Abstract
Separation of concentrated bile acids from hepatic parenchymal cells is a key function of the bile duct epithelial cells (BDECs) that form intrahepatic bile ducts. Using coimmunostaining, we found that tissue factor (TF), the principal activator of coagulation, colocalized with cytokeratin 19, a marker of BDECs in the adult mouse liver. BDEC injury induced by xenobiotics such as α-naphthylisothiocyanate (ANIT) causes cholestasis, inflammation, and hepatocellular injury. We tested the hypothesis that acute ANIT-induced cholestatic hepatitis is associated with TF-dependent activation of coagulation and determined the role of TF in ANIT hepatotoxicity. Treatment of mice with ANIT (60 mg/kg) caused multifocal hepatic necrosis and significantly increased serum biomarkers of cholestasis and hepatic parenchymal cell injury. ANIT treatment also significantly increased liver TF expression and activity. ANIT-induced activation of the coagulation cascade was shown by increased plasma thrombin-antithrombin levels and significant deposition of fibrin within the necrotic foci. ANIT-induced coagulation and liver injury were reduced in low-TF mice, which express 1% of normal TF levels. The results indicate that ANIT-induced liver injury is accompanied by TF-dependent activation of the coagulation cascade and that TF contributes to the progression of injury during acute cholestatic hepatitis.
Keywords: cholestasis, α-naphthylisothiocyanate
a key function of hepatocytes is the synthesis and transport of bile acids, which are critical regulators of lipid metabolism. However, in high concentrations several bile acids are hepatotoxic (42, 48). Of importance, the liver architecture is such that high levels of bile acids are normally separated from the sinusoidal blood. Canaliculi between hepatocytes coalesce into intrahepatic bile ducts lined by bile duct epithelial cells (BDECs). This cellular barrier separates these toxic bile constituents from the liver microcirculation. Damage to intrahepatic bile ducts causes disruption of bile flow (cholestasis), exposes other nonparenchymal cells and hepatocytes to toxic concentrations of bile acids, and elicits hepatocyte injury (56). In humans, altered BDEC homeostasis or injury occurs in multiple diseases including primary biliary cirrhosis, primary sclerosing cholangitis, and graft vs. host disease. In addition, infection and exposure to various drugs and xenobiotics can induce BDEC injury (56).
Several mouse models have been used to study the mechanisms of liver injury during cholestasis. For example, damage to intrahepatic bile ducts is modeled by the treatment of rodents with the chemical α-naphthylisothiocyanate (ANIT) (45). ANIT is metabolized in hepatocytes and transported into the bile as a glutathione (GSH)-conjugate by the transporter MRP2 (8, 16, 49). The instability of the ANIT-GSH conjugate and recycling rounds of ANIT metabolism results in high ANIT concentration in the bile. This causes BDEC damage and the formation of foci of hepatic necrosis characterized by dead hepatocytes and accumulated bile. Indeed, large doses of ANIT cause marked cholestasis, inflammation, and hepatocellular injury in mice and rats (6, 34, 49). The mixed cholestatic-hepatocellular injury caused by ANIT in rodents resembles injury induced by some drugs and xenobiotics (45, 47). To this end, ANIT is a useful model compound to study the mechanisms of liver injury caused by acute intrahepatic bile duct injury.
ANIT-induced BDEC damage and hepatic necrosis formation provokes various mechanisms of injury progression involving extrahepatic factors. For example, ANIT-treated BDECs released a chemotactic factor for neutrophils (27), and neutrophils accumulate within foci of hepatic necrosis (14, 34). Neutrophil depletion and deficiency in the adhesion molecule CD18 protected against ANIT-induced liver damage (14, 34). Other extrahepatic cell types may also contribute to the progression of ANIT-induced hepatotoxicity. For example, platelet depletion inhibited ANIT-induced liver injury (3). This indicates that extrahepatic factors are required for the full manifestation of ANIT-induced liver damage. The role of platelets in ANIT-induced liver injury is not completely understood but may involve activation of the coagulation cascade.
The liver is a critical organ in the regulation of blood coagulation. Hepatocytes synthesize and release numerous coagulation factors into the blood, including fibrinogen and prothrombin, as well as anticoagulant factors such as protein C. Of importance, several hepatotoxic chemicals and drugs have been shown to activate the coagulation cascade (11, 22, 24, 44). This is reflected by thrombin generation, decreased plasma fibrinogen and deposition of fibrin in the liver sinusoids. Moreover, anticoagulants afforded protection against the liver damage induced by diverse xenobiotics (11, 24, 44). This indicates that coagulation contributes significantly to the liver damage induced by some xenobiotics. However, the role of coagulation in ANIT-induced intrahepatic cholestasis is not known. Moreover, few studies have examined the mechanism of xenobiotic-induced coagulation activation in models of hepatotoxicity.
The coagulation cascade is initiated by tissue factor (TF) expressed on the extracellular membrane of various cells. The cell type-specific distribution of TF expression particularly around blood vessels reduces bleeding after injury (39). TF is also expressed in a tissue-specific manner. The brain, heart, lung, and kidney express high levels of TF, whereas organs such as spleen and liver express lower levels of TF (39, 40). Indeed, the liver architecture allows for the regular exposure of various cells, including hepatocytes, to components of the plasma. To this end, the low level of TF expression in the liver may prevent inappropriate activation of coagulation. We have generated mice that express very low levels of TF (43). These so-called “low-TF mice” have normal livers, suggesting that TF is not required for liver hemostasis.
Few studies have examined the expression of TF in the liver and its contribution to hepatic coagulation and injury. Isolated hepatocytes expressed low levels of TF activity (52). Moreover, TF expression is inducible in stellate cells and Kupffer cells (2, 5). Xenobiotic induction or activation of hepatic TF could contribute significantly to coagulation and tissue injury. For example, we have shown that acetaminophen-induced coagulation in mice was TF dependent and that this contributed to the hepatotoxicity in a model of acetaminophen overdose (24). In other studies, TF antisense oligonucleotide treatment or administration of a TF inhibitor called tissue factor pathway inhibitor reduced hepatic ischemia-reperfusion-induced liver injury (41, 57). Accordingly, TF may play an important role in coagulation and hepatotoxicity.
In the present study, we sought to characterize the expression of TF in normal liver using immunofluorescence and to determine the role of TF in coagulation induced by ANIT exposure. Our results indicated that in normal liver TF is expressed by BDECs. Accordingly, we used a genetic approach to test the hypothesis that coagulation and liver injury are TF dependent in a model of ANIT-induced intrahepatic cholestasis.
MATERIALS AND METHODS
Mice.
Male, wild-type C57Bl/6J mice used for these studies were purchased from the Jackson Laboratory. The embryonic lethality of murine TF deficiency (mTF−/−) was rescued by the expression of human TF (hTF) at 1% of normal levels from a transgene. The generation of these low-TF mice (mTF−/−hTF+) has been described previously (43). Male low-TF mice (mTF−/−hTF+ mice) and heterozygous littermate control mice (mTF+/−hTF+ mice) backcrossed six generations onto a C57Bl/6J background between the ages of 8 and 12 wk were used for these studies. Mice were maintained in an AAALAC-accredited facility at the University of Kansas Medical Center. Mice were housed at an ambient temperature of 22°C with alternating 12-h light-dark cycles and allowed water and rodent chow ad libitum (Teklad 8604; Harlan, Indianapolis, IN). All animal procedures were performed according to the guidelines of the American Association for Laboratory Animal Science and were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
ANIT hepatotoxicity model.
Fasted mice were treated with ANIT (Sigma-Aldrich, St. Louis, MO) dissolved in corn oil (60 mg/kg po) or corn oil alone [control (vehicle) treatment] at 10 ml/kg. The dose-response curve for acute ANIT-induced cholestatic liver injury in mice is steep, and doses between 50 and 80 mg/kg produce similar liver histopathology in mice (34, 49, 58). Food was returned after treatment with ANIT or vehicle. The mice were anesthetized with isoflurane 24 or 48 h after ANIT treatment for the collection of blood and liver samples. Blood was collected from the retroorbital venous plexus and allowed to clot, and serum was collected by centrifugation. Blood was also collected from the caudal vena cava into a syringe containing sodium citrate (final concentration 0.38%). Plasma was collected from this blood by centrifugation. Sections of liver from the left lateral lobe were fixed in 10% neutral-buffered formalin. The right medial lobe was affixed to a cork with optimal cutting temperature compound and frozen for 3 min in liquid nitrogen-chilled isopentane. The remaining liver was snap frozen in liquid nitrogen.
Immunofluorescent staining.
For TF staining, frozen, isopentane-fixed livers were sectioned and fixed in 75% acetone-25% ethanol at room temperature for 5 min, washed with phosphate-buffered saline (PBS), and then blocked with 10% goat serum, 3% bovine serum albumin, and 2% mouse serum in PBS for 1 h at room temperature. The sections were then incubated with the monoclonal rat anti-mouse TF antibody 1H1 (33) (10 μg/ml in block solution) overnight at 4°C. Sections were washed with PBS and then incubated with a goat anti-rat IgG-Alexa 488-conjugated antibody (1:500 in block buffer; Invitrogen, Carlsbad, CA) for 3 h at room temperature and then washed with PBS. For CK19 costaining, sections were then incubated for 1 h at room temperature with a rabbit anti-mouse cytokeratin 19 (CK19) antibody (IgNex, Portland, OR) diluted 1:1,000 in block buffer. The sections were washed with PBS and then incubated for 3 h at room temperature with a donkey anti-rabbit IgG-Alexa 594 conjugated antibody (Invitrogen) diluted 1:500 in block buffer. The slides were then washed with PBS, and fluorescent staining in the livers sections was visualized by using an Olympus BX41 microscope (Olympus, Lake Success, NY). Images were captured with an Olympus DP70 and merged by use of the Olympus DP Manager software. Immunofluorescent staining of cultured BDECs was accomplished by using an identical protocol with the addition of DAPI-containing Prolong anti-fade solution (Invitrogen) prior to visualization. Fibrin staining was performed using a rabbit anti-human fibrinogen antibody (Dako) as described previously (24).
Clinical chemistry and thrombin-antithrombin measurement.
The serum activities of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were determined by use of commercially available reagents (Pointe Scientific, Canton, MI). The level of bile acids in serum was determined by using a commercial kit (Bio-quant, San Diego, CA). Thrombin-antithrombin levels in plasma were determined with a commercial ELISA kit (Siemens Healthcare Diagnostics, Deerfield, IL).
Single-stage clotting.
Snap frozen liver was homogenized in a saline solution containing 15 mM n-octyl-β-d-glucopyranoside and 25 mM HEPES and incubated for 15 min at 37°C. The homogenate was diluted with 25 mM HEPES to achieve a final concentration of 5 mM n-octyl-β-d-glucopyranoside, and the procoagulant activity of each homogenate was measured by using a single-stage clotting assay as described previously (24) by use of a Start4 coagulation analyzer (Diagnostica Stago) and mouse plasma (Innovative Research, Southfield, MI). Clotting times were converted to procoagulant activity by comparison with a standard curve established with mouse brain extract. The procoagulant activity of each sample was normalized to total protein concentration determined by a Bio-Rad DC protein assay (Bio-Rad). The TF-dependent procoagulant activity of each homogenate was determined by performing each assay in the presence of 50 μg/ml anti-TF antibody (33).
Histopathology.
Formalin-fixed livers were processed routinely, sectioned at 5 μm, stained with hematoxylin and eosin, and evaluated by light microscopy. Three sections of liver were evaluated from each animal. Sections were ∼1–1.5 cm long and 0.3–0.5 cm wide (at the widest part). Each section was evaluated in its entirety. The liver slides were scored on a five-point system, without foreknowledge of the slide number or the treatment group: 0 = no lesions; 1 = a few individualized necrotic hepatocytes or a few small foci of hepatocellular necrosis; 2 = many small, <100-μm diameter foci of hepatocellular necrosis; 3 = mainly small, <100-μm diameter foci of hepatocellular necrosis, but a few foci larger than 100-μm diameter; 4 = many prominent, >100-μm diameter foci of hepatocellular necrosis; and 5 = confluent, extensive necrosis.
Fibrin Western blotting.
Frozen liver (50 mg) was homogenized in sodium phosphate buffer (pH 7.5) containing 100 mM ɛ-aminocaproic acid, 5 mM EDTA, 10 U/ml heparin, 2 mM PMSF, and protease inhibitors (Roche). The homogenate was rotated end over end overnight at 4°C and then subjected to centrifugation at 10,000 g for 10 min at 4°C. The resulting pellet was washed with the extraction buffer and the centrifugation was repeated. The resulting pellet was then resuspended in 1 ml of 3 M urea, rotated end over end for 2 h at 37°C, and then subjected to centrifugation at 14,000 g for 15 min at 4°C. The pellet was then resuspended in 200 μl of 2× SDS sample buffer (Invitrogen) and reduced by boiling for 5 min in the presence of 2-mercaptoethanol. The protein samples were separated by electrophoresis on NuPAGE Novex 4–12% Bis-Tris gels (Invitrogen) and transferred to Immobilon-P membrane (Millipore). The levels of thrombin-cleaved fibrin were determined by using the 59D8 antibody (kindly provided by Dr. Charles Esmon, Oklahoma Medical Research Foundation), which specifically detects fibrin formed by thrombin cleavage (54). Membranes were incubated overnight at 4°C with a 1:1,000 dilution of the 59D8 antibody followed by incubation for 1 h at room temperature with a secondary anti-mouse IgG-horseradish peroxidase-conjugated Ab diluted at 1:2,000 (Cell Signaling Technology). Membranes were washed and incubated with Supersignal West Pico substrate (Pierce Biotechnology) solution and exposed to Classic Blue Film BX (Midwest Scientific). Densitometry was performed by using Gel Pro 4.5 Gel Blot Analysis software outfitted with an Epson Expression 1,680 transparency scanner.
RNA isolation, cDNA synthesis, and real-time PCR.
RNA was isolated from 75 mg of snap-frozen liver using TRI reagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer's protocol. One microgram of RNA was utilized for the synthesis of cDNA by using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems) and MyCycler thermal cycler (Bio-Rad). Levels of TF and GAPDH mRNA were determined by using TaqMan gene expression assays and TaqMan gene expression master mix (Applied Biosystems) on an ABI Prism 7300 sequence detection system (Applied Biosystems). The expression of TF was normalized relative to GAPDH expression levels, and relative expression level determined by the comparative cycle threshold method.
Hepatocyte isolation.
Under pentobarbital anesthesia (50 mg/kg ip), the abdominal cavity of the mouse was opened, and the inferior vena cava cannulated with an intravenous catheter (24 gauge × 3/4”, Terumo Medical, Elkton, MD). Infusion tubing connected to a pump was inserted into the catheter, and the liver was perfused with 50 ml of calcium- and magnesium-free Hanks' balanced salt solution (HBSS, Sigma Chemical) supplemented with 0.5 mM EGTA, 5.5 mM glucose, and penicillin-streptomycin (Sigma Chemical). At this time, the portal vein was cut and the anterior vena cava between the heart and diaphragm clamped with a small hemostat. The liver was then perfused with 40 ml of calcium- and magnesium-free HBSS supplemented with 1.5 mM calcium chloride, 5.5 mM glucose, penicillin-streptomycin, and 3,595 units of type IV collagenase (Sigma Chemical). The liver was removed and the digested product centrifuged at 50 g for 2 min to pellet the hepatocytes. The hepatocytes were washed three times with Williams' medium E (Invitrogen) and then cultured in Williams' medium E containing 10% FBS and penicillin-streptomycin. After a 3-h attachment period, the medium with unattached cells was removed, and fresh medium was added. Typically, 98% of the cells in the final preparation were hepatocytes, and the viability of the isolated hepatocytes was >90% by the criterion of Trypan blue (Sigma Chemical) exclusion.
BDEC isolation.
BDECs were isolated as described previously (27). Briefly, mouse livers were perfused as described above for hepatocyte isolation. After perfusion with collagenase-containing buffer, the liver was removed and combed to remove hepatocytes. The remaining tissue was transferred to a 100-mm dish, minced with scissors, and digested in 50 ml of HBSS containing 12,365 U collagenase, 5 mg DNAse (Sigma Chemical), 90 mg hyaluronidase (Sigma Chemical), and 50 mg soybean trypsin inhibitor for 1 h at 37°C. The solution was centrifuged at 50 g for 2 min to remove undigested material, and the remaining supernatant was centrifuged for 6 min at 850 g. The resulting pellet was resuspended in 6 ml of 32% isotonic Percoll (Sigma Chemical). This solution was overlaid with 6 ml of 90% isotonic Percoll and centrifuged for 6 min at 1,250 g. The cells contained at the interface of the two Percoll solutions were removed, diluted to 50 ml with 1× phosphate-buffered saline, and centrifuged for 6 min at 850 g. The resulting pellet was resuspended in RPMI-1640 (Sigma Chemical) containing 10% FBS and penicillin-streptomycin. The cells were cultured on collagen-coated BD Falcon culture slides overnight prior to fixation and immunofluorescent staining.
Statistics.
Comparison of two groups was performed by Student's t-test. Comparison of three or more groups was performed by one-way analysis of variance and Tukey's post hoc test. The criterion for statistical significance was P < 0.05.
RESULTS
Tissue factor expression in normal liver.
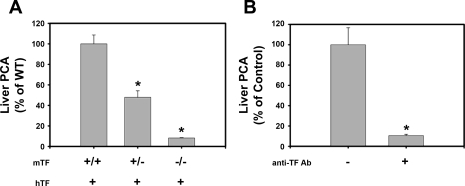
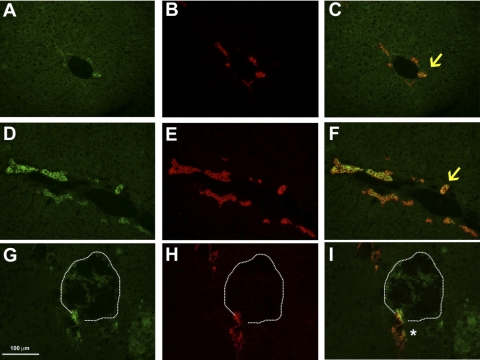
First, we determined the effect of genetically reducing TF levels on the procoagulant activity of liver extracts from wild-type mice. Compared with mTF+/+hTF+ mice, liver procoagulant activity (PCA) was reduced by about 50% in mTF+/−hTF+ mice and 90% in livers from mTF−/−hTF+ mice (low-TF mice) (Fig. 1A). In agreement with this result, liver PCA in wild-type mice was inhibited by preincubation of liver extracts with 50 μg/ml rat anti-mouse TF antibody called 1H1 (Fig. 1B). Immunofluorescent staining of liver sections from naive wild-type mice revealed TF staining localized to portal areas of the liver (Fig. 2A). TF staining was not observed around central veins (not shown). Costaining of liver sections revealed colocalization of TF and cytokeratin 19 (CK19), a selective marker of BDECs in liver (Fig. 2, B and C). TF staining was also observed on some vessels in periportal areas. In agreement with the expression of TF by BDECs in vivo, colocalization of TF and CK19 staining was also observed in isolated primary mouse BDECs (Supplemental Fig. S1, which is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website).
Fig. 1.
Tissue factor (TF)-dependent procoagulant activity (PCA) in normal mouse liver. A: procoagulant activity of liver homogenates from mice with various murine TF (mTF) genotypes. *Significantly different from wild-type (WT) mice. B: effect of the inhibitory 1H1 anti-TF antibody (50 μg/ml) or control antibody (rat IgG2a isotype control) on the procoagulant activity of liver homogenates from wild-type mice. *Significantly different from wild type with control antibody. Procoagulant activity was determined using a single-stage clotting assay. Data are expressed as means ± SE; n = 4–8 mice per group.
Fig. 2.
Effect of α-naphthylisothiocyanate (ANIT) treatment on TF and CK19 staining in liver. Mice were treated with vehicle (A–C) and livers were removed 48 h later or were treated with ANIT (60 mg/kg po) and livers were removed 24 h (D–F) or 48 h (G–I) later. Representative photomicrographs (×400 magnification) showing immunofluorescent staining of liver sections stained for TF (green) (A, D, and G) and CK19 (red) (B, E, and H) are shown. Images were digitally merged to determine colocalization of TF and CK19 staining (yellow) (C, F, and I). Arrows, colocalization of TF and CK19 staining on bile duct epithelial cells. *Discontinuous but colocalized TF and CK19 staining in proximity of a focus of hepatic necrosis (dashed line) 48 h after ANIT treatment.
ANIT-induced liver injury in mice.
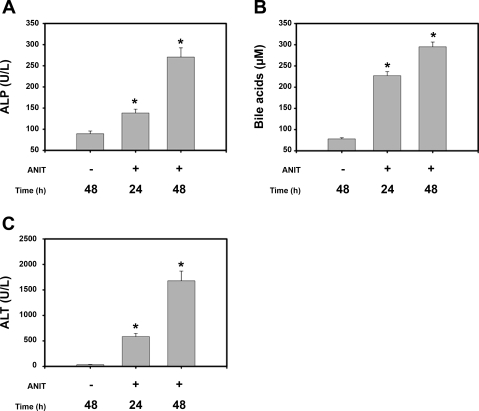
Our finding that BDECs may be a source of TF in the liver prompted us to determine whether injury to BDECs affects liver TF activity and coagulation. To this end, we established a model of ANIT-induced acute liver injury. In agreement with other studies, serum ALP activity and bile acid levels were significantly increased 24 h after ANIT treatment and continued to increase at 48 h (Fig. 3, A and B). Serum ALT activity was significantly increased 24 h after ANIT treatment and increased further at 48 h (Fig. 3C).
Fig. 3.
ANIT-induced bile duct epithelial cell (BDEC) and parenchymal cell injury in mice. Mice were treated with vehicle or ANIT (60 mg/kg po) and the serum alkaline phosphatase (ALP) activity (A), bile acid concentration (B), and alanine aminotransferase (ALT) activity (C) determined 24 and 48 h later. Levels of all biomarkers in mice given vehicle were not different between 24 and 48 h. Data from 48 h are shown. Data are expressed as means ± SE; n = 10–13 mice per group. *Significantly different from vehicle-treated mice. P < 0.05.
Mice treated with vehicle had no necrotic lesions. As previously reported with the ANIT model in rodents (18, 25, 45), lesions at 24 h after ANIT administration consisted of necrosis of intrahepatic bile ducts and sharply demarcated multifocal hepatic parenchymal necrosis, generally in periportal areas. The multifocal necrotic foci contained free erythrocytes, proteinaceous debris, and pyknotic cells. Neutrophils infiltrated the portal triads and the necrotic parenchymal foci, and occasional neutrophils infiltrated the unaffected areas of parenchyma. The affected portal areas were edematous. Throughout the sections, hepatocytes were depleted of glycogen. At 48 h after dosing, the necrotic foci in the hepatic parenchyma were larger and more numerous. In portal areas, the neutrophilic infiltrate was more pronounced. At both 24 and 48 h, some of the remaining, nonnecrotic BDECs were hypertrophic and hyperplastic, consistent with proliferation, and a few mitotic figures were present. One low-TF mouse was not scored because of an underlying background lesion (peliosis hepatitis).
TF expression in ANIT-induced liver injury.
ANIT-induced liver injury induces both BDEC death and proliferation (35). Our results indicate that the BDECs express TF. Accordingly, we determined whether ANIT treatment impacted hepatic TF levels. Liver TF mRNA levels were transiently increased 24 h after ANIT treatment (Fig. 4A). Liver TF activity increased significantly 48 h after ANIT treatment (Fig. 4B). A slight increase was also observed at 24 h, although this did not achieve statistical significance. Intense TF immunostaining associated with areas of marked CK19-positive BDEC proliferation was observed 24 h after ANIT treatment (Fig. 2, D–F). At 48 h after ANIT treatment, CK19 and TF staining was colocalized but discontinuous near areas of hepatic necrosis (Fig. 2, G–I).
Fig. 4.
TF expression in livers of ANIT-treated mice. Mice were treated with vehicle or ANIT (60 mg/kg po), and liver TF mRNA levels (A) and liver TF activity (B) were determined 24 and 48 h later. Data are expressed as means ± SE; n = 5–8 mice per group. *Significantly different from vehicle-treated mice. P < 0.05.
ANIT treatment induces coagulation.
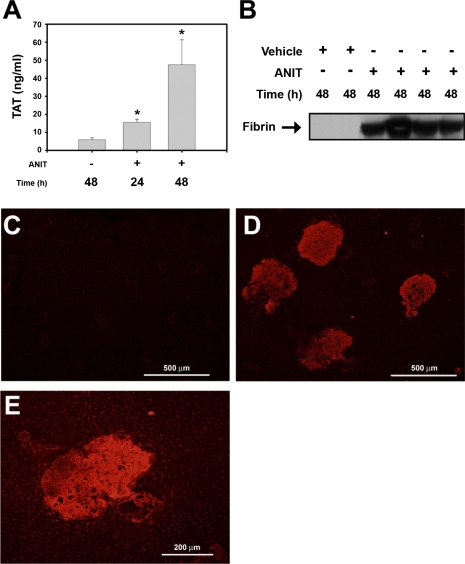
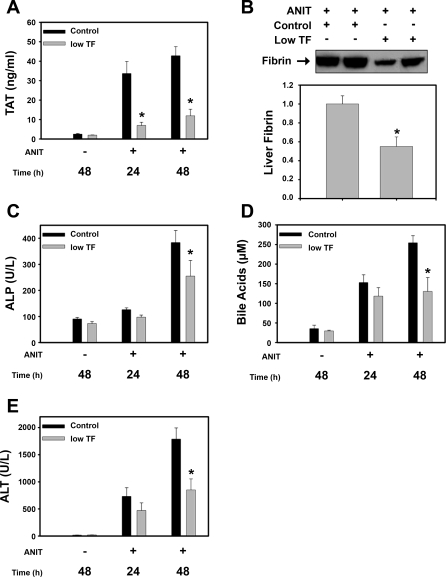
The colocalization of discontinuous TF and CK19 staining in the livers of ANIT-treated mice suggests that damage to intrahepatic bile ducts exposes sinusoidal blood to TF expressed by the BDECs. Accordingly, we determined whether ANIT treatment of mice activated the coagulation cascade. ANIT treatment increased the plasma levels of thrombin-antithrombin (TAT), a biomarker of coagulation activation, 2.5-fold after 24 h and 8-fold by 48 h (Fig. 5A). Increased hepatic fibrin levels in liver extracts from ANIT-treated mice were determined by Western blotting using the monoclonal antibody 59D8, that specifically detects fibrin generated by thrombin cleavage (Fig. 5B). Next, we used immunofluorescence to determine the localization of insoluble fibrin in liver sections. Minimal fibrin deposition was observed in livers of vehicle-treated mice (Fig. 5C). In contrast, marked fibrin deposition was observed within areas of hepatocellular necrosis 48 h after ANIT treatment (Fig. 5, D and E). Taken together, the results indicate that ANIT treatment activates the coagulation cascade and causes hepatic fibrin deposition in mice.
Fig. 5.
Coagulation system activation in ANIT-treated mice. Mice were treated with vehicle or ANIT (60 mg/kg po), and plasma and liver were collected 24 or 48 h later. A: plasma levels of thrombin-antithrombin (TAT) were determined by ELISA. B: levels of liver fibrin were determined by Western blotting. *Significantly different from vehicle-treated mice, P < 0.05; n = 6–10 mice per group. C: representative photomicrograph (×100 magnification) showing minimal fibrin staining in a liver section from a vehicle-treated mouse. Representative photomicrographs (D, ×100 magnification; E, ×200 magnification) showing extensive fibrin staining (red) in livers of ANIT-treated mice.
Role of TF in ANIT-induced coagulation and hepatotoxicity.
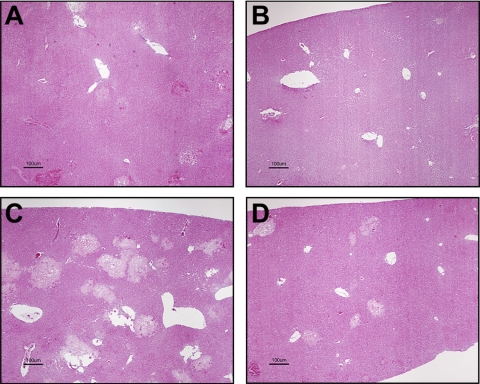
We used a genetic approach to determine the role of TF in ANIT-induced coagulation and hepatotoxicity. Plasma TAT levels were increased at 24 h and remained elevated at 48 h in ANIT-treated mTF+/−hTF+ mice (control mice). Plasma TAT levels were significantly reduced in the ANIT-treated low-TF mice at both time points (Fig. 6A). Consistent with the reduced coagulation, hepatic fibrin levels were significantly reduced at 48 h in the ANIT-treated low-TF mice compared with ANIT-treated control mice (Fig. 6B). ANIT treatment increased the serum levels of ALP activity, bile acids and ALT activity at 24 and 48 h in control mice. Serum ALP activity and bile acid levels were significantly reduced in ANIT-treated low-TF mice at 48 h, but not at 24 h, compared with ANIT-treated control mice (Fig. 6, C and D). Moreover, serum ALT activity did not significantly increase between 24 and 48 h in the low-TF mice (Fig. 6E). Assessment of liver histopathology indicated that the severity of hepatocellular necrosis was reduced in ANIT-treated low-TF mice compared with ANIT-treated control mice (Table 1 and Fig. 7).
Fig. 6.
Effect of TF-deficiency on ANIT-induced coagulation and hepatotoxicity. Control mice (mTF+/−hTF+ mice) and low-TF mice were treated with vehicle or ANIT (60 mg/kg po), and blood and liver samples were collected 24 or 48 h later. A: plasma levels of TAT determined by ELISA. B: liver fibrin levels were determined 48 h after ANIT treatment. A representative Western blot is shown above densitometry (n = 4–6 mice per group). Serum ALP activity (C), bile acid concentration (D), and ALT activity (E) were determined 24 and 48 h after ANIT treatment. *Significantly different from ANIT-treated control mice at that time, P < 0.05; n = 8–16 mice per group.
Table 1.
Severity of hepatic necrosis in ANIT-treated mice
| Mice | Time Point | Average Score |
Necrosis Score (Number of Mice) |
||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||
| Control | 24 h | 3 | 1 | 3 | 1 | ||
| Low TF | 24 h | 2.2 | 1 | 2 | 2 | ||
| Control | 48 h | 3.7 | 3 | 8 | |||
| Low TF | 48 h | 3 | 2 | 4 | 2 | ||
Control mice (mTF±hTF+ mice) and low-tissue factor (TF) mice (mTF−/−hTF+ mice) were treated with α-naphthylisothiocyanate (ANIT; 60 mg/kg) and livers were collected 24 or 48 h later. The severity of necrosis in liver sections was scored as follows: 0 = no lesions, 1 = a few individualized necrotic hepatocytes or a few small foci of hepatocellular necrosis, 2 = many small, <100 μm diameter foci of hepatocellular necrosis, 3 = mainly small foci of hepatocellular necrosis, but a few larger foci, 4 = many prominent, >100 μm diameter foci of hepatocellular necrosis, and 5 = confluent, extensive necrosis.
Fig. 7.
Hepatic periportal necrosis is attenuated in low-TF mice. Control mice (mTF+/−hTF+ mice) (A and C) and low-TF mice (B and D) were treated with ANIT (60 mg/kg po) and livers were collected 24 (A and B) or 48 (C and D) h later. Liver sections were stained with hematoxylin and eosin. Representative histopathological photomicrographs show periportal necrosis. Bar = 100 μm.
DISCUSSION
ANIT hepatotoxicity in mice was associated with activation of the coagulation cascade as indicated by increased plasma TAT levels and deposition of fibrin in areas of hepatic necrosis. ANIT-induced coagulation was inhibited by TF-deficiency, indicating that TF contributes significantly to ANIT-induced coagulation. Of importance, the inhibition of TF-dependent coagulation was associated with a reduction of ANIT-induced cholestatic liver injury and damage to hepatic parenchymal cells. Taken together, our results suggest that TF-dependent coagulation contributes to ANIT hepatotoxicity in mice.
Previous studies showed that TF levels were significantly lower in the liver compared with other tissues (40). Despite this relatively low level of TF, our data indicate that the liver expresses detectable TF-dependent procoagulant activity. The basal expression of TF by various cell types in the liver has not been systematically evaluated. However, it has been suggested that TF is expressed by hepatic parenchymal cells (52, 55). Moreover, several studies have shown that TF expression is inducible in hepatic nonparenchymal cells including Kupffer cells and stellate cells (2, 5). Our data indicate that TF is expressed by BDECs that constitute intrahepatic bile ducts and that TF expression increases during cholestasis. Immunostaining suggesting TF expression on BDECs in human livers has been reported by some (21) but not all studies (17). The expression of TF by BDECs is consistent with the expression of TF by epithelial cells in other organs such as the kidney and lung (4, 37). Further studies are required to elucidate the physiological significance of TF expressed by BDECs. The expression of TF by these cells prompted us to elucidate whether injury to these cells elicited a procoagulant response in the liver.
Administration of high doses of ANIT or feeding a diet containing 0.1% ANIT to mice induces BDEC proliferation, which reflects a compensatory response to the damaging effects of ANIT (1, 35, 36). We found that TF staining was associated with areas of BDEC proliferation after ANIT treatment. ANIT treatment induced a transient increase in liver TF mRNA levels at 24 h and a persistent increase in liver TF activity at 48 h. These results suggest that ANIT-induced bile duct proliferation is associated with an increase in liver TF expression and activity, although the expression of TF by other cells, such as hepatocytes, during ANIT-induced cholestasis deserves further examination. The progression of ANIT-induced injury included marked bile duct damage as indicated by increased serum ALP activity and discontinuous CK19 staining near areas of hepatic necrosis. Of importance, TF staining was scattered throughout the liver parenchyma near damaged bile ducts. This suggests that ANIT-induced liver injury exposes TF to the sinusoidal blood and causes necrosis. The finding that mouse BDECs express TF in vivo and in vitro suggests that, as an important cellular target of ANIT toxicity in the liver, TF expressed by BDECs may contribute to ANIT-induced coagulation. However, we also determined that isolated primary mouse hepatocytes express a low level of TF-dependent procoagulant activity (not shown) Given that even low levels of TF are sufficient to induce pathological activation of coagulation, additional investigation is required to elucidate the contribution of TF expressed by different cell types to coagulation induced by ANIT in vivo.
The liver is a key organ in the regulation of coagulation as hepatocytes synthesize numerous coagulation factors and anticoagulant proteins (51). To this end, coagulation activation is likely under strict control. One important question is how local TF-induced coagulation is inhibited in the normal liver. Previous studies have shown that deficiency in various anticoagulant proteins including tissue factor pathway inhibitor, antithrombin, or protein C in mice results in embryonic or perinatal hepatic fibrin deposition (28, 30, 32). Another possibility is that the TF expressed by BDECs and other liver cells is not exposed to blood under normal conditions. For instance, TF expression may be restricted to the apical membrane of BDECs. In agreement with our TF immunostaining, bile duct injury and/or injury to the peribiliary plexus may result in the exposure of BDEC TF to the hepatic blood supply and activate coagulation. Alternatively, TF activity rather than its spatial distribution may be a key regulatory factor. Indeed, in polarized intestinal epithelial cells the basolateral levels of anionic phospholipids and TF activity were lower compared with the apical membrane (26). To our knowledge, this has not been characterized in detail in polarized cholangiocytes. However, it is interesting to note that the phospholipid levels are higher in apical cholangiocyte membranes compared with the basolateral membrane (53). Further studies are required to determine the exact mechanism of TF expression/activation in the ANIT model of intrahepatic cholestasis.
Although our results suggest a role for TF expressed by BDECs in ANIT-induced coagulation, we cannot rule out a role for TF expressed by other cell types. Previous studies indicated that TF is expressed by human hepatocytes (52, 55). It seems likely that TF expressed by hepatocytes is normally inactive, as hepatocytes synthesize coagulation factors and are persistently exposed to components of the plasma. Interestingly, the TF activity in human hepatocytes was inhibited by N-acetyl cysteine, an antioxidant and precursor of GSH (52). Liver GSH is critical for the detoxification of several xenobiotics and plays a key role in defending the liver against oxidant-induced injury (15). A recent study indicated that GSH conjugation to extracellular sulfhydryl groups on TF may impair its procoagulant activity (46). Thus xenobiotic-induced GSH depletion may be a key step in TF-dependent coagulation in liver. In agreement with this hypothesis, we have shown that two xenobiotics that induce marked GSH depletion in hepatocytes (8, 31), acetaminophen and ANIT, induce TF-dependent coagulation in the liver. Further studies are required to determine whether GSH depletion or hepatocyte TF are important for xenobiotic activation of TF expressed by BDECs or hepatocytes.
The coagulation cascade could contribute to ANIT hepatotoxicity through multiple mechanisms. One possibility is that TF-dependent hepatic fibrin deposition aggravates hepatic parenchymal cell injury. Anticoagulation has been shown to reduce liver hypoxia in models of hepatotoxicity (10, 38), suggesting that fibrin can disrupt sinusoidal blood flow. Another possibility is that fibrin clots act as a scaffold for the recruitment of other cells to the area of necrosis. Two candidate cell types known to participate in ANIT-induced hepatotoxicity are platelets and neutrophils. Platelet depletion reduced ANIT hepatotoxicity (3), although the mechanism of this protection is not known. Fibrin(ogen) binding to platelet integrins localizes and activates platelets (7). This process could recruit and activate platelets within the area of necrosis in ANIT-treated mice. Platelets could contribute to injury by facilitating coagulation but have also been shown to contribute to liver damage independent of coagulation (29). Another possibility is that ANIT-induced fibrin formation recruits and activates neutrophils. Several studies have shown that neutrophils contribute significantly to ANIT-induced cell injury (14, 34). Deficiency in the adhesion molecule CD18 significantly reduced ANIT hepatotoxicity in mice (34). The CD18-ICAM-1 interaction is required for neutrophil activation in some models. Of importance, fibrin(ogen) binding to Mac-1 (CD11b/CD18) on neutrophils also activates intracellular signaling and induces cell activation (50). This interaction has been shown to play a key role in leukocyte activation and the innate immune response (19, 20). Accordingly, the TF-dependent fibrin generation may contribute to ANIT hepatotoxicity by multiple mechanisms.
Another possibility is that thrombin contributes to ANIT-induced liver injury by activation of the tethered ligand G protein-coupled receptor protease-activated receptor-1 (PAR-1) (12). PAR-1 is expressed by several cell types in the liver including sinusoidal endothelial cells, Kupffer cells, and stellate cells (9, 23). PAR-1 activates intracellular signaling pathways that induce the expression of several proinflammatory genes, including neutrophil chemokines and adhesion molecules (13). Although the contribution of PAR-1 to neutrophil-dependent hepatotoxicity is not completely understood, one study suggested that PAR-1 activation is a permissive event for neutrophil transmigration and activation. Perfusion of livers from LPS-primed rats with a PAR-1 agonist was sufficient to elicit neutrophil-dependent hepatotoxicity (9). Accordingly, PAR-1 activation may play an important role in ANIT hepatotoxicity by activating neutrophils.
In summary, our results indicate that TF is expressed by BDECs and that acute intrahepatic cholestasis induced by ANIT causes TF-dependent activation of the coagulation cascade. TF deficiency inhibited both ANIT-induced biliary injury and hepatic parenchymal cell injury. The results suggest that TF plays an important role in liver injury induced by disruption of intrahepatic bile ducts.
GRANTS
The project described was supported by grant number P20 RR016475 from the National Center for Research Resources (NCRR) and COBRE Grant P20 RR021940.
Supplementary Material
Acknowledgments
The authors thank Huina Cai for technical assistance and Alyson Baker for critical reading of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alpini G, Lenzi R, Zhai WR, Slott PA, Liu MH, Sarkozi L, Tavoloni N. Bile secretory function of intrahepatic biliary epithelium in the rat. Am J Physiol Gastrointest Liver Physiol 257: G124–G133, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Arai M, Mochida S, Ohno A, Ogata I, Obama H, Maruyama I, Fujiwara K. Blood coagulation equilibrium in rat liver microcirculation as evaluated by endothelial cell thrombomodulin and macrophage tissue factor. Thromb Res 80: 113–123, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Bailie MB, Pearson JM, Lappin PB, Killam AL, Roth RA. Platelets and alpha-naphthylisothiocyanate-induced liver injury. Toxicol Appl Pharmacol 129: 207–213, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Bastarache JA, Wang L, Wang Z, Albertine KH, Matthay MA, Ware LB. Intra-alveolar tissue factor pathway inhibitor is not sufficient to block tissue factor procoagulant activity. Am J Physiol Lung Cell Mol Physiol 294: L874–L881, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bataller R, Gabele E, Parsons CJ, Morris T, Yang L, Schoonhoven R, Brenner DA, Rippe RA. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology 41: 1046–1055, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Becker BA, Plaa GL. The nature of alpha-naphthylisothiocyanate-induced cholestasis. Toxicol Appl Pharmacol 7: 680–685, 1965. [DOI] [PubMed] [Google Scholar]
- 7.Brass LF, Zhu L, Stalker TJ. Novel therapeutic targets at the platelet vascular interface. Arterioscler Thromb Vasc Biol 28: s43–s50, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter-Deyo L, Marchand DH, Jean PA, Roth RA, Reed DJ. Involvement of glutathione in 1-naphthylisothiocyanate (ANIT) metabolism and toxicity to isolated hepatocytes. Biochem Pharmacol 42: 2171–2180, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Copple BL, Moulin F, Hanumegowda UM, Ganey PE, Roth RA. Thrombin and protease-activated receptor-1 agonists promote lipopolysaccharide-induced hepatocellular injury in perfused livers. J Pharmacol Exp Ther 305: 417–425, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Copple BL, Roth RA, Ganey PE. Anticoagulation and inhibition of nitric oxide synthase influence hepatic hypoxia after monocrotaline exposure. Toxicology 225: 128–137, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Copple BL, Woolley B, Banes A, Ganey PE, Roth RA. Anticoagulants prevent monocrotaline-induced hepatic parenchymal cell injury but not endothelial cell injury in the rat. Toxicol Appl Pharmacol 180: 186–196, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Coughlin SR How the protease thrombin talks to cells. Proc Natl Acad Sci USA 96: 11023–11027, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coughlin SR, Camerer E. PARticipation in inflammation. J Clin Invest 111: 25–27, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahm LJ, Schultze AE, Roth RA. An antibody to neutrophils attenuates alpha-naphthylisothiocyanate-induced liver injury. J Pharmacol Exp Ther 256: 412–420, 1991. [PubMed] [Google Scholar]
- 15.DeLeve LD, Kaplowitz N. Glutathione metabolism and its role in hepatotoxicity. Pharmacol Ther 52: 287–305, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RP. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology 167: 73–81, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol 134: 1087–1097, 1989. [PMC free article] [PubMed] [Google Scholar]
- 18.Eliakim M, Eisner M, Ungar H. Experimental intrahepatic obstructive jaundice following ingestion of alphanaphthyl-iso-thiocyanate. Bull Res Counc Isr Sect E Exp Med 8E: 7–17, 1959. [PubMed] [Google Scholar]
- 19.Flick MJ, Du X, Degen JL. Fibrin(ogen)-alpha M beta 2 interactions regulate leukocyte function and innate immunity in vivo. Exp Biol Med (Maywood) 229: 1105–1110, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest 113: 1596–1606, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flossel C, Luther T, Muller M, Albrecht S, Kasper M. Immunohistochemical detection of tissue factor (TF) on paraffin sections of routinely fixed human tissue. Histochemistry 101: 449–453, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Fujiwara K, Ogata I, Ohta Y, Hirata K, Oka Y, Yamada S, Sato Y, Masaki N, Oka H. Intravascular coagulation in acute liver failure in rats and its treatment with antithrombin III. Gut 29: 1103–1108, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaca MD, Zhou X, Benyon RC. Regulation of hepatic stellate cell proliferation and collagen synthesis by proteinase-activated receptors. J Hepatol 36: 362–369, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Ganey PE, Luyendyk JP, Newport SW, Eagle TM, Maddox JF, Mackman N, Roth RA. Role of the coagulation system in acetaminophen-induced hepatotoxicity in mice. Hepatology 46: 1177–1186, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Goldfarb S, Singer EJ, Popper H. Experimental cholangitis due to alpha-naphthyl-isothiocyanate (ANIT). Am J Pathol 40: 685–698, 1962. [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen CB, van Deurs B, Petersen LC, Rao LV. Discordant expression of tissue factor and its activity in polarized epithelial cells. Asymmetry in anionic phospholipid availability as a possible explanation. Blood 94: 1657–1664, 1999. [PubMed] [Google Scholar]
- 27.Hill DA, Jean PA, Roth RA. Bile duct epithelial cells exposed to alpha-naphthylisothiocyanate produce a factor that causes neutrophil-dependent hepatocellular injury in vitro. Toxicol Sci 47: 118–125, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Huang ZF, Higuchi D, Lasky N, Broze GJ Jr. Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice. Blood 90: 944–951, 1997. [PubMed] [Google Scholar]
- 29.Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, Chisari FV, Ruggeri ZM, Guidotti LG. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med 11: 1167–1169, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishiguro K, Kojima T, Kadomatsu K, Nakayama Y, Takagi A, Suzuki M, Takeda N, Ito M, Yamamoto K, Matsushita T, Kusugami K, Muramatsu T, Saito H. Complete antithrombin deficiency in mice results in embryonic lethality. J Clin Invest 106: 873–878, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci 89: 31–41, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Jalbert LR, Rosen ED, Moons L, Chan JC, Carmeliet P, Collen D, Castellino FJ. Inactivation of the gene for anticoagulant protein C causes lethal perinatal consumptive coagulopathy in mice. J Clin Invest 102: 1481–1488, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirchhofer D, Moran P, Bullens S, Peale F, Bunting S. A monoclonal antibody that inhibits mouse tissue factor function. J Thromb Haemost 3: 1098–1099, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Kodali P, Wu P, Lahiji PA, Brown EJ, Maher JJ. ANIT toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. Am J Physiol Gastrointest Liver Physiol 291: G355–G363, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Kossor DC, Goldstein RS, Ngo W, DeNicola DB, Leonard TB, Dulik DM, Meunier PC. Biliary epithelial cell proliferation following alpha-naphthylisothiocyanate (ANIT) treatment: relationship to bile duct obstruction. Fundam Appl Toxicol 26: 51–62, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Lesage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, Phinizy JL, Francis H, Alpini G. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol 281: G182–G190, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Luther T, Flössel C, Mackman N, Bierhaus A, Kasper M, Albrecht S, Sage EH, Iruela-Arispe L, Grossmann H, Ströhlein A, Zhang Y, Nawroth PP, Carmeliet P, Loskutoff DJ, Müller M. Tissue factor expression during human and mouse development. Am J Pathol 149: 101–113, 1996. [PMC free article] [PubMed] [Google Scholar]
- 38.Luyendyk JP, Shaw PJ, Green CD, Maddox JF, Ganey PE, Roth RA. Coagulation-mediated hypoxia and neutrophil-dependent hepatic injury in rats given lipopolysaccharide and ranitidine. J Pharmacol Exp Ther 314: 1023–1031, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Mackman N Tissue-specific hemostasis in mice. Arterioscler Thromb Vasc Biol 25: 2273–2281, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Mackman N, Sawdey MS, Keeton MR, Loskutoff DJ. Murine tissue factor gene expression in vivo: tissue and cell specificity and regulation by lipopolysaccharide. Am J Pathol 143: 76–84, 1993. [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura K, Okamoto M, Akioka K, Matsuyama M, Yoshimura R, Ushigome H, Kadotani Y, Ohmori Y, Yoshimura N. Effect of antisense oligonucleotides for tissue factor on hepatic ischemia-reperfusion injury in the rat. Transplant Proc 33: 3707–3708, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Palmeira CM, Rolo AP. Mitochondrially-mediated toxicity of bile acids. Toxicology 203: 1–15, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Parry GCN, Erlich JH, Carmeliet P, Luther T, Mackman N. Low levels of tissue factor are compatible with development and hemostasis in mice. J Clin Invest 101: 560–569, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearson JM, Schultze AE, Schwartz KA, Scott MA, Davis JM, Roth RA. The thrombin inhibitor, hirudin, attenuates lipopolysaccharide-induced liver injury in the rat. J Pharmacol Exp Ther 278: 378–383, 1996. [PubMed] [Google Scholar]
- 45.Plaa GL, Priestly BG. Intrahepatic cholestasis induced by drugs and chemicals. Pharmacol Rev 28: 207–273, 1976. [PubMed] [Google Scholar]
- 46.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest 118: 1110–1122, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez-Garay EA Cholestasis: human disease and experimental animal models. Ann Hepatol 2: 150–158, 2003. [PubMed] [Google Scholar]
- 48.Rolo AP, Palmeira CM, Holy JM, Wallace KB. Role of mitochondrial dysfunction in combined bile acid-induced cytotoxicity: the switch between apoptosis and necrosis. Toxicol Sci 79: 196–204, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Roth RA, Dahm LJ. Neutrophil- and glutathione-mediated hepatotoxicity of alpha-naphthylisothiocyanate. Drug Metab Rev 29: 153–165, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Rubel C, Fernandez GC, Dran G, Bompadre MB, Isturiz MA, Palermo MS. Fibrinogen promotes neutrophil activation and delays apoptosis. J Immunol 166: 2002–2010, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Senzolo M, Burra P, Cholongitas E, Burroughs AK. New insights into the coagulopathy of liver disease and liver transplantation. World J Gastroenterol 12: 7725–7736, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stephenne X, Vosters O, Najimi M, Beuneu C, Dung KN, Wijns W, Goldman M, Sokal EM. Tissue factor-dependent procoagulant activity of isolated human hepatocytes: relevance to liver cell transplantation. Liver Transpl 13: 599–606, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Tietz P, Levine S, Holman R, Fretham C, LaRusso NF. Characterization of apical and basolateral plasma membrane domains derived from cultured rat cholangiocytes. Anal Biochem 254: 192–199, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Weiler-Guettler H, Christie PD, Beeler DL, Healy AM, Hancock WW, Rayburn H, Edelberg JM, Rosenberg RD. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest 101: 1983–1991, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willingham AK, Matschiner JT. The activation of factor X by hepatocyte plasma membranes. Cell Mol Biol 35: 421–429, 1989. [PubMed] [Google Scholar]
- 56.Xia X, Demorrow S, Francis H, Glaser S, Alpini G, Marzioni M, Fava G, Lesage G. Cholangiocyte injury and ductopenic syndromes. Semin Liver Dis 27: 401–412, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimura N, Kobayashi Y, Nakamura K, Yamagishi H, Oka T. The effect of tissue factor pathway inhibitor on hepatic ischemic reperfusion injury of the rat. Transplantation 67: 45–53, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Zidek N, Hellmann J, Kramer PJ, Hewitt PG. Acute hepatotoxicity: a predictive model based on focused illumina microarrays. Toxicol Sci 99: 289–302, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.