deoxycytidine triphosphate-labeled cDNA probes as described previously (62). Primer sequences used to generate cDNA probes are reported in Supplementary Table S1. Signal was detected by use of a PhosphorImager (Amersham Biosciences, Piscataway, NJ), and band intensities were quantified by using ImageQuant software (Amersham Biosciences) and normalized to the L32 ribosomal subunit mRNA.
Glutathione measurements.
Total glutathione and oxidized glutathione (GSSG) were measured using a Glutathione Assay Kit (Cayman Chemical, Ann Arbor, MI). Briefly, 300 mg of jejunum was rinsed and homogenized in cold MES buffer. After centrifugation, the supernatant was deproteinated with metaphosphoric acid and treated with triethanolamine. Half of each sample was then treated with 2-vinylpyridine to derivatize the reduced glutathione (GSH), and this portion was used to measure GSSG. Both samples were further processed according to the manufacturer's instructions. GSSG levels were subtracted from total glutathione to obtain concentrations of GSH.
Statistics.
Comparison of survival curves, which were prepared by the Kaplan-Meier procedure, was done by log rank analysis with a post hoc Holm-Sidak test for multiple pairwise comparisons. Comparisons between the expected Mendelian genotype ratios and observed ratios were done by χ2 analysis. Comparisons of two groups were made by using a Student's t-test. Comparisons among multiple groups were made by using a 1-way ANOVA with a post hoc Tukey's t-test. Statistics are represented as means ± SE; P < 0.05 was considered significant.
RESULTS
Genotype ratios and survival of offspring of Cftr+/−Nhe3+/− breeders.
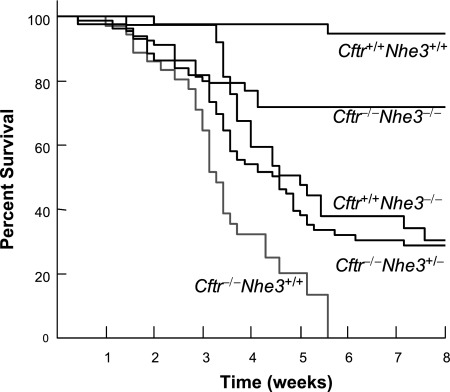
Breeding of double heterozygous mice yielded offspring of all nine genotypes in a normal Mendelian ratio (Table 1). A significant increase in mortality was not observed for any genotype during the first week after birth, but about a third of the Cftr−/−Nhe3+/+ mice died by weaning (3 wk) and none of them survived beyond 6 wk of age (Fig. 1), consistent with previous reports (54). Cftr+/+Nhe3−/− mice exhibited a high mortality rate beginning just after weaning, with ∼30% surviving to adulthood. The high death rate of Nhe3-null mice after weaning was sharply reduced by the presence of the Cftr-null mutation. Partial loss of NHE3 increased survival of CF mice (Cftr−/−Nhe3+/− genotype) to adulthood from 0 to 28%, whereas 66% of the Cftr−/−Nhe3−/− mice survived to adulthood (Fig. 1). After 8 wk of age the incidence of death among CF mice that were heterozygous or homozygous for the Nhe3 mutant allele was very low, with many of them surviving beyond 1 yr of age.
Table 1.
Mendelian ratios of offspring from Cftr+/−Nhe3+/− breeders
| Cftr | Nhe3 | No. of Mice | Frequency |
|---|---|---|---|
| +/+ | +/+ | 49 (48) | 0.063 (0.063) |
| +/+ | +/− | 110 (97) | 0.142 (0.125) |
| +/+ | −/− | 55 (48) | 0.071 (0.063) |
| +/− | +/+ | 110 (97) | 0.142 (0.125) |
| +/− | +/− | 156 (193) | 0.202 (0.250) |
| +/− | −/− | 97 (97) | 0.125 (0.125) |
| −/− | +/+ | 43 (48) | 0.056 (0.063) |
| −/− | +/− | 95 (97) | 0.123 (0.125) |
| −/− | −/− | 58 (48) | 0.075 (0.063) |
Values represent observed distributions with expected Mendelian distributions in parenthesis. P > 0.05 for all genotypes based on χ2 analysis.
Fig. 1.
Survival of offspring from Cftr+/−Nhe3+/− breeders. Double heterozygous mice were bred and survival of mice for 5 of the resulting 9 genotypes (see Table 1) was analyzed. The numbers of mice and genotypes analyzed were: 44 Cftr+/+Nhe3+/+, 39 Cftr+/+Nhe3−/−, 44 Cftr−/−Nhe3−/−, 81 Cftr−/−Nhe3+/−, and 36 Cftr−/−Nhe3+/+. Cftr−/−Nhe3+/+ mice exhibited 100% mortality by 6 wk of age, but survival improved dramatically in Cftr−/−Nhe3+/− and Cftr−/−Nhe3−/− mice. All curves, except those for Cftr−/−Nhe3+/− and Cftr+/+Nhe3−/− mice, were significantly different (P < 0.009) from each other based on a Kaplan-Meier log rank test.
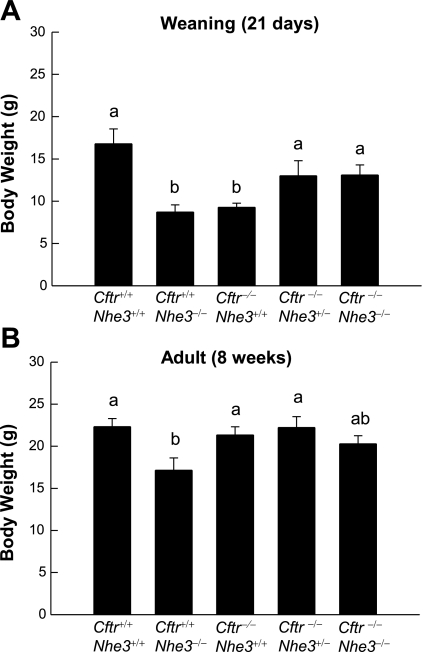
At weaning, the body weights of Cftr−/−Nhe3+/+ (9.3 ± 0.5 g) and Cftr+/+Nhe3−/− (8.7 ± 0.8 g) mice were significantly less than that of wild-type mice (16.8 ± 1.8 g) (Fig. 2A). Although Cftr−/−Nhe3−/− mice that survived to weaning were larger than the single-null mice, those that died before weaning tended to be quite small and sickly. Although slightly smaller than wild-type mice, the body weights of adult Cftr−/−Nhe3+/− and Cftr−/−Nhe3−/− mice did not differ significantly from those of wild-type mice (Fig. 2B). By 8 wk of age, only the Cftr+/+Nhe3−/− mice were significantly smaller than wild-type mice, and Cftr−/−Nhe3+/+ mice that were maintained on Colyte were able to attain normal body weights (Fig. 2B).
Fig. 2.
Effects of Cftr- and Nhe3-null mutations on body weight. Average body weights of mice of the 5 genotypes shown were analyzed at weaning (A) and adulthood (B). The weight of Cftr−/−Nhe3+/+ and Cftr+/+Nhe3−/− mice was significantly lower than that of wild-type (WT) mice at weaning, and this was moderated in Cftr−/−Nhe3+/− and Cftr−/−Nhe3−/− mice. At 8 wk of age, all mice, except Cftr+/+Nhe3−/− mice, had relatively normal body weights. The Cftr−/−Nhe3+/+ mice in B were maintained on the osmotic laxative Colyte, which prevents desiccation of intestinal contents. In each graph, n = 6–16 mice per genotype. Groups with the same letters (a or b) did not differ significantly; those with different letters were significantly different (P < 0.05) based on a 1-way ANOVA with a post hoc Tukey's t-test.
Adult Cftr−/−Nhe3−/− mice could be recognized by their swollen abdomen and diarrhea; however, adult Cftr−/−Nhe3+/− mice were indistinguishable in appearance and behavior from wild-type mice. When three male Cftr−/−Nhe3+/− mice were bred with wild-type females, all of them were fertile beyond 8 mo of age, which has also been observed in Colyte-treated Cftr−/−Nhe3+/+ male mice (L. L. Clarke, unpublished observation). Death of Cftr−/−Nhe3+/− mice, when it occurred, appeared to be due to intestinal obstructions, but obstructions did not occur in Cftr−/−Nhe3−/− mice.
Diarrhea phenotype and increased intestinal fluidity predominates in Cftr−/−Nhe3−/− mice.
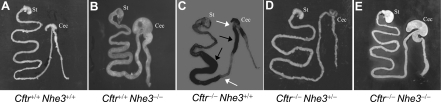
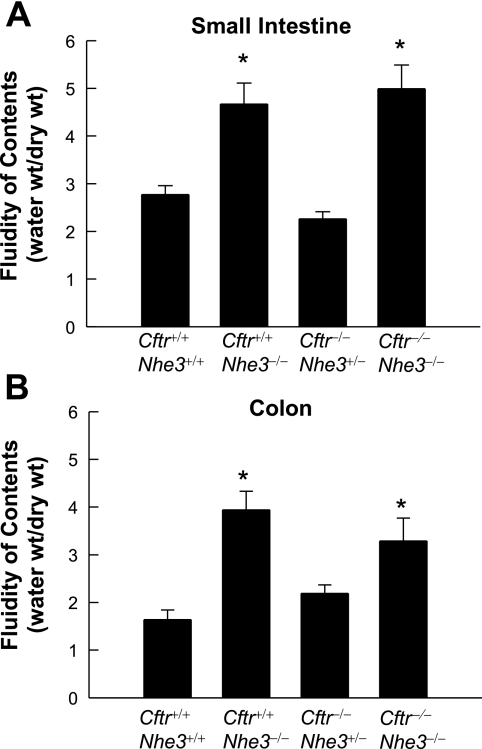
As reported previously (51), Cftr+/+Nhe3−/− mice had a swollen intestinal tract (compare Fig. 3, A and B), enlarged ceca (Fig. 3B, Supplementary Fig. S1), high fluidity of intestinal and colonic contents (Fig. 4), and chronic diarrhea, whereas Cftr−/−Nhe3+/+ mice developed lethal obstructions in the ileum and at the ileal-cecal junction (Fig. 3C) and had characteristic corkscrew-shaped ceca (Fig. 3C, Supplementary Fig. S1). Despite the complete loss of CFTR, Cftr−/−Nhe3−/− mice did not develop impactions, and they had enlarged ceca and expanded cecal volumes (Fig. 3E), increased fluidity in the small intestine and colon (Fig. 4), and diarrhea. Cftr−/−Nhe3+/− mice had a much lower incidence of impactions than Cftr−/−Nhe3+/+ mice and the fluidity of their small intestinal and colonic contents was relatively normal (Fig. 4).
Fig. 3.
Gross anatomy of the intestinal tract of Nhe3 and Cftr mutants. Note the swollen intestine, cecum, and colon in mice lacking NHE3 (B and E) and the abnormal “corkscrew” ceca (see Supplementary Fig. S1) in Cftr−/−Nhe3+/+ (C) and Cftr−/−Nhe3+/− mice (D). The midpoint of 2 impactions (dark arrows) and the points at which the blockages occurred (white arrows) are shown in the Cftr−/−Nhe3+/+ intestine (C); one blockage occurred in the proximal ileum and the other near the ileal-cecal junction. The Nhe3−/− phenotype, characterized by high fluidity of intestinal, cecal, and colonic contents and expansion of the cecum, is dominant in Cftr−/−Nhe3−/− mice. In each image, the stomach (St) is oriented in the upper left and the cecum (Cec) in the upper right. All mice were 7–8 wk of age except in C, which was 3 wk old.
Fig. 4.
Fluidity of intestinal and colonic contents is increased in adult mice lacking NHE3. Luminal contents of the small intestine and colon were collected, and both wet weight and dry weight of the contents were determined. Fluidity was calculated as the weight ratio of water content to dry content (water wt/dry wt) in small intestine (A) and colon (B). *P < 0.05 compared with WT based a 1-way ANOVA with a post hoc Tukey's t-test; n = 6–9 mice per genotype.
Nhe3 deficiency reduces goblet cell hyperplasia in CF mice.
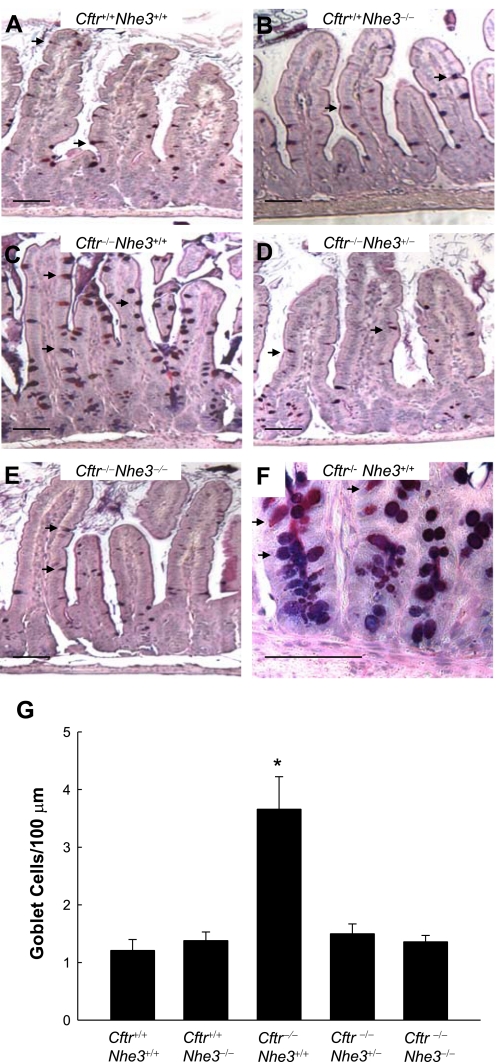
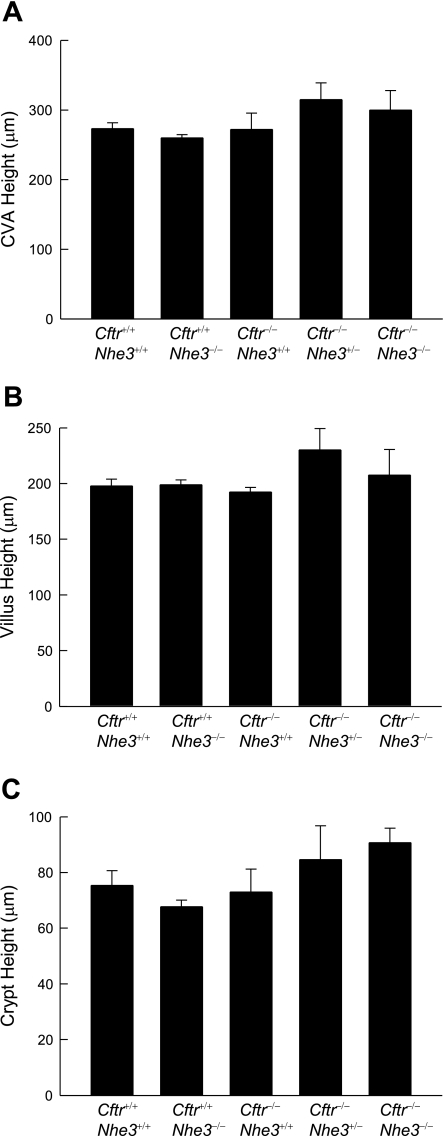
The intestinal tract of CF mice is known to exhibit a number of histological abnormalities, including goblet cell hyperplasia, accumulation of eosinophilic granules in the crypt lumen (5, 24), and increased proliferation of crypt cells (18). Goblet cell hyperplasia in the villi was apparent in Cftr−/−Nhe3+/+ mice, as expected, but this phenotype was eliminated in CF mice lacking one or both copies of the Nhe3 gene. Goblet cell numbers per unit length of villus epithelium (Fig. 5) or expressed as a percent of total villus epithelial cells (Supplementary Fig. S2) were increased approximately three- to fourfold in 25-day-old CF mice that were wild-type with respect to Nhe3 but were normal in CF mice lacking one or both copies of the Nhe3 gene. This was due to an absolute increase in the number of goblet cells since there were no significant differences in heights of the CVA, villi, or crypts (Fig. 6).
Fig. 5.
Goblet cell hyperplasia of the ileal villi of cystic fibrosis (CF) mice is corrected by loss of one or both copies of the Nhe3 gene. Representative Alcian blue and periodic acid-Schiff (AB/PAS)-stained sections of ileum from Cftr+/+Nhe3+/+ (A), Cftr+/+Nhe3−/− (B), Cftr−/−Nhe3+/+ (C), Cftr−/−Nhe3+/− (D), and Cftr−/−Nhe3−/− (E) mice. Arrows indicate goblet cells, which appear dark blue (containing acidic mucins) or magenta (containing neutral or a mix of neutral and acidic mucins). Normal goblet cell numbers were observed in Cftr+/+Nhe3+/+ (A) and Cftr+/+Nhe3−/− (B) mice. In contrast, an increase in both the numbers and apparent size of the goblet cells is evident in Cftr−/−Nhe3+/+ mice (C). Goblet cell hyperplasia is corrected in Cftr−/−Nhe3+/− (D) and Cftr−/−Nhe3−/− (E) mice. F: Cftr−/−Nhe3+/+ crypts also exhibit severe goblet cell hyperplasia. Scale bars represent 50 μm. G: cell counts of villus goblet cells (normalized to 100 μm) indicate a ∼3-fold increase in goblet cell numbers in Cftr−/−Nhe3+/+ mice relative to other genotypes. *P < 0.05 compared with WT based on a 1-way ANOVA with a post hoc Tukey's t-test; n = 3–6 mice per genotype.
Fig. 6.
Crypt and villus heights do not differ significantly among WT and mutant mice. Measurements of the heights of crypt-villus axes (CVA; A), villi (B), and crypts (C) in the ileum of 25-day-old mice revealed no significant differences among genotypes. Only those axes in which both the villus and its adjacent crypt were full length were measured; a minimum of 10 crypt-villus axes were measured for each mouse. *P < 0.05 compared with WT based on a 1-way ANOVA with a post hoc Tukey's t-test; n = 4–6 mice per genotype.
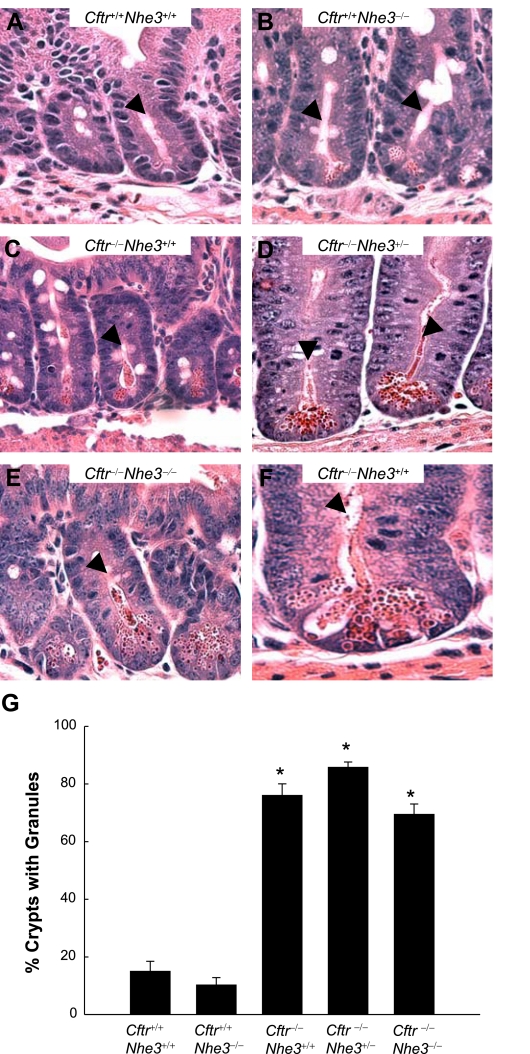
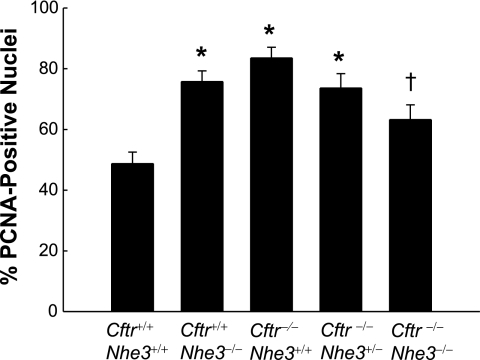
Despite the correction of goblet cell hyperplasia in CF mice lacking one or two copies of the Nhe3 gene, the phenotype involving Paneth cell granules was not corrected and there was only a small effect on cell proliferation. The crypts of all three CF genotypes (wild-type, heterozygous, or homozygous with respect to the Nhe3-null allele) had undissolved eosinophilic granules, which are secreted by Paneth cells (Fig. 7), and the Nhe3 genotype had no apparent effect on the magnitude of this defect. The number of PCNA-positive cells in the crypts, indicative of cell proliferation, was significantly increased in both Cftr-null and Nhe3-null mice that were wild-type for the other transporter; however, loss of two Nhe3 alleles in Cftr−/− mice led to a moderate but statistically significant reduction in cell proliferation (Fig. 8).
Fig. 7.
Paneth cell granules accumulate in the crypts of mice lacking CFTR, even in the absence of NHE3. Hematoxylin and eosin-stained sections of crypts from ileum of Cftr+/+Nhe3+/+ (A), Cftr+/+Nhe3−/− (B), Cftr−/−Nhe3+/+ (C), Cftr−/−Nhe3+/− (D), and Cftr−/−Nhe3−/− mice (E). F: higher magnification of a crypt from a Cftr−/−Nhe3+/+ mouse ileum. Arrowheads point to crypt lumens; undissolved granules are highly eosinophilic (dark red). Original images were taken at ×40 magnification. G: the percent of crypts containing undissolved granules was greatly increased in all Cftr−/− mice, regardless of Nhe3 genotype. Crypts were analyzed in at least 10 ×400 fields of view; n = 3–6 mice per genotype. *P < 0.05 compared with WT based on a 1-way ANOVA with a post hoc Tukey's t-test.
Fig. 8.
Increased cell proliferation in crypts of Cftr-null and Nhe3-null mice. Cell proliferation in duodenal crypts was assessed by quantitation of cells staining positive for proliferating cell nuclear antigen (PCNA), a nuclear marker of proliferation. Bars represent the number of PCNA-positive nuclei as a fraction of the total number of nuclei. Nuclei were counted in at least 10 whole crypts per mouse; n = 4–6 mice per genotype. *P < 0.05 compared with WT and †P < 0.05 compared with Cftr−/−Nhe3+/+ based on a 1-way ANOVA with a post hoc Tukey's t-test.
Microarray analysis of changes in gene expression.
Altered expression of genes involved in xenobiotic metabolism, energy metabolism, and inflammation has been reported in CF mouse small intestine (42). Thus it was of interest to determine whether the additional loss of NHE3 in CF mouse intestine might reverse these changes. Three independent sets of microarray analyses were performed. In each set, gene expression levels in small intestine of mutant (either Cftr−/−Nhe3+/+, Cftr+/+Nhe3−/−, or Cftr−/−Nhe3−/−) mice were compared with their respective wild-type (Cftr+/+Nhe3+/+) controls. When Cftr−/−Nhe3+/+ and Cftr+/+Nhe3+/+ samples were analyzed, significant upregulation of 103 elements and downregulation of 132 elements were observed in the CF mouse intestine. Separate comparisons revealed 134 upregulated and 132 downregulated elements in Cftr+/+Nhe3−/− intestine and 107 upregulated and 128 downregulated elements in Cftr−/−Nhe3−/− intestine, relative to Cftr+/+Nhe3+/+ controls. The complete data sets have been deposited in GEO (see methods).
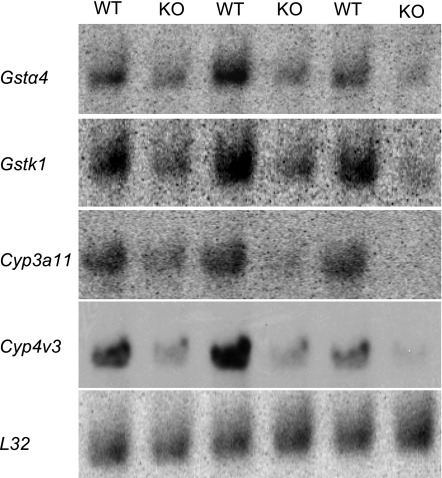
A consistent pattern in all three sets of data was downregulation of genes involved in phase I and phase II xenobiotic metabolism (Table 2). The only gene of this group that was upregulated was glutathione peroxidase 2 (Gpx2), a phase II enzyme. Among drug-metabolism genes with statistically significant changes in expression, only cytochrome P-450 3a11 (Cyp3a11) and epoxide hydrolase 2 (Ephx2) were in common among all three arrays. However, most of the genes that were significantly changed in one set of experiments exhibited similar changes in the other experiments; for comparison, these values have been included in Table 2. Confirmatory Northern blot analysis of two of the cytochrome P-450s (phase I enzymes) and two of the glutathione S-transferases (GSTs, phase II enzymes) showed a similar degree of downregulation as that observed in the microarray analyses (Fig. 9).
Table 2.
Differentially expressed genes involved in xenobiotic metabolism
| Symbol | Name | Cftr−/−Nhe3+/+ | Cftr+/+Nhe3−/− | Cftr−/−Nhe3−/− |
|---|---|---|---|---|
| Phase I | ||||
| Cyp2c29 | cytochrome P-450 2c29 | −4.44* | −2.09 | −1.37† |
| Cyp2c40 | cytochrome P-450 2c40 | −1.63 | NA | −1.92* |
| Cyp2c55 | cytochrome P-450 2c55 | −4.87* | NA | −1.24 |
| Cyp2c65 | cytochrome P-450 2c65 | −1.77* | −3.42 | −2.45* |
| Cyp2c66 | cytochrome P-450 2c66 | NA | NA | −2.22*† |
| Cyp2d26 | cytochrome P-450 2d26 | −1.36† | −1.94* | −2.56*† |
| Cyp2j5 | cytochrome P-450 2j5 | −2.11* | NA | NA |
| Cyp2j6 | cytochrome P-450 2j6 | −1.89*† | −1.56* | 1.10 |
| Cyp2j9 | cytochrome P-450 2j9 | NA | −1.58* | NA |
| Cyp3a11 | cytochrome P-450 3a11 | −2.69* | −3.27* | −3.65* |
| Cyp3a13 | cytochrome P-450 3a13 | −1.79* | −1.42 | −1.22† |
| Cyp3a16 | cytochrome P-450 3a16 | −2.16* | −1.79 | −1.15 |
| Cyp3a25 | cytochrome P-450 3a25 | NA | NA | −2.68*† |
| Cyp3a41 | cytochrome P-450 3a41 | −2.97* | −2.89 | −2.40*† |
| Cyp4v3 | cytochrome P-450 4v3 | NA | NA | −2.87* |
| Cyp27a1 | cytochrome P-450 27a1 | −1.43 | −1.06 | −1.52*† |
| Aadac | acrylacetamide deacetylase | −1.36 | −2.21* | −1.93* |
| Adh1 | alcohol dehydrogenase 1 | −1.85*† | −1.59† | −2.61 |
| Aldh1a1 | aldehyde dehydrogenase 1A1 | −1.59* | −2.20* | −2.54* |
| Cat | catalase | −1.65 | −2.38 | −2.21* |
| Ces3 | carboxylesterase 3 | −1.63† | −1.09 | −2.52* |
| Ces6 | carboxylesterase 6 | −2.80* | −1.41 | −1.63 |
| Ephx2 | epoxide hydrolase 2 | −1.66* | −1.76* | −1.92* |
| Pon2 | paraoxonase | −1.58* | −1.54 | −1.21 |
| Phase II | ||||
| Gsta1 | glutathione S-transferase alpha 1 | −1.61 | −2.02* | −1.90 |
| Gsta2 | glutathione S-transferase alpha 2 | −1.92* | −3.60 | −2.12† |
| Gsta4 | glutathione S-transferase alpha 4 | −1.47 | −1.77 | −2.24* |
| Gstk1 | glutathione S-transferase kappa 1 | 1.01† | −1.31*† | −2.64*† |
| Gstm1 | glutathione S-transferase mu 1 | −5.51* | −2.47 | 1.33† |
| Gstm2 | glutathione S-transferase mu 2 | −2.21* | NA | −1.17† |
| Gstm3 | glutathione S-transferase mu 3 | −2.85 | −1.94* | −1.81 |
| Gpx2 | glutathione peroxidase 2 | 1.59 | 1.78* | 2.17* |
| Gclm | glutamate-cysteine ligase, modifier | −1.39 | −1.39 | −1.72* |
| Sult1d1 | sulfotransferase 1D1 | −1.41† | −1.66* | −1.61* |
| Ugt1a1 | UDP glucuronosyltransferase 1A1 | −1.33 | NA | −1.38*† |
| Ugt1a12 | UDP glucuronosyltransferase 1A9 | −1.17 | −1.50* | −1.99*† |
| Ugt2b5 | UDP glucuronosyltransferase 2b5 | −2.99† | −1.35*† | −1.41 |
| Phase III | ||||
| Abcc2 | ATP-binding cassette C2 | −2.37* | −1.33 | −1.19† |
Three sets of microarray analyses were performed with small intestine RNA from the indicated genotypes compared with wild-type controls; data are presented as fold changes (see methods). Phase I enzymes are listed first, beginning with the cytochrome P-450s and followed by other phase I enzymes in alphabetical order. These are followed by phase II enzymes, beginning with the glutathione transferases and ending with the UDP glucuronosyltransferases. Abcc2, a transport protein, functions as part of the phase III system for xenobiotic metabolism.
Achieved significance based on stringent statistical analysis (see methods).
Average fold change of multiple elements. NA, not available.
Fig. 9.
Northern blots verify the downregulation of several xenobiotic metabolism genes identified by microarray analysis. Because similar patterns of gene expression changes were identified in all 3 sets of microarrays, expression changes for the genes indicated on the left were verified by using WT and Cftr−/−Nhe3−/− (KO) small intestine total RNA (10 μg/lane, 3 mice of each genotype). The blots were hybridized with PCR-amplified cDNA probes, and expression was normalized to the L32 ribosomal subunit mRNA. All four genes were significantly altered (GSTα4, −2.35 ± 0.07; GSTκ1, −2.90 ± 0.05; Cyp3a11, −3.17 ± 0.07; Cyp4v3, −4.24 ± 0.59; P < 0.05 compared with WT based on a Student's t-test).
With regard to proteins involved in energy metabolism, Cftr−/−Nhe3+/+, Cftr+/+Nhe3−/−, and Cftr/Nhe3 double-null intestines exhibited statistically significant differential expression of 9, 2, and 19 genes, respectively. For comparison, the values for these genes in all three genotypes are included in Table 3. Enzymes and transporters involved in lipid metabolism were the major group represented, but there were also several genes involved in carbohydrate metabolism. The changes were relatively modest and there were few genes for which expression changed in opposite directions in the single knockouts. Overall, the changes tended to be greater in double-null intestines than in Cftr−/−Nhe3+/+ or Cftr+/+Nhe3−/− intestines.
Table 3.
Differentially expressed genes involved in energy metabolism
| Symbol | Name | Cftr−/−Nhe3+/+ | Cftr+/+Nhe3−/− | Cftr−/−Nhe3−/− |
|---|---|---|---|---|
| Hao1 | hydroxyacid oxidase 1, liver | 3.53* | NA | −1.11 |
| Fdps | farnesyl diphosphate synthetase | 1.58 | 1.07 | 1.76*† |
| Sqle | squalene epoxidase | 1.48 | 1.37 | 1.84*† |
| Pmvk | phosphomevalonate kinase | 1.28† | 1.07 | 1.66* |
| Hk2 | hexokinase 2 | 1.24 | 1.06 | 1.82* |
| Cyp51 | lanosterol 14alpha-demethylase | 1.15 | NA | 1.68*† |
| Acat2 | acetyl-Coenzyme A acetyltransferase 2 | 1.14† | 1.08 | 1.62*† |
| Pgs1 | phosphatidylglycerophosphate synthase1 | 1.12 | 1.08 | 1.49*† |
| Acat3 | acetyl-Coenzyme A acetyltransferase 3 | 1.11 | NA | 1.80* |
| Dld | dihydrolipamide dehydrogenase | −1.02 | −1.55* | 1.17† |
| Fabp4 | fatty acid binding protein 4, adipocyte | −1.05† | −1.13† | −1.81* |
| Apoc3 | apolipoprotein C-III | −1.18 | −1.15 | −1.84* |
| Cd36 | fatty acid translocase | −1.20† | −1.45*† | −3.07* |
| Phyh | phytanoyl-CoA hydroxylase | −1.27 | −1.57 | −1.70* |
| Scd1 | stearoyl-Coenzyme A desaturase 1 | −1.36 | NA | −2.35* |
| G6pc | glucose-6-phosphatase, catalytic | −1.37 | 1.17 | −2.00* |
| Cyp27a1 | cholesterol 27 hydroxylase | −1.43 | −1.06 | −1.52*† |
| Slc27a2 | fatty acid transport protein 2 | −1.46 | −1.76 | −1.97*† |
| Mte1 | mitochondrial acyl-CoA thioesterase 1 | −1.52* | −1.76 | −1.30 |
| Hsd17b9 | hydroxysteroid dehydrogenase 9 | −1.53* | −2.19 | −1.46 |
| Pck1 | phosphoenolpyruvate carboxykinase 1 | −1.56 | −1.20 | −2.75*† |
| Hpgd | hydroxyprostaglandin dehydrogenase 15 | −1.84* | −1.13 | −1.79*† |
| Scdr9 | hydroxysteroid dehydrogenase 13 | −2.03* | NA | 1.33 |
| Cach | cytosolic acetyl-CoA hydrolase | −2.21* | −1.42 | −1.21 |
| Abcc2 | ATP-binding cassette C2 | −2.37* | −1.33 | −1.08† |
| Nsdhl | NAD(P) dependent steroid dehydrogenase-like | −2.85 | 1.02 | 1.42*† |
| Fabp6 | Gastrotropin | −3.78* | 1.57 | −1.02 |
| Nr4a1 | Nuclear receptor subfamily 4A1 | −6.31* | −4.10 | NA |
Data are presented as fold changes and are from the same 3 sets of microarray experiments described in Table 2 (see methods). Genes are listed according to changes (in descending order) in Cftr+/−Nhe3+/+ intestines.
Achieved significance based on stringent statistical analysis (see methods).
Average fold change of multiple elements.
Inflammation occurs in the intestine of human CF patients (3, 19) and is a prominent feature of the small intestines of untreated CF mice (54) and of CF mice maintained on the Peptamen elemental liquid diet (42). In contrast, our histological analyses (Figs. 5 and 7) revealed no evidence of increased inflammation in the small intestine of CF mice carrying a null mutation in the Nhe3 gene, and a previous study found little evidence of inflammation in Colyte-treated CF mice (9). To search for molecular evidence of inflammation, we compared expression levels of genes involved in immune and inflammatory responses for the CF models in the present study to the levels observed in a previous study (42) of CF mice maintained on a Peptamen liquid diet. It should be noted that the Colyte-treated mice in the present study and the Peptamen-treated mice of the previous study (42) were both on the same background strain (C57BL/6J) and had the same CFTR truncation mutation, thus ruling out gene expression differences based on background effects or specific CF mutation. As shown in Table 4, there was little evidence of an intestinal inflammatory response in either Colyte-treated Cftr−/−Nhe3+/+ mice or in Cftr−/−Nhe3−/− mice, which were on the FVB/N background. Although muclin (Dmbt1) and Reg3γ, which have been considered as possible inflammatory markers (42), were significantly induced, a direct role for these proteins in inflammation has not been demonstrated.
Table 4.
Genes previously reported as markers of inflammation in CF mouse intestine
| Symbol | Name | Cftr−/− (Ref. 42) | Cftr−/−Nhe3+/+ | Cftr−/−Nhe3−/− |
|---|---|---|---|---|
| Retnlb | resistin-like molecule β | 256 | 1.58 | 1.53 |
| HemT1 | hematopoietic cell transcript 1 | 80 | −1.06 | 1.02† |
| Mcpt1 | mast cell protease 1 | 45 | −1.02 | −6.69* |
| Mcpt2 | mast cell protease 2 | 30 | 1.36 | −3.37 |
| Cfi | complement factor I 1 | 19 | 1.30† | NA |
| Lrg | leucine-rich 2 glycoprotein | 14 | −1.14 | 1.37 |
| Saa3 | serum amyloid A3 | 13 | 1.28 | 1.75 |
| Cpa3 | mast cell carboxypeptidase A3 | 11 | −1.32 | −1.44† |
| Cma2 | mast cell chymase 2 | 4.9 | 4.9 | −1.79 |
| Saa2 | serum amyloid A2 | 3.6 | −1.06 | 2.70 |
| Mcptl | mast cell protease-like | 3.6 | NA | −4.15 |
| Socs3 | suppressor of cytokine signaling 3 | 3.3 | 1.42† | 2.05 |
| Cfh | complement factor H | 2.8 | 1.06† | 1.04† |
| Blnk | B cell linker | 2.8 | 1.25 | 1.27† |
| Reg 3γ | regenerating islet-derived 3γ | 2.5 | 2.24 | 7.26* |
| Dmbt1 | muclin | 2.4 | 1.81† | 2.33* |
| Pglyrp1 | peptidoglycan recognition protein 1 | 2.2 | 1.34 | 1.12 |
| Nfkbiz | molecule possessing ankyrin-repeats induced by LPS | 2.2 | 1.17 | 1.24 |
Microarray data reported previously (42) for upregulation of inflammatory markers in small intestines of cystic fibrosis (CF) mice maintained on Peptamen are compared with data from 2 of the microarray experiments performed in the present study (see Tables 2 and 3). Data are presented as fold changes and genes are listed according to changes (in descending order) reported previously (42). The Peptamen-treated mice in Ref. 42 were of the same background (C57BL/6) and carried the same CF truncation mutation as the Cftr+/−Nhe3+/+ mice in the present study, which were maintained on Colyte.
Achieved significance based on stringent statistical analysis (see methods).
Average fold change of multiple elements.
Intracellular glutathione levels are increased in Cftr- and Nhe3-null intestines.
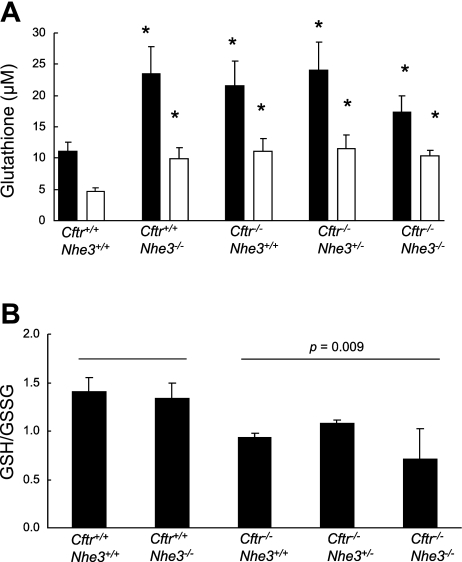
Because the microarray data showed differential expression of numerous genes that utilize glutathione or are involved in glutathione metabolism and because recent studies have shown that CFTR is capable of regulating glutathione efflux from the cell (33, 36), we measured intracellular glutathione levels. In jejunum of all mutant mice analyzed, both total glutathione and GSSG were significantly increased relative to that of wild-type tissue (Fig. 10A). The ratio of GSH to GSSG, indicative of the intracellular redox status, was highly variable even within genotypes. However, a comparison of all mice lacking CFTR to mice that had both copies of the Cftr gene showed a statistically significant decrease in the GSH-to-GSSG ratio, suggesting an increased intracellular oxidative potential (Fig. 10B).
Fig. 10.
Intestinal glutathione levels. Total and oxidized (GSSG) glutathione levels were measured in samples of intestine from mice of each of the indicated genotypes (n = 3–5 mice per genotype). A: tissue levels of total glutathione (solid bars) and GSSG (hatched bars) were increased in all mutant mice compared with WT controls; *P < 0.05 compared with WT based on a Student's t-test. B: when all 3 CF genotypes were pooled, the ratio of GSH to GSSG in mice lacking a functional Cftr gene (and WT, heterozygous, or null mutant with respect to Nhe3) was significantly different (P = 0.009 based on a Student's t-test) compared with pooled values from mice that were WT with respect to Cftr (and either WT or null mutant with respect to Nhe3).
DISCUSSION
Reduced NHE3-mediated Na+ absorption counters the desiccation of intestinal contents in CF mice.
Death from intestinal obstructions in CF mice and morbidity from MI or DIOS in CF patients is due, in large part, to desiccation of the intestinal contents (39, 54). Although the primary defect is loss of CFTR-mediated anion secretion with accompanying fluid, the fluidity of the luminal contents is also modulated by NHE3-mediated absorption (21, 51). This was first indicated by the severe diarrhea and high fluidity of intestinal contents in mice lacking NHE3 (51). In contrast, loss of the NHE2 Na+/H+ exchanger, which is also expressed on apical membranes in the intestine (27), caused no apparent intestinal phenotype (50) and did not worsen the diarrhea state of Nhe3-null mice (34). Subsequent Ussing chamber analyses using tissues from Nhe2- and Nhe3-null mouse models showed that NHE3, but not NHE2, serves a major absorptive function (22, 52). These findings agreed well with earlier studies using a Na+/H+ exchange inhibitor, which suggested that NHE3, but not NHE2, was responsible for Na+ and water absorption in dog ileum (38). More recent studies showed reductions of CFTR activity in Nhe3−/− intestine and NHE3 activity in Cftr−/− intestine (21), indicating that secretion and absorption are regulated in parallel to maintain luminal fluid homeostasis. Thus it seemed possible that reduced NHE3-mediated Na+ absorption might reverse some of the CF pathologies arising from desiccation of intestinal contents. This was confirmed by the increased survival among CF mice that were homozygous or heterozygous for the Nhe3-null allele and the long life span of those that survived to adulthood. Intestinal fluidity was sharply increased in CF mice lacking NHE3 and was relatively normal in Cftr−/−Nhe3+/− mice.
Inhibition of absorption and hydration therapy in CF.
The therapeutic effect of Nhe3 ablation in mice lacking Cftr suggests that inhibition of NHE3 could be used to improve fluidity and reduce the incidence of intestinal obstructions in CF. Such an approach would be similar to that being developed for CF lung disease, in which poor hydration of the luminal surface of airway epithelia leads to inefficient clearance of mucus (2), with obstruction of airways and chronic infections. Inhalation therapy involving inhibition of Na+ absorption via the amiloride-sensitive Na+ channel and/or direct rehydration via delivery of hypertonic saline to airway surfaces have been tested as therapeutic strategies (11, 15). Although earlier studies showed little therapeutic benefit from amiloride (11), which had a very short duration of action and may have had effects other than inhibition of the Na+ channel, recent studies using a potent amiloride derivative indicate that this approach might be beneficial, particularly when combined with rehydration using hypertonic saline (26).
In the intestinal tract, hydration therapy involving osmotic laxatives is useful in treating DIOS and constipation in human CF patients (39) and leads to normal survival in CF mice (6). Despite the value of this therapy in improving fluidity of the bulk contents of the intestine, NHE3-mediated absorption occurring in the absence of secretion might still allow desiccation of the luminal contents immediately adjacent to the epithelial surface. The effects of inhibitors of Na+ absorption have not been tested in CF intestinal disease; however, oral delivery of talniflumate, which has a number of activities, including inhibition of apical Cl−/HCO3− exchangers of the Slc26a family, has been shown to improve survival of CF mice (58). Inhibition of Cl−/HCO3− exchange by itself would not have an immediate effect on net anion absorption but would reduce exchange of intracellular HCO3− for luminal Cl−. With reduced Cl−/HCO3− exchange, NHE3-mediated Na+/H+ exchange would lead to luminal acidification, which would then inhibit Na+/H+ exchange and fluid absorption. Inhibition of NHE3 alone has a major effect on fluid absorption but also leads to luminal alkalinization. A potential pharmacological therapy for CF intestinal pathologies might be partial inhibition of both NHE3-mediated Na+/H+ exchange and apical Cl−/HCO3− exchange, as this would allow reduced absorption while maintaining normal pH of intestinal contents. On the other hand, partial inhibition of NHE3 alone might be sufficient, as the pH of the intestinal contents of Cftr−/−Nhe3+/− mice, which exhibited a substantial improvement in the intestinal phenotype, was not significantly different than that of wild-type controls (data not shown). Drug therapy could also be combined with mild osmotic laxative treatment to optimize luminal fluidity.
Results of the present and previous studies with Nhe3 heterozygous mice indicate that partial inhibition of NHE3 could be therapeutic for small intestine CF disease without causing chronic diarrhea or adverse effects in kidney. Diarrhea does not occur in Nhe3+/− mice (51), but the colonic H+-K+-ATPase, which is required for maximum Na+ absorption via the epithelial Na+ channel (55), was upregulated (51), indicating that compensation for a deficit in absorption occurred in distal colon, downstream of the ileum where blockages commonly occur. In kidney, where NHE3 serves as the major mechanism for HCO3−, NaCl, and fluid absorption (51, 59, 60), Nhe3 heterozygosity caused a reduction in fluid absorption in the proximal tubule (37); however, compensation occurred via increased absorption in the thick ascending limb (37) and there were no apparent alterations in blood pressure, fluid-volume homeostasis, or glomerular filtration rate, as seen in Nhe3−/− mice (51). Thus it is possible that partial inhibition of NHE3, perhaps as part of a combination therapy, could contribute to a more optimal hydration state of intestinal contents while avoiding a severe absorptive defect that might cause diarrhea and perturbations of fluid-volume and acid-base homeostasis. Such problems, if dosage were not well controlled, would likely be easily recognized and tolerated over short time periods. It is uncertain, of course, whether a useful therapeutic dose of an NHE3 inhibitor in humans would be sufficiently below doses that might exceed the capacity of compensatory mechanisms in the distal nephron and colon that would prevent excess fluid, HCO3−, and salt excretion.
Reduced absorption eliminates goblet cell hyperplasia but not crypt pathology.
Previous studies have shown that in CF mice the terminal ileum and colon exhibit goblet cell hyperplasia (54). The lack of ileal goblet cell hyperplasia in Cftr−/−Nhe3+/− mice, which have normal intestinal fluidity, and in Cftr−/−Nhe3−/− mice, which have increased intestinal fluidity and diarrhea, indicates that the increased number of goblet cells may be secondary to impaired hydration of the intestinal contents, rather than being intrinsic to the loss of CFTR. Increased mucus production is often characteristic of conditions in which there is irritation or inflammation of the epithelium. In the lung, this is evident in asthma (16), chronic obstructive pulmonary disease (29), and cystic fibrosis. In the intestine, dietary changes using coarse, high-fiber food that damages the epithelial surface have been shown to increase the number of goblet cells and mucus production in the colon (49). In the CF mouse intestine, it is possible that the high viscosity of the inspissated mucous and intestinal contents, which accumulate not only in the crypts but also between the villi, provide a stimulus for increased goblet cell and mucus production. Under conditions of normal secretion and absorption, increased mucus production might be protective, but in the CF intestine, in which normal hydration via CFTR-mediated anion secretion is defective, absorption via NHE3-mediated Na+/H+ exchange would tend to desiccate the mucus and slurry of food residue trapped against the mucosal surface. Alternatively, the reduction in goblet cell numbers in the Cftr−/−Nhe3+/− and Cftr−/−Nhe3−/− mice may reflect other changes that are secondary to increased luminal fluidity, such as a decrease in either bacterial overgrowth or inflammation, which correlate with reduced mucus accumulation in CF mice (10).
Because goblet cells arise in the crypts it initially seemed surprising that reducing absorption in epithelial cells of the villi would prevent goblet cell hyperplasia; however, transgenic expression of CFTR using a fatty acid binding protein promoter, which is restricted to the villi, also eliminated goblet cell hyperplasia (65). Thus improved luminal fluidity via either reduced absorption or increased anion secretion along the villi can correct this aspect of the phenotype. In contrast to the effects of NHE3 deficiency on goblet cell hyperplasia, accumulation of Paneth cell granules in the crypts of Cftr-null mice was not ameliorated by one or even both copies of the Nhe3 gene. This suggests that the loss of CFTR-mediated secretion has a significant impact on the normal flushing, hydration, and pH of the local crypt environment. The data also indicate that reduced levels of NHE3, which is expressed at much higher levels in the upper villus than in the lower villus (1), have little effect on the fluid status of the crypts and the ability to dissolve and clear granules from the lumen.
The effects of Nhe3 ablation on increased cell proliferation in the crypts of CF mice (18) were less clear. Nhe3-null mice exhibited an increase in cell proliferation that was similar in magnitude to that of CF mice, but when combined with the Cftr-null background the absence of both Nhe3 alleles appeared to have a moderating effect on this aspect of the phenotype. However, the effect of complete Nhe3 ablation was modest and loss of a single Nhe3 allele in CF mice did not cause a significant decrease in cell proliferation, suggesting that therapy involving partial inhibition of NHE3 would have little effect on cell proliferation in the crypts.
Glutathione regulation is abnormal in the CF intestine.
Dysregulation of glutathione efflux has been identified as a possible factor in the pathogenesis of CF lung disease (4, 28); however, its potential role in the intestinal pathology of CF has not been studied. A number of investigators have shown that CFTR facilitates glutathione efflux into luminal fluids, either directly by serving as a glutathione channel (33, 36) or indirectly by affecting the activity of other glutathione transporters (19), with the luminal glutathione serving an antioxidant function. Previous studies have demonstrated that glutathione efflux is reduced in cells lacking a functional CFTR (19, 20), and reduced levels of GSH have been reported in extracellular bronchoalveolar lavage fluid from both CF patients (7, 47) and mice (8, 30, 57).
In CF models that were either wild-type or homozygous-null with respect to Nhe3 we observed downregulation of mRNAs encoding GST isoforms, suggesting that there might be alterations in glutathione metabolism. Tissue levels of total glutathione were elevated in jejunum, consistent with a reduction in glutathione efflux from enterocytes lacking CFTR, and the GSH-to-GSSG ratio was decreased, consistent with a more oxidative intracellular environment. Although increased synthesis could be responsible for the increase in total glutathione, mRNA for the glutamate-cysteine ligase modifier subunit, which is involved in GSH synthesis (64), was reduced. A possible discrepancy in this interpretation is that the Cftr+/+Nhe3−/− intestine also had increased levels of total glutathione, despite the presence of intact CFTR. It should be noted, however, that maximally stimulated CFTR activity, as measured in Ussing chamber experiments, is significantly decreased in response to reductions in NHE3-mediated absorption (21), and it is conceivable that CFTR activity is sharply reduced in vivo in response to increased luminal fluidity and diarrhea caused by loss of NHE3. Additional studies will be needed to understand the mechanisms underlying the alterations in glutathione metabolism in the CF intestine and whether this might be related to the altered expression of drug metabolism genes in both Cftr-null and Nhe3-null small intestine.
Gene expression changes in CF small intestine and effects of Nhe3 ablation.
The present microarray data, and those of previous studies (5, 42), revealed downregulation of genes involved in phase I (e.g., cytochrome P-450s, carboxylesterases, and alcohol and aldehyde dehydrogenases) and phase II (e.g., GSTs, UGTs) drug metabolism. Surprisingly, the patterns were similar in Cftr-null, Nhe3-null, and Cftr/Nhe3 double-null mice. The basis for these similarities is unclear, but could be related, at least in part, to alterations in glutathione metabolism secondary to loss of Cftr-mediated glutathione efflux. Although the liver is the primary site of xenobiotic metabolism, the importance of the small intestine, which is a major site of drug absorption, is being increasingly recognized (31, 43). In CF patients, metabolic clearance of some drugs has been reported to be increased (32, 44, 45), although those studies focused almost exclusively on hepatic clearance. Given the discrepancy between the downregulation of drug metabolism genes in the CF mouse intestine and the seemingly contradictory increase in drug clearance in CF patients, additional studies will be needed to understand the mechanisms underlying the changes in drug metabolism in CF and the relative roles of liver and intestinal xenobiotic metabolism.
The microarray experiments also revealed differential expression of genes involved in lipid and energy metabolism, as observed previously in CF mice maintained on Peptamen (42). Contrary to the earlier study (42), however, we observed no evidence of inflammation, and five mast cell protease mRNAs that were sharply elevated in Peptamen-treated mice were reduced in Cftr−/−Nhe3−/− intestine, suggesting that the number of mast cells was reduced. The changes in lipid and energy metabolism genes, which occurred in both Cftr−/−Nhe3+/+ mice maintained on Colyte and in Cftr−/−Nhe3−/− mice, were relatively modest and somewhat lower than the changes identified previously (42). It is known that CF mice have inherent abnormalities in cholesterol processing (23, 61), and Nhe3−/− mice may also have a lipid metabolism defect because they accumulate little, if any, visceral fat (G. E. Shull, unpublished observations). This may explain why a greater number of lipid and energy metabolism genes were altered in the double knockouts. Regarding the absence of an inflammatory response in the CF models studied here, it has been shown that inflammation in Peptamen-treated CF mice is due to bacterial overgrowth and that this did not occur in Colyte-treated CF mice (9). Although Cftr−/−Nhe3+/− and double-null mice were smaller than wild-type controls at weaning, by 8 wk of age there was no significant difference in body weight. Adult CF mice maintained on Colyte also were comparable in size to wild-type controls. These data suggest that establishing appropriate fluidity of the intestinal tract and/or limiting inflammation can, despite modest alterations in the expression of energy metabolism genes, permit CF mice to attain relatively normal adult body weights.
Concluding comments.
The results of these studies using CF mice suggest that inhibition of NHE3-mediated absorption has the potential to improve intestinal fluid homeostasis in CF patients. This in turn could have a therapeutic effect on some of the intestinal pathologies, including goblet cell hyperplasia with overproduction of mucus, inflammation, and obstructions. Such a strategy would be similar to that being pursued for treatment of CF lung pathology (11, 15) but with a different transporter as a target. The finding that Nhe3 heterozygosity was effective in reducing pathology suggests that partial inhibition of NHE3 could be sufficient to maintain intestinal fluid homeostasis and, given the known compensatory mechanisms in the kidney (37) and distal colon (51, 55), such partial inhibition would likely be well tolerated. Although our studies focused on the intestinal phenotype, it is also possible that NHE3 inhibition could reduce CF pathology in other organs as well. CF mice on a liquid elemental diet develop multiorgan pathology by 8 mo of age, including liver and pancreatic disease (13), similar to that observed in CF patients. Both NHE3 and CFTR are expressed in apical membranes of cholangiocytes (40) and pancreatic ducts (35), where NHE3 appears to serve absorptive functions that counter CFTR-mediated secretion. It is therefore possible that inhibition of absorption might also prevent or reduce some of the other pathologies that occur in the bile and pancreatic ducts.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-50594 and DK-48816.
Supplementary Material
Acknowledgments
We thank Jeffrey A. Whitsett for providing the CF mice on the FVB/N background.
Present address for M. A. Sartor: Center for Computational Medicine and Biology, University of Michigan, Ann Arbor, MI 48109.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB. Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine. Expression and localization. J Clin Invest 93: 106–113, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher RC Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med 13: 231–240, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bruzzese E, Raia V, Gaudiello G, Polito G, Buccigrossi V, Formicola V, Guarino A. Intestinal inflammation is a frequent feature of cystic fibrosis and is reduced by probiotic administration. Aliment Pharmacol Ther 20: 813–819, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Childers M, Eckel G, Himmel A, Caldwell J. A new model of cystic fibrosis pathology: lack of transport of glutathione and its thiocyanate conjugates. Med Hypotheses 68: 101–112, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Clarke LL, Gawenis LR, Bradford EM, Judd LM, Boyle KT, Simpson JE, Shull GE, Tanabe H, Ouellette AJ, Franklin CL, Walker NM. Abnormal Paneth cell granule dissolution and compromised resistance to bacterial colonization in the intestine of CF mice. Am J Physiol Gastrointest Liver Physiol 286: G1050–G1058, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci 46: 612–618, 1996. [PubMed] [Google Scholar]
- 7.Dauletbaev N, Viel K, Buhl R, Wagner TO, Bargon J. Glutathione and glutathione peroxidase in sputum samples of adult patients with cystic fibrosis. J Cyst Fibros 3: 119–124, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Day BJ, van Heeckeren AM, Min E, Velsor LW. Role for cystic fibrosis transmembrane conductance regulator protein in a glutathione response to bronchopulmonary pseudomonas infection. Infect Immun 72: 2045–2051, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Lisle RC, Roach E, Jansson K. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol 293: G577–G584, 2007. [DOI] [PubMed] [Google Scholar]
- 10.De Lisle RC, Roach EA, Norkina O. Eradication of small intestinal bacterial overgrowth in the cystic fibrosis mouse reduces mucus accumulation. J Pediatr Gastroenterol Nutr 42: 46–52, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med 354: 241–250, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Dray X, Bienvenu T, Desmazes-Dufeu N, Dusser D, Marteau P, Hubert D. Distal intestinal obstruction syndrome in adults with cystic fibrosis. Clin Gastroenterol Hepatol 2: 498–503, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Durie PR, Kent G, Phillips MJ, Ackerley CA. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol 164: 1481–1493, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckman EA, Cotton CU, Kube DM, Davis PB. Dietary changes improve survival of CFTR S489X homozygous mutant mouse. Am J Physiol Lung Cell Mol Physiol 269: L625–L630, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 354: 229–240, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Fahy JV Remodeling of the airway epithelium in asthma. Am J Respir Crit Care Med 164: S46–S51, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Field M, Rao MC, Chang EB. Intestinal electrolyte transport and diarrheal disease (1). N Engl J Med 321: 800–806, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher AM, Gottlieb RA. Proliferation, not apoptosis, alters epithelial cell migration in small intestine of CFTR null mice. Am J Physiol Gastrointest Liver Physiol 281: G681–G687, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Gao L, Broughman JR, Iwamoto T, Tomich JM, Venglarik CJ, Forman HJ. Synthetic chloride channel restores glutathione secretion in cystic fibrosis airway epithelia. Am J Physiol Lung Cell Mol Physiol 281: L24–L30, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Gao L, Kim KJ, Yankaskas JR, Forman HJ. Abnormal glutathione transport in cystic fibrosis airway epithelia. Am J Physiol Lung Cell Mol Physiol 277: L113–L118, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Gawenis LR, Hut H, Bot AG, Shull GE, de Jonge HR, Stien X, Miller ML, Clarke LL. Electroneutral sodium absorption and electrogenic anion secretion across murine small intestine are regulated in parallel. Am J Physiol Gastrointest Liver Physiol 287: G1140–G1149, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Gawenis LR, Stien X, Shull GE, Schultheis PJ, Woo AL, Walker NM, Clarke LL. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol 282: G776–G784, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Gentzsch M, Choudhury A, Chang XB, Pagano RE, Riordan JR. Misassembled mutant DeltaF508 CFTR in the distal secretory pathway alters cellular lipid trafficking. J Cell Sci 120: 447–455, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Grubb BR, Gabriel SE. Intestinal physiology and pathology in gene-targeted mouse models of cystic fibrosis. Am J Physiol Gastrointest Liver Physiol 273: G258–G266, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi H, Szaszi K, Coady-Osberg N, Furuya W, Bretscher AP, Orlowski J, Grinstein S. Inhibition and redistribution of NHE3, the apical Na+/H+ exchanger, by Clostridium difficile toxin B. J Gen Physiol 123: 491–504, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsh AJ, Zhang J, Zamurs A, Fleegle J, Thelin WR, Caldwell RA, Sabater JR, Abraham WM, Donowitz M, Cha B, Johnson KB, St George JA, Johnson MR, Boucher RC. Pharmacological properties of N-(3,5-diamino-6-chloropyrazine-2-carbonyl)-N′-4-[4-(2,3-dihydroxypropoxy) phenyl]butyl-guanidine methanesulfonate (552-02), a novel epithelial sodium channel blocker with potential clinical efficacy for cystic fibrosis lung disease. J Pharmacol Exp Ther 325: 77–88, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol Gastrointest Liver Physiol 270: G29–G41, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Hudson VM New insights into the pathogenesis of cystic fibrosis: pivotal role of glutathione system dysfunction and implications for therapy. Treat Respir Med 3: 353–363, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC, Fahy JV. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest 130: 1102–1108, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Kariya C, Leitner H, Min E, van Heeckeren C, van Heeckeren A, Day BJ. A role for CFTR in the elevation of glutathione levels in the lung by oral glutathione administration. Am J Physiol Lung Cell Mol Physiol 292: L1590–L1597, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato M Intestinal first-pass metabolism of CYP3A4 substrates. Drug Metab Pharmacokinet 23: 87–94, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Kearns GL, Crom WR, Karlson KH Jr, Mallory GB Jr, Evans WE. Hepatic drug clearance in patients with mild cystic fibrosis. Clin Pharmacol Ther 59: 529–540, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Kogan I, Ramjeesingh M, Li C, Kidd JF, Wang Y, Leslie EM, Cole SP, Bear CE. CFTR directly mediates nucleotide-regulated glutathione flux. EMBO J 22: 1981–1989, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ledoussal C, Woo AL, Miller ML, Shull GE. Loss of the NHE2 Na+/H+ exchanger has no apparent effect on diarrheal state of Nhe3-deficient mice. Am J Physiol Gastrointest Liver Physiol 281: G1385–G1396, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Lee MG, Ahn W, Choi JY, Luo X, Seo JT, Schultheis PJ, Shull GE, Kim KH, Muallem S. Na+-dependent transporters mediate HCO3− salvage across the luminal membrane of the main pancreatic duct. J Clin Invest 105: 1651–1658, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linsdell P, Hanrahan JW. Glutathione permeability of CFTR. Am J Physiol Cell Physiol 275: C323–C326, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in Nhe3-deficient mice. Am J Physiol Renal Physiol 277: F447–F453, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Maher MM, Gontarek JD, Jimenez RE, Donowitz M, Yeo CJ. Role of brush border Na+/H+ exchange in canine ileal absorption. Dig Dis Sci 41: 651–659, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Mascarenhas MR Treatment of gastrointestinal problems in cystic fibrosis. Curr Treat Options Gastroenterol 6: 427–441, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Mennone A, Biemesderfer D, Negoianu D, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Aronson PS, Boyer JL. Role of sodium/hydrogen exchanger isoform NHE3 in fluid secretion and absorption in mouse and rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 280: G247–G254, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Muller T, Wijmenga C, Phillips AD, Janecke A, Houwen RH, Fischer H, Ellemunter H, Fruhwirth M, Offner F, Hofer S, Muller W, Booth IW, Heinz-Erian P. Congenital sodium diarrhea is an autosomal recessive disorder of sodium/proton exchange but unrelated to known candidate genes. Gastroenterology 119: 1506–1513, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Norkina O, Kaur S, Ziemer D, De Lisle RC. Inflammation of the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol 286: G1032–G1041, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Paine MF, Oberlies NH. Clinical relevance of the small intestine as an organ of drug elimination: drug-fruit juice interactions. Expert Opin Drug Metab Toxicol 3: 67–80, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Parker AC, Pritchard P, Preston T, Smyth RL, Choonara I. Enhanced drug metabolism in young children with cystic fibrosis. Arch Dis Child 77: 239–241, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rey E, Treluyer JM, Pons G. Drug disposition in cystic fibrosis. Clin Pharmacokinet 35: 313–329, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Robert A, Nezamis JE, Lancaster C, Hanchar AJ, Klepper MS. Enteropooling assay: a test for diarrhea produced by prostaglandins. Prostaglandins 11: 809–828, 1976. [DOI] [PubMed] [Google Scholar]
- 47.Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol 75: 2419–2424, 1993. [DOI] [PubMed] [Google Scholar]
- 48.Sartor MA, Tomlinson CR, Wesselkamper SC, Sivaganesan S, Leikauf GD, Medvedovic M. Intensity-based hierarchical Bayes method improves testing for differentially expressed genes in microarray experiments. BMC Bioinformatics 7: 538, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt-Wittig U, Enss ML, Coenen M, Gartner K, Hedrich HJ. Response of rat colonic mucosa to a high fiber diet. Ann Nutr Metab 40: 343–350, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest 101: 1243–1253, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Seidler U, Rottinghaus I, Hillesheim J, Chen M, Riederer B, Krabbenhoft A, Engelhardt R, Wiemann M, Wang Z, Barone S, Manns MP, Soleimani M. Sodium and chloride absorptive defects in the small intestine in Slc26a6 null mice. Pflügers Arch 455: 757–766, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Smyth GK Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science 257: 1083–1088, 1992. [DOI] [PubMed] [Google Scholar]
- 55.Spicer Z, Clarke LL, Gawenis LR, Shull GE. Colonic H+-K+-ATPase in K+ conservation and electrogenic Na+ absorption during Na+ restriction. Am J Physiol Gastrointest Liver Physiol 281: G1369–G1377, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Vaandrager AB Structure and function of the heat-stable enterotoxin receptor/guanylyl cyclase C. Mol Cell Biochem 230: 73–83, 2002. [PubMed] [Google Scholar]
- 57.Velsor LW, van Heeckeren A, Day BJ. Antioxidant imbalance in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Physiol Lung Cell Mol Physiol 281: L31–L38, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Walker NM, Simpson JE, Levitt RC, Boyle KT, Clarke LL. Talniflumate increases survival in a cystic fibrosis mouse model of distal intestinal obstructive syndrome. J Pharmacol Exp Ther 317: 275–283, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Wang T, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Giebisch G, Aronson PS. Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol Renal Physiol 277: F298–F302, 1999. [DOI] [PubMed] [Google Scholar]
- 60.Wang T, Yang CL, Abbiati T, Shull GE, Giebisch G, Aronson PS. Essential role of NHE3 in facilitating formate-dependent NaCl absorption in the proximal tubule. Am J Physiol Renal Physiol 281: F288–F292, 2001. [DOI] [PubMed] [Google Scholar]
- 61.White NM, Jiang D, Burgess JD, Bederman IR, Previs SF, Kelley TJ. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 292: L476–L486, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Woo AL, Gildea LA, Tack LM, Miller ML, Spicer Z, Millhorn DE, Finkelman FD, Hassett DJ, Shull GE. In vivo evidence for interferon-gamma-mediated homeostatic mechanisms in small intestine of the NHE3 Na+/H+ exchanger knockout model of congenital diarrhea. J Biol Chem 277: 49036–49046, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Woo AL, Noonan WT, Schultheis PJ, Neumann JC, Manning PA, Lorenz JN, Shull GE. Renal function in Nhe3-deficient mice with transgenic rescue of small intestinal absorptive defect. Am J Physiol Renal Physiol 284: F1190–F1198, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm−/− knockout mouse. Novel model system for a severely compromised oxidative stress response. J Biol Chem 277: 49446–49452, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA, Whitsett JA. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science 266: 1705–1708, 1994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.