Abstract
Zinc metabolism during chronic disease is dysregulated by inflammatory cytokines. Experiments with IL-6 knockout mice show that LPS regulates expression of the zinc transporter, Zip14, by a mechanism that is partially independent of IL-6. The LPS-induced model of sepsis may occur by a mechanism signaled by nitric oxide (NO) as a secondary messenger. To address the hypothesis that NO can modulate Zip14 expression, we treated primary hepatocytes from wild-type mice with the NO donor S-nitroso N-acetyl penicillamine (SNAP). After treatment with SNAP, steady-state Zip14 mRNA levels displayed a maximal increase after 8 h and a concomitant increase in the transcriptional activity of the gene. Chromatin immunoprecipitation documented the kinetics of activator protein (AP)-1 and RNA polymerase II association with the Zip14 promoter after NO exposure, indicating a role of AP-1 in transcription of Zip14. We then stimulated the primary murine hepatocytes with IL-1β, an LPS-induced proinflammatory cytokine and a potent activator of inducible NO synthase (iNOS) and NO production. In support of our hypothesis, IL-1β treatment led to a threefold increase in Zip14 mRNA and enhanced zinc transport, as measured with a zinc fluorophore, in wild-type but not iNOS−/− hepatocytes. These data suggest that signaling pathways activated by NO are factors in the upregulation of Zip14, which in turn mediates hepatic zinc accumulation and hypozincemia during inflammation and sepsis.
Keywords: liver, activator protein-1, inflammation
zinc is essential for numerous catalytic, structural, and regulatory roles in cells (20). Thus organisms require specific and efficient transport mechanisms to maintain cellular zinc homeostasis. The movement of zinc into and out of cells and subcellular organelles is mediated by zinc transport proteins. Specifically, the Zip (Zrt/Irt-like) family of transport proteins includes members that mediate the zinc uptake into cells (9). This family includes Zip1–8 and Zip14, which all have functional zinc transport activities in mammals (4, 5, 30). In rodent models of inflammation, plasma concentrations of zinc are transiently decreased (6). In response to cytokine treatment, zinc is redistributed among various tissues, particularly the liver (5). This redistribution is accompanied by an increase of hepatic metallothionein-bound zinc. However, the mechanism of this redistribution and the role and physiological significance of hypozincemia in response to inflammation are not well understood. These events chronologically are similar to dysregulated iron metabolism referred to as the anemia of chronic disease (46).
We have recently identified Zip14 (Slc39a14) as a zinc transport protein involved in hepatic zinc uptake during murine models of inflammation (31). In the sterile abscess model of inflammation, upregulation of Zip14 is the mechanism responsible for hypozincemia. In this model, experiments with IL-6−/− mice demonstrated that steady-state mRNA levels and transport activities of Zip14 are dependent on the production of IL-6. LPS-stimulated hypozincemia does follow changes in plasma cytokine levels (12). However, companion experiments with IL-6−/− mice suggest that LPS regulates Zip14 expression via a mechanism that is partially independent of IL-6. IL-1β, an LPS-induced proinflammatory cytokine, is a potent activator of inducible nitric oxide synthase (iNOS) and NO production (13). Consequently, the LPS-induced increase in Zip14 expression may occur by a mechanism signaled by NO as a secondary messenger.
NO has demonstrated the ability to both upregulate and downregulate the expression of genes through various mechanisms. In iron homeostasis, NO activates iron regulatory protein (IRP)1 through interaction with its Fe-S center, thus regulating iron influx and storage through binding of IRP1 to iron-responsive elements on mRNAs of transferrin receptor (TfR) and ferritin (19, 35, 36). Zinc-finger proteins are another major target of NO regulation. Nitrosylation of zinc-thiolate clusters leads to transient impairment of the DNA-binding activities of some zinc-finger transcription factors such as Sp1, p53, NF-κB, and activator protein (AP)-1 (reviewed in Ref. 23). However, activation of these same transcription factors by NO has also been observed (1, 11, 38, 39).
The purpose of the present experiments was to determine whether NO mediates the upregulation Zip14 expression and how this regulation occurs. The results show that IL-1B induction of Zip14 is fully prevented in hepatocytes from iNOS−/− vs. wild-type (WT) mice. Augmentation of cellular NO levels with an NO donor, however, produced full induction of Zip14 in the iNOS−/− hepatocytes. Furthermore, NO augmented the transcriptional activity of Zip14, and this upregulation led to an increase in intracellular labile zinc as detected by fluorescence. Finally, chromatin immunoprecipitation analysis shows that NO increases binding of the transcription factor AP-1 to the Zip14 promoter.
MATERIALS AND METHODS
Animals.
The iNOS−/− and control strain C57BL/6 mice were purchased from Jackson Laboratory. Metallothionein knockout (MT−/−) mice were breed in house from founder mice purchased from the Jackson Laboratory. The control mice for the MT−/− strain are 129S3/SvImJ mice. Six- to eight-wk-old male mice were used in all experiments. Mice were given free access to tap water and received commercial rodent diets (Harlan Teklad) with a 12-h light/dark cycle. Protocols were approved by the University of Florida Institutional Animal Care and Use Committee.
Hepatocyte isolation and culture.
Mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (60 mg/kg). Isolation of hepatocytes began with an infusion of a calcium-free solution containing 140 mM NaCl, 7 mM KCl, and 10 mM HEPES buffer (pH 7.3) via the subhepatic inferior vena cava (31). Next, a solution containing 67 mM NaCl, 7 mM KCl, 5 mM CaCl2, 100 mM HEPES buffer (pH 7.3), and 0.04% (wt/vol) collagenase (Sigma type IV collagenase, C-5138), at a flow rate of 7 ml/min for 14 min, was allowed to perfuse the liver. Upon completion of perfusion, the liver was excised rapidly and transferred to 15 ml of the collagenase solution, and the liver cells were aseptically liberated. The cells were passed through a 100-μm cell strainer, suspended in a buffered wash medium [William's Medium E (WME) + 10 mM HEPES, pH 7.3], and collected by centrifugation. Cells were then washed in the same buffer twice, and the final cell pellet was resuspended in attachment medium [WME supplemented with 10% FBS (vol/vol), 100 nM insulin, 100 nM dexamethasone, 100 U/ml penicillin, and 100 mg/ml streptomycin]. Only suspensions with >95% viability as evaluated by Trypan Blue were used in experiments. Cells were seeded at 2.5 × 105 cells/well in 12-well culture plates, 5 × 105 cells/well in six-well culture plates, and 2 × 105 cells/chamber in four-chamber glass microscopy slides. After being plated, the cells were allowed to attach for 3 h (37°C, 5% CO2). Following selective attachment of parenchymal cells, medium in each well was exchanged, and these culture conditions continued for 18–22 h. The fresh medium contained LPS (0.1 μg/ml), IL-1β (100 U/ml), or S-nitroso-N-acetylpenicillamine (SNAP) at 100 μM all from Sigma.
Cell culture.
AML12 mouse hepatocytes (American Type Culture Collection) were grown in DMEM/F-12 containing 10% (vol/vol) FBS, 40 ng/ml dexamethasone, and insulin/Tf/selenium supplement (BD Biosciences). Medium also contained penicillin, streptomycin, and amphotericin B (Sigma). These hepatocytes were maintained at 37°C in 5% CO2. SNAP was added as described above.
Antibodies and Western blot analysis.
The following antibodies were purchased from Santa Cruz Biotechnology: c-Fos (K-25), sc-253 (K-25), RNA Pol II (polymerase II), sc-899, and normal rabbit IgG, sc-2027. The ZIP14 antibody was developed as previously described (31). Total cell lysates, PAGE, and subsequent Western blotting were as previously described (31). To confirm equivalent loading, blots were initially stained with Ponceau Red and, after probing for Zip14, the membranes were stripped and reprobed with mouse monoclonal anti-tubulin clone B-5–1-2 (Sigma), followed by horseradish peroxidase-conjugated goat anti-mouse IgG (Zymed). Visualization was by enhanced chemiluminescence (Pierce) and X-ray film.
Immunofluorescent localization of mZip14.
C57BL/6 WT hepatocytes were fixed with 4% (wt/vol) paraformaldehyde, blocked with PBS containing 3% (wt/vol) BSA for 1h, then incubated for 1 h with 10 μg/ml affinity-purified anti-mZIP14 antibody. Cells were then washed three times with PBS and incubated for 45 min in 3% (wt/vol) BSA with anti-Alexa Fluor 594 (Invitrogen) conjugated to anti-rabbit IgG. ZIP14 localization was visualized as described previously (31).
Zinc uptake and NO production by hepatocytes.
iNOS−/− or C57BL/6 hepatocytes were incubated with 5 μM FluoZin-3AM (Invitrogen), a cell-permeable zinc fluorophore, in serum-free medium for 30 min. These cells had been incubated with 40 μM zinc before addition of the fluorophore. Intracellular zinc accumulation was measured as previously described (31). The Griess Reaction was used to determine the synthesis of NO by measuring the nitrite content of the culture supernatant (26). The absorbance was measured at 570 nm, and nitrite concentrations were calculated by comparison with a standard curve prepared using NaNO2.
Transcription rate, steady-state mRNA, and chromatin immunoprecipitation analysis (ChIP).
Total RNA was isolated from AML12 cells using TRIzol (Invitrogen) and treated with DNaseI to eliminate any DNA contamination. To measure the transcriptional activity from Zip14, primers spanning Zip14 exon 5 and intron 5 junction were used to measure the short-lived, unspliced heterogeneous nuclear RNA (hnRNA). This procedure for measuring transcriptional activity was adapted from Palii et al. (34) and is based on that described by Lipson and Baserga (28). The levels of hnRNA were determined by quantitative real-time PCR (qPCR) using SYBR Green (Applied Biosystems). Reactions with no reverse-transcriptase were used as negative controls for assessment of genomic DNA contamination. The primers for amplification were: sense primer, 5′-TCCTGGTGGTTGCCTTGC-3′, and antisense primer, 5′-AGAGGAAACCGTACCCCCATA-3′. The PCR reaction conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 60 s, and one final cycle at 60°C for 60 s. After PCR, melting curves were acquired by a stepwise increase of temperature from 55 to 95°C to ensure that a single product was amplified during the reactions.
qPCR was used to determine the relative amount of Zip14 mRNA in each of the same samples basically as described above. Primers amplified exon 10 region of Zip14 mRNA and were as follows: sense primer, 5′-GTAAACCTTGAGCTGCACTTAGC-3′, and antisense primer, 5′-TGCAGCCGCTTCATGGT-3′. Transcript abundances were normalized to 18s rRNA (sense primer, 5′-AGTCCCTGCCCTTTGTACACA-3′, and antisense primer, 5′-GATCCGAGGGCCTCACTAAAC-3′) and based on an RNA standard curve. PCR reactions were performed in duplicate for each sample, and samples were collected from at least three independent experiments.
ChIP analysis was performed as described by Chen et al. (3). The reaction mixtures were incubated at 95°C for 10 min, followed by 40 cycles of amplification at 95°C for 15 s and 60°C for 60 s. The Zip14 promoter primers were: (c-Fos) sense primer, 5′-TGGTTGGCTGGGGTAGGCAAA-3′, and antisense primer, 5′-TCGCTCCTGAGGGAGAGTGCC-3′, (RNA Pol II) sense primer, 5′-TTGGCCAGGGTAACGACGCT-3′, and antisense primer, 5′-CATGCCCGGCCATATACCCT-3′.
Statistical analysis.
Data are presented as the means ± SD and were analyzed by two-way ANOVA. Bonferroni's post hoc test was used for multiple comparisons. Statistical significance was set at P < 0.05.
RESULTS
Induction of Zip14 expression in mouse IL-1β is NO dependent.
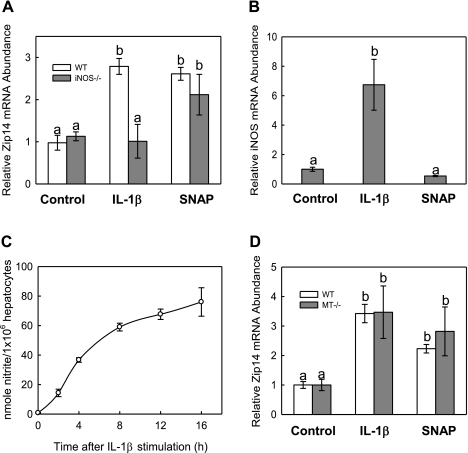
LPS stimulates IL-6, TNF-α, and IL-1β (8). Of the proinflammatory mediators, IL-1β signals the production of NO. Since we found that LPS induces hepatic Zip14 expression in mice (23), we have examined which of these cytokines regulates Zip14 in primary mouse hepatocytes. We exposed primary hepatocytes from WT (C57BL/6) mice to 100 U/ml of IL-1β. After 8 h of treatment, IL-1β caused an approximate twofold increase in relative Zip14 mRNA levels (Fig. 1A). However, with primary hepatocytes from iNOS−/− mice, IL-1β was unable to stimulate an increase in Zip14 expression. When hepatocytes from the WT mice were incubated with 100 U/ml of IL-1β for up to 16 h, the iNOS mRNA increased to a level sevenfold higher than untreated cells (Fig. 1B). Increased iNOS expression corresponded with a major increase in nitrite production upon treatment with IL-1β (Fig. 1C). As further proof of an NO-mediated response, 100 μM SNAP was added to iNOS−/− hepatocytes and caused a significant twofold increase in Zip14 expression (Fig. 1A). In contrast, no differences in Zip14 mRNA levels were observed between hepatocytes from WT and MT−/− mice (Fig. 1D), indicating that NO increased Zip14 expression independent of MT.
Fig. 1.
Influence of IL-1β and nitric oxide (NO) on Zip14 expression. A: hepatocytes from inducible NO synthase (iNOS)−/− and C57BL/6 wild-type (WT) mice were treated with the NO donor IL-1β (100 U/ml) or S-nitroso-N-acetylpenicillamine (SNAP) (100 μM) for 8 h, and Zip14 mRNA was measured by quantitative real-time PCR (qPCR) B: hepatocytes from C57BL/6 mice were exposed to either IL-1β (100 U/ml) or SNAP (100 μM) for up to 16 h. Relative iNOS mRNA abundance was measured. Similar results were achieved with hepatocytes from 129S3/SvImJ mice. C: WT hepatocytes were exposed to IL-1β (100 U/ml) for up to 16 h. The nitrite concentration of the medium in response to IL-1β was measured by using the Griess reaction and normalized to the total cell number per well. D: contribution of metallothionein (MT) to regulation of Zip14 by NO was investigated by incubating MT−/− and corresponding control strain hepatocytes with or IL-1β (100 U/ml) or SNAP (100 μM) for 8 h. Values are means ± SD of 3 independent experiments. Values with different letters are significantly different (P < 0.001).
Transcription of the Zip14 gene.
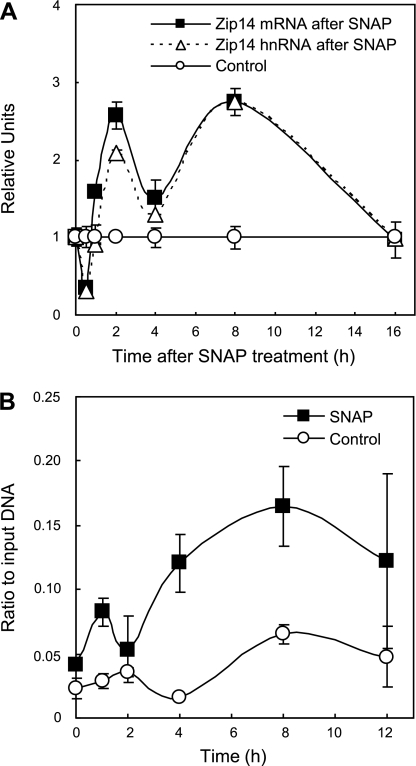
Hepatocytes from C57BL/6 mice were incubated with 100 μM SNAP for up to 16 h, and the steady-state mRNA level for Zip14 was measured for three independent experiments by qPCR. Similar results were obtained when the AML12 hepatocyte cell line was used in a separate independent experiment (data not shown). An initial twofold increase of Zip14 mRNA occurred 2 h after treatment and was sustained for 8 h (Fig. 2A). To assess the transcriptional activity of Zip14, the same samples used for mRNA quantification were used to measure the short-lived hnRNA. An almost identical biphasic response to Zip14 hnRNA as the mRNA strongly suggests a transcriptional control mechanism for NO regulation of Zip14. Further evidence of Zip14 transcriptional activity was revealed by treating AML12 hepatocytes for 12 h with 100 μM SNAP, followed by RNA Pol II ChIP analysis of the Zip14 promoter (Fig. 2B). Collectively, these results indicate that RNA Pol II is recruited to the Zip14 promoter and carries out active transcription following NO exposure.
Fig. 2.
Effect of NO on Zip14 steady-state mRNA levels and transcriptional activity. Primary hepatocytes from WT (C57BL/6) mice were incubated with SNAP (100 μM) for up to 16 h. At the times indicated, total RNA was isolated and analyzed by qPCR. A: transcriptional activity of the Zip14 gene was assessed by measurement of the heterogeneous nuclear RNA (hnRNA), utilizing primers corresponding to the junction of exon 4 and intron 4. From the same samples, steady-state mRNA levels for Zip14 were determined as in Fig 1. The Zip14 hnRNA and mRNA abundance is plotted as arbitrary units normalized to 18s rRNA, relative to control values, and based on an RNA standard curve. Each data point represents the mean ± SD of 3 independent experiments. B: murine AML12 hepatocytes were used to provide further evidence for transcriptional activity of the Zip14 gene. The cells were treated with SNAP (100 μM) for 0–12 h, and chromatin immunoprecipitation (ChIP) analysis was performed using RNA Pol II antibody. Relative binding of Pol II to the Zip14 promoter was analyzed by qPCR. Data were plotted as the ratio immunoprecipitated DNA to a 1:20 dilution of input DNA. Background immunoprecipitation values were obtained by using a nonspecific rabbit IgG and never achieved a ratio higher than 0.01 to input DNA. Each data point represents the mean ± SE for 3 replicate experiments.
Transcription factor c-Fos associates with the Zip14 promoter in response to NO.
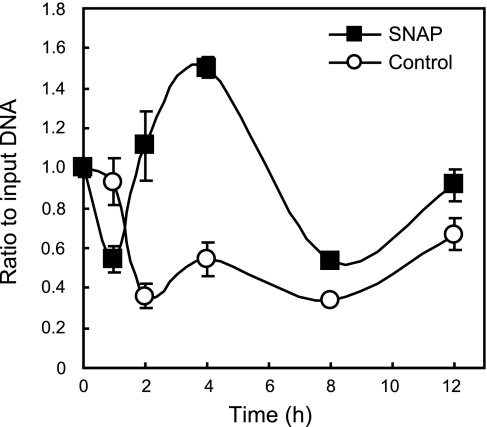
One candidate transcription factor that might be involved in the NO-responsiveness of the Zip14 gene was AP-1 (c-Fos/c-Jun heterodimer) (23). By using the MatInspector bioinformatics program, we were able to map a putative AP-1 binding site to −486/−477 of the Zip14 promoter (37). Analysis of this potential c-Fos/c-Jun binding site was carried out by ChIP followed by qPCR to amplify a region of the Zip14 promoter containing the −486 to −477 (5′-CGTGAGTCAAG-3′) proposed AP-1 binding site. AML12 hepatocytes were treated with 100 μM SNAP for 12 h. Immunoprecipitation of protein/DNA complexes was performed with an anti-c-Fos antibody or nonspecific rabbit IgG (negative control) (Fig. 3). The data show that c-Fos is significantly enriched at the Zip14 promoter beginning after 2 h of SNAP treatment, with the greatest amount of enrichment occurring after 4 h. Analysis of promoter DNA from the negative control IgG by qPCR always resulted in a quantity of at least 10-fold less than with the c-Fos antibody.
Fig. 3.
ChIP analysis shows that c-Fos binds to the Zip14 promoter in response to NO. Murine AML12 hepatocytes were incubated with SNAP (100 μM) for 12 h. ChIP analysis was performed using an anti-c-Fos antibody, followed by qPCR. A nonspecific rabbit IgG was used as a negative control. Data were plotted as the ratio of immunoprecipitated DNA to a 1:20 dilution of input DNA. Background immunoprecipitation levels were always below a ratio of 0.01 (to input DNA). Each data point represents the mean ± SE for 3 independent experiments.
NO increases Zip14 protein expression and function at the plasma membrane of hepatocytes.
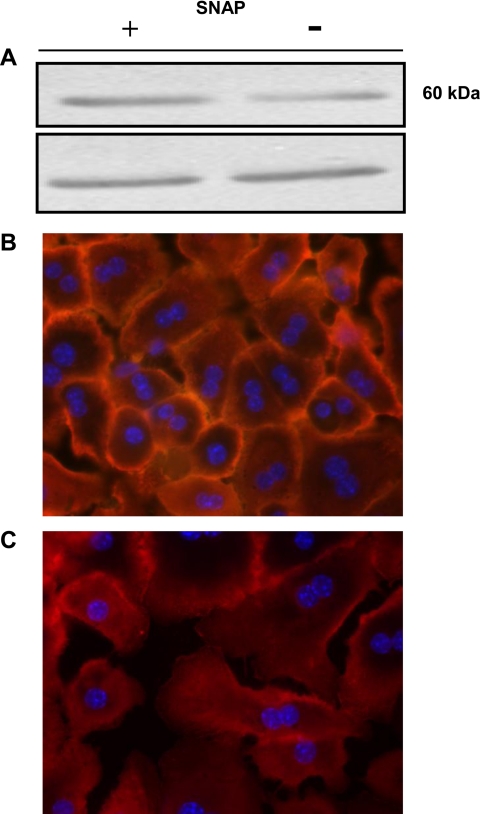
WT hepatocytes were treated with SNAP for 12 h, and expression of ZIP14 was analyzed by Western blotting. Two immunoreactive bands of ∼50 kDa are increased after SNAP treatment (Fig. 4A, top). Densitometric analysis of these immunoreactive bands showed an approximate twofold change in ZIP14 abundance after SNAP treatment (2.75 for SNAP-treated cells vs. 1 for control). Immunofluorescence revealed a greater concentration of ZIP14 at the plasma membrane in SNAP-treated cells (Fig. 4B) than in untreated hepatocytes (Fig. 4C). These results were attained with nonpermeabilized hepatocytes; however, similar results were achieved when permeabilized hepatocytes were used. Identical gain was used for both images.
Fig. 4.
NO upregulates ZIP14 protein expression in liver parenchymal cells. Hepatocytes from WT (C57BL/6) mice were incubated with SNAP for 8 h. A: total cell lysates were separated by SDS-PAGE, and ZIP14 was detected by Western blot analysis using an affinity-purified ZIP14 antibody. Top: increased ZIP14 expression produced by SNAP. Bottom: tubulin loading control. B: nonpermeabilized primary hepatocytes were stained with 4′,6-diamidino-2-phenylindole (DAPI) for visualization of the nucleus, and an affinity-purified ZIP14 antibody was used for immunolocalization of ZIP14. C: untreated cells were used as a control. Representative images from SNAP-treated and untreated cells using identical gain settings are shown.
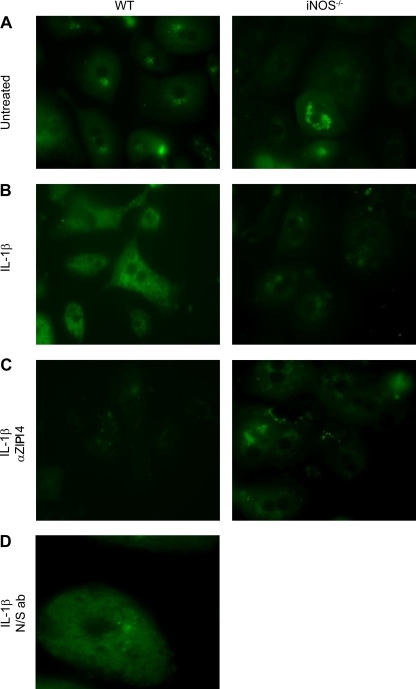
Hepatocytes were isolated from WT and iNOS−/− mice and treated with IL-1β, followed by incubation with or without the ZIP14 antibody. IL-1β stimulation of WT hepatocytes caused an increase in intracellular labile zinc, as measured by an increase in fluorescence from FluoZin-3AM that was not seen in iNOS−/− hepatocytes (Fig. 5A vs. 5B). The peptide used to generate the antibody is from an extracellular epitope and should block ZIP14-mediated zinc uptake. Note that no increase in fluorescence was observed in response to IL-1β when hepatocytes were preincubated with the Zip14 antibody before measurement of zinc uptake (Fig. 5C). Furthermore, when WT hepatocytes were preincubated with an antibody not directed against ZIP14, uptake of zinc was not blocked (Fig. 5D). These functional studies show that the increase in intracellular zinc was dependent on upregulation of Zip14 by an IL-1β-induced mechanism.
Fig. 5.
Fluorescent detection of NO-mediated zinc uptake in hepatocytes from WT and iNOS−/− mice using FluoZin3-AM. Hepatocytes were treated without (A) or with (B) Il-1β for 8 h, followed by incubation with 40 μM zinc for 5 min, and then immediately visualized by fluorescence microscopy. C: hepatocytes were incubated with IL-1β for 8 h, and then an anti-ZIP14 antibody was added before addition of zinc to the medium. D: hepatocytes were treated as above, but a nonspecific antibody (N/S ab) was used instead of an anti-ZIP14 antibody.
DISCUSSION
The results presented in the present study describe an NO-induced mechanism for increasing liver zinc uptake during hepatic inflammation. We have previously shown that Zip14 may be the major zinc transporter responsible for the hypozincemia associated with inflammation and the acute-phase response (31). Our prior research utilized two different model systems to examine Zip14 expression. In the turpentine model of inflammation, IL-6 was necessary for the in vivo induction of Zip14 expression and hypozincemia, whereas the LPS model did not show an absolute requirement for IL-6. These differences in Zip14 regulation may be related to the cytokines produced by each stimulus. Turpentine administration, for the most part, leads to an IL-6-specific response, whereas LPS cytokine induction is more complex, involving TNF-α, IL-1α, IL-1β, leptin, and IL-6 (10). Therefore, since increased NO production is a downstream response to IL-1β, we hypothesized that stimulation of hepatic NO by IL-1β may be another mechanism for upregulation of Zip14 expression.
NO regulates a broad spectrum of physiological responses (18). These NO-activated responses are mediated by signaling cascades that elicit changes in blood pressure, neurotransmission, smooth muscle contraction, and mineral metabolism (17). NO may also regulate genes to modulate immune responses and inflammation and promote or inhibit tumor progression and metastasis (reviewed in Ref. 11). The exact mechanisms underlying these NO-induced effects are not entirely known. However, certain signal transducers and transcription factors have been identified that are necessary for regulating these genes in response to NO, including hypoxia inducible factor-1-α, NF-κB, phosphatidylinositol-3-kinase, PKC, p53, and the Ras-Raf-MEK-ERK pathway, which leads to activation of AP-1 (17; reviewed in Ref. 11).
Genetic and pharmacological techniques have revealed both protective and toxic roles of NO on liver cell injury depending on the NO concentration (22). However, the level of NO donors used here are not likely to produce nitrosative stress or cell injury. Previous studies have indicated that primary hepatocytes are resistant to nitrosative stress with greater than 90% viability (LA Lichten and RJ Cousins, unpublished observations). It has been shown that, at high concentrations of NO donors, nitrosative stress results in the release of intracellular zinc (24). Therefore, the present experiments may present a more physiological view of the NO-Zn interaction at the level of zinc transport.
The transcription complex, AP-1, continuously appears in screens of genes that are activated by NO (11, 23, 38, 39). The AP-1 family of transcription factors consists of three main groups: the Fos proteins (c-Fos, FosB, Fra1, and Fra2), the Jun proteins (c-Jun, JunB, and JunD), and the activating transcription factors (ATF2, ATF3, and B-ATF) (4). These various family members can form homo- or heterodimers that make up the active AP-1 complex. The DNA-binding and transactivation potential of the AP-1 complex is not only regulated by the dimer composition but by transcription of the genes and posttranslational protein modifications (41, 44). Of importance is the observation that c-Fos transcription and AP-1 activation occur quickly in response to NO (17). The mouse Zip14 gene promoter harbors two putative AP-1 binding sites 5′-TGAGTCA-3′ (16), the first of which lies at position −481 relative to the transcription start site. Phylogenetic footprinting analysis of this promoter region shows conservation between mice and humans (28). We therefore investigated whether AP-1 was involved in regulation of Zip14 gene expression by ChIP analysis. In these experiments, we focused on c-Fos because of the documented quality of data produced with the c-Fos antibody used here. Significant enrichment of c-Fos was observed at the Zip14 promoter post-SNAP treatment, suggesting that AP-1 is involved in activation of the Zip14 gene after NO exposure.
Upregulation of zinc transporters may have positive or negative physiological consequences depending on the stimuli involved and/or cellular location of the transporter. Interestingly, exposure of dendritic cells to LPS affects expression of many of the zinc transport proteins, resulting in a net increase in Zn transport out of cells (reviewed in Ref. 33). Overexpression of Zip6 (Slc39a6), whose abundance is reduced by LPS, suppresses dendritic cell maturation most likely by increasing intracellular zinc levels (21). Examination of the closest evolutionary neighbor to the Zip14 gene, Zip8 (Slc39a8), reveals both protective and cytotoxic effects of this transporter. An increase in the expression of Zip8 in primary human lung epithelia by TNF-α causes an increase in intracellular zinc levels, which leads to protection against TNF-α-induced cytotoxicity (2). However, expression of Zip8 is associated with sensitivity to cadmium (Cd) toxicity specifically in vascular endothelial cells of the testis (7). Similarly, ZIP14 was shown to have a high affinity for cadmium, and this ability to transport Cd displaces manganese Mn and Zn2+ when expressed in mouse fetal fibroblasts, leading to unwanted cell death attributable to the toxic nature of Cd (14). Although ZIP14 was shown to transport Cd, on a physiological basis we have observed that expression of Zip14 does not seem to be deleterious to cells, and even overexpression in human embryonic kidney cells, AML12, or SF9 insect cells is not cytotoxic (31, 29). Although no significant cellular stress was observed here (data not shown), upregulation of Zip14 by NO may serve a protective purpose by increasing intracellular zinc and metallothionein. Numerous reports have documented the beneficial effects of both zinc and/or MT on liver function and hepatocyte survival after exposure to deleterious agents such as ethanol (22, 43, 47), Cd, carbon-tetrachloride (CCl4) (6), radiation, and oxidative damage and may contribute to control of cellular proliferation and apoptosis (22, 25). The role of MT in apoptosis has been extensively studied, with the majority of studies showing that MT plays a protective role with respect to apoptosis (42). In this regard, ZIP14 may be characterized as a positive acute phase protein, possibly protecting the liver during inflammation.
In reference to metal metabolism, the present report is not the first demonstration of NO regulating the transcription of a metal transporter gene. The transport protein involved in intestinal uptake and cellular iron release, divalent metal transporter-1 (DMT-1), is downregulated in response to NO (36). The mechanism for downregulation of DMT-1 is not at the posttranscriptional level as is usual for regulation of the gene by iron (15). Rather, NO increases binding of the transcription factor NF-κB to the DMT-1 promoter leading to transcriptional repression of the gene in neuronal cells. Of possible relevance is transcriptional upregulation of DMT-1 in respiratory epithelial cells by modulators of the inflammatory response such as LPS, IFN-γ, and TNF-α (45).
Hypoferremia is a primary marker of the anemia of inflammation, and is thought to occur via increased tissue iron uptake, specifically liver uptake, and decreased intestinal and macrophage iron export (reviewed in Ref. 32). We recently reported that ZIP14 mediates non-Tf-bound iron uptake into hepatocytes, which would be consistent with inflammation and NO increasing the expression of this transporter (29). Furthermore, the transport of zinc from plasma to hepatocytes during inflammation is related to ZIP14 expression and is fully (turpentine-induced inflammation), or partially (LPS-induced inflammation) controlled by IL-6 (31). In the present report, we demonstrate that NO could functionally activate ZIP14, thereby increasing zinc uptake. In agreement with that hypothesis, IL-1β increased fluorescently labeled intracellular zinc but required both iNOS expression and functional ZIP14 to do so. Of particular importance is the predicted topology of the ZIP14 protein. By using bioinformatics and experimental data, we predicted that the histidine-rich region contained within the large peptide loop connecting transmembrane domains 3 and 4 was extracellular (31). The ability of the ZIP14 antibody to block zinc transport results supports our predicted topology. However, when immunofluorescence studies are conducted on permeabilized, rather than nonpermeabilized, cells a greater fluorescent intensity from Alexa Fluor 594-labeled ZIP14 is observed (data not shown). Therefore, we cannot rule out the possibility that the histidine-rich loop may become cytoplasmic during a transition state. Collectively, our results show that IL-1β can stimulate NO production and elevate ZIP14 expression via signaling pathways leading to AP-1 activation, which in turn leads to hepatic zinc accumulation. Overall, regulation of the zinc transporter Zip14 by NO adds a new dimension to our understanding of hepatic zinc homeostasis in health and disease.
GRANTS
This research was funded by National Institutes of Health Grant DK 31127, Boston Family Endowment Funds (to R. Cousins), and College of Agriculture and Life Sciences Alumni Fellowship (to L. Lichten).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Blanchette J, Pouliot P, Olivier M. Role of protein tyrosine phosphatases in the regulation of interferon-[gamma]-induced macrophage nitric oxide generation: implication of ERK pathway and AP-1 activation. J Leukoc Biol 81: 835–844, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Beseker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell DL. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol Lung Cell Mol Physiol 294: L1127–L1136, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Pan YX, Dudenhausen EE, Kilberg MS. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localized histone acetylation. J Biol Chem 279: 50829–50839, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Chinenov Y, Kerppola TK. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20: 2438–2452, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Cousins RJ, Leinart AS. Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin 1. FASEB J 2: 2884–2890, 1988. [DOI] [PubMed] [Google Scholar]
- 6.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem 281: 24085–24089, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, Chang X, Baxter CS, Nebert DW. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci USA 102: 3401–3406, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis SR, Cousins RJ. Metallothionein expression in animals: a physiological perspective on function. J Nutr 130: 1085–1088, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Eide DJ Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta 1763: 711–722, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1β mediates leptin induction during inflammation. Am J Physiol Regul Integr Comp Physiol 274: R204–R208, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer 6: 521–534, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Gaetke LM, McClain CJ, Talwalkar RT, Shedlofsky SI. Effects of endotoxin on zinc metabolism in human volunteers. Am J Physiol Endocrinol Metab 272: E952–E956, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Geller DA, de Vera ME, Russell DA, Shapiro RA, Nussler AK, Simmons RL, Billiar TR. A central role for IL-1 beta in the in vitro and in vivo regulation of hepatic inducible nitric oxide synthase IL-1 beta induces hepatic nitric oxide synthesis. J Immunol 155: 4890–4898, 1995. [PubMed] [Google Scholar]
- 14.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol 73: 1413–1423, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, Hediger MA. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett 509: 309–316. [DOI] [PubMed]
- 16.Halazonetis TD, Georgopoulos K, Greenberg ME, Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell 55: 917–924, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Hemish J, Nakaya N, Mittal V, Enikolopov G. Nitric oxide activates diverse signaling pathways to regulate gene expression. J Biol Chem 278: 42321–2329, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol 34: 879–886, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Kim S, Ponka P. Nitrogen monoxide-mediated control of ferritin synthesis: implications for macrophage iron homeostasis. Proc Natl Acad Sci USA 99: 12214–12219, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King JC, Cousins RJ. Modern Nutrition in Health and Disease, 10th ed. Shils ME, Shike M, Ross AC, Caballero B, and Cousins RJ, eds. Baltimore, MD: Lippincott Williams & Wilkins, 2005, pp. 271–285.
- 21.Kitamura H, Morikawa H, Kamon H, Iguchi M, Shinatro H, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol 7: 971–977, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol 39: 267–294, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Kröncke KD Nitrosative stress and transcription. Biol Chem 384: 1365–1377, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Kröncke KD, Klotz LO, Suschek CV, Sies H. Comparing nitrosative versus oxidative stress toward zinc finger-dependent transcription. Unique role for NO. J Biol Chem 277: 13494–13501, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Lazo JS, Kuo SM, Pitt BR. The protein thiol metallothionein as an antioxidant and protectant against antineoplastic drugs. Chem Biol Interact 111: 255–262, 1998. [DOI] [PubMed] [Google Scholar]
- 26.LeClaire RD, Kell WM, Sadik RA, Downs MB, Parker GW. Regulation of staphylococcal enterotoxin B-elicited nitric oxide production by endothelial cells. Infect Immun 63: 539–546, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Billiar TR. Determinants of nitric oxide protection and toxicity in liver. Am J Physiol Gastrointest Liver Physiol 276: G1069–G1073, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Lipson KE, Baserga R. Transcriptional activity of the human thymidine kinase gene determined by a method using the polymerase chain reaction and an intron-specific probe. Proc Natl Acad Sci USA 86: 9774–9777, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liuzzi JP, Aydemir Nam H F, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA 103: 13612–13617, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr 24: 151–172, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA 102: 6843–6848, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moshage H Cytokines and the hepatic acute phase response. J Pathol 181: 257–266, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Murakami M, Hirano T. Intracellular zinc homeostasis and zinc signalling. Cancer Sci 99: 1515–1522, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palii SS, Thiaville MM, Pan YX, Zhong C, Kilberg MS. Characterization of the amino acid response element within the human sodium-coupled neutral amino acid transporter 2 (SNAT2) System A transporter gene. Biochem J 395: 517–512, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pantopoulos K Iron metabolism and the IRE/IRP regulatory system: an update. Ann NY Acad Sci 1012: 1–13, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Paradkar PN, Roth JA. Nitric oxide transcriptionally down-regulates specific isoforms of divalent metal transporter (DMT1) via NF-kappaB. J Neurochem 96: 1768–1777, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res 23: 4878–4884, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salazar-Montes A, Ruiz-Corro L, Sandoval-Rodriguez A, Lopez-Reyes A, Armendariz-Borunda J. Increased DNA binding activity of NF-kappaB, STAT-3, SMAD3 and AP-1 in acutely damaged liver. World J Gastroenterol 12: 5995–6001, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlieper A, Anwar M, Heger J, Piper HM, Euler G. Repression of anti-apoptotic genes via AP-1 as a mechanism of apoptosis induction in ventricular cardiomyocytes. Pflügers Arch 454: 53–61, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder JJ, Cousins RJ. Maintenance of zinc-dependent hepatic functions in rat hepatocytes cultured in medium without added zinc. J Nutr 121: 844–853, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Sheerin A, Thompson KS, Goyns MH. Altered composition and DNA binding activity of the AP-1 transcription factor during the ageing of human fibroblasts. Mech Ageing Dev 122: 1813–1824, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Shimoda R, Achanzar WE, Qu W, Nagamine T, Takagi H, Mori M, Waalkes MP. Metallothionein is a potential negative regulator of apoptosis. Toxicol Sci 73: 294–300, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Tomita K, Azuma T, Kitamura N, Tamiya G, Ando S, Nagata H, Kato S, Inokuchi S, Nishimura T, Ishii H, Hibi T. Leptin deficiency enhances sensitivity of rats to alcoholic steatohepatitis through suppression of metallothionein. Am J Physiol Gastrointest Liver Physiol 287: G1078–G1085, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Trøen G, Nygaard V, Jenssen T, Ikonomou IM, Tierens A, Matutes E, Gruszka-Westwood A, Catovsky D, Myklebost O, Lauritzsen G, Hovig E, Delabie J. Constitutive expression of the Ap-1 transcription factors c-jun, junD, junB, and c-fos and the marginal zone B-cell transcription factor Notch2 in splenic marginal zone lymphoma. J Mol Diagn 6: 297–307, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Garrick MD, Yang F, Dailey LA, Piantadosi CA, Ghio AJ. TNF, IFN-γ, and endotoxin increase expression of DMT1 in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 289: L24–L33, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Weiss G Pathogenesis and treatment of anaemia of chronic disease. Blood Rev 16: 87–96, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol 166: 1681–1690, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]