Abstract
Experimental hepatopulmonary syndrome (HPS) after common bile duct ligation (CBDL) in rat is accompanied by increased lung vascular endothelial endothelin B (ETB) receptor expression and increased circulating levels of endothelin-1 (ET-1). The onset of HPS is hypothesized to be triggered by ET-1/ETB receptor activation of endothelial nitric oxide synthase (eNOS)-derived NO production in the pulmonary endothelium. However, whether functional pulmonary vascular ETB receptors are required for the development of experimental HPS is not defined. We evaluated the effects of vascular ETB receptor deficiency on the development of experimental HPS. The molecular and physiological alterations of HPS were compared in 2-wk CBDL wild-type and ETB receptor-deficient (transgenic sl/sl) rats. Relative to wild-type rats, basal hepatic and plasma ET-1 levels were elevated in sl/sl controls although, unlike wild-type animals circulating ET-1 levels, did not increase further after CBDL in sl/sl animals. In contrast to wild-type animals, ETB receptor-deficient rats did not develop increased Akt and eNOS expression and activation and did not develop gas exchange abnormalities of HPS after CBDL. There was a similar degree of pulmonary intravascular monocyte accumulation in both 2-wk CBDL sl/sl and wild-type animals. In conclusion, ETB receptor deficiency inhibits lung Akt/eNOS activation and prevents the onset of experimental HPS after CBDL. This effect is independent of inhibition of pulmonary intravascular monocyte accumulation. These results demonstrate that ET-1/ETB receptor signaling plays a key role in the initiation of experimental HPS.
Keywords: common bile duct ligation, endothelin-1, endothelial nitric oxide synthase, intravascular monocyte
the hepatopulmonary syndrome (HPS) results when pulmonary microvascular dilation causes hypoxemia in cirrhosis (22). It is found in 15–30% of such patients, and its presence significantly increases mortality. Presently, there are no effective medical therapies (22, 26).
The pathogenesis of intrapulmonary vasodilatation in HPS is an area of active investigation. Biliary cirrhosis induced by common bile duct ligation (CBDL) in the rat reproduces the pulmonary vascular and gas exchange abnormalities of human HPS and serves as a model system (1). In CBDL animals, hepatic production and circulating levels of endothelin-1 (ET-1) increase within 1 wk after ligation (18). Although classically recognized as a vasoconstrictor acting through endothelin A (ETA) receptors on vascular smooth muscle cells, increased expression of endothelin B (ETB) receptors have been found on pulmonary vascular endothelium within 2 wk after CBDL (18). Activation of these ETB receptors by circulating ET-1 appears to initiate vasodilation and HPS through Akt and endothelial nitric oxide synthase (eNOS) activation and NO production (16, 18). Chronic infusion of a selective ETB receptor antagonist in animals after CBDL decreases pulmonary eNOS levels and improves HPS, thereby supporting a role for ET-1/ETB receptor signaling in this process (16).
However, whether functional pulmonary microvascular ETB receptor is required for the development of experimental HPS is unknown. To explore this question, we employed the spotting-lethal (sl) rat, which carries a naturally occurring deletion in the ETB receptor gene that prevents expression of functional ETB receptors in the vasculature (5). These rats have diminished ETB receptor-mediated pulmonary vasodilatation and exhibit exaggerated pulmonary vasopressor response to acute hypoxia and exogenous ET-1 (10, 11, 24). Therefore, we compared the development of HPS in wild-type and transgenic sl/sl rats that underwent CBDL.
MATERIALS AND METHODS
Animal models.
Male wild-type and ETB receptor-deficient sl/sl Wister rats (obtained from Dr. David Pollock, Vascular Biology Center Medical College of Georgia) underwent sham surgery or CBDL as previously described (1, 16). Body weight, mean systemic arterial pressure (MSAP), portal venous pressure (PVP), and spleen weight were measured to evaluate systemic effects and liver abnormalities. The study was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and conforms to National Institutes of Health guidelines on the care and use of laboratory animals. Five to eight animals were used in each group.
Arterial blood gas analysis.
Arterial blood drawn at rest was analyzed on an ABL 520 radiometer (Radiometer America, Westlake, OH) in the Clinical Laboratory, UAB Hospital as previously described (1, 16, 28). The alveolar-arterial oxygen difference (AaPo2) was calculated as 150 − (Pco2/0.8) − Po2.
Measurement of plasma and hepatic ET-1 levels.
ET-1 concentrations in plasma and liver were measured with a commercially available radioimmunoassay kit (Phoenix Pharmaceuticals, Mountain View, CA) as previously described (17) following the manufacturer's instructions.
Western blot analysis.
Tissue was homogenized in RIPA buffer in the presence of protease inhibitors. Equal amounts of protein from homogenates were fractionated on SDS-PAGE gel and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ). Incubation with primary antibodies was followed by addition of horseradish peroxidase-conjugated secondary antibodies, and detection with enhanced chemiluminescence (ECL, Amersham). Antibodies for total eNOS (BD Biosciences, San Jose, CA) and its phosphorylation at Ser1177 (peNOSser1177, Cell Signaling Technology, Danvers, MA), ETA receptor (EMD Biosciences, San Diego, CA), ectodermal dysplasia 1 (ED1) (specific marker for monocytes/macrophages; Santa Cruz Biotechnology, Santa Cruz, CA), heme oxygenase-1 (HO-1; Stressgen Bioreagents, Ann Arbor, MI), and total and phosphorylation of Akt (pAktser473, Cell Signaling Technology) were used to measure their levels in lung from sham and CBDL animals. The results were normalized by GADPH and expressed as fold control.
Statistical analysis.
All measurements are expressed as means ± SE. Data were analyzed using Student's t-test or ANOVA with Bonferroni correction for multiple comparisons between groups. P < 0.05 was considered statistically significant.
RESULTS
Systemic and portal hemodynamics and ET-1 levels in wild-type and transgenic sl/sl animals.
Systemic and portal hemodynamic changes were assessed in all groups (Table 1). Mean arterial blood pressure and PVP were similar in sham-operated wild-type and transgenic sl/sl rats. After CBDL, wild-type rats developed elevated PVP and spleen weight and a reduction in MSAP as previously observed (1, 16, 28). Sl/sl rats had similar increases in PVP and spleen weight compared with wild-type rats after CBDL. However, there was a trend for the MSAP to fall less in sl/sl rats relative to wild-type animals after CBDL rats. Liver histology was similar in wild-type and sl/sl rats and revealed biliary proliferation and bridging fibrosis 2 wk after CBDL (data not shown).
Table 1.
Systemic and portal hemodynamics in wild-type and transgenic sl/sl animals
|
Control |
2-wk CBDL
|
|||
|---|---|---|---|---|
| Wild-type | sl/sl | Wild-type | sl/sl | |
| MSAP, mmHg | 103.5±6.5 | 108.8±9.6 | 92.8±3.3* | 100.2±16.6 |
| PVP, mmHg | 5.1±0.2 | 5.8±0.4 | 9.1±0.5* | 9.3±0.5‡ |
| Spleen weight, % body weight | 1.6±0.1 | 1.8±0.1 | 2.3±0.1* | 2.9±0.3‡ |
Values are means ± SE, n = 4–5 animals each group. CBDL, common bile duct ligation; MSAP, mean systemic arterial pressure; PVP, portal vein pressure.
P < 0.05 relative to wild-type control animals;
P < 0.05 relative to sl/sl control animals.
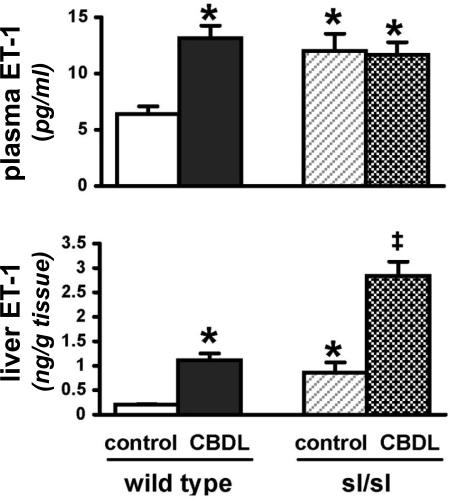
Elevated circulating ET-1 levels have been reported in sl/sl rats (8, 10). Therefore, we measured ET-1 levels in plasma and liver because hepatic production appears to contribute to the increase in circulating levels after CBDL (Fig. 1). We found that sl/sl rats had high basal ET-1 levels in plasma and liver compared with wild-type rats. In addition, both sl/sl and wild-type animals had a similar increase (about fourfold) in hepatic production of ET-1 relative to their respective controls after CBDL. However, in contrast to wild-type CBDL animals where circulating ET-1 levels increased after CBDL, there was no further increase in plasma ET-1 levels in CBDL sl/sl animals.
Fig. 1.
Circulating and hepatic endothelin-1 (ET-1) levels in wild-type and sl/sl animals after common bile duct ligation (CBDL). Values are means ± SE, n = 4–5 animals each group. *P < 0.05 relative to wild-type control animals, ‡P < 0.05 relative to sl/sl control animals.
Development of HPS in wild-type and transgenic sl/sl rats after CBDL.
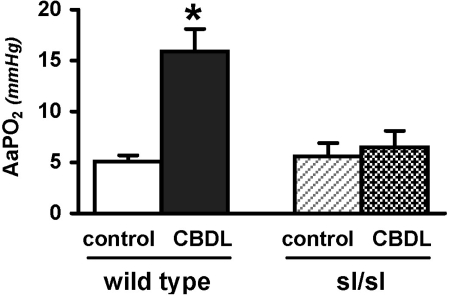
To define whether ETB receptor deficiency in the pulmonary vasculature influences the development of HPS, we performed arterial blood gas analysis and calculated arterial-alveolar oxygen gradients in wild-type and sl/sl animals after CBDL (Fig. 2). Wild-type rats developed abnormal gas exchange reflected by increased (A-a)Po2 after CBDL, relative to the control group consistent with prior studies. In contrast, in sl/sl animals there was no significant difference in (A-a)Po2 in control and CBDL animals, indicating that HPS did not develop.
Fig. 2.
Arterial blood gases in wild-type and sl/sl animals after CBDL. Values are means ± SE, n = 4–5 animals each group. AaPO2, arterial-alveolar oxygen gradients. *P < 0.05 relative to wild-type control animals.
Lung Akt and eNOS expression and activation in wild-type and transgenic sl/sl rats.
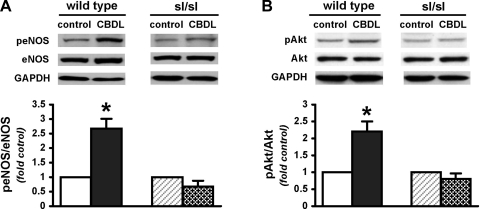
To explore whether established ETB-related signaling pathways are influenced after CBDL in ETB receptor-deficient rats, we measured pulmonary protein levels of Akt and eNOS expression/activation using Western blotting (Fig. 3). We first compared the basal levels of eNOS and Akt phosphorylation (peNOS at Ser1177 and pAkt at Ser473) among sham-operated wild-type and sl/sl rats and did not find any significant difference. After CBDL, wild-type animals had significant (two- to threefold) increases in lung peNOS and pAkt levels, which were not seen in sl/sl CBDL rats.
Fig. 3.
Lung endothelial nitric oxide synthase (eNOS) (A) and Akt (B) expression and phosphorylation (p) in wild-type and sl/sl animals after CBDL. Values are means ± SE, n = 4–5 animals each group. *P < 0.05 relative to wild-type control animals.
Pulmonary intravascular monocyte accumulation and HO-1 and ETA receptor expression in wild-type and transgenic sl/sl rats.
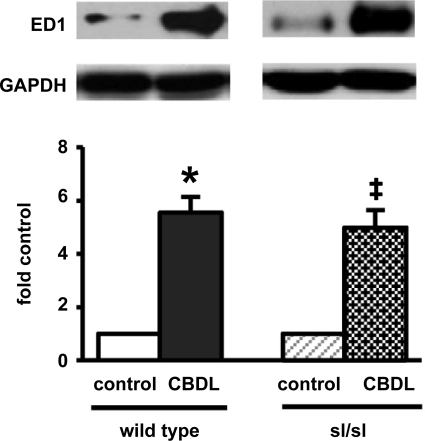
To assess whether other pathways recognized to contribute to intrapulmonary vasodilation after CBDL (pulmonary intravascular monocyte accumulation and HO-1 production) or that could influence ET-1 signaling (compensatory changes in ETA receptor expression) are altered in ETB receptor-deficient rats, we evaluated these parameters in our models (Fig. 4). Pulmonary intravascular monocyte accumulation, quantified by measuring levels of the monocyte marker ED1, increased significantly and to a similar degree in both wild-type and sl/sl animals. No increase in HO-1 expression or ETA receptor levels were observed in any of the groups (data not shown).
Fig. 4.
Pulmonary monocyte accumulation in wild-type and sl/sl animals after CBDL. Values are means ± SE, n = 4–5 animals each group. *P < 0.05 relative to wild-type control animals. ‡P < 0.05 relative to sl/sl control animals. ED1, ectodermal dysplasia 1.
DISCUSSION
We have previously observed that pulmonary vascular endothelial ETB receptor expression is increased in normal rats undergoing CBDL and is accompanied by alterations in vascular eNOS production and activation and intravascular monocyte accumulation (16, 18). In the present study, we employed homozygous ETB receptor-deficient sl/sl and wild-type rats to directly test the hypothesis that ET-1/ETB receptor-mediated eNOS activation plays a central role in development of experimental HPS. We found that, unlike wild-type animals, sl/sl rats lacking functional ETB receptors did not increase eNOS expression or activation and did not develop gas exchange abnormalities of HPS after CBDL. However, there was a similar degree of pulmonary intravascular monocyte accumulation in both 2-wk CBDL sl/sl and wild-type animals. Together, these findings confirm a direct role for ET-1/ETB receptor signaling in the development of experimental HPS.
The spotting lethal rat used in the present study carries a naturally occurring deletion in the ETB receptor gene that renders it nonfunctional (5). Since homozygous sl/sl rats do not live beyond 1 mo because of congenital intestinal aganglionosis and resulting intestinal obstruction, a dopamine β-hydroxylase (DβH) promoter has been used to direct ETB transgene expression in sl/sl rats to support normal enteric nervous system development (5). These transgenic sl/sl rats live into adulthood and are healthy, expressing ETB receptors in adrenal glands and other adrenergic neurons (5, 12). They are ETB-deficient in vascular endothelium and smooth muscles (4). Thus, the “rescued” ETB receptor-deficient rats are useful in determining the pathophysiological role of ETB receptors in vascular tissues. These animals generally exhibit high circulating ET-1 levels and alterations in systemic blood pressure under certain pathophysiological conditions (8, 10, 11, 21).
In the present study, our findings support that ETB receptor deficiency directly inhibits the changes in lung eNOS expression and signaling seen after CBDL. However, indirect effects of or compensatory responses to ETB receptor deficiency could also influence the development of experimental HPS. For example, ET-1 is recognized to contribute to hepatic injury and may also influence systemic and splanchnic hemodynamics (3, 9, 26a, 26b). Either of these effects could in turn influence pulmonary microvascular alterations (3, 11). We explored whether hepatic histology and/or systemic or splanchnic pressures were different in sl/sl animals after CBDL. We found that histology and portal pressures were similar in wild-type and ETB receptor-deficient animals, supporting that the degree of liver injury was not different in the two groups. This observation is in line with studies using selective ET receptor antagonists in experimental cirrhosis (16). We also found that baseline MAP was similar in wild-type and ETB receptor-deficient animals as previously reported (24) although basal increases have been observed in other studies particularly on high-salt diets (8, 10, 19). After CBDL, MAP in sl/sl CBDL animals appeared to be intermediate between that seen in wild-type sham and wild-type CBDL animals. These findings support a role for ETB/NO-mediated systemic vasodilation in cirrhosis (20, 23), a finding consistent with results in human cirrhosis (2, 15, 23, 25, 27).
Changes in circulating ET-1 levels and ETA receptor signaling have also been observed in ETB receptor-deficient animals (8, 10, 11, 19) and could influence pulmonary alterations after CBDL. We did find that basal circulating ET-1 levels were significantly higher in sl/sl rats than in wild-type animals in line with prior work (8, 10). However, there was no further increase in circulating ET-1 levels after CBDL in sl/sl animals in contrast to wild-type animals where circulating levels are established to significantly increase (16, 17). Therefore, both the failure of circulating ET-1 levels to increase as well as the loss of functional ETB receptors may both contribute to the reduced lung Akt/eNOS/NO expression and activation in sl/sl animals. Either enhanced or reduced ETA receptor-mediated vasoconstriction has also been reported in ETB receptor-deficient rats (11, 13, 24). For example, exaggerated ET-1/ETA receptor-induced pulmonary and systemic vasoconstriction has been reported in DOCA salt-sensitive hypertension (11). In other studies, either downregulation of ETA receptor expression in the pulmonary and systemic vasculature or impaired ETA receptor-mediated vascular signaling have been reported (14, 24). We found that pulmonary ETA receptor protein levels remained unchanged among all groups in the present study, supporting that altered expression is not a contributor to the lack of vasodilatation after CBDL in sl/sl rats. However, we have not directly assessed ETA receptor binding or function, and it would be anticipated that unopposed or enhanced ETA receptor function in the absence of ETB receptor-mediated eNOS activation would contribute to the maintenance of pulmonary microvascular tone.
The precise mechanisms involved in pulmonary intravascular monocyte accumulation after CBDL and their role in the development of HPS remain incompletely defined. In prior studies, we have found that selective blockade of ETB receptors inhibits pulmonary intravascular monocyte accumulation after CBDL (16) and that intravenous ET-1 administration in partial portal vein-ligated animals, where pulmonary ETB receptor levels are increased, enhances the recruitment (18). These findings support an important role for ET-1/ETB receptor signaling in monocyte adherence. In addition, inhibition of TNF-α production after CBDL also reduces monocyte influx, supporting a role for inflammatory cytokines in this process (29). Whether ET-1 and TNF-α-mediated pathways overlap in modulating monocyte-endothelial interactions has not been studied. In the present study, we find that pulmonary intravascular monocyte accumulation occurs in sl/sl rats after CBDL, despite the absence of functional ETB receptors, but that monocyte accumulation alone is insufficient to trigger HPS. These results suggest either that the accumulation of monocytes after CBDL is not solely dependent on functional ETB receptors or that compensatory changes in sl/sl animals result in persistent accumulation. Further studies are needed to more clearly define the role of monocyte accumulation in experimental HPS.
In summary, our findings provide direct support for a key role for ET-1/ETB receptor signaling in the onset of experimental HPS. They also demonstrate that the onset of HPS is independent of the accumulation of pulmonary intravascular monocytes. Defining whether ET-1 plays a similar role in human HPS and understanding the mechanisms involved in increased circulating ET-1 levels and pulmonary ETB receptor overexpression are important areas for future investigation.
GRANTS
This work was supported by Grants AHA 0735468N (to J. Zhang) and NIH DK0203 (to M. B. Fallon).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Fallon MB, Abrams GA, McGrath JW, Hou Z, Luo B. Common bile duct ligation in the rat: a model of intrapulmonary vasodilatation and hepatopulmonary syndrome. Am J Physiol Gastrointest Liver Physiol 272: G779–G784, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284: R1–R12, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Galie N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res 61: 227–237, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Gariepy C, Williams S, Richardson J, Hammer R, Yanagisawa M. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest 102: 1092–1101, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gariepy CE, Cases DT, Yanagisawa M. Null mutation of endothelin receptor type B gene in spotting lethal rats causes aganglionic megacolon and white coat color. Proc Natl Acad Sci USA 93: 867–872, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest 105: 925–933, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iglarz M, Schiffrin E. Role of endothelin-1 in hypertension. Curr Hypertens Rep 5: 144–148, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Ivy DD, McMurtry IF, Yanagisawa M, Gariepy CE, Cras L, Gebb TD, Morris SA, Wiseman KG, Abman IC, Steven H. Endothelin B receptor deficiency potentiates ET-1 and hypoxic pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol 280: L1040–L1048, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Ivy DD, Yanagisawa M, Gariepy CE, Gebb SA, Colvin KL, McMurtry IF. Exaggerated hypoxic pulmonary hypertension in endothelin B receptor-deficient rats. Am J Physiol Lung Cell Mol Physiol 282: L703–L712, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Kapur RP, Sweetser DA, Doggett B, Siebert JR, Palmiter RD. Intercellular signals downstream of endothelin receptor-B mediate colonization of the large intestine by enteric neuroblasts. Development 121: 3787–3795, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Kawanishi H, Hasegawa Y, Nakano D, Ohkita M, Takaoka M, Ohno Y, Matsumura Y. Involvement of the endothelin ET(B) receptor in gender differences in deoxycorticosterone acetate-salt-induced hypertension. Clin Exp Pharmacol Physiol 34: 280–285, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Kuc RE, Maguire J, Davenport AP. Quantification of endothelin receptor subtypes in peripheral tissues reveals downregulation of ETA receptors in ETB-deficient mice. Exp Biol Med (Maywood) 231: 741–745, 2006. [PubMed] [Google Scholar]
- 15.La Villa G, Barletta G, Pantaleo P, Del Bene R, Vizzutti F, Vecchiarino S, Masini E, Perfetto F, Tarquini R, Gentilini P, Laffi G. Hemodynamic, renal, and endocrine effects of acute inhibition of nitric oxide synthase in compensated cirrhosis. Hepatology 34: 19–27, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Ling Y, Zhang J, Luo B, Song D, Liu L, Tang L, Stockard CR, Grizzle WE, Ku DD, Fallon MB. The role of endothelin-1 and the endothelin B receptor in the pathogenesis of hepatopulmonary syndrome in the rat. Hepatology 39: 1593–1602, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Luo B, Abrams GA, Fallon MB. Endothelin-1 in the rat bile duct ligation model of hepatopulmonary syndrome: correlation with pulmonary dysfunction. J Hepatol 29: 571–578, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Luo B, Liu L, Tang L, Zhang J, Stockard CR, Grizzle WE, Fallon MB. Increased pulmonary vascular endothelin B receptor expression and responsiveness to endothelin-1 in cirrhotic and portal hypertensive rats: a potential mechanism in experimental hepatopulmonary syndrome. J Hepatol 38: 556–563, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Matsumura Y, Kuro T, Kobayashi Y, Konishi F, Takaoka M, Wessale JL, Opgenorth TJ, Gariepy CE, Yanagisawa M. Exaggerated vascular and renal pathology in endothelin-B receptor-deficient rats with deoxycorticosterone acetate-salt hypertension. Circulation 102: 2765–2773, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Niederberger M, Martin P, Ginès P, Morris K, Tsai P, Xu DL, McMurtry I, Schrier RW. Normalization of nitric oxide production corrects arterial vasodilation and hyperdynamic circulation in cirrhotic rats. Gastroenterology 109: 1624–1630, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Ohuchi T, Kuwaki T, Ling G, Dewit D, Ju K, Onodera M, Cao W, Yanagisawa M, Kumada M. Elevation of blood pressure by genetic and pharmacological disruption of the ETB receptor in mice. Am J Physiol Regul Integr Comp Physiol 276: R1071–R1077, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Palma DT, Fallon MB. The hepatopulmonary syndrome. J Hepatol 45: 617–625, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Pateron D, Tazi KA, Sogni P, Heller J, Chagneau C, Poirel O, Philippe M, Moreau R, Lebrec D. Role of aortic nitric oxide synthase 3 (eNOS) in the systemic vasodilation of portal hypertension. Gastroenterology 119: 196–200, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Perry MG, Molero M, Giulumian AD, Katakam PVG, Pollock JS, Pollock DM, Fuchs LC. ETB receptor-deficient rats exhibit reduced contraction to ET-1 despite an increase in ETA receptors. Am J Physiol Heart Circ Physiol 281: H2680–H2686, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Pinzani M, Milani S, De Franco R, Grappone C, Caligiuri A, Gentilini A, Tosti-Guerra C, Maggi M, Failli P, Ruocco C, Gentilini P. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology 110: 534–548, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB, ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 24: 861–880, 2004. [DOI] [PubMed] [Google Scholar]
- 26a.Schiffrin EL Endothelin: a role in experimental hypertension. J Cardiovasc Pharmacol 35: S33–S35, 2000. [DOI] [PubMed] [Google Scholar]
- 26b.Schiffrin EL Role of endothelin-1 in hypertension and vascular disease. Am J Hypertens 14: 83s–89s, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Uemasu J, Matsumoto H, Kawasaki H. Increased plasma endothelin levels in patients with liver cirrhosis. Nephron 60: 380, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Ling Y, Luo B, Tang L, Ryter SW, Stockard CR, Grizzle WE, Fallon MB. Analysis of pulmonary heme oxygenase-1 and nitric oxide synthase alterations in experimental hepatopulmonary syndrome. Gastroenterology 125: 1441–1451, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Ling Y, Tang L, Luo B, Chacko BK, Patel RP, Fallon MB. Pentoxifylline attenuation of experimental hepatopulmonary syndrome. J Appl Physiol 102: 949–955, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]