Abstract
Cyclic AMP protects against hepatocyte apoptosis by a protein kinase A-independent cAMP-GEF/phosphoinositide-3-kinase (PI3K)/Akt signaling pathway. However, the signaling pathway coupling cAMP-GEF with PI3K is unknown. The aim of this study was to investigate the role of Src tyrosine kinases (Src-TYK) and PI3K-p110 isoforms in this pathway. Studies were done in rat hepatocytes using the hydrophobic bile acid glycochenodeoxycholic acid (GCDC) to induce apoptosis. cAMP-binding guanine nucleotide exchange factors (cAMP-GEFs) were selectively activated by using 4-(4-chloro-phenylthio)-2′-O-methyladenosine-3′-5′-cyclic monophosphate (CPT-2-Me-cAMP), which sequentially phosphorylated Src-TYK (within 1 min) followed by Akt (within 5 min). The Src inhibitors PP2 and SU6656 inhibited basal and CPT-2-Me-cAMP-mediated Src and Akt phosphorylation. These inhibitors had no effect on CPT-2-Me-cAMP-mediated activation of Rap GTPases. CPT-2-Me-cAMP induced transient Src dependent autophosphorylation of the epidermal growth factor receptor (EGFR). Inhibition of the EGFR with AG 1478 partially inhibited the ability of CPT-2-Me to phosphorylate Akt. Whereas PP2 completely abolished the protective effect of CPT-2-Me-cAMP in GCDC induced apoptosis, AG 1478 partially inhibited the cytoprotective effect. CPT-2-Me-cAMP treatment resulted in Src-dependent activation of the p110 β and α subunits of PI3K, but only the latter was sensitive to inhibition with AG 1478. In conclusion, activation of cAMP-GEFs results in phosphorylation of Src-TYK and Akt and activation of the p110 β/α subunits of PI3K. Maximal cAMP-GEF-mediated Akt phosphorylation as well as protection from bile acid-induced apoptosis requires activation of Src-TYK and the EGFR. These studies support the existence of two pathways: cAMP-GEF/Rap/Src/PI3Kβ/Akt and cAMP-GEF/Rap/Src/EGFR/PI3Kα/Akt, both of which are necessary for maximal cytoprotective effect of cAMP-GEFs in hepatocytes.
Keywords: bile acids, Rap, Akt, EGF receptor, cAMP-binding guanine nucleotide exchange factors
cyclic AMP is an important prosurvival signaling molecule in the liver. Increases in cAMP, such as occur during times of metabolic stress when circulating glucagon levels are high, protect cultured hepatocytes from apoptosis due to several stimuli including bile acids, Fas, and TNF-α (12, 21, 36, 61). In vivo, oral administration of phosphodiesterase inhibitors (18, 32) or β2-adrenergic agonists (1), agents that increase hepatic cAMP levels, or parenteral administration of glucagon (24) or cell-permeable cAMP analogs (28, 57) protect against toxic and ischemic hepatic injury in laboratory animals. Furthermore, it is known that the diseased liver develops dysregulations in cAMP signaling that contribute to increased hepatobiliary damage (15, 19, 20, 27, 35). Despite cAMP's important role in maintaining hepatocyte health, the downstream signaling events involved in its prosurvival signaling in hepatocytes have not been fully characterized.
cAMP transduces intracellular signals through three pathways: 1) the serine threonine protein kinase, protein kinase A (PKA); 2) cyclic nucleotide-gated channels in the brain and heart; and 3) the cAMP-binding guanine nucleotide exchange factors (cAMP-GEFs, also known as exchange proteins regulated by cAMP) (26). cAMP-GEFs catalyze the formation of the active GTP-bound form of the small GTPases, Rap. Over the last 10 years since their discovery the cAMP-GEF signaling pathways have been shown to regulate a growing array of cellular functions including integrin-mediated cell adhesion, gap junction formation, calcium-mediated exocytosis, and cell survival (12, 26, 33, 34, 41, 56, 59).
cAMP/cAMP-GEF/Rap signaling pathways exist in hepatocytes, and studies show that cAMP-GEF activation is responsible for cAMP's prosurvival effect in hepatocytes (12, 56). We have reported that cytoprotection by cAMP-GEFs in hepatocytes is dependent on activation of the lipid kinase phosphoinositide-3-kinase (PI3K) (12, 61). PI3K is a well-known survival kinase in a number of cell types including hepatocytes (6, 12, 45, 53, 54, 62). The mechanism whereby cAMP-GEF activation is coupled to PI3K activation is unknown. Src tyrosine kinases (Src-TYK) are a family of kinases several of which are ubiquitously expressed (60). In nonhepatic cells, cAMP can activate Src-TYK (30), and Src-TYK are known to upregulate PI3K/Akt signaling (2, 9, 38, 39, 48). In addition, Src-TYK are well-known prosurvival signaling molecules in many cell types including hepatocytes (5, 14, 44, 55). Thus Src-TYK may also be involved in cAMP-GEF-mediated cytoprotection and PI3K activation in hepatocytes.
The PI3Ks are a family of lipid kinases grouped into three classes (I–III) (16, 17). The class I PI3Ks are primarily responsible in vivo for the generation of phosphatidylinositol-3,4,5-trisphosphate (PIP3), which is coupled to Akt activation. The class 1A PI3Ks are heterodimers that consist of a p85 regulatory subunit and a p110 catalytic subunit. The p85 regulatory subunit is involved in activation of the catalytic subunit by receptor and nonreceptor tyrosine kinases. There are 3 class 1A catalytic p110 isoforms, p110 α, p110 β, and p110 δ. The class 1B PI3K is a heterodimer of a p101 regulatory subunit and a catalytic p110 γ subunit. The p110 γ subunit is activated by G protein-coupled receptors through direct interaction with Gβγ subunits of trimeric G proteins. The p110 α and β subunits are the predominant enzymes in hepatocytes with lesser amounts of p110 γ (31, 42).
Emerging evidence suggests that distinct PI3K-p110 isoforms couple different early signaling events to ultimately lead to different biological responses (16). For example, p110 α mediates insulin sensitivity in skeletal muscle (16), p110 β regulates aggregation in platelets (29) and p110 γ mediates inflammatory responses in white blood cells (52). With the recent introduction of p110 isoform-selective PI3K inhibitors and their potential for therapeutic application in diabetes mellitus, thrombosis, and inflammatory disorders, an understanding of isoform-specific activation becomes increasingly important. Despite this, the role of specific PI3K-p110 isoforms in cell survival and hepatocyte cell biology is poorly characterized. It is presently unknown which p110 isoform is activated by cAMP-GEFs in hepatocytes.
The overall objective of our studies was to determine the role of Src-TYK in cAMP-GEF signaling and cytoprotection in hepatocytes and to elucidate whether cAMP-GEFs mediate isoform-specific activation of PI3K-p110. To study cAMP-GEF function we used a cAMP-GEF specific cAMP analog 4-(4-chlorophenylthio)-2′-O-methyladenosine-3′5′cyclic monophosphate (CPT-2-Me-cAMP) to selectively activate cAMP-GEF in hepatocytes and examined this analog's prosurvival effect in an in vitro model of cholestatic apoptotic cell death in which rat hepatocytes are exposed to the hydrophobic bile acid glycochenodeoxycholic acid (GCDC). The results show that cAMP-GEF activation in rat hepatocytes results in Src-TYK-dependent activation of the p110 β/α subunit of PI3K and that this activation is essential for cAMP-GEF cytoprotection.
EXPERIMENTAL PROCEDURES
Reagents.
Collagenase, Hoechst 33258, GCDC, phospho-HGFty1230, hepatocyte growth factor (HGF), epidermal growth factor (EGF), and all tissue culture reagents were purchased from Sigma-Aldrich Chemical (St. Louis, MO). The cAMP-GEF specific cAMP analog, 4-(4-chlorophenylthio)-2′-O-methyladenosine-3′5′cyclic monophosphate (CPT-2-Me-cAMP) was from Alexis Biochemical (San Diego, CA). SU 6656, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), 4-amino-7-phenylpyrazol [3,4-d]pyrimidine (PP3), and AG1478 were from Calbiochem (San Diego, CA). [3H]taurocholate and [γ-32P]ATP were from PerkinElmer (Boston, MA). Phosphospecific antibodies to Srcty416 and Aktser473 and total Akt antibodies were obtained from Cell Signaling Technology (Beverly, MA). Total EGF receptor (EGFR), HGF receptor (HGFR), caspase 3, and phospho-EGFRty1173 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Rat hepatocyte cultures.
Rat hepatocytes were isolated from male Wistar rats (200–250 g) as previously described (12). Hepatocytes were plated at 5 × 105 cells/cm2 on tissue culture dishes or coverslips coated with Type I rat tail collagen in minimal Eagle's media with l-glutamine, 100 nM insulin, and 10% heat-inactivated fetal calf serum and incubated at 37°C in a humidified atmosphere of 5% CO2. After 1 h was allowed for cell attachment, apoptosis was initiated by the addition of 50 μM GCDC. Unless otherwise noted, modulators were added at the indicated concentration 30 min prior to the addition of GCDC. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23, revised 1985), and animal use protocols were approved by Tufts University IACUC.
Assessment of apoptosis.
Morphological evaluation of apoptotic cell death was conducted 2 h after the addition of GCDC as previously described (12). Briefly, coverslips were stained with Hoechst 33258 and apoptosis was evaluated with fluorescent microscopy. Apoptotic cells were identified as those whose nucleus exhibited brightly staining condensed chromatin or nuclear fragmentation. Five hundred cells were counted by an observer blinded to the treatment conditions, and the number of apoptotic cells was expressed as a percentage of the total number of cells counted. The presence of the p17-kDa cleavage product of caspase 3 was used as a biochemical indicator of hepatocyte apoptosis. Cell lysates were prepared from hepatocytes treated with GCDC for 1 h in cell lysis buffer (CLB), 20 mM Tris, pH = 7.5, 150 mM NaCl, 1 mm EGTA, 1% Triton, 2 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM glycerolphosphate, 1 mM phenylmethylsulfonyl fluoride, 100 nM okadaic acid, 1 mM sodium orthovanadate, and 10 μg/ml of leupeptin, aprotinin, and pepstatin and separated on SDS-PAGE, and the proteins were transferred to polyvinylidene difluoride (PVDF) membranes. Immunoblotting was performed with a caspase 3 antibody, and equal protein loading was verified by stripping and probing with an actin antibody.
Determination of Rap 1 activation.
Rap 1 and Rap 2 activation were determined by using a commercially available Rap Activation Assay Kit (Upstate Biotechnology, Lake Placid, NY) that measures the amount of activated GTP-bound Rap precipitated by a glutathione S transferase-tagged protein corresponding to the Ral guanine dissociation stimulator binding domain of Rap bound to glutathione-agarose. The GTP-bound Rap is detected by immunoblotting with Rap 1 or Rap 2 antibodies.
Preparation of whole cell lysates and immunoblotting techniques.
Hepatocyte cultures were treated with the indicated modulators and cell lysates prepared at predetermined time points in a CLB. After dilution into SDS gel loading buffer and boiling, 50–100 μg of protein were separated by SDS polyacrylamide gel electrophoresis and proteins transferred to polyvinylidene difluoride (PVDF) membranes. After blocking, membranes were incubated with primary antibody for overnight at 4°C. After washing, blots were incubated with the appropriate peroxidase labeled secondary antibodies (Bio-Rad, Hercules, CA) for 1 h at room temperature. Immunoblots were developed with enhanced chemiluminescence and proteins detected by autoradiography. The blots were scanned into Adobe Photoshop and subjected to computerized densitometric scanning using Sigma Gel.
Bile acid uptake.
The 30-min accumulation of radiolabeled bile acid, 3H-taurocholate (PerkinElmer), in hepatocyte cultures was determined as previously described (61).
Lipid kinase assay.
Hepatocyte cultures were treated with 20 μM CPT-2-ME-cAMP for 1, 5, or 15 min and cell lysates prepared in PI3K buffer (50 mM HEPES pH = 7.4, 1% NP-40, 10% glycerol, 0.5 mM EGTA, with 5 μg/ml of leupeptin, pepstatin, and aprotinin, 0.5 mM phenylmethylsulfonyl fluoride, and 5 mM orthovanadate). Aliquots were immediately frozen at −80°C. Isoform specific lipid kinase assays were performed after immunoprecipitation of the catalytic p110 subunit of PI3K with antibodies to p110 α (7174, Santa Cruz Biotechnology), p110 β (06-568, Upstate Biotechnology), or p110 γ (4252, Cell Signaling Technology). The specificity of these antibodies for the selective immunoprecipitation of PI3K isoforms has been documented in several cell types including hepatocytes (5, 6, 17, 22, 30, 40, 42, 51) and by immunoblotting in our laboratory (data not shown). Immunoprecipitation of the α, β, and γ subunits was performed on 100, 50, or 500 μg of cell lysis protein, respectively, for 2 h at 4°C. Immune complexes were captured by centrifuging for 1 h in 20 μl of protein A/G (Santa Cruz Biotechnology). Additional lipid kinase assays were conducted on anti-phosphotyrosine immunoprecipitates. For these assays 300 μg of cell lysate was incubated with 30 μl of anti-phosphotyrosine antibody-agarose conjugate for 5 h (4G10, 06-101; Upstate Biotechnology) at 4°C. After the immunoprecipitates were washed once with PI3K assay buffer and three times in phosphate-buffered saline, pH = 7.4, lipid kinase assays were performed as previously described (55). The results were expressed as the fold increase in the production of the PI3K-specific reaction product, PIP3.
Immunoprecipitation of phosphotyrosinated proteins.
Hepatocyte cultures were treated for 5 min with 20 μM CPT-2-Me-cAMP or EGF (50 ng/ml) or HGF (50 ng/ml) as positive controls. Cell lysates were prepared and phosphotyrosinated proteins were immunoprecipitated with an anti-phosphotyrosine antibody (clone 4G10, Upstate Biotechnology, Charlottesville, VA). After being washed three times with CLB and resuspended in SDS loading buffer, the immunoprecipitates were subjected to SDS-PAGE and proteins were transferred to PVDF membranes. Immunoblotting was performed with antibodies to EGFR, EGFRtyr1173, and/or HGFRtyr1230.
Statistical evaluation.
All results are expressed as means ± SD and analyzed for statistical significance with Student's t-test or ANOVA, with P values of <0.05 considered significant.
RESULTS
cAMP-GEF-mediated cytoprotection is Src tyrosine kinase dependent.
We have previously demonstrated that cAMP-GEF protects hepatocytes from bile acid-induced apoptosis (12). To determine whether this antiapoptotic effect involves Src-TYK, rat hepatocytes were sequentially treated with a Src-TYK inhibitor, PP2, or its inactive analog, PP3, prior to the addition of CPT-2-Me-cAMP. Apoptosis was then induced with GCDC, and 2 h later the effect of Src-TYK inhibition on apoptosis was determined. The Src-TYK inhibitor PP2 completely reversed the protective effect of CPT-2-Me-cAMP (Fig. 1), whereas the inactive analog PP3 had no effect. Incubation with PP2 alone significantly increased GCDC induced apoptosis by 35%. The protective effect of CPT-2-Me-cAMP in GCDC-induced apoptosis was accompanied by inhibition of caspase 3 cleavage. This inhibitory effect was abolished by pretreatment with PP2, but not PP3 (Fig. 1B). These results suggest that the cytoprotective effect of cAMP-GEF is mediated via Src-TYK.
Fig. 1.
The antiapoptotic effect of cAMP binding guanine nucleotide exchange factor (cAMP-GEF) activation is Src tyrosine kinase dependent. A: hepatocytes were treated with 50 μM of glycochenodeoxycholic acid (GCDC) for 2 h and the amount of apoptosis determined by morphological evaluation of Hoechst-stained cells. Some cultures were pretreated with 20 μM 4-(4-chloro-phenylthio)-2′-O-methyladenosine-3′-5′-cyclic monophosphate (CPT-2-Me-cAMP) for 30 min or sequentially with 10 μM 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) or 10 μM 4-amino-7-phenylpyrazol [3,4-d]pyrimidine (PP3) for 30 min and then CPT-2-Me-cAMP before addition of the apoptotic stimulus. Results of 4 experiments were expressed as the mean % apoptosis ± SD. *Significantly different from value seen in control cells; #significantly different from value in GCDC-treated cells (P < 0.05) B: immunoblots for the p17 cleavage product of caspase 3 (Clv 3). In these experiments hepatocytes were pretreated sequentially with 10 μM PP2 or PP3 followed by 20 μM of CPT-2-Me-cAMP and processed 1 h after the addition of 50 μM GCDC. Equal protein loading was assessed by staining for actin.
cAMP-GEF phosphorylation of Akt requires Src family tyrosine kinases.
We have previously demonstrated that phosphorylation of Akt is necessary for the cytoprotective effect of cAMP-GEFs in rat hepatocytes (12, 61). To determine whether Src-TYK phosphorylation is involved in cAMP-GEF-induced phosphorylation of Akt, we first determined whether treatment of rat hepatocytes with CPT-2-Me-cAMP results in Src phosphorylation and if so how this phosphorylation event is temporally related to Akt phosphorylation. Phosphorylation of Akt and Src were monitored at various time points after the addition of CPT-2-Me-cAMP by using phosphospecific antibodies to Aktser473 and Srcty416. Within 1 min CPT-2-Me-cAMP induced a significant 1.5-fold increase in Srcty416 phosphorylation, which peaks at approximately threefold at 5 min and declines thereafter but still remains significantly elevated at 60 min (Fig. 2). In contrast, the CPT-2-Me-cAMP-mediated phosphorylation of Aktser473 is not detected until 5 min and continues to increase over the 60-min observation period (Fig. 2). A similar time course for CPT-2-Me-cAMP-induced Aktthr308 phosphorylation was seen (data not shown). These results suggest that CPT-2-Me-cAMP-mediated Src phosphorylation precedes Akt phosphorylation.
Fig. 2.
Activation of cAMP-GEF results in time-dependent phosphorylation of Aktser473 and Srctyr416. Whole cell lysates were prepared from control hepatocytes and hepatocytes were treated with 20 μM CPT-2-Me-cAMP for the indicated time period. The amount of phosphorylated Aktser473 and Srctyr416 was determined by immunoblotting with phosphospecific antibodies. A: results of quantitative analysis of at least 4 immunoblots. B: representative immunoblots. *Significantly difference from the value at time 0 (P < 0.05).
To determine whether Src-TYK phosphorylation was necessary for CPT-2-Me-cAMP-mediated Akt activation, hepatocytes were pretreated with either PP2, SU 6556, or PP3. The results show that both Src-TYK inhibitors, PP2 and SU 6656, decrease basal and CPT-2-Me-cAMP-mediated Src (Fig. 3, A and C) and Aktser473 phosphorylation (Fig. 3, B and C). Similar results were seen with Aktthr308 phosphorylation (data not shown). The inactive analog, PP3, had no effect on CPT-2-Me-cAMP phosphorylation of these kinases. This data verifies the Src inhibitory activity of PP2 and SU6656 in rat hepatocytes and demonstrates that Src-TYK activity is needed for cAMP-GEF-mediated Akt phosphorylation.
Fig. 3.
Src inhibitors prevent cAMP-GEF-mediated phosphorylation of Aktser473 and Srctyr416. Whole cell lysates were prepared from control hepatocytes and hepatocytes treated with 20 μM CPT-2-Me-cAMP for 5 min (Src) or 15 min (Akt) with or without pretreatment with 10 μM of the Src kinase inhibitors PP2 or SU6656 or with an inactive analog, PP3. The amount of Srctyr416 (A) or Aktser473 (B) phosphorylation was determined by quantifying immunoblotting with phosphospecific antibodies (n = 3). C: representative immunoblots. *Significantly different from value seen in CPT-2-Me-cAMP-treated cells; #significantly different from value in control cells (P < 0.05).
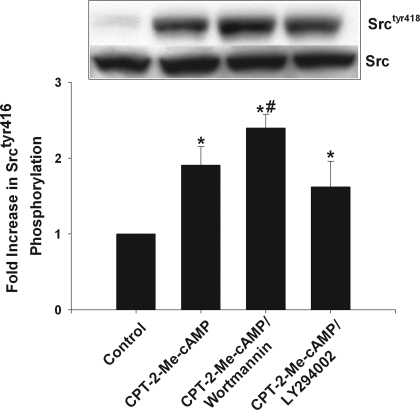
Akt phosphorylation by cAMP-GEF in rat hepatocytes is dependent on PI3K activation (12, 61). To determine whether Srctyr416 phosphorylation is upstream or downstream of PI3K in the Akt activation pathway, hepatocytes were treated for 15 min with one of two PI3K inhibitors, wortmannin (50 nM) or LY294002 (20 μM), prior to the addition of CPT-2-Me-cAMP. Neither wortmannin or LY294002 inhibited cAMP-GEF-induced Srctyr416 phosphorylation (Fig. 4) or had any effect on basal Src phosphorylation (data not shown). These results suggest that cAMP-GEF-induced Srctyr416 phosphorylation is upstream of PI3K and are compatible with a cAMP-GEF/Src/PI3K/Akt signaling pathway.
Fig. 4.
cAMP-GEF-mediated phosphorylation of Srctyr416 is PI3K independent. Whole cell lysates were prepared from control hepatocytes and hepatocytes treated with 20 μM CPT-2-Me-cAMP for 5 min with or without pretreatment with 50 nM wortmannin or 20 μM LY294002. The amount of Srctyr416 phosphorylation was quantified from immunoblotting with phosphospecific antibodies (n = 3). A representative immunoblot is shown. *Significantly different from value in control cells; #significantly different from value in hepatocytes treated with CPT-2-Me-cAMP.
cAMP-GEF-induced activation of rap GTPases is Src independent.
The Rap GTPases are important downstream effectors in cAMP-GEF signaling (23). We have previously shown that cAMP-GEF results in PI3K-independent activation of the Rap 1 GTPase in rat hepatocytes (12). This observation is extended to show that CPT-2-Me-cAMP also activates Rap 2 GTPase in rat hepatocytes (Fig. 5B). To determine whether activation of Src phosphorylation by cAMP-GEF is required for Rap GTPase activation we treated hepatocytes sequentially with the Src inhibitors, PP2 or SU6656, and then with CPT-2-Me-cAMP and determined the effect on Rap 1 and Rap 2 activation. Neither PP2 nor SU6656 had any effect on CPT-2-Me-cAMP-mediated activation of Rap 1 or Rap 2 (Fig. 5, A and B). PP2 alone had no effect on Rap 1 or Rap 2 activity (data not shown). These results indicate that Src-TYK phosphorylation is not necessary for cAMP-GEF-mediated Rap activation and would be compatible with a signaling pathway in which Rap GTPases activation precede Src phosphorylation in a cAMP-GEF/Rap GTPase/Src/PI3K/Akt pathway.
Fig. 5.
cAMP-GEF-mediated activation of Rap GTPases is Src independent. Hepatocytes were treated with 20 μM CPT-2-Me-cAMP for 15 min with or without pretreatment with 10 μM PP2 or SU6656 for 30 min and then assayed for the amount of active GTP bound Rap 1 (A) or Rap 2 (B). The results are the quantification of amount of GTP-Rap 1 or GTP-Rap 2 from 3 separate experiments. A representative immunoblot showing the amount of GTP-bound Rap 1 or Rap 2 from a single experiment is shown. *Significantly different from value seen in control hepatocytes.
cAMP-GEF cytoprotection and transactivation of the HGF and EGF receptor.
Src-TYK can phosphorylate the EGF receptor and lead to its transactivation (8, 23, 64). cAMP and CPT-2-Me-cAMP have been shown to transactivate growth factor receptors as well (3, 4, 47, 63). Both the EGF receptor and HGF receptor are coupled to PI3K/Akt activation in hepatocytes and are known to function as survival kinases in hepatocytes (7, 37, 44, 62). Thus we hypothesized that cAMP-GEF downstream signaling to Src or Akt might involve transactivation of growth factor receptors. To test this hypothesis the effect of cAMP-GEF activation on the EGF and HGF receptor phosphorylation was examined. Since both HGF and EGF receptor activation involves tyrosine phosphorylation, two strategies were used to evaluate receptor phosphorylation: 1) immunoprecipitation of phospho-tyrosinated proteins followed by immunoblotting for the EGF or HGF receptor and 2) immunoblotting of whole cell lysates with phosphospecific antibodies to the major autophosphorylation sites, EGFRtyr1173 and HGFRtyr1230. Our results show that whereas treatment with HGF itself causes a robust receptor phosphorylation that is detected by using both techniques, 1-, 5-, and 30-min treatment with CPT-2-Me-cAMP does not result in phosphorylation of the HGF receptor (data not shown).
When EGFR phosphorylation was examined, cAMP-GEF caused a transient increase in EGFRtyr1173 phosphorylation within 1 min that was undetectable by 15 min posttreatment (Fig. 6, A–C). To further define the role of EGFR, the effect of AG1478, an EGFR tyrosine kinase inhibitor, on cAMP-GEF-mediated Src-TYK and Akt phosphorylation and cytoprotection was determined. The EGFR inhibitor had no effect on CPT-2-Me-cAMP-mediated phosphorylation of Srctyr416 (Fig. 6, D–F). CPT-2-Me-cAMP-induced phosphorylation of Aktser473 was partially inhibited by AG 1478 (Fig. 7, D and E). Thus activation of Akt by cAMP-GEF is partially mediated via EGFR.
Fig. 6.
Transient transactivation of the epidermal growth factor receptor (EGFR) by cAMP-GEF is partially responsible for cAMP-GEF-mediated Aktser473 phosphorylation but has no role in Srctry416 phosphorylation. Cell lysates were prepared from hepatocytes treated with 20 μM CPT-2-Me-cAMP or 100 ng/ml of EGF for the indicated time periods. Whole cell lysates were immunoblotted directly with phosphoantibodies to EGFRtyr1173. A: quantification of at least 3 separate immunoblots. B: representative immunoblots. C: phospho-tyrosinated proteins (Phos-Ty) were immunoprecipitated (IP) from the cell lysates and immunoblotted with total EGFR (TEGFR) antibody. A representative immunoblot of cells treated for 5 min with CPT-2-Me-cAMP or EGF is shown. D: cell lysates were prepared from hepatocytes treated for the indicated time period with 20 μM CPT-2-Me-cAMP or 100 ng/ml EGF with or without pretreatment with 5 μM AG 1478 and then immunoblotted for Srctyr416 or Aktser473. Results of quantitative analysis of 4–8 separate experiments are shown. E: representative immunoblots for each kinase are shown. TSrc, total Src; TAkt, total Akt. F: cell lysates were treated as in D except for pretreatment with 10 μM PP2 alone or in combination with CPT-2-Me-cAMP and then immunoblotted for Aktser473. *Significantly different from value seen in control untreated cells; #different from value seen in CPT-2-Me-cAMP-treated cells.
Fig. 7.
cAMP-GEF-mediated cytoprotection is partially dependent on Src- mediated EGFR transactivation. A: hepatocytes were treated with 50 μM GCDC for 2 h and apoptosis determined by morphological evaluation of Hoechst-stained cells. Some cultures were pretreated with 20 μM CPT-2-Me-cAMP for 30 min or sequentially with 5 μM AG1478 or 10 μM PP2 for 30 min followed by CPT-2-Me-cAMP. Results of 8 experiments were expressed as the mean % apoptosis with or without SD. *Significantly different from value seen in GCDC-treated cells (P < 0.05); #different from value seen in GCDC/CPT-2-Me-cAMP treated cells. B and C: hepatocytes were treated with 20 μM of CPT-2-Me-cAMP for 5 min with or without pretreatment for 30 min with either 5 μM AG 1478 or 10 μM PP2. Whole cell lysates were prepared and immunoblotted for the phosphorylated receptor, EGFRtyr1173. B: quantified results from 4 separate immunoblots. C: representative immunoblot. *Statistically different from value seen in control cells.
The partial inhibition of Akt phosphorylation via EGFR and the fact that cAMP-GEF-mediated Akt phosphorylation is necessary for cAMP's cytoprotection in hepatocytes raised the possibility that cAMP-GEF-induced EGFR activation may be involved in the cytoprotection. Thus the effect of AG 1478 on CPT-2-Me-cAMP's antiapoptotic effect was examined. The EGFR inhibitor partially inhibited the cytoprotective effect of cAMP-GEF (Fig. 7A). These results suggest that a cAMP-GEF/EGFR mediated-pathway to Akt phosphorylation exists in hepatocytes and that activation of this pathway may be necessary for maximal activation of Akt and cytoprotection from GCDC apoptosis.
cAMP-GEF-mediated transactivation of the EGFR has been reported to occur through Src (63). To determine whether this was the case in our system, we pretreated hepatocytes with PP2 prior to treatment with CPT-2-Me-cAMP and compared this with the effect of pretreatment with the EGFR inhibitor, AG 1478. The results show that inhibition of Src-TYK blocked cAMP-GEF-induced phosphorylation of the EGFR as effectively as the EGFR inhibitor AG1478 (Fig. 7, B and C). These results are compatible with the presence of a cAMP-GEF/RapGTPase/Src/EGFR/AKT signaling pathway in hepatocytes.
CPT-2-Me-cAMP results in Src tyrosine kinase-dependent activation of the p110 β and p110 α catalytic subunits of PI3K.
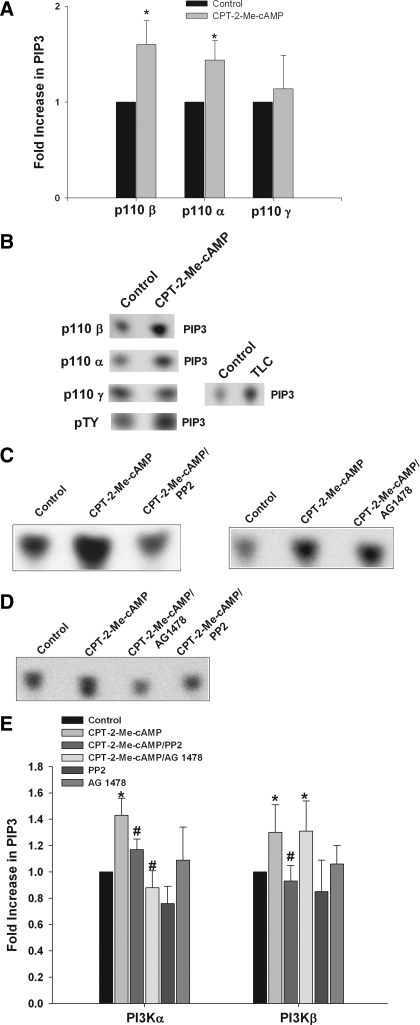
We have previously reported that cAMP treatment of hepatocytes results in an increase in PI3K activity in p85 immunoprecipitates (61). To investigate the isoform-specific activation of PI3K-p110 by cAMP-GEFs, lipid kinase assays were conducted by immunoprecipitating the active catalytic subunits of PI3K, p110 α, β, or γ and then subjecting the immunoprecipitates to lipid kinase assays. In these kinase assays the production of PIP3 in vitro was used as an indication of class I PI3K activation. Treatment of hepatocytes with CPT-2-Me-cAMP resulted in activation of the p110 β and p110 α but not the p110 γ catalytic subunit (Fig. 8, A and B). Note that TLC, used as a positive control (25), activated p110 γ (Fig. 8B). Since the p110 β subunit can be activated either by tyrosine phosphorylation of the p85 PI3K subunit or by Gβγ subunits, the effect of CPT-2-Me-cAMP on PI3K activity in phosphotyrosine immunoprecipitates was investigated. CPT-2-Me-cAMP activated PI3K in these immunoprecipitates (Fig. 8B) indicating PI3K-p85 mediated activation of p110 β.
Fig. 8.
cAMP-GEF activates the p110 β and p110 α catalytic subunit of phosphoinositide-3-kinase (PI3K). Whole cell lysates were prepared from hepatocytes treated with 20 μM CPT-2-Me-cAMP for 5 min. PI3K was immunoprecipitated from the lysates with antibodies specific for either the p110 α, β, or γ catalytic subunit of PI3K. After the washing, lipid kinase assays were performed by using phosphatidylinositol-3,4,5-trisphosphate (PIP3) as a substrate with [γ-32P] ATP. The amount of PIP3 generated was determined by autoradiography after thin layer chromatography (TLC) of the reaction products. A: fold increase in PIP3 seen in the lipid kinase assay with immunoprecipitates of each isoform, representing the results of at least 5 separate assays. B: representative autoradiographs of lipid kinase assays on p110 α, β, γ, and phosphotyrosinated protein immunoprecipitates are shown. Positive control lipid kinase assays with 25 μM taurolithocholic acid (TLC) (15 min) are shown for the p110 γ isoform-specific assay. The effect of Src and EGFR inhibition on p110 β and α activation was assessed by pretreatment with 10 μM PP2 or 5 μM AG1478 for 15 min alone or followed by stimulation with 20 μM CPT-2-Me-cAMP for 5 min. Lipid kinase assays were done with p110 β (C) and p110 α (D) immunoprecipitates, and the result of at least 5 separate experiments are presented graphically (E). *Significantly different than in control cells; #significantly different than in CPT-2-Me-cAMP treated cells.
To determine whether CPT-2-Me-cAMP-induced activation of PI3K-p110 β/α was mediated via Src-TYK, the effect of Src inhibition on PI3K activity was determined. Pretreatment with PP2 for 15 min prior to stimulation with CPT-2-Me-cAMP for 5 min inhibited activation of both p110 β and α isoforms (Fig. 8, C–E). These results are consistent with a cAMP-GEF/Src/p110 β/α PI3K signaling pathway.
The EGFR can activate PI3K via two mechanisms: 1) by binding and activating the p85 regulatory subunit, which in turn could activates the p110 α or β subunit, and 2) by activating Ras, which has been linked only to activation of the p110 α subunit (50). To dissect the role of the EGFR receptor in p110 activation, we determined the effect of the EGFR inhibitor AG1473 on the activation of p110 α/β in response to CPT-2-Me-cAMP. The results (Fig. 8, C–E) show that AG1473 inhibited p110 α activation, but not p110 β activation.
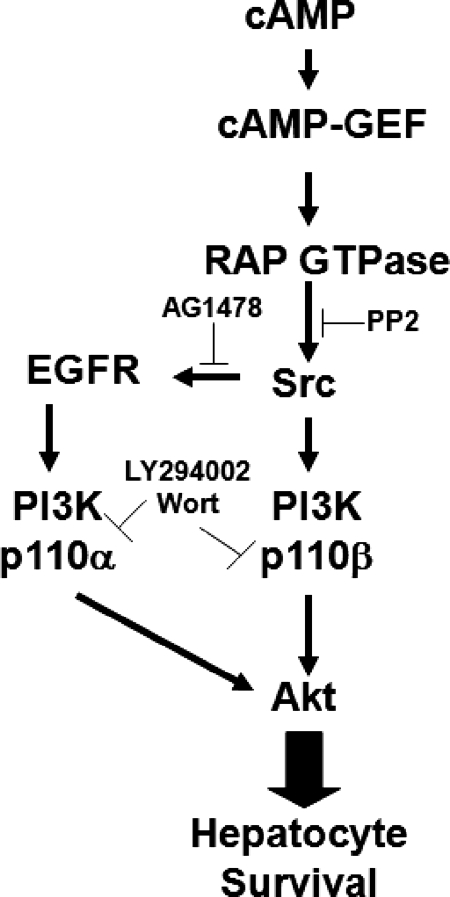
Collectively these results suggest that cAMP-GEF activates the class 1A p110 α and β isoforms of PI3K through divergent Src dependent mechanisms (Fig. 9). The p110 α isoform is activated through a cAMP-GEF/Rap/Src/EGFR pathway and the p110 β isoform through a cAMP-GEF/Rap/Src pathway.
Fig. 9.
Putative antiapoptotic cAMP-GEF survival signaling pathways in hepatocytes. cAMP activates cAMP-GEFs, which promote hepatocyte survival. Both cAMP-GEF activation of PI3K/Akt and cAMP-GEF cytoprotection are prevented by PI3K inhibition with either LY 294002 or wortmannin. Since these PI3K inhibitors have no effect on cAMP-GEF activation of Rap GTPases or Src tyrosine kinases, these events are upstream of cAMP-GEF-mediated PI3K. Inhibition of Src tyrosine kinases with PP2 prevents cAMP-GEF activation of PI3K p110 β and p110 α and thus Akt and prevents cAMP-GEFs antiapoptotic effect, but it has no effect on cAMP-mediated Rap activation. cAMP-GEF results in Src-dependent phosphorylation of the EGFR, which is necessary for p110 α activation, but not for p110 β activation. This transactivation is necessary for both maximal Akt activation and cytoprotection by cAMP-GEFs.
Effect of kinase inhibitors on bile acid accumulation in hepatocytes.
To verify that the observed effects of kinase inhibitors were not influenced by alterations in bile acid uptake, the accumulation of [3H]taurocholate in hepatocytes treated with 10 μM PP2, 10 μM PP3, 5 μM AG1478, or 10 μM SU6656 was determined. The accumulation of taurocholate in PP2, PP3, and AG 1478 treated hepatocytes was 99 ± 6, 96 ± 3.5, and 101 ± 7.6% (n = 3) of that seen in control hepatocytes, respectively. In hepatocytes treated with SU6656, accumulation of taurocholate was mildly, but significantly, decreased to 75 ± 9.5% of that seen in control cells. Since bile acids must enter hepatocytes to cause apoptosis, these results with SU6656 precluded its use in hepatocyte apoptosis assays. We have previously shown that PI3K inhibition has no effect on the 30-min accumulation of taurocholate (61).
DISCUSSION
The aim of this study was to determine the role of Src-TYK in cAMP-GEF signaling and cytoprotection in hepatocytes and to elucidate whether cAMP-GEFs mediate isoform-specific activation of PI3K-p110. Our results show that cAMP-GEF activation in hepatocytes results in phosphorylation of Src-TYK, which in turn activates PI3K/Akt and is necessary for cAMP-GEF cytoprotection from bile acid-induced apoptosis. In addition, we show that cAMP-GEF results in Src-dependent isoform-specific activation of the p110 β and α catalytic subunits of PI3K by two divergent pathways: a cAMP-GEF/Rap-GTPase/Src/EGFR/PI3K p110 α pathway and a cAMP-GEF/Rap-GTPase/SrcTYK/PI3K p110 β pathway (Fig. 9).
Although a mechanistic link between growth factor signaling and Src-TYK activation of PI3K/Akt has been established in several cell types, this report is the first demonstration that cAMP-mediated PI3K/Akt activation occurs through cAMP-GEF-induced phosphorylation of Src-TYK in hepatocytes. A recent study demonstrated a similar cAMP-GEF/Src/PI3K/Akt pathway in mesangial cells (63). Activation of Src-TYK requires both autophosphorylation of Tyr 418 and dephosphorylation of the autoinhibitory site, Tyr 527 (58, 60). This is achieved by protein-protein interactions between Src's SH2 or SH3 domains and phosphorylated tyrosine residues or proline-rich sequences bearing a PxxP motif, respectively. In addition, each Src family member possesses a unique NH2-terminal domain that can influence activation status. Previous studies have demonstrated that cAMP acting through PKA can phosphorylate Src on serine 17 in this unique region, resulting inhibition (46). The mechanism whereby cAMP/cAMP-GEF activates Src-TYK is unknown. Structural analysis of cAMP-GEFs, however, suggests that direct binding to Src-TYKs is unlikely and thus activation may involve as yet unknown intermediate signaling molecules.
Although cAMP has been shown to activate PI3K in a variety of cell types, little information is available on which PI3K p110 catalytic subunits are involved. We show in this study that cAMP-GEF mediates Src-dependent activation of the p110 β and p110 α subunits in hepatocytes. cAMP-GEF-mediated activation of the p110 α subunit requires transactivation of the EGFR because it is completely blocked by inhibition of EGFR tyrosine kinase activity. In contrast, p110 β activation proceeds in the face of EGFR inhibition (Fig. 9). These results might be explained if the activation of p110 α proceeds through EGFR activation of Ras since the activation of Ras by tyrosine kinase receptors has only been linked to the activation of the p110 α and not the p110 β isoform (50). Our studies are the first to demonstrate that cAMP working through a PKA-independent cAMP-GEF pathway can transactivate the EGFR in hepatocytes and that this transactivation is coupled to activation of the of PI3K.
cAMP-GEF activation of the p110 β catalytic subunit is Src-TYK dependent but independent of the EGFR and HGFR. Src-TYKs are known to upregulate PI3K through multiple mechanisms including recruitment of PI3K to activated membrane compartments, activation of the p85 PI3K regulatory subunit, and inhibition of the lipid phosphatase PTEN (2, 9, 38, 48). Our data provide some insight into the mechanism of action of Src-TYK. If Src-TYKs were acting through activation of the p85 subunit then the p110 α and β subunits should have responded similarly in our experiments. Similarly, if Src-TYKs were inhibiting PTEN then one would have expected to see a generalized increase in the activity of all three p110 isoforms. Thus it is more likely that the Src-TYKs may selectively influence the movement of the p110 β isoform to membrane, although the exact mechanism whereby the Src-TYK results in the activation of p110 β isoform in hepatocytes remains to be elucidated.
Several studies have identified a role for cAMP-GEF signaling in cell survival (12, 33, 34, 56). In rodent hepatocytes activation of cAMP-GEF protects against Fas, TNF-α, and bile acid-induced apoptosis (12, 61). Studies in diverse cell types have demonstrated that cAMP-GEF activates a PI3K/Akt pathway (12, 33, 41, 43), and this activation has been linked to its prosurvival effect in hepatocytes, cardiac myocytes, and macrophages (12, 33, 43). Our previous studies in rat hepatocytes (12) and recent studies in mouse hepatocytes (56) have demonstrated a mechanistic link between cAMP-GEF/PI3K/Akt signaling and survival. Our present studies extend these observations to show that phosphorylation of Src-TYK, transactivation of the EGFR, and subsequent activation of p110 β and α are necessary for maximal activation of PI3K/Akt and protection from bile acid-induced apoptosis.
There is evidence in the literature suggesting that cAMP/PKA-dependent pathways may also be involved in PI3K activation (13, 31). In Cos 7 and WIF B cells cAMP activation of PI3K is sensitive to PKA inhibition; however, this observation is based on the use of a relatively nonselective PKA inhibitor, KT 5720, which also is known to inhibit PDK1 (31). More recent studies in fibroblast and thyroid cells have convincingly demonstrated cAMP/PKA-dependent serine 83 phosphorylation of p85 regulatory subunit of PI3K (13). This phosphorylation amplifies formation of a Ras-PI3K complex that stimulates PI3K catalytic activity. Although it is unknown whether this cAMP-PKA-PI3K signaling pathway is present in hepatocytes, our studies clearly show that cAMP activates PI3K via cAMP-GEF in hepatocytes.
Most of the downstream actions of cAMP-GEFs are mediated by the Rap family of small GTP proteins, Rap 1 and Rap 2 (26). Our previous studies showing that cAMP-GEFs activate Rap 1 in hepatocytes (12), and our present observation that they also activate Rap 2 verifies the presence of these cAMP/cAMP-GEF/Rap signaling pathway in hepatocytes. The fact that cAMP-GEF activation of Rap GTPases is not prevented by Src and PI3K inhibition suggests that Rap activation is upstream of these kinases in a cAMP-GEF/Src/PI3K/Akt activation pathway (Fig. 9). This is consistent with studies in nonhepatic cells showing that cAMP-GEF activation of PI3K/Akt requires Rap GTPases (41, 59). In addition, a connection between cAMP/Ras and PI3K activation has been established in COS 7 cells (31). However, the possibility that Rap activation may not be involved in cAMP-GEF signaling to PI3K/Akt in hepatocytes cannot be ruled out. Some actions of cAMP-GEF are Rap independent, including activation of Ras GTPases and interactions with microtubule and secretory associated proteins (26). Additional studies are necessary to more clearly define the role of Rap GTPases in a cAMP-GEF/Src/PI3K/Akt pathway.
Src-TYK are a family of nine enzymes (58). Of these family members, Src, Yes, Fyn, and Lck are known to be expressed in hepatocytes. Although we show in this study that Src-TYKs are necessary for cAMP-GEF survival in bile acid-induced apoptosis, which member of the Src family is involved in this effect remains to be elucidated. Of the family members, the role of c-Src in cell survival has been well documented. In hepatocytes Src-TYK are necessary for glycine-induced hepatocyte resistance to hypoxia, adenosine-mediated hepatic preconditioning, and hepatitis B virus X protein and TGF-β-stimulated survival (5, 6, 44, 55). Similar to this study's finding, these Src-mediated survival effects have been linked to activation of PI3K/Akt.
Activation of Src-TKY may also be proapoptotic in hepatocytes (49). In primary hepatocytes, apoptosis induced by Fas ligand or hydrophobic bile acids, including GCDC, requires selective phosphorylation of the Src TYK Yes. Yes phosphorylation transactivates the EGF receptor, facilitating its association with and activation of the Fas death receptor. Inhibition of Yes activation with SU6656, but not PP2, prevents both bile acid-induced Yes activation and apoptosis. We have also seen that SU 6656 pretreatment prevents GCDC-induced apoptosis and initially attributed this largely to the fact that this inhibitor partially blocks bile acid uptake, but some of the protective effect of SU 6656 may also be explained by selective Yes inhibition. Thus Src-TYK kinase activation may be pro- or antiapoptotic in hepatocytes, and the choice between these two responses may be dictated by different Src family members. Divergent effects by different Src effectors may be generated by differential recruitment of Src family members to the cellular membranes and/or interactions with different substrates and binding partners.
cAMP is an important second messenger in hepatic signaling and is strongly associated with cytoprotective properties in hepatocytes. The mechanisms and relevant players mediating cAMP effects on hepatocyte health are still being discovered. The results of this present study help to clarify some of the effectors and support the existence of both cAMP-GEF/Src/PI3K p110 β/Akt and cAMP-GEF/Src/EGFR/PI3K p110 α/Akt survival pathways in hepatocytes, both of which must be activated for maximal survival effects. Further dissection of these prosurvival pathways could lead to the identification of target molecules that can be exploited for the development of therapeutic strategies to slow the progression of chronic hepatopathies.
GRANTS
This worked was supported by the following grants from the National Institutes of Health: NIH R01 DK-065975 to C. R. L. Webster and NIH R01 DK-033436 awarded to M. S. Anwer.
Acknowledgments
We thank Irwin Arias and Lyuba Varticovski from the National Institutes of Health for advice and for sharing expertise in performing the PI3K lipid kinase assays.
Present address for S. Hohenester: AMC Liver Center, Department of Gastroenterology & Hepatology, University of Amsterdam, Amsterdam, The Netherlands.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andre C, Couton D, Gaston J, Erraji L, Renia L, Varlet P, Brian P, Guillet J. Alpha 2-adrenergic receptor selective agonist clenbuterol prevents Fas mediated liver apoptosis and death in mice. Am J Physiol Gastrointest Liver Physiol 276: G647–G654, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Arcaro A, Aubert M, Espinosa del Hierro ME, Khanzada UK, Angelidou S, Tetley TD, Bittermann AG, Frame MC, Seckl M. Critical role for lipid raft associated Src kinases in activation of PI3K-Akt signaling. Cell Signal 19: 1081–1092, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Barbier AJ, Poppleton HM, Yigzaw Y, Mullenix JB, Wiepz GJ, Bertics PJ, Patel TB. Transmodulation of epidermal growth factor receptor function by cyclic AMP-dependent protein kinase. J Biol Chem 274: 14067–14073, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Bertelsen LS, Barrett KE, Keely SJ. Gs protein-coupled receptor agonists induce transactivation of the epidermal growth factor receptor in T84 cells: implications for epithelial secretory responses. J Biol Chem 279: 6271–6279, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Beuers U, Denk GU, Soroka CJ, Wimmer R, Rust C, Paumgartner G, Boyer JL. Taurolithocholic acid exerts cholestatic effects via phosphatidylinositol 3-kinase dependent mechanisms in perfused rat livers, and rat hepatocytes couplets. J Biol Chem 278: 17810–17818, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Brachmann SM, Yballe CM, Innocenti M, Deane JA, Fruman DA, Thomas SM, Cantley LC. Role of phosphoinositide 3-kinase regulatory isoforms in development, and actin rearrangement. Essential role of PI3K-p110 beta in cell growth, metabolism and tumorigenesis. Mol Cell Biol 25: 2593–2606, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carini R, Alchera E, Baldanzi G, Piranda D, Splendores R, Grazia De Cesaris M, Caraceni P, Grasiani A, Albano E. Role of p38 map kinase in glycine-induced hepatocyte resistance to hypoxic injury. J Hepatol 46: 692–699, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Carini R, Grazia de Cesaris M, Splendore R, Baldanzi G, Nitti MP, Alchera E, Filigheddu N, Domenicotti C, Pronzato MA, Graziani A, Albano E. Role of phosphatidylinositol 3-kinase in the development of hepatocyte preconditioning. Gastroenterology 127: 914–923, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Carmona-Cuenca I, Herrera B, Ventura JJ, Roncero C, Fernandez M, Fabregat I. EGF blocks NADPH oxidase activation by TGF-beta in fetal rat hepatocytes, impairing oxidative stress, and cell death. J Cell Physiol 207: 322–330, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Chen K, Vita JA, Berk BC, Keaney JF. c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves Src-dependent epidermal growth factor receptor transactivation. J Biol Chem 276: 16045–16050, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Cuevas B, Lu Y, Mao M, Zhang J, LaPushin R, Siminovitch K, Mills GB. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphoinositol 3-kinase. J Biol Chem 276: 27455–27461, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Cullen K, McCool J, Anwer MS, Webster CRL. Activation of cAMP-guanine exchange factor (cAMP-GEF) confers protein kinase A independent protection from hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol 287: G334–G343, 2004. [DOI] [PubMed] [Google Scholar]
- 13.De Gregorio G, Coppa A, Cosentino C, Ucci S, Messina S, Nicolussi A, D'Inzeo S, DiPardo A, Avvedimento EV, Porcellini A. The p85 regulatory subunit of PI3K mediates TSH-cAMP-PKA growth and survival signals. Oncogene 26: 2039–2047, 2007. [DOI] [PubMed] [Google Scholar]
- 14.DeToni EN, Kuntzen C, Gerbes AL, Thasler WE, Sonoc N, Mucha SR, Camaj P, Bruns C, Goke B, Eichhorst ST. p60-c-src suppresses apoptosis through inhibition of caspase 8 activation in hepatoma cells, but not in primary hepatocytes. J Hepatol 46: 682–691, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Diehl A, Yang S, Wand G. Chronic ethanol consumption disturbs G-protein expression and inhibits cyclic AMP dependent signaling in regenerating liver. Hepatology 16: 1212–1219, 1992. [PubMed] [Google Scholar]
- 16.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7: 606–619, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Critical role for the p110 alpha phosphoinositide 3-OH kinase in growth and metabolic regulation. Nature 441: 366–370, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Gantner F, Küsters S, Wendel A, Hatzelmann A, Schudt C, Tiegs G. Protection from T cell mediated murine liver failure by phosphodiesterase inhibitors. J Pharmacol Exp Ther 280: 53–60, 1997. [PubMed] [Google Scholar]
- 19.Glaser S, Alvaro D, Francis H, Ueno Y, Marucci L, Benedetti DeMorrow S A, Marzioni M, Mancino MG, Phinizy JL, Reichenback R, Fava G, Summers R, Venter J, Alpini G. Adrenergic receptor agonists prevent bile duct injury by adrenergic denervation by increased cAMP levels and activation of Akt. Am J Physiol Gastrointest Liver Physiol 290: G813–G826, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Gobejishvili L, Barve S, Joshi-Barve S, Uriarte S, Song Z, McClain C. Chronic ethanol mediated decreases in cAMP primes macrophages to enhanced LPS inducible NF-κB activity and TNF expression: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 291: G681–G688, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Graf D, Reinehr R, Kurz AK, Fishcer R, Haussinger D. Inhibition of taurolithocholate 3-sulfate-induced apoptosis by cyclic AMP in rat hepatocytes involves protein kinase A-dependent and -independent mechanisms. Arch Biochem Biophys 415: 34–42, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B. Angiogenesis selectively requires the p110α isoform of PI3K to control cell migration. Nature 453: 662–666, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Han C, Bowen WC, Michalopoulos GK, Wu T. Alpha-1 adrenergic receptor transactivates signal transducer and activator of transcription-3 (Stat3) through activation of Src and epidermal growth factor receptor (EGFR) in hepatocytes. J Cell Physiol 216: 486–497, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harbrecht BG, Perpetua M, Fulmer M, Zhang B. Glucagon regulates hepatic inducible nitric oxide synthesis in vivo. Shock 22: 157–162, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Hohenester S, Gates A, Beuers U, Anwer S, Webster CRL. Differential activation of Class IA phosphoinositide-3 kinase p110 isoforms by bile acids: role of p110 γ activation in hepatocyte apoptosis (Abstract). Hepatology 46: 254, 2007.17596850 [Google Scholar]
- 26.Holz GC, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of Epac. J Physiol 577: 5–15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikegami T, Kriloy L, Meng J, Patel B, Chapin-Kennedy K, Bouscarel B. Decreased glucagon responsiveness by bile acids: a role for protein kinase C alpha and glucagon receptor phosphorylation. Endocrinology 147: 5294–5302, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa H, Jin MB, Ogata T, Taniguchi M, Sukuki T, Shimamura T, Magata S, Horiuchi H, Ogata K, Masuko H, Fujita M, Furukawa H, Todo S. Role of cyclic nucleotides in ischemia and reperfusion injury of canine livers. Transplantation 73: 1041–1048, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, Kenche V, Anderson KE, Dopheide SM, Yuan Y, Sturgeon SA, Prabaharan H, Thompson PE, Smith GD, Shepherd PR, Daniele N, Kulkarni S, Abbott B, Saylik D, Jones C, Lu L, Giuliano S, Hughan SC, Angus JA, Robertson AD, Salem HH. PI3-kinase p110 beta: a new target for anti-thrombotic therapy. Nat Med 11: 507–514, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ. Essential roles of PI(3)K-p110β in cell growth, metabolism, and tumorigenesis. Nature 454: 776–779, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagawa T, Varticovski L, Sai Y, Arias IM. Mechanism by which cAMP activates PI3-kinase and increases bile acid secretion in WIF-B9 cells. Am J Physiol Cell Physiol 283: C1655–C1666, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Kume M, Banafsche R, Yamamoto Y, Yamoaka Y, Nobiling R, Gebhard MM, Klar E. Dynamic changes of post-ischemic hepatic microcirculation improved by a pretreatment of phosphodiesterase-3 inhibitor, milrinone. J Surg Res 136: 209–218, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Kwak JHJ, Park KM, Choi HE, Chung KS, Lim HJ, Park HY. PDE4 inhibitor, roflumilast protects cardiomyocytes against NO-induced apoptosis via activation of PKA and Epac dual pathways. Cell Signal 20: 803–814, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Kwon G, Pappan KL, Marshall C, Schaffer JE. cAMP dose-dependently prevents palmitate induced apoptosis by both protein kinase A and cAMP guanine nucleotide exchange factor dependent pathways in beta cells. J Biol Chem 279: 8938–8945, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Lesma E, Grande V, Di Giulio AM, Castellana P, Crosignani A, Cerri A, Gorio A. G-protein mediated signal transduction is affected in primary biliary cirrhosis. Hepatol Res 35: 45–52, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Yang S, Billiar TR. Cyclic nucleotides suppress tumor necrosis factor alpha-mediated apoptosis by inhibiting caspase activation and cytochrome c release in primary hepatocytes via a mechanism independent of Akt activation. J Biol Chem 275: 13026–13034, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Mizuno S, Nakamura T. Anti-necrotic and anti-apoptotic effects of hepatocyte growth factor on cholestatic hepatitis in a mouse model of bile-obstructive diseases. Am J Physiol Gastrointest Liver Physiol 292: G639–G646, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Marengere LE, Koch CA, Pawson T. The v-Src SH3 domain binds phosphatidylinositol 3′-kinase. Mol Cell Biol 13: 5225–5232, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, McMurrany JS, Fang X, Yung WK, Siminovitch KA, Mills GB. Src family protein tyrosine kinases alter the function of PTEN to regulate phosphatidyl 3-kinase/Akt cascades. J Biol Chem 278: 40057–40066, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Macrez N, Mironneau C, Carricaburu V, Quignard JF, Babich A, Czupalla C, Nürnberg B, Mironneau J. Phosphoinositide 3-kinase isoforms selectively couple receptors to vascular L-type Ca (2+) channels. Circ Res 89: 692–699, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Mei F, Qiao J, Tsygankova O, Meinkoth F, Quilliam L, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP dependent protein kinase on protein kinase B activation. J Biol Chem 277: 11497–11504, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Misra S, Varticovski L, Arias I. Mechanism by which cAMP increases bile acid secretion in rat liver and canalicular membrane vesicles. Am J Physiol Gastrointest Liver Physiol 285: G316–G324, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Misra UK, Kaczowka S, Pizzo SV. The cAMP-activated GTP exchange factor, Epac1 upregulates plasma membrane and nuclear Akt kinase activities in 8-CPT-2-O-Me-cAMP-stimulated macrophages: gene silencing of the cAMP-activated GTP exchange Epac1 prevents 8-CPT-2-O-Me-cAMP activation of Akt activity in macrophages. Cell Signal 20: 1459–1470, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murillo MM, del Castillo G, Sanchez A, Fernandez M, Fabregat I. Involvement of EGF receptor and c-Src in the survival signals induced by TGF-beta1 in hepatocytes. Oncogene 24: 4580–4587, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Nitta T, Kim JS, Mohunczy D, Behrns KE. Murine cirrhosis induces hepatocytes epithelial mesenchymal transition and alterations in survival signaling pathways. Hepatology 48: 909–919, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obara Y, Labudda K, Dillon TJ, Stork PJ. PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J Cell Sci 117: 6085–6094, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Piiper A, Dikic I, Lutz MP, Leser J, Kronenberger B, Elez R, Cramer H, Müller-Esterl W, Zeuzem S. Cyclic AMP induces transactivation of the receptors for epidermal growth factor and nerve growth factor, thereby modulating activation of MAP kinase, Akt, and neurite outgrowth in PC12 cells. J Biol Chem 277: 43623–43630, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Pleiman CM, Hertz WM, Cambier J. Activation of phosphatidylinositol-3′ kinase by Src family kinase SH3 binding to p85 subunit. Science 263: 1609–1612, 1994. [DOI] [PubMed] [Google Scholar]
- 49.Reinehr R, Becker S, Wettstein M, Haussinger D. Involvement of the Src family kinase yes in bile salt-induced apoptosis. Gastroenterology 127: 1540–1557, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Viciana P, Sabatier C, McCormick F. Signaling specificity by Ras family GTPases is determined by the full spectrum of effectors they regulate. Mol Cell Biol 24: 4943–4954, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rohrbach A, Rubio I, Bulgay-Moerschel M, Koenig C, Poehlmann TG, Markert UR, Gruen M. Selective downregulation of phosphoinositide 3-kinase alpha in leukocytes during pregnancy. Am J Reprod Immunol 61: 130–135, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Ruckle T, Schwarz MK, Rommel C, Clump DA, Oazi IH, Sudol M, Flynn DC. PI3Kgamma inhibition: towards an 'aspirin of the 21st century'? Nat Rev Drug Discov 5: 903–918, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Schoemaker MH, Conde de la Rosa L, Buist-Homan M, Vrenken TE, Havinga R, Poelstra K, Haisma HJ, Jensen PL, Moshage H. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology 39: 1563–1573, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Schulze-Bergkamen H, Brenner D, Krueger A, Suess D, Fas SC, Frey CR, Dax A, Zink D, Büchler P, Müller M, Krammer PH. Hepatocyte growth factor induces Mcl-1 in primary human hepatocytes and inhibits CD95-mediated apoptosis via Akt. Hepatology 39: 645–654, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Shih WL, Kuo ML, Chuang SE, Cheng AL, Doong SL. Hepatitis B virus X protein activates a survival signaling by linking SRC to phosphatidylinositol 3-kinase. J Biol Chem 278: 31807–31813, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Sinclair EM, Yusta B, Streutker C, Baggio LL, Koehler J, Charron MJ, Drucker DJ. Glucagon receptor signaling is essential for control of murine hepatocyte survival. Gastroenterology 135: 2096–2106, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Takano M, Arai T, Mokuno Y, Nishimura H, Nimura Y, Yoshikai Y. Dibutyryl cyclic adenosine monophosphate protects mice against tumor necrosis factor induced hepatocyte apoptosis accompanied by increased heat shock protein 70 expression. Cell Stress Chaperones 3: 109–116, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas S, Brugge J. Cellular function regulated by Src family kinase. Annu Rev Cell Dev Biol 13: 513–609, 1997. [DOI] [PubMed] [Google Scholar]
- 59.Tiwari S, Felekkis K, Moon EY, Sherr DH, Lerner A. Among circulating hematopoietic cells, B-CLL, uniquely expresses functional EPAC1, but EPAC 1-mediated Rap 1 activation does not account for PDE4 inhibitor induced apoptosis. Blood 103: 2661–2667, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Trevino JG, Summy JM, Gallick GE. SRC inhibitors as potential therapeutic agents for human cancers. Mini Rev Med Chem 6: 681–687, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Webster CRL, Usechak P, Anwer MS. cAMP inhibits bile acid induced apoptosis by blocking caspase activation and cytochrome c release. Am J Physiol Gastrointest Liver Physiol 283: G727–G738, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Webster CR, Anwer MS. Phosphoinositide 3-kinase, but not mitogen-activated protein kinase, pathway is involved in hepatocyte growth factor-mediated protection against bile acid-induced apoptosis in cultured rat hepatocytes. Hepatology 33: 608–615, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Yano N, Suzuki D, Endoh M, Zhao TC, Padbury JF, Tseng YT. A novel phosphoinositide 3-kinase-dependent pathway for angiotensin II/AT-1 receptor-mediated induction of collagen synthesis in MES-13 mesangial cells. J Biol Chem 282: 18819–18830, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Yano N, Ianus V, Zhao TC, Tseng A, Padbury JF, Tseng YT. A novel signaling pathway for β-adrenergic receptor mediated activation of phosphoinositide 3-kinase in H9c2 cardiomyocytes. Am J Physiol Heart Circ Physiol 293: H385–H393, 2007. [DOI] [PubMed] [Google Scholar]