Abstract
Repetitive strain stimulates intestinal epithelial migration across fibronectin via focal adhesion kinase (FAK), Src, and extracellular signal-related kinase (ERK) although how these signals act and interact remains unclear. We hypothesized that PI3K is central to this pathway. We subjected Caco-2 and intestinal epithelial cell-6 cells to 10 cycles/min deformation on flexible fibronectin-coated membranes, assayed migration by wound closure, and signaling by immunoblots. Strain stimulated PI3K, AKT, glycogen synthase kinase (GSK), and p38 phosphorylation. Blocking each kinase prevented strain stimulation of migration. Blocking PI3K prevented strain-stimulated ERK and p38 phosphorylation. Blocking AKT did not. Downstream, blocking PI3K, AKT, or ERK inhibited strain-induced GSK-Ser9 phosphorylation. Upstream of AKT, reducing FAK or Rac1 by siRNA blocked strain-stimulated AKT phosphorylation, but inhibiting Src by PP2 or siRNA did not. Transfection with FAK point mutants at Tyr397, Tyr576/577, or Tyr925 demonstrated that only FAK925 phosphorylation is required for strain-stimulated AKT phosphorylation. Myosin light chain activation by strain required FAK, Rac1, PI3K, AKT, GSK, and ERK but not Src or p38. Finally, blebbistatin, a nonmuscle myosin II inhibitor, blocked the motogenic effect of strain downstream of myosin light chain. Thus strain stimulates intestinal epithelial migration across fibronectin by a complex pathway including Src, FAK, Rac1, PI3K, AKT, GSK, ERK, p38, myosin light chain, and myosin II.
Keywords: intestinal epithelium, mechanical deformation, phosphatidylinositol 3-kinase
the intestinal mucosa is subjected to repetitive deformation by several sources, including peristalsis (23), shear stresses from endoluminal chyme (41), and villous motility (69). Such deformation can activate tyrosine kinase activity within the mucosa in vivo (6) as well as intracellular kinases such as extracellular signal-related kinase (ERK) (16). In vitro, repetitive deformation stimulates Caco-2 and intestinal epithelial cell (IEC)-6 migration across a fibronectin matrix but inhibits migration across a collagen I matrix (74), suggesting that deformation stimulates the intestinal mucosa in a complex fashion depending on fibronectin levels. Induction of migration in primary colonic lamina propria fibroblasts isolated from patients with inflamed Crohn's disease is also fibronectin dependent (9). Fibronectin is deposited in tissue in settings of chronic inflammation such as Crohn's disease (66) and increased in the plasma in other conditions where gut motility is altered, such as sepsis (32).
During normal gut function, the intestinal mucosa continuously experiences injury that must be repaired (42) to maintain the mucosal barrier. The first phase of healing is restitution, in which sheet migration of the epithelium occurs across the wound, independently of cell proliferation. In conditions of chronic inflammation or sepsis, mucosal injury is increased, and failure of mucosal healing with subsequent bacterial translocation may contribute to disease pathogenesis (13, 44, 72).
Alterations in the mechanical forces acting on the intestinal epithelium or the intestinal epithelial responses to such forces could contribute to failure of wound healing. Indeed, previous in vivo work from our laboratory suggests that mechanical forces, such as that seen in a partial bowel obstruction, modulate wound closure (15). In vitro work has shown that repetitive deformation of intestinal epithelial monolayers stimulates Caco-2 or IEC-6 intestinal epithelial wound closure across a fibronectin substrate in an ERK-dependent manner (74). ERK activation by repetitive deformation appears influenced by Src, focal adhesion kinase (FAK), and Rac1 (11), but little else is known about the signals that mediate this motogenic effect. Both ERK and p38 have been implicated in mucosal wound healing in vivo (30, 60). Furthermore, PI3K, AKT, and p38 have been reported to influence epithelial migration in the absence of repetitive deformation (25). Glycogen synthase kinase-3β (GSK), a downstream substrate of AKT, influences microtubule alignment and bundling (35) and thus might also be involved in motility. The PI3K/AKT pathway itself has been shown to be involved in the ERK-induced stimulation of cell migration in uveal melanoma cells (71), fibroblasts (10), and intestinal epithelial cells not exposed to strain (12). We therefore sought to evaluate the possible role of the PI3K-AKT axis and p38 in strain-induced cell migration.
MATERIAL AND METHODS
Materials.
DMEM, oligofectamine, lipofectamine, Plus reagent, and GAPDH antibody were obtained from Invitrogen (Carlsbad, CA), Western stripping reagent from Chemicon International (Temecula, CA), human transferrin and mouse monoclonal hemagglutinin (HA) antibody (clone 12C5) for immunoprecipitation from Roche Applied Science (Indianapolis, IN), and trypsin and horseradish peroxidase-conjugated rabbit anti-mouse IgG from Sigma (St. Louis, MO). Phosphospecific antibodies to p44/p42 (pERK1/2), AKT, p38, GSK-3β, (myosin light chain) MLC, phospho-tyrosine, phosphospecific antibody to AKT, horseradish peroxidase-conjugated anti-mouse IgG, and total antibodies to the same molecules were from Cell Signaling (St. Louis, MO). HA antibody was from Covance (Berkeley, CA), protein G-Sepharose from GE Healthcare (Piscataway, NJ), antibody to the PI3K p85 subunit from Upstate Cell Signaling Solutions (Charlottesville, VA), and MLC antibody from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture.
Human intestinal epithelial Caco-2BBE cells (48) and nontransformed rat intestinal IEC-6 epithelial cells (American Type Culture Collection, Manassas, VA) were maintained at 37°C as previously described (74).
Matrix and inhibitors.
We precoated Flexwell amino plates with 12.5 μg/ml tissue fibronectin (Sigma) as described (8). Cells were seeded at 300,000/well and studied after confluence. PD98059, PP2, LY294002, AKT inhibitor IV, SB203580 (Calbiochem, La Jolla, CA), SB415286, and blebbistatin (Biomol International, Plymouth Meeting, PA) were each dissolved in DMSO, diluted immediately before use, and added to the cells 1 h before exposure to strain.
Strain application.
Confluent monolayers on Flexwell plates were subjected to mechanical deformation using the Flexcell Strain Unit (FX-3000; Flexcell, McKeesport, PA) as described (74). Briefly, cells maintained at 37°C humidified incubator with 5% CO2 were subjected to cyclic strain by a computer-controlled vacuum manifold using a 20-kPa vacuum at 10 cycles/min, producing an average 10% strain. Controls were in the same incubator but not deformed. Nonuniformity of strain was addressed by placing a Plexiglas ring in the center to exclude this area, where strain is less uniform. Cells experience reproducible elongation and relaxation in parallel with the repetitively deformed membrane with this technique (7).
Migration.
Confluent Caco-2 or IEC-6 monolayers on fibronectin-precoated Flexwell plates were subjected to 0–24 h of repetitive deformation after 24-h serum starvation and induction of circular wounds using a 1,000-μl pipette tip as described (74). Wound areas were serially photographed on an inverted light microscope, measured on a Kodak Image Station (Perkin Elmer, Boston, MA), and compared with migration without strain.
Immunoblot and immunoprecipitation.
Confluent cells serum starved for 24 h were subjected to 0–60 min of cyclic strain, lysed on ice with protease inhibitors, and assayed for protein by bicinchoninic acid analysis (Pierce Chemical, Rockford, IL). Equal protein aliquots were either first immunoprecipitated with appropriate antibodies and Sepharose-G or resolved directly on 10% SDS-PAGE or 4–20% gradient gels (Lonza, Rockland, ME), transferred to nitrocellulose (Hybond-ECL; Amersham Biosciences, Piscataway, NJ), blocked in 5% bovine serum albumin with 1 ml/l Tween-20, immunoblotted, and detected with ECL Plus (Amersham Biosciences) on the Kodak Image Station 440 CF PhosphoImager (Kodak Scientific Imaging Systems, Rochester, NY) as described (11). All exposures used for densitometry were within the linear range.
siRNA transfection.
30–40% confluent Caco-2 cells were transfected with nontargeting siRNA (NT1) or siRNA to AKT1, AKT2, FAK, Rac1, or Src (Dharmacon, Lafayette, CO) as described previously (68). Under these conditions, we routinely obtain ≈90% transfection efficiency, as demonstrated by parallel studies using fluorescently tagged siRNA sequences (11). Cells were exposed to serum-free media 24 h before study. Parallel immunoblots for AKT1, AKT2, FAK, Rac1, and Src demonstrated ≈50–80% protein reduction.
FAK-AKT cotransfection.
Caco-2 cells were plated at 70% confluence and cotransfected as described with HA-tagged FAK plasmids point mutated to change tyrosines (Y) to phenylalanines (F) at Y397, Y576/577, and Y925 (11). After 6 h, media was replaced with normal media for 24 h, followed by serum starvation for 24 h before study. Transfection efficiency is ≈20% with this method (62).
Cell adhesion assay.
Cell adhesion assays under pressure were done as previously described (68). Briefly, cells were seeded in six-well plates under ambient and increased pressure conditions using an airtight pressure box for 30 min after 60-min incubation with SB415286 vs. vehicle control (DMSO). Nonadherent cells were washed away, and adherent cells were counted microscopically to determine level of adhesion.
Statistics.
We compared results using Student's t-test with Bonferroni correction for multiple comparisons or Wilcoxon signed ranks test for nonparametric data and considered P < 0.05 statistically significant. Data was expressed as the means ± SE of at least three independent similar experiments.
RESULTS
Mechanical strain stimulates PI3K, AKT, GSK, and p38 phosphorylation.
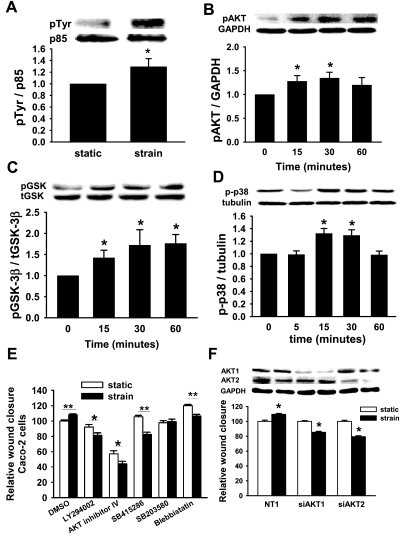
Repetitive deformation of Caco-2 cells for 30 min increased tyrosine phosphorylation of the PI3K p85 subunit 30 ± 14% (n = 4, P < 0.03, Fig. 1A). Similarly, repetitive deformation induced in a time-dependent manner AKT-ser473 (n = 10, P < 0.02, Fig. 1B), GSK-3β-ser9 (n = 6, P < 0.01, Fig. 1C), and p38 phosphorylation (n = 6, P < 0.01, Fig. 1D).
Fig. 1.
Role of PI3K, AKT, glycogen synthase kinase (GSK), and p38 in the motogenic effect of strain. A: strain stimulates phosphorylation of the p85 subunit of PI3K at 30 min in Caco-2 cells (n = 4, *P < 0.03). Phosphorylation of AKT (B), GSK (C), and p38 (D) occurs as early as 15 min of strain (n ≥ 6, *P < 0.02). Inhibiting these signals in Caco-2 cells with the PI3K inhibitor LY294002 (20 μM), AKT inhibitor IV (1 μM), GSK inhibitor SB415286 (20 μM), or p38 inhibitor SB203580 (20 μM) (E) blocked the motogenic effect of strain (solid bars) similarly to nonmuscle myosin II inhibition with blebbistatin (85.5 μM) compared with wound closure in static cells (open bars). Data summarize results of 6–36 holes per experiment from at least 3 independent experiments (*P < 0.05, **P < 0.001). F: isoform-specific siRNA reduced AKT1 or AKT2 78 and 65%, respectively (blots). Reducing either AKT1 or AKT2 reversed the motogenic effect of strain across fibronectin (graph, n ≥ 25, *P < 0.0001). t-, total; p-, phospho; NT1, nontargeting control SiRNA.
PI3K, AKT, GSK, p38, and nonmuscle myosin II are required for strain stimulation of migration.
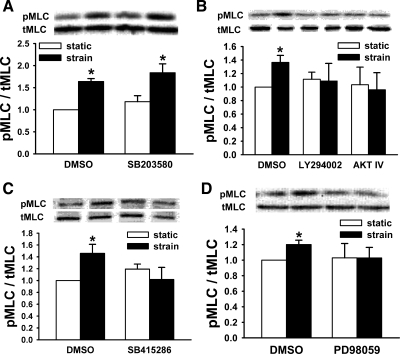
ERK has been previously reported to inhibit strain-induced migration in Caco-2 cells across fibronectin (74), so we next investigated the contribution of PI3K, AKT, GSK, and p38 to the motogenic effect of strain across Caco-2 cells. For these 24-h studies, 10 μM AKT inhibitor IV proved toxic, so we used 1 μM, which still prevented strain-induced AKT phosphorylation (not shown). We assessed migration after pretreatment with the PI3K inhibitor LY294002 (20 μM), AKT inhibitor IV (1 μM), the GSK inhibitor SB415286 (20 μM), the p38 inhibitor SB203580 (10 μM), the nonmuscle myosin II inhibitor blebbistatin (85.5 μM), or DMSO vehicle controls. Blocking p38 prevented the motogenic effect of strain, whereas blocking PI3K, AKT, GSK, or nonmuscle myosin II reversed it (n ≥ 25, P < 0.05 for each, Fig. 1E). Further studies used specific siRNA to reduce either AKT1 or AKT2, using nontargeting NT1 siRNA as a control. Transfected cells exhibited a specific ≈60–75% reduction in AKT1 or AKT2. Reducing either AKT1 or AKT2 also reversed strain-stimulated migration (n ≥ 25, P < 0.01, Fig. 1F). Since AKT also mediates the stimulation of cell adhesion by extracellular pressure in a matrix-independent manner (68), we assessed the effects of GSK blockade on Caco-2 adhesion to collagen in parallel. Interestingly, SB415286 did not prevent the stimulation of adhesion by increased pressure (28 ± 10% and 21 ± 4% for DMSO and SB415286, respectively, n = 6, P < 0.001), suggesting a difference between pressure-mediated signaling in suspended cells before adhesion and strain-mediated signaling in already adherent cells.
PI3K but not AKT is required for ERK and p38 activation.
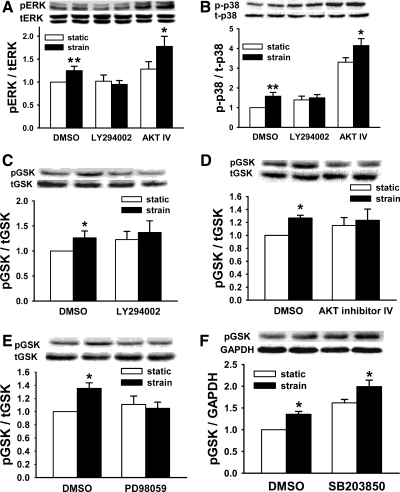
Since the downstream MAPK ERK and p38 are each required for the motogenic effect of strain, we sought to determine whether PI3K and AKT are required for strain activation of these MAPK. PI3K inhibition blocked both ERK and p38 activation by strain, but AKT blockade did not prevent activation of either MAPK (n ≥ 8, P < 0.05, Fig. 2, A and B).
Fig. 2.
Signal interaction in response to strain. Downstream signals were studied in Caco-2 cells under static (open bars) and cyclic strain (solid bars) conditions during upstream signal inhibition. A: blocking PI3K blocks strain-induced extracellular signal-related kinase (ERK) activation. Blocking AKT does not (n = 10, *P < 0.05, **P < 0.01). B: similarly, PI3K inhibition prevents strain-induced p38 activation, whereas AKT inhibition does not (n ≥ 8, *P < 0.05, **P < 0.01). Furthermore, strain-induced GSK phosphorylation was blocked by PI3K inhibition (C, n = 9, *P < 0.01), AKT inhibition (D, n = 8, *P < 0.0001), and ERK inhibition (E, n = 10, *P < 0.0001). F: p38 inhibition did not block GSK phosphorylation (n = 6, *P < 0.001).
PI3K, AKT, and ERK phosphorylate GSK, whereas p38 does not.
Blocking PI3K, AKT, or ERK each prevented GSK phosphorylation (n ≥ 8, P < 0.01, Fig. 2, C–E). p38 Inhibition with SB203580 did not prevent GSK phosphorylation (n = 5, P < 0.03, Fig. 2F).
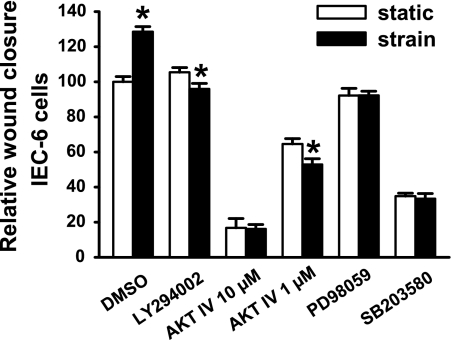
IEC-6 cells respond similarly to Caco-2 cells.
To ensure that the observed signals were not important only in Caco-2 cells, we performed parallel studies in nontransformed rat intestinal IEC-6 cells. Similarly to Caco-2 cells shown here and previously (74), IEC-6 cells exhibited a 28 ± 3% increase in wound closure in response to repetitive deformation. This response was similarly reversed by PI3K or AKT blockade and ablated by ERK or p38 blockade (n ≥ 16, P < 0.05, Fig. 3).
Fig. 3.
Intestinal epithelial cell (IEC)-6 migration. Inhibiting PI3K, AKT, ERK, and p38 prevents strain stimulation of IEC-6 migration across fibronectin (n = 16, *P < 0.00001). Open bars represent static conditions, and solid bars 24 h of strain.
FAK and Rac1 are upstream of PI3K and AKT, whereas Src is not.
Since FAK, Src, and Rac1 influence migration in response to strain (11), we studied PI3K and AKT after blocking these kinases. PI3K blockade inhibited AKT phosphorylation in response to strain (n = 9, P < 0.001, Fig. 4A), but neither Src blockade (n = 6, P < 0.05, Fig. 4B) nor ≈60% Src reduction (n = 10, P < 0.05, Fig. 4C) prevented strain-induced AKT activation. However, reducing FAK or Rac1 by siRNA (≈60–80%) prevented the increased AKT phosphorylation in NT1-transfected cells (n = 5, P < 0.05, Fig. 4D). PI3K activation by strain was also prevented by reducing FAK or Rac (n = 7, P < 0.001, Fig. 4E). Figure 4F shows siRNA knockdown efficiency.
Fig. 4.
Upstream control of AKT activation. PI3K inhibition (A) blocked AKT activation (n = 9, *P < 0.001), but neither Src inhibition (B, n = 6, *P < 0.04) nor Src reduction by siRNA (C) prevented strain-induced AKT activation (n = 10, *P < 0.05) in Caco-2 cells. However, reducing focal adhesion kinase (FAK) or Rac1 by siRNA prevented strain-induced phosphorylation of AKT (D, n = 5, *P < 0.05) and PI3K p85 subunit (E, n = 7, *P < 0.001). siRNA transfection achieved ∼60–80% knockdown as shown by immunoblot (F).
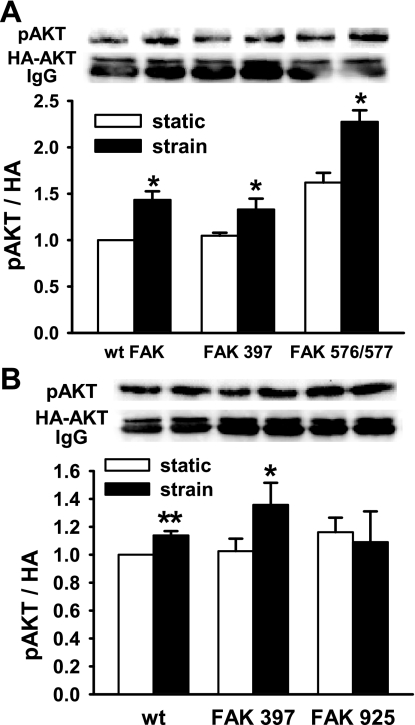
AKT phosphorylation is FAK925 dependent but FAK397 and 576/577 independent.
To identify the FAK phosphorylation site responsible for this effect, we cotransfected wild-type FAK, FAKY397F, FAKY576/577F, or FAKY925F mutants with HA-tagged AKT2 at a 3:1 lower concentration to ensure that the HA-tagged AKT2 would be expressed in cells also expressing the relevant FAK construct. Strain stimulated phosphorylation of the coexpressed AKT in cells cotransfected with wild-type FAK, FAKY397F, or FAKY576/577F (n = 12, P < 0.05, Fig. 5A). However, cotransfection with FAKY925F, unphosphorylatable at Y925, prevented the downstream phosphorylation of AKT (n = 3, P < 0.05, Fig. 5B).
Fig. 5.
Specific FAK phosphorylation sites selectively control AKT activation by strain. Caco-2 cells were cotransfected with HA-tagged wild-type (wt) or point-mutated FAK constructs and HA-tagged AKT2 and subjected to static or cyclic strain conditions before immunoprecipitation of the HA antigen and immunoblot for phospho-AKT. Cotransfection with FAK Y397F or Y576/577F did not alter strain-induced AKT activation (A, n = 12, *P < 0.03), but FAK Y925F cotransfection blocked strain-induced AKT activation (B, n = 3, *P < 0.05, **P < 0.01). HA, hemagglutinin.
Role of MLC kinase in the motogenic effect.
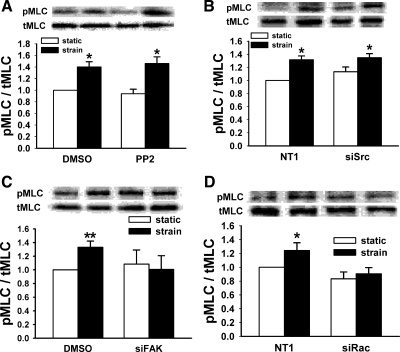
MLC is phosphorylated in response to deformation, and MLC kinase (MLCK) inhibition prevents this activation (57). We sought to determine whether PI3K, AKT, GSK, p38, or ERK are required for MLC phosphorylation. p38 Inhibition did not alter strain stimulation of MLC phosphorylation at Thr18/Ser19 (n = 4, P < 0.05, Fig. 6A). However, PI3K, AKT, GSK, or ERK inhibition each blocked the strain-associated increase in pMLC observed in vehicle controls (In ≥ 4, P < 0.01 for control, Fig. 6, B–D). Neither Src inhibition by PP2 (n = 9, P < 0.002, Fig. 7A) nor reducing Src by siRNA (n = 12, P < 0.05, Fig. 7B) prevented strain-induced stimulation of MLC phosphorylation. In contrast, reducing FAK or Rac1 by specific siRNA each blocked strain-induced MLC phosphorylation (n ≥ 7, P < 0.01 for controls, Fig. 7, C and D).
Fig. 6.
The role of myosin light chain (MLC) kinase in the motogenic effect of strain. A: p38 inhibition (SB203580) did not block MLC phosphorylation in response to strain (n = 4, *P < 0.03), but inhibiting PI3K (LY294002) or AKT (B), GSK (SB415286, C) or ERK (PD98059, D) blocked strain-associated MLC phosphorylation (n ≥ 4, *P < 0.002) in Caco-2 cells.
Fig. 7.
Effects of upstream kinases on MLC phosphorylation. Caco-2 MLC phosphorylation was not prevented by Src inhibition by PP2 (A, n = 9, *P < 0.002) or Src reduction by siRNA (B, n = 12, *P < 0.05), but reducing FAK (C) or Rac1 (D) by siRNA prevented strain-stimulated MLC phosphorylation (n ≥ 7, *P < 0.01, **P < 0.0001) in Caco-2 cells.
DISCUSSION
Constant mucosal injury during normal bowel function requires repair to maintain the intestinal mucosal barrier (41). Intestinal epithelial signaling may be affected by mechanical forces during digestion, such as peristaltic contraction, shear stress, and villous motility, whereas the mucosal barrier deteriorates in settings of sepsis, postsurgical ileus, and inflammatory bowel disease when such forces are altered (1, 18, 28). Alterations in the forces applied to IEC can regulate intestinal epithelial motility (11, 74), critical for mucosal wound healing, consistent with work in vascular endothelium and bone suggesting that physical forces regulate cell migration and other cellular biology (29, 40, 51). Although Src, FAK, and ERK mediate strain stimulation of intestinal epithelial migration, the remainder of the pathway is poorly understood. In this study, we delineate a complex web of signals involving the PI3K/AKT/GSK pathway upstream of ERK. The pathway seems to diverge and then converge on the MAPK and includes a separate and distinct previously unappreciated role for p38 in mediating the motogenic effects of strain. Although Src activation contributes to the stimulation of migration by repetitive strain, Src does not function via ERK (11). FAK involvement at the focal adhesion regulates migration through the PI3K/AKT axis via its Tyr925 site, whereas the Tyr397 autophosphorylation site and the Tyr576/577 Src-dependent phosphorylation site function through Src and control migration independently of PI3K and AKT. Finally, the downstream phosphorylation of MLC involves the PI3K/AKT/GSK pathway as well as ERK but is independent of p38.
PI3K and AKT were activated by strain and required for its motogenic effect. PI3K and AKT have previously been implicated in intestinal epithelial motility in the absence of deformation (61) and influence airway myocyte motility in response to strain (20). AKT has numerous downstream substrates, including GSK-3β, BAD, and MDM2, and its regulation is complex (39, 70). Although our observations do not rule out a role for such other AKT substrates, GSK seems critical. GSK is active at baseline but inactivated when phosphorylated at serine-9. GSK has diverse functions, including microtubule destabilization (35). Inactive GSK cannot phosphorylate adenomatous polyposis coli, a microtubule-associated protein, which then can bundle microtubules (5). In LoVo colon cancer cells, parathyroid hormone-related protein stimulation activates PI3K and AKT with phosphorylation of GSK at serine-9 (inactivation) and subsequent increased cell migration (52). This same pattern of GSK inactivation by AKT increases integrin expression and migration in fibroblasts (50). Our data suggest a similar mechanism in which GSK is phosphorylated at serine-9 in response to strain by AKT, thus facilitating migration. In contrast, AKT activation with subsequent GSK inactivation may decrease IEC-6 migration although this was in response to polyamine depletion (65), not to strain.
Reducing either AKT1 or AKT2 reversed the strain effect. AKT1 and AKT2 are ubiquitous; AKT3 is not expressed in the intestine (68). Although AKT1 and AKT2 are highly homologous, they may play different roles. AKT1 but not AKT2 mediates the stimulation of cancer cell adhesion by increased extracellular pressure (63), whereas AKT2 but not AKT1 mediates the stimulation of macrophage phagocytosis by increased extracellular pressure (54). In mouse fibroblasts, AKT1 knockouts migrate more slowly, whereas AKT2 knockouts migrate more rapidly (75). In contrast, downregulation of AKT2 inhibits epidermal growth factor (EGF)-stimulated breast epithelial cell migration, whereas downregulation of AKT1 stimulates migration (26). AKT1 inhibition was sufficient to block migration in ovarian cancer cells though AKT2 was not investigated (43). The blockade of strain stimulation of migration in our model by reduction in either isoform suggests either that both isoforms are individually required or that the effect is isoform independent but that total AKT levels were reduced sufficiently by reduction of either isoform to block strain-induced migration. We previously reported that total AKT is reduced by 30 or 70%, respectively, by siRNA to AKT1 or AKT2 in Caco-2 cells using the protocols employed here (63). Interestingly, although pressure-stimulated Caco-2 cell adhesion is blocked by AKT1 reduction, it is not blocked by either AKT2 reduction or GSK blockade. The disparity between the effects of pressure on Caco-2 adhesion and the effects of strain on Caco-2 motility suggest that different physical forces may act by different pathways in the same cell.
Although ERK had previously been implicated in the regulation of intestinal epithelial migration by strain (74), p38 had not previously been investigated. p38 Does not mediate strain stimulation of intestinal epithelial proliferation on collagen (37), but our results suggest that p38 is required for strain stimulation of intestinal epithelial migration across fibronectin. In vascular endothelium from the pulmonary artery, ERK and p38 are each activated in response to strain, but neither seems required for strain regulation of endothelial migration (33). In contrast, vascular myocyte p38 is phosphorylated in response to strain and stimulates migration (36). Thus the role of p38 in mediating strain effects seems likely to be cell type and matrix specific. p38 Stimulates migration in intestinal cells subjected to some other stimuli as well, including insulin-like growth factor binding protein (17), E-selectin, and adhesion (64). However, lansoprazole-stimulated gastric epithelial migration requires ERK but not p38 (58).
AKT is often viewed as the critical downstream substrate of PI3K, but PI3K modulates several different independent downstream mediators, including Ras, phospholipase D, and Rho (55). Our data suggest that intestinal epithelial MAPK activation by strain on fibronectin requires PI3K but not AKT. Although AKT activation is also required for the motogenic effect of strain, the requirement for AKT activation appears independent of the MAPK. When stem cells on fibronectin differentiate, AKT decreases, whereas ERK increases (22). In vascular endothelial cells, PI3K also regulates ERK-induced migration independently of AKT (49). Our data suggest that the intestinal epithelial motogenic signal pathway induced by strain diverges here. PI3K activates the MAPK, and AKT phosphorylates GSK.
We have previously reported that Src, as well as FAK and Rac1, contributes to strain-induced cell migration across fibronectin (11). Although Src is required for strain to stimulate migration across fibronectin, Src does not function through either AKT or FAK Tyr-925 in this system. Src has been reported to mediate AKT activation in monocyte chemotaxis (3) and breast cancer cell migration (27). However, although Src and AKT are each increased following colonic epithelial exposure to EGF and both cooperate to promote Rac activation, increased cell motility in response to EGF receptor activation is independent of AKT (14). Our results suggest that Src contributes to strain-induced migration independently of PI3K or AKT. Similarly, Src has been reported to function through FAK, which influences migration in the absence of deformation (21). However, we have previously reported that ERK activation by strain in intestinal epithelial cells on fibronectin requires FAK phosphorylation at Tyr-925 that occurs independently of Src although FAK phosphorylation at Tyr-397 and Tyr-576/577 in response to the same stimulus is Src dependent (11, 73). AKT phosphorylation in response to strain is prevented by mutating the Tyr-925 phosphorylation site of FAK but not affected by mutating the Tyr-397 or Tyr-576/577 sites. Phosphorylation of FAK at Tyr-397 and Tyr-576/577 by Src influences migration by regulating integrins and cell-to-cell interactions in the absence of strain (47). Thus Src may influence motility in response to strain via its effect on FAK Tyr-397 and Tyr-576/577 phosphorylation, but our previous results (11), taken together with the present observations, seem to exclude a role for Src in the FAK Tyr-925 ERK-dependent pathway that we trace here.
Rac1 inhibition also blocks AKT activation with strain, suggesting that this is also a cooperative signaling pathway. This is similar to previous reports that Rac-induced fibroblast migration is blocked by PI3K or MAPK inhibition, suggesting Rac is upstream of these molecules (2). Our data suggest that Rac is upstream of both PI3K and AKT.
MLCK phosphorylation of MLC is a critical event in the activation of nonmuscle myosin (24) and is increased during strain stimulation of intestinal epithelial migration (74). In a previous preliminary study, we observed increased MLC phosphorylation in response to deformation even in the presence of the MEK inhibitor PD98059. However, in that study, the MEK inhibitor markedly attenuated basal ERK activity but did not completely prevent ERK stimulation by strain. In the present study, longer PD98059 preincubation completely blocked ERK (not shown) and prevented MLC phosphorylation in response to strain. This suggests that ERK is upstream of MLC in this pathway. Our present results also demonstrate that blocking p38 does not inhibit MLC phosphorylation. However, reducing FAK or blocking PI3K, AKT, or GSK each prevent increased phosphorylation of MLC in response to strain. Thus PI3K may play multiple roles in the motogenic effect of strain, leading to both an AKT-dependent, GSK-dependent activation of myosin and an AKT-independent activation of ERK and p38. The demonstration that the nonmuscle myosin inhibitor blebbistatin prevents strain stimulation of migration suggests the importance of MLC phosphorylation. This suggests another divergence in the pathway. FAK, Rac1, and PI3K are required for MAPK activation, but FAK, PI3K, AKT, GSK, and ERK are required for MLC phosphorylation, each of which are independently required for strain to stimulate motility. Although this is a poorly understood area, it is consistent with literature in some cancer and fibroblast models (53, 59).
Although stimulation of migration by strain may seem modest, these results are highly statistically significant. Such apparently small changes may very well be important in an intricately and tightly regulated biological system that is experiencing constant injury to the mucosal layer. We (7, 11) and others (4, 19, 34, 67) have studied changes in cell migration, proliferation, or cell signaling in intestinal epithelial cells of similar magnitudes in response to other stimuli. Such differences in the speed of gut epithelial restitution could be enough to determine whether a mucosal wound heals or whether the mucosal barrier breaks down in vivo. Furthermore, strain effects are amplitude dependent (7). The mucosa in vivo experiences more than 10% deformation and would likely exhibit greater effects of strain in vivo though the in vitro strain amplitude is limited by the experimental apparatus available.
Although studies confirming that this signal pathway mediates a motogenic effect of strain in vivo would be beyond the scope of the present manuscript, previous in vivo observations by our group and others seem consistent with this concept. The gut mucosa is subject to constant repetitive injuries that it normally heals without difficulty to maintain the mucosal barrier that prevents the movement of enteric bacteria into the body (41). However, the mucosal barrier frequently fails in critically ill patients with chronic hypomotile ileus and often elevated plasma fibronectin levels, leading to bacterial translocation and the development of chronic inflammatory response syndromes (32, 66, 72). Although the etiology of this barrier failure is likely to be multifactorial, in vitro observations like the present manuscript suggest that the decreased patterns of mucosal strain associated with such ileus may impede healing and contribute to this pathology. Repetitive intestinal deformation is associated increased tyrosine kinase activity in small and large intestinal mucosa in vivo (6), and strain also stimulates enterocyte proliferation and mucosal hypertrophy in vivo (56). Murine partial small bowel obstruction at a defunctionalizing Roux-en-Y anastomosis is associated with increased luminal pressure and retarded healing of experimental mucosal ulcers independently of luminal flow (16). In vitro studies (16) suggest that increases in extracellular pressure slow intestinal epithelial cell migration, so these observations are consistent with the concept that a physical force can influence intestinal epithelial healing in vivo without contradicting the prediction that repetitive deformation can influence mucosal healing in a different direction. Different physical forces can often have different effects (62, 74). Although experimental difficulties have impeded the precise delineation of force-activated motogenic signal pathways in the intact intestine in vivo, the signals implicated here in the motogenic effects of repetitive deformation have also been demonstrated to regulate intestinal epithelial wound healing in vivo in other settings. For instance, PI3K signaling is required for prostaglandin-induced mucosal healing after ischemia (38), and ERK has also been strongly implicated in mucosal ulcer healing (31, 45, 46, 60). The extrapolation that activation of these signals by repetitive deformation also stimulates mucosal healing therefore seems plausible.
Taken together with our previous observations on the role of Src and FAK, these results both implicate a series of signals in mediating the stimulation of intestinal epithelial cell migration by mechanical deformation and, by deduction from the effects of blocking each signal upon the others, begin to delineate the complex pathway that regulates this effect. Upstream, Src controls migration through a mechanism separate from PI3K that involves FAK Tyr-397 and Tyr-576/577 phosphorylation, whereas Tyr-925 phosphorylation and Rac1 work through PI3K to subsequently activate AKT and ERK independently. AKT phosphorylates GSK and subsequently activates MLC to promote migration, whereas p38 acts independently of this subpathway. The pathway reconverges because ERK seems to also regulate GSK phosphorylation. Our data suggest that both AKT and ERK activation are each independently required for GSK phosphorylation in response to deformation. Although the mechanism by which deformation promotes intestinal epithelial motility is not completely delineated, targeting the elements of the pathway identified here could promote mucosal healing in patients with prolonged ileus, sepsis, or inflammatory bowel disease and preserve the mucosal barrier.
GRANTS
This work was supported in part by a Veterans Affairs Merit Award and by NIH RO1 DK067257 (each to M. Basson) and by NIH 2 T32 GM008420 (M. Basson and C. Gayer).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Al-Jurf AS, Younoszai MK, Chapman-Furr F. Effect of nutritional method on adaptation of the intestinal remnant after massive bowel resection. J Pediatr Gastroenterol Nutr 4: 245–252, 1985. [DOI] [PubMed] [Google Scholar]
- 2.Alahari SK Nischarin inhibits Rac induced migration and invasion of epithelial cells by affecting signaling cascades involving PAK. Exp Cell Res 288: 415–424, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Amin MA, Ruth JH, Haas CS, Pakozdi A, Mansfield PJ, Haghshenas J, Koch AE. H-2g, a glucose analog of blood group H antigen, mediates mononuclear cell recruitment via Src and phosphatidylinositol 3-kinase pathways. Arthritis Rheum 58: 689–695, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Antico G, Lingen MW, Sassano A, Melby J, Welch RW, Fiore S, Pilon AL, Miele L. Recombinant human uteroglobin/CC10 inhibits the adhesion and migration of primary human endothelial cells via specific and saturable binding to fibronectin. J Cell Physiol 207: 553–561, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Barth AI, Caro-Gonzalez HY, Nelson WJ. Role of adenomatous polyposis coli (APC) and microtubules in directional cell migration and neuronal polarization. Semin Cell Dev Biol 19: 245–251, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basson MD, Coppola CP. Repetitive deformation and pressure activate small bowel and colonic mucosal tyrosine kinase activity in vivo. Metabolism 51: 1525–1527, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Basson MD, Li GD, Hong F, Han O, Sumpio BE. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J Cell Physiol 168: 476–488, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Basson MD, Modlin IM, Madri JA. Human enterocyte (Caco-2) migration is modulated in vitro by extracellular matrix composition and epidermal growth factor. J Clin Invest 90: 15–23, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenmoehl J, Lang M, Hausmann M, Leeb SN, Falk W, Scholmerich J, Goke M, Rogler G. Evidence for a differential expression of fibronectin splice forms ED-A and ED-B in Crohn's disease (CD) mucosa. Int J Colorectal Dis 22: 611–623, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Cao C, Sun Y, Healey S, Bi Z, Hu G, Wan S, Kouttab N, Chu W, Wan Y. EGFR-mediated expression of aquaporin-3 is involved in human skin fibroblast migration. Biochem J 400: 225–234, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaturvedi LSP, Gayer CP, Marsh HM, Basson MD. Repetitive deformation activates Src-independent FAK-dependent ERK motogenic signals in human Caco-2 intestinal epithelial cells. Am J Physiol Cell Physiol 294: C1350–C1361, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dambacher J, Beigel F, Seiderer J, Haller D, Goke B, Auernhammer CJ, Brand S. Interleukin 31 mediates MAP kinase and STAT1/3 activation in intestinal epithelial cells and its expression is upregulated in inflammatory bowel disease. Gut 56: 1257–1265, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Hertogh G, Aerssens J, Geboes KP, Geboes K. Evidence for the involvement of infectious agents in the pathogenesis of Crohn's disease. World J Gastroenterol 14: 845–852, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dise RS, Frey MR, Whitehead RH, Polk DB. Epidermal growth factor stimulates Rac activation through Src and phosphatidylinositol 3-kinase to promote colonic epithelial cell migration. Am J Physiol Gastrointest Liver Physiol 294: G276–G285, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Flanigan TL, Owen CR, Gayer C, Basson MD. Supraphysiologic extracellular pressure inhibits intestinal epithelial wound healing independently of luminal nutrient flow. Am J Surg 196: 683–689, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu P, Thompson JA, Bach LA. Promotion of cancer cell migration: an insulin-like growth factor (IGF)-independent action of IGF-binding protein-6. J Biol Chem 282: 22298–22306, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Gorostiza E, Poullain MG, Marche C, Gobert JG, Broyart JP, Macry J, Cezard JP. Effect of fasting and refeeding on the adaptation of the small intestine in rats. A model for physiopathologic studies. Gastroenterol Clin Biol 9: 790–796, 1985. [PubMed] [Google Scholar]
- 19.Han O, Li GD, Sumpio BE, Basson MD. Strain induces Caco-2 intestinal epithelial proliferation and differentiation via PKC and tyrosine kinase signals. Am J Physiol Gastrointest Liver Physiol 275: G534–G541, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Hasaneen NA, Zucker S, Lin RZ, Vaday GG, Panettieri RA, Foda HD. Angiogenesis is induced by airway smooth muscle strain. Am J Physiol Lung Cell Mol Physiol 293: L1059–L1068, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Hauck CR, Sieg DJ, Hsia DA, Loftus JC, Gaarde WA, Monia BP, Schlaepfer DD. Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor-stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res 61: 7079–7090, 2001. [PubMed] [Google Scholar]
- 22.Hayashi Y, Furue MK, Okamoto T, Ohnuma K, Myoishi Y, Fukuhara Y, Abe T, Sato JD, Hata R, Asashima M. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells 25: 3005–3015, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol 517: 575–590, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herring BP, El-Mounayri O, Gallagher PJ, Yin F, Zhou J. Regulation of myosin light chain kinase and telokin expression in smooth muscle tissues. Am J Physiol Cell Physiol 291: C817–C827, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollborn M, Bringmann A, Faude F, Wiedemann P, Kohen L. Signaling pathways involved in PDGF-evoked cellular responses in human RPE cells. Biochem Biophys Res Commun 344: 912–919, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol 171: 1023–1034, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jallal H, Valentino ML, Chen G, Boschelli F, Ali S, Rabbani SA. A Src/Abl kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo. Cancer Res 67: 1580–1588, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Jaskiewicz K, Lemmer E. Histological findings in gastroduodenal mucosa in patients with Crohn's disease. Any diagnostic significance? Pol J Pathol 47: 115–118, 1996. [PubMed] [Google Scholar]
- 29.Jessop HL, Rawlinson SC, Pitsillides AA, Lanyon LE. Mechanical strain and fluid movement both activate extracellular regulated kinase (ERK) in osteoblast-like cells but via different signaling pathways. Bone 31: 186–194, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Karrasch T, Steinbrecher KA, Allard B, Baldwin AS, Jobin C. Wound-induced p38MAPK-dependent histone H3 phosphorylation correlates with increased COX-2 expression in enterocytes. J Cell Physiol 207: 809–815, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Kawanaka H, Tomikawa M, Jones MK, Pai R, Szabo IL, Sugimachi K, Sarfeh IJ, Tarnawski AS. Portal hypertensive gastric mucosa has reduced activation of MAP kinase (ERK2) in response to alcohol injury: a key to impaired healing? FASEB J 15: 574–576, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Kiener JL, Saba TM, Cho E, Blumenstock FA. Clearance and tissue distribution of fibronectin in septic rats: relationship to synthetic rate. Am J Physiol Regul Integr Comp Physiol 251: R724–R734, 1986. [DOI] [PubMed] [Google Scholar]
- 33.Kito H, Chen EL, Wang X, Ikeda M, Azuma N, Nakajima N, Gahtan V, Sumpio BE. Role of mitogen-activated protein kinases in pulmonary endothelial cells exposed to cyclic strain. J Appl Physiol 89: 2391–2400, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J 25: 713–726, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase-3—an overview of an over-achieving protein kinase. Curr Drug Targets 7: 1377–1388, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Li C, Hu Y, Sturm G, Wick G, Xu Q. Ras/Rac-Dependent activation of p38 mitogen-activated protein kinases in smooth muscle cells stimulated by cyclic strain stress. Arterioscler Thromb Vasc Biol 20: E1–E9, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Duzgun A, Sumpio BE, Basson MD. Integrin and FAK-mediated MAPK activation is required for cyclic strain mitogenic effects in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 280: G75–G87, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Little D, Dean RA, Young KM, McKane SA, Martin LD, Jones SL, Blikslager AT. PI3K signaling is required for prostaglandin-induced mucosal recovery in ischemia-injured porcine ileum. Am J Physiol Gastrointest Liver Physiol 284: G46–G56, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumoto T, Yung YC, Fischbach C, Kong HJ, Nakaoka R, Mooney DJ. Mechanical strain regulates endothelial cell patterning in vitro. Tissue Eng 13: 207–217, 2007. [DOI] [PubMed] [Google Scholar]
- 41.McNeil PL, Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology 96: 1238–1248, 1989. [DOI] [PubMed] [Google Scholar]
- 42.McNeil PL, Ito S. Molecular traffic through plasma membrane disruptions of cells in vivo. J Cell Sci 96: 549–556, 1990. [DOI] [PubMed] [Google Scholar]
- 43.Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal 18: 2262–2271, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Nazli A, Yang PC, Jury J, Howe K, Watson JL, Soderholm JD, Sherman PM, Perdue MH, McKay DM. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am J Pathol 164: 947–957, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owen CR, Yuan L, Basson MD. Smad3 knockout mice exhibit impaired intestinal mucosal healing. Lab Invest 88: 1101–1109, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pai R, Ohta M, Itani RM, Sarfeh IJ, Tarnawski AS. Induction of mitogen-activated protein kinase signal transduction pathway during gastric ulcer healing in rats. Gastroenterology 114: 706–713, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Parri M, Buricchi F, Giannoni E, Grimaldi G, Mello T, Raugei G, Ramponi G, Chiarugi P. EphrinA1 activates a Src/focal adhesion kinase-mediated motility response leading to rho-dependent actino/myosin contractility. J Biol Chem 282: 19619–19628, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Peterson MD, Bement WM, Mooseker MS. An in vitro model for the analysis of intestinal brush border assembly. II. Changes in expression and localization of brush border proteins during cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci 105: 461–472, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Qiao M, Shapiro P, Kumar R, Passaniti A. Insulin-like growth factor-1 regulates endogenous RUNX2 activity in endothelial cells through a phosphatidylinositol 3-kinase/ERK-dependent and Akt-independent signaling pathway. J Biol Chem 279: 42709–42718, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Roberts MS, Woods AJ, Dale TC, Van Der Sluijs P, Norman JC. Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins. Mol Cell Biol 24: 1505–1515, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosales OR, Isales CM, Barrett PQ, Brophy C, Sumpio BE. Exposure of endothelial cells to cyclic strain induces elevations of cytosolic Ca2+ concentration through mobilization of intracellular and extracellular pools. Biochem J 326: 385–392, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen X, Mula RV, Evers BM, Falzon M. Increased cell survival, migration, invasion, and Akt expression in PTHrP-overexpressing LoVo colon cancer cell lines. Regul Pept 141: 61–72, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shien T, Doihara H, Hara H, Takahashi H, Yoshitomi S, Taira N, Ishibe Y, Teramoto J, Aoe M, Shimizu N. PLC and PI3K pathways are important in the inhibition of EGF-induced cell migration by gefitinib ('Iressa', ZD1839). Breast Cancer 11: 367–373, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Shiratsuchi H, Basson MD. Akt2, but not Akt1 or Akt3 mediates pressure-stimulated serum-opsonized latex bead phagocytosis through activating mTOR and p70 S6 kinase. J Cell Biochem 102: 353–367, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Sotsios Y, Ward SG. Phosphoinositide 3-kinase: a key biochemical signal for cell migration in response to chemokines. Immunol Rev 177: 217–235, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Spencer AU, Sun X, El-Sawaf M, Haxhija EQ, Brei D, Luntz J, Yang H, Teitelbaum DH. Enterogenesis in a clinically feasible model of mechanical small-bowel lengthening. Surgery 140: 212–220, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299: 1743–1747, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki H, Masaoka T, Minegishi Y, Motosugi Y, Miura S, Ishii H. Lansoprazole promotes gastric mucosal cell proliferation and migration by activating p44/p42 mitogen-activated protein kinase. Wound Repair Regen 12: 93–99, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Swant JD, Rendon BE, Symons M, Mitchell RA. Rho GTPase-dependent signaling is required for macrophage migration inhibitory factor-mediated expression of cyclin D1. J Biol Chem 280: 23066–23072, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tarnawski AS, Pai R, Wang H, Tomikawa M. Translocation of MAP (Erk-1 and -2) kinases to cell nuclei and activation of c-fos gene during healing of experimental gastric ulcers. J Physiol Pharmacol 49: 479–488, 1998. [PubMed] [Google Scholar]
- 61.Tetreault MP, Chailler P, Beaulieu JF, Rivard N, Menard D. Epidermal growth factor receptor-dependent PI3K-activation promotes restitution of wounded human gastric epithelial monolayers. J Cell Physiol 214: 545–557, 2008. [DOI] [PubMed] [Google Scholar]
- 62.Thamilselvan V, Basson MD. Pressure activates colon cancer cell adhesion by inside-out focal adhesion complex and actin cytoskeletal signaling. Gastroenterology 126: 8–18, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Thamilselvan V, Craig DH, Basson MD. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. FASEB J 21: 1730–1741, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Tremblay PL, Auger FA, Huot J. Regulation of transendothelial migration of colon cancer cells by E-selectin-mediated activation of p38 and ERK MAP kinases. Oncogene 25: 6563–6573, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Vaidya RJ, Ray RM, Johnson LR. Akt-mediated GSK-3beta inhibition prevents migration of polyamine-depleted intestinal epithelial cells via Rac1. Cell Mol Life Sci 63: 2871–2879, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verspaget HW, Biemond I, Allaart CF, van Weede H, Weterman IT, Gooszen HG, Pena AS, Lamers CB. Assessment of plasma fibronectin in Crohn's disease. Hepatogastroenterology 38: 231–234, 1991. [PubMed] [Google Scholar]
- 67.Von Offenberg Sweeney N, Cummins PM, Cotter EJ, Fitzpatrick PA, Birney YA, Redmond EM, Cahill PA. Cyclic strain-mediated regulation of vascular endothelial cell migration and tube formation. Biochem Biophys Res Commun 329: 573–582, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Wang S, Basson MD. Identification of functional domains in AKT responsible for distinct roles of AKT isoforms in pressure-stimulated cancer cell adhesion. Exp Cell Res 314: 286–296, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Womack WA, Barrowman JA, Graham WH, Benoit JN, Kvietys PR, Granger DN. Quantitative assessment of villous motility. Am J Physiol Gastrointest Liver Physiol 252: G250–G256, 1987. [DOI] [PubMed] [Google Scholar]
- 70.Woodgett JR Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol 17: 150–157, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Ye M, Hu D, Tu L, Zhou X, Lu F, Wen B, Wu W, Lin Y, Zhou Z, Qu J. Involvement of PI3K/Akt signaling pathway in hepatocyte growth factor-induced migration of uveal melanoma cells. Invest Ophthalmol Vis Sci 49: 497–504, 2008. [DOI] [PubMed] [Google Scholar]
- 72.Yu P, Martin CM. Increased gut permeability and bacterial translocation in Pseudomonas pneumonia-induced sepsis. Crit Care Med 28: 2573–2577, 2000. [DOI] [PubMed] [Google Scholar]
- 73.Zeng L, Si X, Yu WP, Le HT, Ng KP, Teng RM, Ryan K, Wang DZ, Ponniah S, Pallen CJ. PTP alpha regulates integrin-stimulated FAK autophosphorylation and cytoskeletal rearrangement in cell spreading and migration. J Cell Biol 160: 137–146, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Owen CR, Sanders MA, Turner JR, Basson MD. The motogenic effects of cyclic mechanical strain on intestinal epithelial monolayer wound closure are matrix dependent. Gastroenterology 131: 1179–1189, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Zhou GL, Tucker DF, Bae SS, Bhatheja K, Birnbaum MJ, Field J. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J Biol Chem 281: 36443–36453, 2006. [DOI] [PubMed] [Google Scholar]