Abstract
human equilibrative nucleoside transporter-3 (hENT3) was recently reported as a pH-dependent, intracellular (lysosomal) transporter capable of transporting anti-human immunodeficiency virus (HIV) dideoxynucleosides (ddNs). Because most anti-HIV ddNs (e.g., zidovudine, AZT) exhibit clinical mitochondrial toxicity, we investigated whether hENT3 facilitates transport of anti-HIV ddNs into the mitochondria. Cellular fractionation and immunofluorescence microscopy studies in several human cell lines identified a substantial presence of hENT3 in the mitochondria, with additional presence at the cell surface of two placental cell lines (JAR, JEG3). Mitochondrial or cell surface hENT3 expression was confirmed in human hepatocytes and placental tissues, respectively. Unlike endogenous hENT3, yellow fluorescent protein (YFP)-tagged hENT3 was partially directed to the lysosomes. Xenopus oocytes expressing NH2-terminal-deleted hENT3 (expressed at the cell surface) showed pH-dependent interaction with several classes of nucleosides (anti-HIV ddNs, gemcitabine, fialuridine, ribavirin) that produce mitochondrial toxicity. Transport studies in hENT3 gene-silenced JAR cells showed significant reduction in mitochondrial transport of nucleosides and nucleoside drugs. Our data suggest that cellular localization of hENT3 is cell type dependent and the native transporter is substantially expressed in mitochondria and/or cell surface. hENT3-mediated mitochondrial transport may play an important role in mediating clinically observed mitochondrial toxicity of nucleoside drugs. In addition, our finding that hENT3 is a mitochondrial transporter is consistent with the recent finding that mutations in the hENT3 gene cause an autosomal recessive disorder in humans called the H syndrome.
Keywords: mitochondrial transport, lysosomes, anti-human immunodeficiency virus drugs
the human equilibrative nucleoside transporter (ENT) family comprises three members, ENT1–3 (4). These transporters are expressed in multiple tissues, are Na+ independent, and transport both purines and pyrimidines (4). While ENT1 and ENT2 are expressed in the plasma membrane of cells, ENT3 has been reported by Baldwin et al. (5) to be a pH-dependent intracellular transporter with partial localization in late endosomes/lysosomes. A possible fourth member of the family, ENT4, may not be a nucleoside transporter, because our studies have shown that it is a monoamine transporter (11) with a poor ability to transport adenosine at physiological pH (6).
A number of nucleoside drugs such as the anti-human immunodeficiency virus (HIV) dideoxynucleosides (ddNs; zidovudine or AZT, zalcitabine or ddC, didanosine or ddI, stavudine or d4T) and anti-hepatitis B (fialuridine; FIAU) and anticancer (gemcitabine) drugs demonstrate moderate to severe mitochondrial toxicity (15, 20, 23, 24, 26). For example, a phase I/II clinical trial of FIAU in patients with hepatitis B resulted in life-threatening hepatic failure because of mitochondrial toxicity (26). Similarly, long-term use of AZT, ddC, ddI, and d4T in HIV-infected patients results in mitochondrial toxicity manifesting as lactacidosis, hepatotoxicity, myopathy, pancreatitis, and peripheral neuropathy (15, 20, 23, 24). For example, 20–60% and 29% of HIV-1-infected patients treated with one or more of the anti-HIV ddNs exhibit elevated plasma lactate concentration and peripheral neuropathy, respectively (7, 10, 18). The mechanism of mitochondrial toxicity of nucleoside drugs is thought to be inhibition of the mitochondrion-specific DNA replicase (polymerase γ) by the triphosphates of the nucleoside drugs (15, 20, 23, 24). However, it is not clear how the hydrophilic nucleoside triphosphates gain entry into the mitochondrial compartment to produce toxicity.
Earlier we reported that, besides expression on the plasma membrane, human ENT1 (hENT1) is also expressed in the mitochondrial membrane and transports FIAU into the mitochondria where it can be phosphorylated. Therefore, we postulated and showed that hENT1 facilitates the mitochondrial toxicity of FIAU (21, 22). However, this finding does not explain the mitochondrial toxicity of the anti-HIV ddNs, because they have a poor affinity for hENT1 (and hENT2) because of the absence of 3′-oxygen group in the sugar ring (8, 25, 39). In contrast, hENT3 can transport anti-HIV ddNs (e.g., AZT, ddC, and ddI) and is highly expressed in the liver (5). While Baldwin et al. (5) showed that hENT3 is an intracellular transporter that partially colocalizes to the late endosomes/lysosomes, the localization of the majority of the remaining intracellular hENT3 was not characterized. On closer examination of the hENT3 sequence, we identified a putative mitochondria targeting signal at the NH2 terminus of hENT3 (Supplemental Fig. S1).1 Therefore, we investigated whether hENT3 is localized to the mitochondria and, if so, whether it can transport nucleosides/nucleoside drugs into the mitochondria. Here we report a detailed characterization of hENT3 expression and subcellular distribution in multiple human cell types including hepatic cells (5, 37). Our findings show that hENT3 is predominantly a mitochondrial transporter that can transport ddNs into the mitochondria. These findings suggest that hENT3 may be involved in the mitochondrial toxicity of nucleoside drugs including the anti-HIV ddNs. In addition, our finding that hENT3 is a mitochondrial transporter is consistent with an important finding that mutations of the hENT3 gene cause an autosomal recessive disorder in humans called the H syndrome (27).
MATERIALS AND METHODS
Antibodies and reagents.
A polyclonal antibody against hENT3 was generated by immunizing rabbits with a peptide epitope corresponding to Y259 to G309 of hENT3 (third intracellular loop) with a fusion construct pMAL-hENT3. Goat polyclonal antibodies against NH2 and COOH terminus hENT3 regions (sc-48149 and sc-48147) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). EEA-1, lamp2, and calreticulin rabbit polyclonal antibodies and hsp70 mouse monoclonal antibodies were obtained from Affinity Bioreagents (Golden, CO). A monoclonal β-actin antibody was obtained from Sigma. Alexa 488 and 594-conjugated goat anti-rabbit, anti-mouse, and anti-rat and Alexa 594-conjugated donkey anti-goat secondary antibodies were obtained from Molecular Probes-Invitrogen (Carlsbad, CA).
All radiolabeled substrates were obtained either from Moravek Radiochemicals (Brea, CA) or from American Radiolabeled Chemicals, (St. Louis, MO). Uridine, thymidine, inosine, nitrobenzylthioinosine, EGTA, dexamethasone, 4′,6′-diamidino-2-phenylindole (DAPI), Trypan blue, epidermal growth factor (EGF), cholera toxin, insulin, hydrocortisone, hexadimethrine bromide, and other chemicals were from Sigma. Bicinchoninic acid (BCA) protein assay reagent was from Pierce Chemical (Rockford, IL). Fetal bovine serum (FBS) and horse serum were from Hyclone Laboratories (Logan, UT). Fluorescent antifade mounting reagent, insulin-transferrin-selenium (ITS), and penicillin-streptomycin were obtained from Molecular Probes-Invitrogen.
Plasmid construction.
Full-length hENT3 was PCR cloned from a human intestinal cDNA library with the following primers: forward primer 5′-CAATAGATCTCCACCATGGCCGTTGTCTCAGAGGA, reverse primer 3′-GATTGGTACCCGAGTGAGGTGCACCAGGAGGGTAG. The PCR product was digested and subcloned into BglII and KpnI sites of pEYFP-C1 (Clontech), and the resulting plasmid construct was designated as pEYFP-hENT3. Human ENT3-LLAA and ΔN36-hENT3 mutant constructs were generated as described previously (5) except that a 5′ BglII site and a 3′ KpnI site were used to subclone the mutants into pEYFP-C1 vector. The resulting plasmid constructs were designated as pEYFP-hENT3-LLAA and pEYFP-hENT3-ΔN36, respectively.
For Xenopus oocyte expression, full-length hENT3 was PCR amplified with pEYFP-hENT3 as a template and the following primers: forward primer 5′-CAATAATGGCCGTTGTCTCAGAGGA-3′, reverse primer 5′-CTAGATGAGGTGCACCAGGAGGGTA-3′. The amplified PCR product was first subcloned into Topo 2.1 (Invitrogen) and subsequently into pOX Xenopus vector with a 5′ HindIII and a 3′ XbaI site. The resulting construct was designated as pOX-hENT3. For Xenopus oocyte expression of Δ36hENT3, a mutant construct was generated by PCR with pEYFP-hENT3 as a template and the following primers: forward primer 5′-CGATAAGCTTCAATAATGGACCGCCCGCCCCCTGGCC-3′, reverse primer 5′CGCGTCTAGACTAGATGAGGTGCACCAGGAGGGTAGA-3′. The resulting PCR product was cloned into HindIII-XbaI sites of pOX Xenopus vector and designated as pOX-Δ36hENT3. Inserts in all generated constructs were verified by sequencing at both ends.
Cell lines and tissues.
Primary human hepatocytes in suspension were kindly donated by Cellzdirect (Pittsboro, NC) and cultured as described previously (13). JAR, BeWo, JEG3 (placental choriocarcinoma cell lines), and HepG2 (hepatic carcinoma cell lines) were obtained from American Type Culture Collection (ATCC), and HeLa (cervical carcinoma cells) were obtained from Dr. Rodney Ho (University of Washington, Seattle, WA). JAR cells were maintained in RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 10 mM HEPES, and 1.0 mM sodium pyruvate. JEG-3, HepG2, and HeLa cells were maintained in MEM supplemented with 10% FBS, 1.5 g/l sodium bicarbonate, and 1 mM sodium pyruvate. BeWo cells were maintained in Ham's F-12K medium supplemented with 10% FBS, 2 mM l-glutamine, and 1.5 g/l sodium bicarbonate. Breast carcinoma cell lines were obtained from Dr. Keith Johnson (University of Nebraska Medical Center, Omaha, NE) and maintained in MEM with 10% FBS (MCF-7 and BT-20) at 5% CO2 (37°C) or Leibovitz L-15 with 10% FBS (MDA-MB-231) at 1% CO2 (37°C). MCF-10A (also obtained from Dr. Keith Johnson) was maintained in DMEM + F12 containing 5% horse serum, 20 ng/ml EGF, 100 ng/ml cholera toxin, 10 μg/ml insulin, and 500 ng/ml hydrocortisone.
The collection and use of human tissues (liver and placenta) for research was approved by the University of Washington Human Subjects Review Board. Human liver samples (n = 3) were obtained from an existing bank maintained by the University of Washington School of Pharmacy (Seattle, WA).
Lipofectamine-mediated gene transfer into mammalian cells.
FuGENE 6 transfection reagent was used to transfect cells with pEYFP-hENT3, pEYFP-hENT3-LLAA, or pEYFP-Δ36hENT3 plasmids according to the manufacturer's instructions (Roche). Briefly, cells (5 × 104) were seeded on 100-mm dishes containing 10 ml of complete medium and incubated at 37°C. After 16 h, cells were transfected with various plasmids and 24 μl of FuGENE 6 reagent: 8 μg of DNA complex in 800 μl of serum-free medium. The transfection complex was preincubated for at least 15 min at room temperature before transfection. The medium was replaced by fresh medium 5 h after transfection, and cells were selected with G418 (400 μg/ml, active) for 2–3 wk. Polyclonal cultures were maintained in G418 (200 μg/ml).
Real-time PCR analysis.
Validated TaqMan probes and primers for various ENTs and concentrative nucleoside transporters (CNTs) were described previously (13). Probes and primers for hENT3 (Hs00983219_m1) were purchased from Applied Biosystems. Amplication efficiency analysis of various primers and real-time PCR analysis of samples were performed as described previously (13).
Expression in Xenopus laevis oocytes.
cRNA of hENT3 and Δ36hENT3 were synthesized with T3 polymerase mix and the mMESSAGE mMACHINE (Ambion) transcription system per manufacturer's instructions. Fifty nanoliters (200–400 ng/μl) of cRNA was injected into defolliculated oocytes as described previously (38) except that injections were made on the day of defolliculation. Uptake of 3H-/14C-labeled substrates was measured in groups of 10–20 oocytes 48 h after injection at room temperature in transport buffer (in mM: 100 NaCl, 2 KCl, 1 CaCl2, 1 MgCl2, and 10 HEPES, pH 7.4). Uptake was terminated by washing oocytes five times with ice-cold buffer containing 20 mM uridine. Individual oocytes were then solubilized in 500 μl of 10% SDS, and the radioactivity was quantified by liquid scintillation counting.
Immunocytochemical and immunohistochemical analysis.
Cells grown on glass coverslips were gently rinsed in phosphate-buffered saline (PBS; 37°C) and fixed in 2% paraformaldehyde. Cells were blocked and permeabilized with a solution containing 1% goat/horse serum or BSA (as appropriate) and 0.25% Triton X-100. Cells were then immunostained with one or two primary antibodies. Incubation with antibodies was performed in a solution containing 1% goat/horse serum or BSA and 0.25% Tween 20 for 1 h at room temperature. Secondary antibodies conjugated with Alexa 488 or Alexa 594 (Molecular Probes-Invitrogen, Carlsbad, CA) were used as appropriate. Cells were washed three times for 15 min, after both primary antibody and secondary antibody incubation steps. Nuclei were counterstained with DAPI (Molecular Probes-Invitrogen). The cells were finally rinsed briefly with water (to remove salts), and the coverslips were mounted on glass slides with fluorescent antifade mounting reagent (Molecular Probes). Images of immunostained cells were acquired with an Olympus inverted IX70 fluorescence microscope equipped with a charge-coupled device camera. Images were captured with Softmax-pro software and deconvoluted with the DV Linux image analysis system. Immunohistochemical analysis of frozen placental tissue sections were performed as described previously (12), and images were acquired with a Leica TCS NT laser-scanning confocal microscope equipped with a krypton/argon laser as the light source.
Subcellular fractionation by differential centrifugation and density gradient centrifugation.
Enriched light and heavy lysosomal fractions from cultured cells were obtained by density gradient centrifugation subsequent to differential centrifugation with the Lysosomal Isolation Kit (LYSISO1, Sigma). A crude lysosomal fraction (CLF) containing a mixture of lysosomes, mitochondria, peroxisomes, and endoplasmic reticulum was first prepared from cultured cells by homogenization with a Dounce homogenizer followed by a series of centrifugation steps (low speed 1,000 g; high speed 20,000 g). Further purification of CLF was carried out with Option A described in the technical bulletin of the Lysosome Isolation Kit. Fractions were collected from the top of the gradient as 50-μl aliquots, and a portion (20 μl) from each of the aliquots was subjected to Western blotting analysis. Blots were analyzed for the expression of various subcellular marker proteins by serially probing (after stripping the blots) with various antibodies against each of the marker proteins.
Mitochondrial isolation from cultured cells and tissues.
An enriched mitochondrial fraction was obtained from cultured cells and human liver tissues with Cell and Tissue Mitochondrial Isolation Kits (MITOISO2 and MITOISO1; Sigma), respectively. Briefly, 50 mg of human liver tissue was homogenized with a Dounce homogenizer, and cultured cells from a T75 flask were lysed by a detergent-lysis step. Aliquots of these samples were saved as “crude lysate” fractions. A purified heavy mitochondria fraction (with minimal contamination with lysosomes and peroxisomes) was obtained with low- and high-speed centrifugation steps at 1,000 g and 3,500 g per manufacturer's instructions. Pellets obtained after the final spin (3,500 g) were reconstituted with 50 μl of lysis buffer (see below) and saved as enriched “mitochondrial” fractions. Aliquots of supernatant from this step were saved as the corresponding “nonmitochondrial” fractions. Ten micrograms [assayed by BCA reagent (Pierce)] of “total” and 20 μg of “nonmitochondrial” and “mitochondrial” samples were subjected to Western blotting analysis.
Western blotting analysis.
Lysis of cultured cells was carried out by incubating cells with TNE lysis buffer [10 mM Tris·HCl, pH 8.0, 0.5% Nonidet P-40, 1 mM EDTA, 2 mM PMSF, and protease inhibitor cocktail (Roche Applied Science)] on ice for 15 min. Subsequently, cells were homogenized by passing them through a 18-gauge needle followed by a 23-gauge needle. Extracts were centrifuged at 2,900 g for 15 min at 4°C, and the supernatant was collected. Hepatic tissue (100 mg) was homogenized with lysis buffer and a Teflon-glass homogenizer. The cell debris was pelleted by centrifugation at 13,000 g for 5 min, and 20 μg of samples was boiled for 4 min with 1× SDS loading buffer. The samples were then separated on a 12% SDS gel, and proteins were transferred to a 0.2-μm nitrocellulose membrane (Immobilon FL, Millipore) with a blotting apparatus (Bio-Rad). The membrane was washed, blocked (Odyssey blocking buffer), and incubated with one or two primary antibodies, followed by washing and incubation with IR Dye 800-conjugated anti-mouse IgG (H & L) (Rockland) and/or Alexa Fluor 680 goat anti-rabbit IgG (H & L) (Molecular Probes, Invitrogen) secondary antibodies. Bound antibodies were detected and quantified with the Odyssey Infrared Imaging System per manufacturer's instructions (LI-COR Biosciences).
Nucleoside transport into cells and mitochondria.
Cellular and mitochondrial transport experiments were carried out as described previously (13, 21).
RNA interference studies.
The following three small interfering RNA (siRNA) duplex sequences obtained from Ambion (Austin, TX) were used: siRNA ID no. 120031: 5′-GCGAGAUGCAAGCAAAUGCtt-3′ (sense), 5′-GCAUUUGCUUGCAUCUCGCTG-3′ (antisense); siRNA ID no. 120032: 5′-GGGAUCAAGCAUGUCUGGCtt-3′ (sense), 5′-GCCAGACAUGCUUGAUCCCtt-3′ (antisense); siRNA ID no. 115763: 5′-GCAAAUGCUCAGCUCUCCUtt-3′ (sense), 5′-AGGAGAGCUGAGCAUUUGCTT-3′ (antisense).
For long-term production of siRNA, inserts were generated for expression of hairpin RNA by annealing two oligonucleotide strands (55–60 mers) for each of the duplex pair mentioned above and ligated into BamHI and HindIII sites of pSilencer 5.1 Retro vector. The pSilencer expression vector insert design tool (Ambion) was used to design the oligonucleotide strands. Recombinant infectious virus particles were produced by transfecting the pSilencer 5.1 Retro constructs into a packaging cell line (PA317). JAR cells were infected three times with the three types of recombinant retroviruses starting at 24 h from the time of seeding and subsequently selected with puromycin (1,000 ng/ml; after 48 h of seeding). Selection was carried out for several weeks, and individual puromycin-resistant cell clones were isolated with glass cylinders and subsequently expanded. Cells were maintained in medium containing 500 ng/ml of puromycin. A pSilencer 5.1-H1 retro-scrambled construct and a cyclophilin A construct were used as controls. Semiquantitative RT-PCR (see below) and Western blotting analysis were performed to quantify mRNA and protein expression of hENT3.
Semiquantitative RT-PCR was performed with the following primers: 5′-CTGCTTGTCAACAGGGTTGC-3′ and 5′-GAACAGGCCTCATGTAGTAC-3′. Glyceraldehyde-3-phosphate dehydrogenase primers (5′-CGTATTGGGCGCCTGGTCACCAGGGCTGCT-3′ and 5′-TTGAGGGCAATGCCAGCCCCAGCGTCGAAG-3′) were used as an internal control. Amplification conditions were 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. hENT3 and glyceraldehyde-3-phosphate dehydrogenase amplification was performed for 20 cycles. The resulting products were run on a 1% agarose gel, and the intensity of the bands was quantified with Scion Imaging Software (Scion, Frederick, MD).
RESULTS
Specificity of a polyclonal anti-hENT3 antibody.
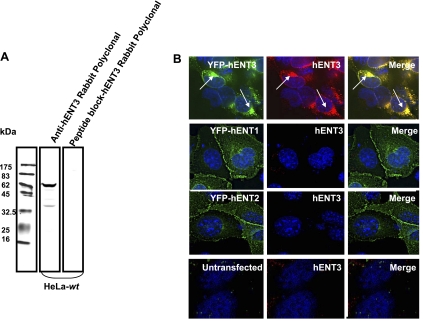
All three hENT3 antibodies, the in-house-generated rabbit polyclonal antibody (Fig. 1A), as well as two commercial antibodies directed against the NH2 and COOH termini of hENT3 (data not shown) recognized a single major band in HeLa cell lysates at ∼65 kDa in SDS-PAGE gels. This migration pattern is consistent with an N-glycosylated form of the transporter (predicted molecular mass 52 kDa) and is in agreement with an earlier report (5). A synthetic peptide corresponding to the epitope region used to generate the polyclonal antibody, but not an irrelevant peptide (an epitope of hCNT2), competitively inhibited hENT3 immunoreactivity in Western blots (Fig. 1A, 3rd lane from left). In addition, the polyclonal antibody detected hENT3 expression in mouse 3T3 fibroblasts transfected with pEYFP-hENT3 (Fig. 1B, row 1) and cell surface trafficking hENT3 mutant constructs (Supplemental Fig. S2; Ref. 5). In contrast, the polyclonal hENT3 antibody did not cross-react with cells expressing pEYFP-hENT1 or pEYFP-hENT2 (Fig. 1B, rows 2 and 3), but weakly recognized endogenous mEnt3 in mouse 3T3 fibroblasts (data not shown). Collectively, these data show that the generated polyclonal anti-hENT3 antibody is specific to hENT3 with no cross-reactivity with other ENT members (hENT1 and hENT2). Unless otherwise indicated, all subsequent studies were performed with the anti-hENT3 rabbit polyclonal antibody.
Fig. 1.
Specificity of anti-human equilibrative nucleoside transporter (hENT)3 antibodies. A: Western blots showing specificity of anti-hENT3 polyclonal antibody. Immunoblots of total proteins prepared from HeLa cell lysates (20 μg) and probed with 1:2,000 anti-hENT3 polyclonal antibody (2nd lane from left). Note that hENT3 migrates as a major ∼65-kDa band (compare with protein marker, 1st lane). Preincubation with a synthetic peptide corresponding to the epitope region completely abolished this band (3rd lane). wt, Wild type. B: immunolocalization experiments showed that anti-hENT3 polyclonal antibody detects hENT3 but not hENT1 or hENT2 in 3T3 mouse fibroblasts. 3T3 mouse fibroblasts were transfected with pEYFP-hENT3, pEYFP-hENT1, or pEYFP-hENT2 expression plasmid constructs, and immunocytochemical analysis was performed with 1:1,000 anti-hENT3 polyclonal antibody. Note extensive colocalization (yellow in merged images) of yellow fluorescent protein (YFP) fluorescence of the fusion proteins (green) with the hENT3 antibody staining (red) in hENT3-expressing cells (1st row). Also note that the antibody does not recognize YFP-hENT1 or YFP-hENT2 in 3T3 fibroblasts (2nd and 3rd rows). Arrows indicate localization of YFP-hENT3 to the cytoplasmic structures. No distinct staining was observed in untransfected cells (4th row). Nuclei stained with 4′,6′-diamidino-2-phenylindole (DAPI) are blue. Original magnification ×40.
Cell type-dependent localization of native hENT3.
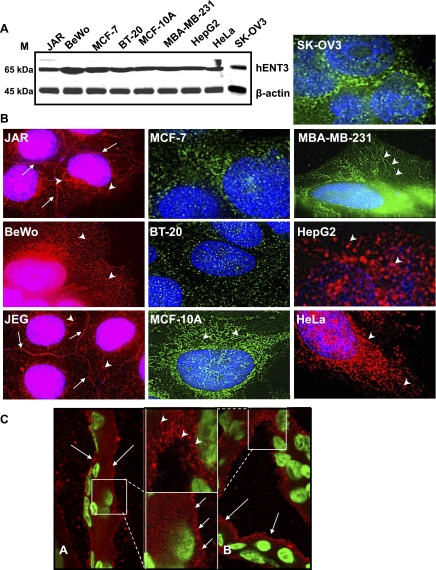
To characterize hENT3 expression and distribution in various human cell types, we analyzed endogenous hENT3 in several human cell lines by Western blotting and immunocytochemical analyses. Three placental choriocarcinoma cell lines (JAR, BeWo, JEG3) were tested because earlier microarray studies (5, 37) identified the human placenta as a tissue with highest hENT3 transcript expression. Western blotting analysis showed abundant expression of native hENT3 (∼65-kDa band) in all cell lines tested (Fig. 2A). Immunostaining analysis with the anti-hENT3 polyclonal antibody showed distinct differences in the localization of hENT3 in these cells (Fig. 2B). In two of three placental choriocarcinoma cell lines (JAR and JEG3), a significant proportion of native hENT3 was found localized at the cell surface (Fig. 2B, arrows), apparently at regions of cell-to-cell contacts. In addition, reticular, elongated tubular, and comma-shaped staining patterns were frequently observed in the cytoplasm of the placental cells (Fig. 2B, left, arrowheads). In four breast cell lines (MCF-7, BT-20, MBA-MB-231, and the noncancerous MCF-10A breast cell line), HepG2, HeLa, BeWo, and SKOV3 cells, hENT3 staining was observed only in the cytoplasm (Fig. 2B, arrowheads). Unlike the previous report on lysosomal localization of hENT3 (in HeLa cells; Ref. 5), our study identified a variable, cell type-dependent distribution of native hENT3 including cell surface localization in two placental cell lines. Furthermore, the intracellular staining pattern was not always vesicular (consistent with lysosomal localization) but was reminiscent of the mitochondrial localization that we observed previously with hENT1 and MitoTracker Red (a marker of mitochondrial localization) (21).
Fig. 2.
Expression and distribution of hENT3. A: immunoblot showing expression of hENT3 in various human cell lines. Total cell lysates (20 μg) prepared from different human cell lines were separated with SDS-PAGE and transferred to a nitrocellulose membrane. The membranes were probed with 1:2,000 anti-hENT3 polyclonal antibody and, after stripping, reprobed with 1:10,000 anti-β-actin monoclonal antibody. Note significant hENT3 expression (∼65-kDa band) in all cell types. β-Actin internal control migrated at ∼45 kDa. M, marker position. B: immunolocalization showing varied distribution of hENT3 in human cell lines. Various cell lines cultured in glass coverslips were fixed and analyzed by immunocytochemical analysis using 1:1,000 anti-hENT3 polyclonal antibody. Note significant variation in the distribution pattern of hENT3 (green and red) with cell surface staining in JAR and JEG3 cells (arrows), curved and tubular cytoplasmic staining in BeWo, MCF-10A, MBA-MB-231, and HeLa cells (arrowheads), and predominantly vesicular or a mixed pattern of staining in other cell types (SKOV3, BT20, HepG2, and MCF-7). Nuclei are stained with DAPI (blue). Original magnification ×60. C: immunohistochemical analysis of hENT3 in human placental sections. Human placental tissues were stained with 1:1,000 anti-hENT3 polyclonal antibody, and the localization of hENT3 was analyzed. Note hENT3 (red) expression at both maternal and fetal sides of syncytiotrophoblasts (thin arrows, top left). Insets in C, A and B, are magnified to emphasize cell surface and cytoplasmic staining of hENT3, respectively. Arrows and arrowheads represent cell surface and intracellular hENT3 staining, respectively. Nuclei were stained with DAPI (green). Original magnification ×60.
To test whether the observed cell surface localization of hENT3 in placental cell lines is reproduced in the human placenta, we also examined hENT3 localization in three human placental tissues (gestational ages 50–55 days) with immunohistochemical analysis and confocal microscopy. Consistent with the high expression of its message (5, 37), hENT3 protein expression appeared to be abundant in the placenta but showed substantial heterogeneity in its subcellular distribution. Some regions showed predominantly cell surface expression (Fig. 2C, arrows), while others showed only discrete punctated or elongated structures in the cytoplasm (Fig. 2C, arrowheads). This expression was detected at both maternal and fetal sides of the syncytiotrophoblastic layers.
Mitochondrial localization of endogenous hENT3 in multiple cell types.
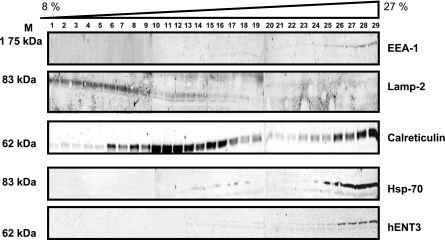
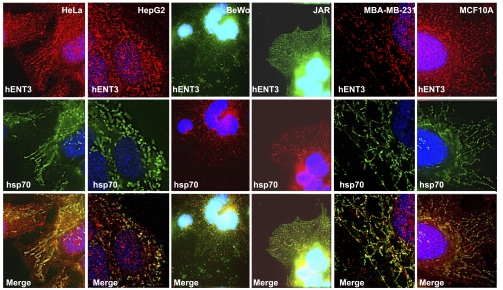
To identify the subcellular compartmentalization of cytoplasmic hENT3, we performed subcellular fractionation studies as well as double-immunolocalization analysis. In HepG2 cells, native hENT3 cofractionated with high-density fractions (fractions 26–29) that contained markers of early endosomes (EEA-1), endoplasmic reticulum (calreticulin), and mitochondria (hsp70) but not appreciably with fractions containing heavy or light lysosomal marker (lamp2) (Fig. 3). Only a low level of hENT3 signal cofractionated with lamp2 in fractions 13–18 (Fig. 3, lamp2 and hENT3 lanes) and was identified on overexposure. hENT3 also substantially colocalized with hsp70 in HeLa, HepG2, MCF10A, MCF-MB-231, JAR, and BeWo cells (Fig. 4, yellow in merged images) but not extensively with lamp2 in HeLa cells (see Fig. 6B, left). Substantial colocalization of hENT3 with dsRed-Mito mitochondrial marker was also observed in HeLa cells (data not shown). Comparison of various anti-hENT3 antibodies showed that the generated polyclonal antibody directed against an epitope corresponding to a larger span (amino acids 259–309 between TM5 and TM6) of a cytoplasmic loop in hENT3 allows effective binding to mitochondrial hENT3 in fixed cells and in denatured lysates (Supplemental Fig. S3). Collectively, these results show that hENT3 is widely expressed in multiple cell types with at least a fraction of endogenous hENT3 localized to the mitochondrial compartment.
Fig. 3.
Compartmentalization of hENT3: hENT3 cofractionation with the mitochondria. Lysates prepared from HepG2 cells were subjected to differential centrifugation in 8–27% sucrose gradient (LYSISO1, Sigma), and the presence of hENT3 in various fractions (collected from the top) was examined by Western blotting analysis. Immunoblot was first probed with anti-hENT3 polyclonal antibody and then stripped and serially probed with various antibodies against markers for various subcellular organelle (see materials and methods). Note significant cofractionation of hENT3 with hsp70, calreticulin, and EEA-1, but only minimally with fractions containing lamp2. Marker positions are indicated on left.
Fig. 4.
Mitochondrial localization of native hENT3. Colocalization of native hENT3 with hsp70 mitochondrial marker in various cell lines. Cells grown on glass coverslips were fixed and immunostained with anti-hENT3 polyclonal (top) and anti-hsp70 (middle) monoclonal antibodies. Merged images show regions of colocalization of hENT3 with mitochondria (bottom, yellow). Note moderate to extensive colocalization of hENT3 with hsp70 depending on the cell type. Nuclei were stained with DAPI (blue). Magnification ×60.
Expression of hENT3 in hepatic tissues.
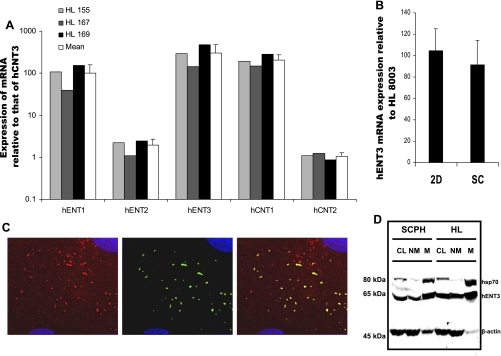
Because mitochondrial nucleoside transporters could facilitate mitochondrial hepatotoxicity of nucleoside drugs (21, 22), we investigated whether hENT3 is expressed in the human liver and specifically in the liver mitochondria. We analyzed the transcript levels of various nucleoside transporters (including hENT3) in three human liver samples with real-time PCR. Of all the ENTs and CNTs, hENT3 transcript expression was present at highest levels in all three human liver samples analyzed. The rank order of expression was hENT3 > hCNT1 ≈ hENT1 >> hENT2 ≈ hCNT2 > hCNT3 (Fig. 5A). In addition, similar hENT3 transcript levels were identified in the cultured primary human hepatocytes that were grown in two-dimensional and sandwich configurations (Fig. 5B). Since our earlier studies (13) also showed that sandwich-cultured hepatocytes are capable of sorting various nucleoside transporters into canalicular and/or sinusoidal membrane domains, we subsequently tested the subcellular localization of hENT3 in sandwich-cultured hepatocytes. Immunostaining analysis for hENT3 identified the exclusive presence of hENT3 in the cytoplasm (Fig. 5C) as observed in the HepG2 hepatic carcinoma cell line (Fig. 2B). hENT3 staining in sandwich-cultured hepatocytes was predominantly observed as vesicular and slightly elongated structures (Fig. 5C). Double immunolocalization studies showed partial colocalization of hENT3 with hsp70 (Fig. 5C, yellow in merged panel). To confirm the mitochondrial localization of hENT3, enriched “heavy mitochondrial” fractions were isolated from sandwich-cultured human hepatocytes and human liver tissue by differential centrifugation steps (see materials and methods) and the abundance of hsp70 and hENT3 was examined. Western blotting analysis of equal amounts (20 μg each) of “mitochondrial” fractions and the corresponding “nonmitochondrial” fractions indicated clear partitioning of hsp70 into the mitochondrial fractions but not in the nonmitochondrial fractions (Fig. 5D, compare NM and M lanes for hsp70). Similar to hsp70, endogenous hENT3 was found significantly enriched in the “mitochondrial” fractions; however, a substantial amount of hENT3 also separated into the “nonmitochondrial” fractions (Fig. 5D, compare NM and M lanes for hENT3). These results suggest that while a substantial fraction of the endogenous hENT3 resides in the mitochondrial compartment, hENT3 is also expressed in other cell organelles.
Fig. 5.
Expression of hENT3 in human liver and primary hepatocytes. A: transcript expression of various nucleoside transporters in 3 human livers (HLs). Relative mRNA levels were quantified by real-time PCR analysis using various gene-specific primers and probes. mRNA level of each of the nucleoside transporters was expressed relative to that of hCNT3 in that liver. Average Ct value for hCNT3 expression in HL samples was 37.5, while in the control samples (no reverse transcriptase) it was either >41.9 or below the level of detection. Open bars are means ± SD of the 3 livers. Note highest level of hENT3 expression in all 3 liver samples. B: real-time analysis of hENT3 mRNA expression in primary human hepatocytes cultured in 2-dimensional and sandwich configurations compared with that in a single liver sample and expressed as % ± SD (n = 3). C: localization of hENT3 in hepatocyte mitochondria. Sandwich-cultured hepatocytes were grown in glass coverslips, fixed, and coimmunostained with 1:1,000 anti-hENT3 polyclonal (left) and 1:2,500 hsp70 monoclonal (center) antibodies and appropriate secondary antibodies. Note partial colocalization of hENT3 (red) with hsp70 (green) in merged panel (yellow; right). Nuclei stained with DAPI are blue. Original magnification ×60. D: enrichment of hENT3 in heavy mitochondrial membranes. Crude lysate (CL; 10 μg), mitochondrial (M; 20 μg), and corresponding nonmitochondrial (NM; 20 μg) fractions were isolated from sandwich-cultured hepatocyte lysates or from a human liver sample and subjected to Western blotting analysis. Immunoblots were probed with 1:2,000 anti-hENT3 polyclonal (∼65 kDa), 1:5,000 anti-hsp70 monoclonal (70 kDa), and 1:10,000 anti-β-actin monoclonal antibodies (∼45 kDa) at the same time. Note presence of hENT3 predominantly in the mitochondrial fraction but some hENT3 partitioning into the nonmitochondrial fraction. Also note hsp70 predominantly in the mitochondrial fraction. β-Actin was used as an internal control. Marker positions are indicated on left. SCPH, sandwich-cultured primary human hepatocytes.
Yellow fluorescent protein epitope tagging of hENT3 hinders mitochondrial localization of hENT3.
Our data that hENT3 is predominantly a mitochondrial protein differs from that of Baldwin et al. (5), who claimed predominant lysosomal localization of yellow fluorescent protein (YFP)-tagged hENT3. Although both studies used HeLa cells, they differ in that the previous report (5) used YFP-tagged hENT3, while we studied the native hENT3. Therefore, we asked whether NH2-terminal tagging of hENT3 with a marker protein (YFP) could misdirect the localization of hENT3. Contrary to the staining pattern noted with native hENT3, YFP-hENT3 was predominantly seen in larger-sized vesicular structures in the cytoplasm in almost all cell types studied (Fig. 6A, right; Supplemental Fig. S3). In JAR, MBA-MB-231, or HeLa cells, unlike native hENT3 (Fig. 6A, left), YFP-hENT3 was not colocalized with hsp70 or E-cadherin, indicating lack of proper trafficking of YFP-fused hENT3 protein to the mitochondria or to the cell surface (data not shown). While the native hENT3 showed no clear or only very minimal colocalization with lamp2 in HeLa cells (Fig. 6B, left, arrowheads), YFP-hENT3 showed substantial colocalization of YFP fluorescence with lamp2 in many vesicular structures in the cytoplasm of HeLa cells (Fig. 6B, middle, arrows). In the same batch of cells, native hENT3 showed substantial colocalization with hsp70 (Fig. 6B, right, yellow). In addition, YFP-hENT3 was not found at the cell surface in JAR cells (Fig. 6A, top right).
Fig. 6.
Mistargeting of YFP-hENT3 to lysosomes. A: lack of replication of endogenous hENT3 staining pattern by YFP-hENT3. Various cell lines grown in glass coverslips were immunostained for native hENT3 with anti-hENT3 polyclonal antibody (left). Another set of cells was transfected with YFP-hENT3 and fixed and visualized directly under the microscope (right). Note that significant native hENT3 (top left), but not YFP-hENT3 (top right), was localized at the cell surface in JAR cells. Also note the staining patterns of native hENT3 in MDA-MB-231 and HeLa cells (middle and bottom left) and YFP-hENT3 (middle and bottom right) were distinctly different. B: substantial colocalization of YFP-hENT3, but not native hENT3, with lamp2. HeLa cells grown on glass coverslips were fixed and immunostained with anti-hENT3 polyclonal antibody (red) and anti-lamp2 monoclonal antibody (green) (left). Note minimal colocalization of hENT3 vesicular structures with lamp2 (arrowheads). HeLa cells were transfected with YFP-hENT3 plasmid construct (green) and immunostained with anti-lamp2 monoclonal antibody (red) (middle). Note substantial colocalization of YFP-hENT3 with lamp2 (arrows). In contrast, HeLa cells immunostained with anti-hENT3 polyclonal antibody (green) and anti-hsp70 monoclonal antibody (red) (right) showed substantial colocalization of native hENT3 with hsp70 (yellow). Nuclei stained with DAPI are blue. Original magnification ×60.
hENT3 transport characteristics in Xenopus oocytes.
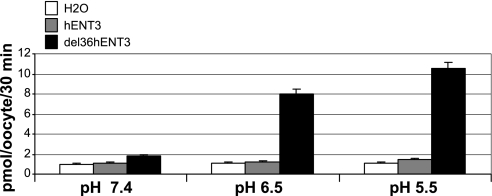
Earlier functional characterization of hENT3 transport was performed in Xenopus oocytes with hENT3 mutants (Δ36hENT3 and LLAAhENT3) that allowed trafficking of hENT3 to the cell surface and transport of hENT3 substrates (5). Hence, we adopted the same strategy to study the transport of [3H]adenosine by Δ36hENT3 RNA-injected Xenopus oocytes. As previously reported, we observed much higher [3H]adenosine transport at pH 5.5 or pH 6.5 than at pH 7.4 (Fig. 7A), and this transport was sodium independent.
Fig. 7.
Transport characteristics of hENT3: pH dependence of NH2-terminal-deleted hENT3 transport activity in Xenopus oocytes. Transport of [3H]adenosine (1 μM, over 30 min) by Xenopus oocytes was measured 24 h after injection of H2O or cRNA of full-length hENT3 (hENT3) or of NH2-terminal-deleted hENT3 (del36hENT3) (n = 2). Measurements were conducted after incubation of oocytes in Na+-free buffers at pH 7.4, 6.5, and 5.5. Note significant transport activity in oocytes expressing del36hENT3 but not in oocytes expressing hENT3 or in control oocytes (H2O). Also note presence of robust del36hENT3 transport activities at pH 6.5 and 5.5 but not at pH 7.4.
To determine the substrate selectivity of hENT3 at pH 6.5, a mitochondrially relevant pH (17, 28), we determined the transport of nucleosides, nucleotides, nucleobases, and organic cations (e.g., monoamines) into Δ36hENT3 RNA-injected Xenopus oocytes. Δ36hENT3 transported a number of anti-HIV ddNs (AZT, ddC, ddI, d4T, 3TC) and the anticancer drug gemcitabine at pH 6.5 (Table 1). Additionally, ribavirin and FIAU were substrates of hENT3 as well as adenosine triphosphate (ATP) and ribavirin triphosphate (RTP). A low level of transport was observed of organic cations such as 1-methyl-4-phenylpyridinium (MPP+) and serotonin neurotransmitter. However, MPP+ transport was indifferent at a pH range of 5.5–7.4 (data not shown).
Table 1.
Transport of natural nucleosides, nucleobases, nucleotides, deoxynucleosides, organic cationic compounds, and nucleoside drugs into Δ36hENT3 cRNA-injected Xenopus oocytes relative to that in H2O-injected oocytes
| Substrate | Fold Increase |
|---|---|
| [3H]adenosine | 7.8±0.5 |
| [3H]cytidine | 2.7±0.4 |
| [3H]thymidine | 5.3±1.0 |
| [3H]guanosine | 7.9±1.4 |
| [3H]inosine | 12.3±0.9 |
| [3H]uridine | 5.4±0.8 |
| [3H]adenine | 0.09±0.0 |
| [3H]cytosine | 0.25±0.0 |
| [3H]thymine | 0.57±0.0 |
| [3H]guanine | 2.5±0.3 |
| [3H]hypoxanthine | 1.0±0.0 |
| [3H]uracil | 1.3±0.1 |
| [3H]ATP | 3.6±1.6 |
| [3H]RTP | 16.3±5.3 |
| 2′-[3H]deoxyadenosine | 25.2±6.0 |
| 2′-[3H]deoxycytidine | 3.5±1.3 |
| [3H]MPP+ | 3.0±0.4 |
| [3H]norepinephrine | 3.1±0.6 |
| [3H]dopamine | 1.7±1.0 |
| [3H]serotonin | 2.8±0.2 |
| [3H]metformin | 1.5±0.4 |
| [3H]tyramine | 1.5±0.5 |
| [3H]acetylcholine | 0.7±0.2 |
| [3H]AZT | 2.8±0.5 |
| [3H]3TC | 17.9±4.1 |
| [3H]d4T | 4.4±1.0 |
| [3H]ddC | 20.3±5.4 |
| [3H]ddI | 6.3±0.2 |
| [3H]ribavirin | 9.0±4.3 |
| [3H]gemcitabine | 9.5±2.2 |
| [3H]FIAU | 13.4±4.7 |
Values represent means ± SD from 2 experiments. hENT3, human equilibrative nucleoside transporter 3; ATP, adenosine triphosphate; RTP, ribavirin triphosphate; MPP+, 1-methyl-4-phenylpyridinium; NE, norepinephrine, AZT, zidovudine; 3TC, stavudine; d4T, lamivudine; ddC, zalcitabine; ddI, didanosine, FIAU, fialuridine. Transport at 30 min of various tracer compounds (1 μM) was measured in Xenopus oocytes 24 h after injection of H2O or cRNA Δ36hENT3. Measurements were conducted after incubation of oocytes in Na+-free buffers at pH 6.5. Background values obtained from uninjected oocytes were subtracted from the values in the injected oocytes, and uptake into Δ36hENT3 cRNA-injected oocytes was expressed as fold change with respect to that observed in H2O-injected oocytes. Values >1 (bold) indicate higher transport activity in Δ36hENT3-cRNA-injected oocytes than H2O-injected oocytes.
Interestingly, certain nucleobases were found to be transported at higher rates into H2O-injected oocytes than Δ36hENT3 cRNA-injected oocytes (Table 1). This is possible if Xenopus oocytes exhibit high levels of endogenous concentrative nucleobase transport activity and the presence of hENT3 allows efflux of the nucleobase (37). To test this hypothesis, we indirectly measured the affinity of nucleobases for hENT3 by studying the uptake of [3H]adenosine in the absence or presence of a high concentration (1 mM) of various nucleobases. Our results showed that adenine-, thymine-, and cytosine-inhibited Δ36hENT3 mediated [3H]adenosine transport into the oocytes by 46.6 ± 9.9%, 62.4 ± 8.8% and 68.7 ± 19%, respectively (Supplemental Fig. S5).
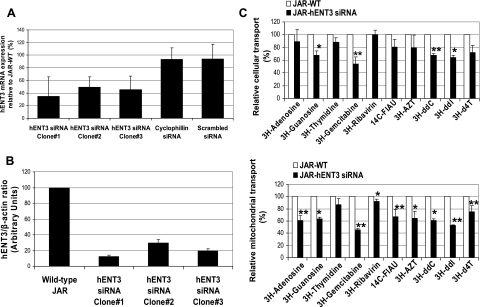
siRNA silencing of hENT3 decreased cellular and/or mitochondrial transport of nucleosides and dideoxynucleoside drugs.
To understand the role of hENT3 in the mitochondrial transport of nucleosides in mammalian cells, we opted to utilize siRNA strategies to knock down endogenous hENT3 activity in JAR placental cells. JAR cells were chosen because of their high levels of hENT3 protein expression both at the cell surface and in the mitochondria. siRNA gene silencing methods produced three clones with significantly reproducible reduction in both hENT3 mRNA (Fig. 8A) and protein (Fig. 8B). hENT3 siRNA JAR cells (clone 1), having the greatest reduction of hENT3 protein (87.5% knockdown at 5 days of culture), were used for further transport studies. Compared with wild-type JAR cells, hENT3-siRNA-expressing JAR cells demonstrated significant reduction in cellular and/or mitochondrial transport of natural nucleosides as well as nucleoside drugs (gemcitabine, FIAU, AZT, ddI, ddC, and d4T) (Fig. 8C).
Fig. 8.
Effect of hENT3 small interfering RNA (siRNA) in cellular and mitochondrial transport of nucleosides and nucleoside drugs. A: hENT3 transcript reduction in hENT3-knockdown JAR cells expressed as % ± SD (n = 2) of that in JAR wt cells. Total RNA was isolated, and transcript levels of hENT3 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; internal control) were analyzed by semiquantitative RT-PCR. Cells infected with retroviruses harboring a scrambled sequence or that expressing cyclophilin A siRNA served as controls. Note significant reduction of hENT3 mRNA in all 3 clones. B: extent of hENT3 protein knockdown in JAR cells. Five micrograms of whole cell extracts was subjected to Western blotting analysis, and immunoblots were probed with 1:2,000 anti-hENT3 polyclonal antibody and 1:10,000 β-actin monoclonal antibody. β-Actin was used as an internal control. Signal intensities of hENT3 and β-actin protein bands were quantified, and average hENT3-to-β-actin ratios (n = 2) were plotted in arbitrary units for comparison. C: reduction in cellular (top) and mitochondrial (bottom) transport of nucleosides in hENT3-knockdown JAR cells expressed as % ± SD (n = 2) of that in control cells. Cellular (pH 7.4) and mitochondrial (pH 6.5) transport experiments with various 3H- and 14C-radiolabeled compounds (1 μM) were conducted in Na+-free buffer containing 10 nM nitrobenzylmercaptopurine riboside (NBMPR) using JAR wt (control, open bars) and hENT3-knockdown (clone 1, filled bars) cells or in 50 μg of purified mitochondrial fractions isolated from these cells. FIAU, fialuridine; AZT, zidovudine; ddC, zalcitabine; ddI, didanosine; d4T, stavudine. *P < 0.05, **P < 0.01 compared with transport in control cells.
DISCUSSION
In this report, we provide multiple independent lines of evidence that support our conclusion of significant functional expression of native hENT3 in the mitochondria. First, discontinuous sucrose density gradient centrifugation identified migration of hENT3 with the mitochondrial fraction (in HepG2 cells). Second, differential centrifugation showed association of a substantial fraction of the native hENT3 with the mitochondrial enriched heavy membranes (in primary human hepatocytes). Third, immunocytochemical analysis demonstrated substantial colocalization of native hENT3 with mitochondrial hsp70 (in several cell lines). Fourth, hENT3 gene silencing resulted in decreased transport of nucleosides and nucleoside drugs into purified mitochondrial fractions (in JAR cells).
These data are in contrast to an earlier report (5) suggesting that hENT3 is a lysosomal nucleoside transporter. While we found only minimal colocalization of lamp2 lysosomal marker with endogenous hENT3, the level of colocalization of lamp2 substantially increased with YFP-hENT3. This discrepancy in the localization of endogenous hENT3 and YFP-hENT3, along with a lack of mitochondrial staining pattern of YFP-hENT3, suggests that the bulky YFP-hENT3 is misdirected to the lysosomes. Such marker protein (e.g., green fluorescent protein) tagging has been reported to cause mislocalization of mitochondrial proteins (e.g., Grb10) (30). Impaired localization of YFP-hENT3 to vesicular structures in the cytoplasm was also observed with JAR placental cells that were capable of recruiting endogenous hENT3 to the cell surface. These results suggest that hENT3 requires a free NH2 terminus for correct targeting and/or localization.
One of the major differences between hENT1/2 and hENT3 is the presence in the latter of a long NH2-terminal extension (∼50 amino acids) before the predicted first transmembrane domain (5, 16). Edmondson helical wheel analysis revealed that a segment from this region forms an amphiphilic helix with positively charged and hydrophobic residues lining the sides of the helix (Supplemental Fig. S1). Since proteins harboring such asymmetric helices can interact with translocases in the outer (TOM) and inner (TIM) mitochondrial membranes (32), we predict that such a region in the hENT3 NH2 terminus is a (or part of a) mitochondrial targeting signal (MTS). It is possible that attachment of YFP to the NH2 terminus of hENT3 could compromise its normal function(s) (e.g., protein-protein interactions), resulting in mislocalization of the fusion protein. Further studies are required to understand the precise role of the NH2 terminus of hENT3 in mitochondrial targeting.
Baldwin et al. (Ref. 5 and confirmed in the present study, Supplemental Fig. S2) showed that the substitution of two leucines at positions 34 and 36 in hENT3 (to alanines) is sufficient to redirect intracellularly localized hENT3 to the plasma membrane (5). Even though leucines in dileucine motifs could signal lysosomal/endosomal targeting, we propose that these leucines can also determine mitochondrial localization because they could be integral to the hydrophobic region of putative MTS on hENT3 (Supplemental Fig. S1). One example in which leucines in a hydrophobic face of an amphiphilic helix govern mitochondrial localization is the rat mitochondrial outer member protein TOM22 (29). Substituting any two out of three leucines (with alanines) in this face of the helix has been shown clearly to perturb the mitochondrial targeting of this protein. Since native hENT3 was localized to both mitochondria and lysosomes, it is also possible that leucine 34 and leucine 36 in hENT3 are integral to an overlapping targeting signal for the mitochondria and the lysosomes. Evidence for multiple subcellular targeting signals within a small stretch of amino acids has been reported for many other organelle proteins (29, 34, 35).
Consistent with the earlier report (5), we found hENT3 to be a Na+-independent, (acidic) pH-dependent transporter with optimal transport activity between pH 5.5 and 6.5. Although such a low-pH environment is physiologically relevant to acidic organelles like lysosomes, the transport of nucleosides by hENT3 at this pH would need to be from the lysosomal lumen to the cytosol. The movement of nucleosides from the cytosol to the lysosomal lumen is not operationally possible because of the extralysosomal (cytosolic) pH of 7.4, at which no hENT3 transport activity (at least for [3H]adenosine) is detectable in Xenopus oocytes. Moreover, adenosine transport into isolated lysosomal preparations was unaffected by pH change from 5.0 to 8.0 (33). Unlike the lysosomes, the mitochondria consist of outer and inner mitochondrial membranes, with the inner membrane forming specialized tubular inner structures (cristae). While the bulk mitochondrial matrix pH in mammalian cells can be slightly alkaline (pH ≥ 7.4), the local pH in the intracristal compartments might be much less than 7.4 (the local pH gradient hypothesis), a condition that would be required for optimal function of the ATP synthase (14, 19). Also, experimental evidence, at least in plant cells, has also shown that the inner mitochondrial membrane region (between outer and inner membranes) has a slightly acidic pH (lowest measured pH of 6.5), creating a microclimatic (acidic) pH gradient across the inner membrane (17, 28). Microclimatic (acidic) pH gradient across mitochondrial membranes has also been demonstrated in mammalian heart mitochondria (1, 2), and we speculate that such a gradient allows optimal operation of hENT3 in the mammalian mitochondria. The robust transport activity of Δ36hENT3 measured in Xenopus oocytes at pH 6.5 and substantial localization of endogenous hENT3 in the mitochondria support a physiological role for hENT3 in transporting nucleosides/deoxynucleosides into the mitochondria. Salvage of nucleosides/deoxynucleosides into the mitochondria and subsequent phosphorylation by intramitochondrial kinases (e.g., deoxyguanosine kinase and thymidine kinase 2) may play an important role in mitochondrial DNA synthesis/repair (3) in nonproliferating and differentiated cells. In this regard, an important recent report describes three mutations (2 missense mutations and 1 single nucleotide deletion) in the sixth exon of hENT3 gene as a cause of an autosomal recessive disorder in humans called the H syndrome (27). Many clinical manifestations of the H syndrome are reminiscent of mitochondrial respiratory chain defects or nuclear/mitochondrial DNA mutations (27). Together, these data support an endogenous function for hENT3 in transporting nucleosides into the mitochondria, possibly facilitating mitochondrial homeostasis including mitochondrial DNA synthesis/repair.
As previously reported (5), both Δ36hENT3 RNA-injected Xenopus oocytes and hENT3 gene-silenced JAR cells showed that hENT3 has broad substrate selectivity, transporting both nucleosides and nucleoside drugs. In addition, we identified novel substrates of hENT3 such as 3TC and d4T (anti-HIV ddNs), FIAU (anti-hepatitis B drug), ribavirin (anti-hepatitis C drug), nucleotides, and certain neuromodulators (MPP+, serotonin). The latter appear to be transported with poor intrinsic activity. Interestingly, unlike nucleoside transport, MPP+ transport by hENT3 at pH 7.4 was not augmented at acidic pH. The mechanism of such substrate-dependent difference in pH-sensitive transport is not clear.
Unlike our Xenopus oocyte transport data, characterization of hENT3 substrates with hENT3 gene-silenced JAR cells showed significant transport of only certain nucleoside drugs (e.g., gemcitabine, ddC, and ddI) but not of (or only minimal transport of) others such as AZT, FIAU, and ribavirin. There are several possible explanations for this discrepancy. First, AZT and FIAU are relatively more lipophilic than the other nucleoside drugs (e.g., gemcitabine, ddC), allowing passive diffusion of these compounds even under hENT3-silenced conditions. Second, knockdown of hENT3 was not complete in JAR-hENT3 siRNA cells, and residual expression of any hENT3 could still allow transport of nucleosides in hENT3-silenced conditions. Third, 10 nM nitrobenzylmercaptopurine riboside (NBMPR), at which concentration transport experiments were conducted, allows operation of cell surface hENT2 (Ki ∼2.8 μM) that can transport AZT and possibly FIAU and ribavirin. We could not use a higher concentration of NBMPR because such a concentration would inhibit hENT3 (5).
Our earlier studies (21) showed that hENT1 localized in the mitochondria of human cells transports FIAU into the mitochondria. Furthermore, hENT1 is also localized in the cell plasma membrane. Thus, in cells that lack hENT3 on the plasma membrane, the entry of FIAU into such cells will be mediated by hENT1. Subsequently, the entry of FIAU into the mitochondria by hENT1 is likely augmented by hENT3. This dual expression of transporters in the mitochondria likely contributes to the fatal mitochondrial toxicity of FIAU. Unlike FIAU, the lack of 3′-oxygen in the sugar ring of the ddNs makes them less likely to be high-affinity substrates for hENT1 (25). Consistent with this, little or no transport of anti-HIV ddNs was observed in Xenopus oocytes or mammalian cells expressing hENT1 (unpublished observations; Ref. 39). Hence, the ability of hENT3 to transport (3′-oxygen lacking) anti-HIV ddNs at significantly higher rates into the mitochondria suggests a strong link between hENT3 expression and anti-HIV ddN mitochondrial toxicity. Recently, anti-HIV ddNs have been implicated in mitochondrial toxicity in the fetus/newborn of pregnant women administered ddNs to reduce maternal-fetal HIV transmission (9). High levels of hENT3 (mRNA) expression in the human placenta and the targeting of hENT3 protein to the cell surface of placental epithelial cells (at both maternal and fetal sides) support the notion that hENT3 is involved in the fetal toxicity of anti-HIV ddNs.
In summary, our study has confirmed hENT3 as a widely expressed, broadly specific, Na+-independent, acidic environment-driven intracellular transporter. However, in contrast to an earlier report (5), we demonstrated significant differences with regard to tissue-specific, subcellular localization and pH-dependent transport characteristics of this transporter. Mitochondrial hENT3 localization was identified in multiple cell types, but cell surface hENT3 localization was detected only in certain placental cells. The pH sensitivity of the hENT3 transport was clearly found to be substrate dependent (nucleosides vs. MPP+). The mitochondrial localization of hENT3 suggests a potential role in mitochondrial toxicity of nucleoside drugs (e.g., FIAU, anti-HIV ddNs) manifesting in the clinic as hepatotoxicity, peripheral neuropathy, pancreatitis, myopathy, and fetal abnormalities.
GRANTS
This study was supported by National Institute of General Medical Sciences (NIGMS) Grants RO1-GM-054447 and RO1-GM-066233. We also acknowledge NIGMS Grant PO1-GM-32165, which supports the maintenance of the human liver bank of the School of Pharmacy, University of Washington, Seattle, WA.
Supplementary Material
Acknowledgments
We thank Dr. Christopher Black, Cellzdirect, Inc., for his support in obtaining human hepatocyte cultures and Christina Shadle for her technical assistance. We appreciate the assistance of the Keck Imaging Center, University of Washington, with the immunofluorescence image capture and analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Addanki S, Cahill D, Sotos JF. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. J Biol Chem 243: 2337–2348, 1968. [PubMed] [Google Scholar]
- 2.Addanki S, Cahill D, Sotos JF. Reliability of the quantitation of intramitochondrial pH and pH gradient of heart mitochondria. Anal Biochem 25: 17–29, 1968. [DOI] [PubMed] [Google Scholar]
- 3.Arner ES, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacol Ther 67: 155–186, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflügers Arch 447: 735–743, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin SA, Yao SY, Hyde RJ, Ng AM, Foppolo S, Barnes K, Ritzel MW, Cass CE, Young JD. Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem 280: 15880–15887, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Barnes K, Dobrzynski H, Foppolo S, Beal PR, Ismat F, Scullion ER, Sun L, Tellez J, Ritzel MW, Claycomb WC, Cass CE, Young JD, Billeter-Clark R, Boyett MR, Baldwin SA. Distribution and functional characterization of equilibrative nucleoside transporter-4, a novel cardiac adenosine transporter activated at acidic pH. Circ Res 99: 510–519, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet 356: 1423–1430, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Chang C, Swaan PW, Ngo LY, Lum PY, Patil SD, Unadkat JD. Molecular requirements of the human nucleoside transporters hCNT1, hCNT2, and hENT1. Mol Pharmacol 65: 558–570, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Divi RL, Leonard SL, Kuo MM, Nagashima K, Thamire C, St Claire MC, Wade NA, Walker VE, Poirier MC. Transplacentally exposed human and monkey newborn infants show similar evidence of nucleoside reverse transcriptase inhibitor-induced mitochondrial toxicity. Environ Mol Mutagen 48: 201–209, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Dragovic G, Jevtovic D. Nucleoside reverse transcriptase inhibitor usage and the incidence of peripheral neuropathy in HIV/AIDS patients. Antivir Chem Chemother 14: 281–284, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem 279: 50042–50049, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Govindarajan R, Bakken AH, Hudkins KL, Lai Y, Casado FJ, Pastor-Anglada M, Tse CM, Hayashi J, Unadkat JD. In situ hybridization and immunolocalization of concentrative and equilibrative nucleoside transporters in the human intestine, liver, kidneys, and placenta. Am J Physiol Regul Integr Comp Physiol 293: R1809–R1822, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Govindarajan R, Endres CJ, Whittington D, Lecluyse E, Pastor-Anglada M, Tse CM, Unadkat JD. Expression and hepatobiliary transport characteristics of the concentrative and equilibrative nucleoside transporters in sandwich-cultured human hepatocytes. Am J Physiol Gastrointest Liver Physiol 295: G570–G580, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hainers TH, Dencher NA. Cardiolipin: a proton trap for oxidative phosphorylation. FEBS Lett 528: 35–39, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Honkoop P, Scholte HR, de Man RA, Schalm SW. Mitochondrial injury: lessons from the fialuridine trial. Drug Saf 17: 1–7, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Hyde RJ, Cass CE, Young JD, Baldwin SA. The ENT family of eukaryote nucleoside and nucleobase transporters: recent advances in the investigation of structure/function relationships and the identification of novel isoforms. Mol Membr Biol 18: 53–63, 2001. [PubMed] [Google Scholar]
- 17.Igamberdiev AU, Kleczkowski LA. Equilibration of adenylates in the mitochondrial intermembrane space maintains respiration and regulates cytosolic metabolism. J Exp Bot 57: 2133–2141, 2006. [DOI] [PubMed] [Google Scholar]
- 18.John M, Moore CB, James IR, Nolan D, Upton RP, McKinnon EJ, Mallal SA. Chronic hyperlactatemia in HIV-infected patients taking antiretroviral therapy. AIDS 15: 717–723, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Khalifat N, Puff N, Bonneau S, Fournier J, Angelova MI. Membrane deformation under local pH gradient: mimicking mitochondrial cristae dynamics. Biophys J 95: 4924–4933, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohler JJ, Lewis W. A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environ Mol Mutagen 48: 166–172, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Lai Y, Tse CM, Unadkat JD. Mitochondrial expression of the human equilibrative nucleoside transporter 1 (hENT1) results in enhanced mitochondrial toxicity of antiviral drugs. J Biol Chem 279: 4490–4497, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Lee EW, Lai Y, Zhang H, Unadkat JD. Identification of the mitochondrial targeting signal of the human equilibrative nucleoside transporter 1 (hENT1): implications for interspecies differences in mitochondrial toxicity of fialuridine. J Biol Chem 281: 16700–16706, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat Med 1: 417–422, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Lewis W Nucleoside reverse transcriptase inhibitors, mitochondrial DNA and AIDS therapy. Antivir Ther 10, Suppl 2: M13–M27, 2005. [PubMed] [Google Scholar]
- 25.Lum PY, Ngo LY, Bakken AH, Unadkat JD. Human intestinal es nucleoside transporter: molecular characterization and nucleoside inhibitory profiles. Cancer Chemother Pharmacol 45: 273–278, 2000. [DOI] [PubMed] [Google Scholar]
- 26.McKenzie R, Fried MW, Sallie R, Conjeevaram H, Di Bisceglie AM, Park Y, Savarese B, Kleiner D, Tsokos M, Luciano C, Pruett T, Stokka JL, Straus SE, Hoofnagle JH. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N Engl J Med 333: 1099–1105, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Molho-Pessach V, Lerer I, Abeliovich D, Agha Z, Abu Libdeh A, Broshtilova V, Elpeleg O, Zlotogorski A. The H syndrome is caused by mutations in the nucleoside transporter hENT3. Am J Hum Genet 83: 529–534, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore AL, Rich PR. Organization of the respiratory chain and oxidative phosphorylation. In: Encyclopedia of Plant Physiology, Vol. 18. Higher Plant Cell Respiration, edited by Douce R, Day DA. Berlin: Springer, 1985, p. 134–172.
- 29.Nakamura Y, Suzuki H, Sakaguchi M, Mihara K. Targeting and assembly of rat mitochondrial translocase of outer membrane 22 (TOM22) into the TOM complex. J Biol Chem 279: 21223–21232, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Nantel A, Huber M, Thomas DY. Localization of endogenous Grb10 to the mitochondria and its interaction with the mitochondrial-associated Raf-1 pool. J Biol Chem 274: 35719–35724, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Neve EP, Ingelman-Sundberg M. Identification and characterization of a mitochondrial targeting signal in rat cytochrome P450 2E1 (CYP2E1). J Biol Chem 276: 11317–11322, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Pfanner N, Geissler A. Versatility of the mitochondrial protein import machinery. Nat Rev Mol Cell Biol 2: 339–349, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Pisoni RL, Thoene JG. Detection and characterization of a nucleoside transport system in human fibroblast lysosomes. J Biol Chem 264: 4850–4856, 1989. [PubMed] [Google Scholar]
- 34.Pujol C, Marechal-Drouard L, Duchene AM. How can organellar protein N-terminal sequences be dual targeting signals? In silico analysis and mutagenesis approach. J Mol Biol 369: 356–367, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Robin MA, Anandatheerthavarada HK, Biswas G, Sepuri NB, Gordon DM, Pain D, Avadhani NG. Bimodal targeting of microsomal CYP2E1 to mitochondria through activation of an N-terminal chimeric signal by cAMP-mediated phosphorylation. J Biol Chem 277: 40583–40593, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shayeghi M, Akerman R, Jarvis SM. Nucleobase transport inopossum kidney epithelial cells and Xenopus laevis oocytes: the characterisation, structure-activity relationship of uracil analogues and oocyte expression studies of sodium-dependent and -independent hypoxanthine uptake. Biochim Biophys Acta 1416: 109–118, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA 99: 4465–4470, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Giacomini KM. Characterization of a bioengineered chimeric Na+-nucleoside transporter. Mol Pharmacol 55: 234–240, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Yao SY, Ng AM, Sundaram M, Cass CE, Baldwin SA, Young JD. Transport of antiviral 3′-deoxy-nucleoside drugs by recombinant human and rat equilibrative, nitrobenzylthioinosine (NBMPR)-insensitive (ENT2) nucleoside transporter proteins produced in Xenopus oocytes. Mol Membr Biol 18: 161–167, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.