Abstract
The incretin hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), play an important role in glucose homeostasis in both health and diabetes. In mice, sucralose, an artificial sweetener, stimulates GLP-1 release via sweet taste receptors on enteroendocrine cells. We studied blood glucose, plasma levels of insulin, GLP-1, and GIP, and gastric emptying (by a breath test) in 7 healthy humans after intragastric infusions of 1) 50 g sucrose in water to a total volume of 500 ml (∼290 mosmol/l), 2) 80 mg sucralose in 500 ml normal saline (∼300 mosmol/l, 0.4 mM sucralose), 3) 800 mg sucralose in 500 ml normal saline (∼300 mosmol/l, 4 mM sucralose), and 4) 500 ml normal saline (∼300 mosmol/l), all labeled with 150 mg 13C-acetate. Blood glucose increased only in response to sucrose (P < 0.05). GLP-1, GIP, and insulin also increased after sucrose (P = 0.0001) but not after either load of sucralose or saline. Gastric emptying of sucrose was slower than that of saline (t50: 87.4 ± 4.1 min vs. 74.7 ± 3.2 min, P < 0.005), whereas there were no differences in t50 between sucralose 0.4 mM (73.7 ± 3.1 min) or 4 mM (76.7 ± 3.1 min) and saline. We conclude that sucralose, delivered by intragastric infusion, does not stimulate insulin, GLP-1, or GIP release or slow gastric emptying in healthy humans.
the interaction of nutrient with the small intestine plays an important role in the regulation of appetite, energy intake and glucose homeostasis. For example, the suppression of energy intake induced by small intestinal fat infusion is much greater than that in response to an equivalent intravenous fat load (46). Exposure of the small intestine to nutrients is also associated with feedback inhibition to slow the rate of gastric emptying (4, 20). Both the slowing of gastric emptying and suppression of appetite are mediated by the secretion of gastrointestinal hormones, including glucagon-like peptide-1 (GLP-1) (26, 40), the release of which is strongly stimulated by carbohydrate (23). GLP-1 is one of the two known “incretin” hormones that stimulate glucose-dependent insulin release (10). In healthy humans, GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) account for at least 50% of the postprandial insulin response (42). GLP-1 in pharmacological doses also inhibits glucagon secretion, slows gastric emptying, and suppresses appetite, leading to weight loss in the long term (31). GLP-1, but not GIP, has preserved insulinotropic effects in patients with type 2 diabetes (30). Therefore, the GLP-1 analogs, such as exenatide and liraglutide, and enzyme dipeptidyl peptidase IV inhibitors that enhance circulating concentrations of active GLP-1, such as sitagliptin, have been developed for therapeutic use (2, 12).
The detection system for sweet taste on the tongue has been established for a decade. Sweet tastants are detected by G protein-coupled receptors (GPCR) of the T1R family, of which T1R2 and T1R3 receptors heterodimerize to form broadly tuned sweet taste receptors. T1R2+T1R3 couples to a G protein, gustducin, and, in turn, to the transient receptor potential ion channel TRPM5 (39). It has recently been established that the α-subunit of gustducin, α-gustducin, is expressed in the mucosa of the murine gastrointestinal tract (34, 44). Expression of α-gustducin is evident throughout the mouse small intestine and, among several cell types, appears to colocalize with GLP-1-secreting L cells. The sweet taste receptor molecules T1R2, α-gustducin, and TRPM5 have now been shown to also be expressed in the human small intestinal mucosa (43).
Artificial sweeteners have been used to replace carbohydrate in the management of diabetes and obesity (1). Sucralose is a noncaloric sweetener derived from sucrose and is ∼600 times sweeter. Although sucralose (1–5 mM) has been shown to stimulate GLP-1 from human L cells in vitro, in a concentration-dependent manner (21), it is not known whether this effect occurs in vivo. A report that long-term (3 mo) dietary supplementation with sucralose (667 mg daily) did not alter glycated hemoglobin in patients with type 2 diabetes (19) argues against a significant effect. However, there is a lack of data about the effects of sucralose on gastric emptying or GLP-1 release in either animals or humans.
The aims of our study were to evaluate the effects of sucralose at a concentration chosen to match the sweetness of a sucrose load (0.4 mM) and at a much higher concentration (4 mM), in the range shown to stimulate GLP-1 release from a human enteroendocrine cell line in vitro, on gastric emptying, GLP-1, GIP, insulin, and blood glucose concentrations in healthy subjects.
MATERIALS AND METHODS
Subjects
Seven healthy subjects (age 24 ± 2 yr; body mass index 21.6 ± 1.2 kg/m2) were studied. None had a history of gastrointestinal disease, was taking medications known to affect gastrointestinal motility or appetite, was a smoker, or habitually consumed more than 20 g of alcohol per day. The study protocol was approved by the Royal Adelaide Hospital Research Ethics Committee, and each subject provided written, informed consent before taking part. The number of subjects was based on power calculations derived from our previous work (15).
Protocol
Each subject attended the Discipline of Medicine at the Royal Adelaide Hospital at ∼0830 h after an overnight fast (14 h for solids, 12 h for liquids) on four occasions, each separated by 3–7 days. Women were studied in the follicular phrase of the menstrual cycle. On each study day, a catheter (external diameter ∼3 mm) was introduced into the stomach via an anesthetized nostril. Its intragastric position was verified by rapid injection of 10 ml air and auscultation over the upper abdomen. An intravenous cannula was inserted into a forearm vein to allow repeated blood sampling. All subjects received an intragastric infusion, over 3 min (t = −3–0 min), of either 1) 50 g sucrose dissolved in water to a total volume of 500 ml (∼290 mosmol/l), 2) 80 mg sucralose (Tate & Lyle, Decatur, IL) in 500 ml normal saline (∼300 mosmol/l, 0.4 mM sucralose, equivalent sweetness to sucrose), 3) 800 mg sucralose in 500 ml normal saline (∼300 mosmol/l, 4 mM sucralose), or 4) 500 ml normal saline (∼300 mosmol/l), in randomized, single-blind fashion. All of the infusates were labeled with 150 mg 13C-acetate, and breath samples were collected immediately before and every 5 min after intragastric infusion in the first hour and every 15 min for a further 3 h (7). Blood was sampled immediately before the infusion (t = −3 min), and at t = 0, 5, 15, 30, 60, 90, 120, 150, 180, and 240 min for measurement of blood glucose and plasma GLP-1, GIP, and insulin. After 240 min, the nasogastric catheter and intravenous cannula were removed, and the subject was offered lunch and then allowed to leave the laboratory.
Gastric emptying.
13CO2 enrichment in the breath samples was measured by mass spectroscopy (ABCA 20–20 mass spectrometer; Europa Scientific, Crewe, UK) to determine the percentage 13CO2 recovery per hour and the cumulative percentage of 13CO2 recovery over 4 h (7). The gastric half-emptying time (t50) and gastric emptying coefficient (GEC) were calculated as measures of the gastric emptying rate (27). Breath tests using the 13C acetate label have been validated against scintigraphy for the measurement of liquid gastric emptying (5, 7).
Blood glucose and plasma GLP-1, GIP, and insulin concentrations.
Blood glucose concentrations were determined immediately using a portable glucometer (Medisense Precision QID; Abbott Laboratories, Bedford, MA). The remainder of each blood sample was placed in a prechilled EDTA tube containing 400 kIU aprotinin (Trasylol; Bayer Australia, Pymble, Australia) per liter of blood and then centrifuged at 3,200 revolution/min for 15 min (4°C). Plasma was separated and samples were stored at −70°C for subsequent analysis (26). Total plasma GLP-1 was measured by radioimmunoassay (GLPIT-36HK; Linco Research, St. Charles, MO). The sensitivity was 3 pmol/l, and the interassay coefficient of variation (CV) was 9.2%. Total plasma GIP was measured by radioimmunoassay (41). The sensitivity was 2 pmol/l, and both the intra- and interassay CVs were 15%. Plasma insulin was measured by solid-phase, two-site chemiluminescent immunometric assay (Immulite 2000 Insulin; Siemens Medical Solutions Diagnostics, Los Angeles, CA). Sensitivity was 2 mU/l, intra-assay CV was 3.9%, and interassay CV was 5.0%.
Statistical Analysis
Blood glucose and plasma hormone concentrations were analyzed by repeated-measures ANOVA (SuperANOVA; Abacus Concepts, Berkeley, CA) with time and treatment as factors. t50 and GEC were also analyzed by ANOVA. Post hoc means comparisons were performed in the event of a significant treatment × time interaction. Statistical significance was accepted at P < 0.05, and data are presented as means ± SE.
RESULTS
The study was well tolerated by all subjects. Fasting blood glucose concentrations and plasma GLP-1, GIP, and insulin concentrations did not differ between the four study days.
Gastric Emptying
There was a significant treatment effect for t50 between the four study days (P < 0.005). t50 was longer for sucrose than saline (87.4 ± 4.1 min vs. 74.7 ± 3.2 min; P < 0.005), whereas there were no differences in t50 between sucralose at 0.4 mM (73.7 ± 3.1 min) or sucralose at 4 mM (76.7 ± 3.1 min) and saline. Accordingly, GEC was less for sucrose compared with saline (4.3 ± 0 vs. 4.7 ± 0.1; P < 0.0005), whereas it was not different between sucralose 0.4 mM (4.8 ± 0.1) or 4 mM (4.6 ± 0.1) and saline.
Blood Glucose Concentrations
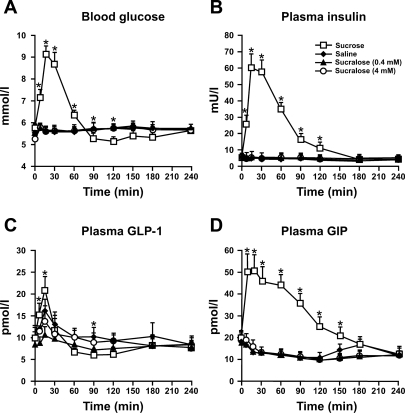
As seen in Fig. 1A, there was a rise in blood glucose after sucrose administration (P = 0.0001), which was evident from t = 5 min and decreased progressively after about 30 min, falling below baseline levels at 90 min. There was a significant treatment × time interaction (P = 0.0001), with the blood glucose concentration being greater after sucrose (P < 0.05) between 5 min and 60 min and lower between 90 min and 120 min (P < 0.05) compared with saline infusion. There were no significant differences in blood glucose between either load of sucralose and saline.
Fig. 1.
Concentrations of blood glucose (A), plasma insulin (B), plasma glucagon-like peptide-1 (GLP-1) (C), and plasma glucose-dependent insulinotropic polypeptide (GIP) (D) in response to intragastric infusion of 500 ml solutions containing either 1) 50 g sucrose, 2) normal saline, 3) 80 mg sucralose (0.4 mM), or 4) 800 mg sucralose (4 mM) in 7 healthy subjects. *Sucrose vs. saline, P < 0.05. Data are means ± SE. There were no significant differences in blood glucose, plasma insulin, plasma GLP-1, and plasma GIP between either load of sucralose and saline.
Plasma Insulin
Figure 1B demonstrates that plasma insulin rose promptly after sucrose administration (P = 0.0001), with a maximum response at 15 min, and then fell to basal levels by t = 240 min. Insulin concentrations were markedly greater after sucrose infusion between 5 min and 120 min compared with those after saline infusion (P < 0.05). Neither sucralose load stimulated an insulin response.
Plasma GLP-1
As seen in Fig. 1C, there was a marked increase in plasma GLP-1 after sucrose administration (P = 0.0001), which was maximal at 15 min followed by a subsequent decline to baseline levels by t = 60 min. The GLP-1 level was greater after sucrose between 5 and 15 min, and less at t = 90 min, compared with saline (P < 0.05). There was no difference between sucralose at 0.4 mM or 4 mM and saline.
Plasma GIP
Figure 1D shows that there was a prompt rise in plasma GIP after sucrose infusion (P = 0.0001). After a peak between 5–15 min, GIP declined progressively to baseline values by 240 min. Plasma GIP was greater between 5 min and 150 min after sucrose, compared with saline infusion (P < 0.05). There was no difference between sucralose at 0.4 mM or 4 mM and saline.
DISCUSSION
This study is the first to evaluate the incretin, insulin, and glycemic responses to sucralose administration and to determine whether this artificial sweetener is capable of generating feedback in the small intestine that slows gastric emptying in healthy humans. In contrast to administration of sucrose, sucralose given by intragastric infusion had no effect on GLP-1, GIP, or insulin secretion or gastric emptying, both at a concentration chosen to match the sweetness of the sucrose load (0.4 mM) and at a much higher concentration (4 mM), in the range shown to stimulate GLP-1 release from a human enteroendocrine cell line in vitro (21). This is consistent with reports that sucralose failed to influence plasma glucose or serum C-peptide in patients with type 1 and 2 diabetes (29) and that the noncaloric sweetener, stevioside, failed to stimulate GLP-1 or GIP in patients with type 2 diabetes (16). Similarly, there was no effect of sucralose on fasting blood glucose or glycated hemoglobin levels over 3 mo in patients with type 2 diabetes (19).
We administered sucralose by intragastric infusion in this study, rather than infusing directly into the small intestine. However, since sucralose is stable in acidic solution (<1% hydrolysis at pH 3 after 1 yr, Ref. 18), it is unlikely that the properties of sucralose would have been altered by exposure to gastric acid before emptying into the small intestine. We do not believe that giving the test solution orally, rather than by intragastric infusion, would have altered the outcome because there is no evidence for an effect of the cephalic phase of digestion on incretin hormone release (3). We selected a higher concentration of sucralose that was toward the upper end of the effective range in vitro (21) and a lower concentration that approximated that used in the food industry and observed no effect of sucralose at either concentration. It should be recognized that, although we cannot be absolutely certain that these concentrations were too high or too low to stimulate a response (21), it would not have been feasible to undertake additional study days in the same volunteers, particularly given the volume of blood sampled. We also did not measure the concentration of sucralose within the small intestinal lumen, but, given that the emptying of both sucralose solutions from the stomach was rapid, we would not anticipate a substantial difference from the concentrations administered intragastrically.
The presence of carbohydrate in the small intestine is a well established stimulus for GLP-1 and GIP secretion, leading to glucose-dependent insulin secretion from the β-cells and feedback that regulates gastric emptying (24). Direct exposure of carbohydrate to the mucosa of the small intestine appears to be an essential requirement for GLP-1 and GIP secretion (10), and the magnitude of the former is dependent on the rate of delivery of glucose into the small intestine (35). Elements of the sweet taste receptor present in the tongue have recently been identified in both rodent (34, 44) and human (43) small intestine. The T1R2+3 heterodimer should, by analogy to the tongue, respond to various sweet-tasting molecules as diverse as sucrose, saccharin, acesulfame K, and sucralose (32, 47). Furthermore, it has been demonstrated in two mouse enteroendocrine cell lines, GLUTag and STC-1, that sweet taste receptors are colocalized with GLP-1 and GIP (22, 37) and that sucralose stimulates secretion of GLP-1 and GIP from GLUTag cells (28). Mice that lack α-gustducin show markedly defective GLP-1 secretion in response to glucose (21). Furthermore, the human L cell line, NCI-H716, expresses α-gustducin and several other taste-signaling elements (21), and GLP-1 release from NCI-H716 cells is stimulated by glucose, sucrose, and sucralose and blocked by the sweet receptor antagonist, lactisole, or siRNA for α-gustducin (21). However, until this study, there has been no information available regarding effects of artificial sweeteners on GLP-1 release in humans in vivo. Moreover, even in mice, only a minority (8%) of L cells coexpress α-gustducin (44); the potential stimulus for GLP-1 release from the remainder is unclear. It should also be noted that major differences between species are evident with regards to incretin hormone release. For example, fructose stimulates the release of GLP-1 in rats (38) and humans (36) but not in dogs (41).
Although sucrose, sucralose, and other sweet tastants all bind to the T1R2+3 heterodimer, they do not act in identical fashion, at least on the tongue. For example, sucrose binds with a different affinity from sucralose (33), whereas absence of T1R3 receptors in knockout mice completely abolishes any taste preference for sucralose but merely diminishes the preference for sucrose (9). Moreover, functional brain imaging in humans indicates differences in central activation between sucrose and identically sweet sucralose solutions (13).
Absorption of monosaccharide may be necessary for GLP-1 or GIP release; this seems to be true for GIP at least (45) since rapidly and slowly digestible carbohydrates differ considerably in their ability to stimulate GIP secretion (45), whereas phloridzin, an inhibitor of the sodium-glucose cotransporter 1 (SGLT1) glucose transporter, suppresses GIP release (14). Absorption of monosaccharide via SGLT1 has also been suggested as a trigger for GLP-1 secretion from L cells (17).
We have not examined whether sucralose has any effect on carbohydrate absorption in humans. Supplementation of the diet with sucralose increases SGLT1 mRNA and glucose absorption in wild-type mice but not in T1R3 or α-gustducin knockout mice (28). Furthermore, sucralose, acesulfame potassium, and saccharin stimulate glucose absorption in rats by enhancing apical insertion of GLUT2 (25). This raises the question as to whether the combination of an artificial sweeter with carbohydrate could have a synergistic effect on incretin release, even if sucralose has no effect alone, and might account for the observation that chronic exposure of mice to oligofructose (a nondigestible sugar) enhances GLP-1 secretion in response to a high-fat diet (6).
It should also be recognized that GPCR other than T1R2 have recently been linked to incretin hormone release. For example, GPR40 and GPR119 are both GPCR that may stimulate incretin release in response to fatty acids, and agonists of these receptors could be therapeutically useful in diabetes (8). Furthermore, sweet taste sensors other than T1R2, such as SGLT3 (11), could be important in the detecting carbohydrate in the small intestine.
In conclusion, we have not been able to demonstrate that sucralose given by intragastric infusion stimulates GLP-1 or GIP release in humans or elicits a feedback response to slow gastric emptying. This implies that artificial sweeteners may have no therapeutic benefit in the dietary management of diabetes, other than as a substitute for carbohydrate.
GRANTS
This study was supported by awards from the Sylvia and Charles Viertel Charitable Foundation and the Faculty of Health Sciences, University of Adelaide, to Dr. Rayner, and by the National Health and Medical Research Council of Australia (NHMRC).
Acknowledgments
We thank Tate & Lyle, Decatur, IL for supplying the sucralose used in this study.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.American Dietetic Association. Position of the American Dietetic Association: use of nutritive, and nonnutritive sweeteners. J Am Diet Assoc 104: 255–275, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Ahren B DPP4 inhibitors. Best Pract Res Clin Endocrinol Metab 21: 517–533, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Ahren B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes 50: 1030–1038, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Andrews JM, Doran S, Hebbard GS, Rassias G, Sun WM, Horowitz M. Effect of glucose supplementation on appetite and the pyloric motor response to intraduodenal glucose and lipid. Am J Physiol Gastrointest Liver Physiol 274: G645–G652, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Braden B, Adams S, Duan LP, Orth KH, Maul FD, Lembcke B, Hor G, Caspary WF. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology 108: 1048–1055, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 55: 1484–1490, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Chew CG, Bartholomeusz FD, Bellon M, Chatterton BE. Simultaneous 13C/14C dual isotope breath test measurement of gastric emptying of solid and liquid in normal subjects and patients: comparison with scintigraphy. Nucl Med Rev Cent East Eur 6: 29–33, 2003. [PubMed] [Google Scholar]
- 8.Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, Pedraza M, Mondala H, Gao H, Bagnol D, Chen R, Jones RM, Behan DP, Leonard J. A role for intestinal endocrine cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology 149: 2038–2047, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850–853, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Deacon CF What do we know about the secretion and degradation of incretin hormones? Regul Pept 128: 117–124, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, Wright EM, Koepsell H. A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci USA 100: 11753–11758, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drucker DJ The role of gut hormones in glucose homeostasis. J Clin Invest 117: 24–32, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank GK, Oberndorfer TA, Simmons AN, Paulus MP, Fudge JL, Yang TT, Kaye WH. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage 39: 1559–1569, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Fushiki T, Kojima A, Imoto T, Inoue K, Sugimoto E. An extract of Gymnema sylvestre leaves and purified gymnemic acid inhibits glucose-stimulated gastric inhibitory peptide secretion in rats. J Nutr 122: 2367–2373, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Gentilcore D, Bryant B, Wishart JM, Morris HA, Horowitz M, Jones KL. Acarbose attenuates the hypotensive response to sucrose and slows gastric emptying in the elderly. Am J Med 118: 1289, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Gregersen S, Jeppesen PB, Holst JJ, Hermansen K. Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism 53: 73–76, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52: 1147–1154, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Grice HC, Goldsmith LA. Sucralose—an overview of the toxicity data. Food Chem Toxicol 38, Suppl 2: S1–S6, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR, Pi-Sunyer FX. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc 103: 1607–1612, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Heddle R, Dent J, Read NW, Houghton LA, Toouli J, Horowitz M, Maddern GJ, Downton J. Antropyloroduodenal motor responses to intraduodenal lipid infusion in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 254: G671–G679, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104: 15069–15074, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kieffer TJ, Huang Z, McIntosh CH, Buchan AM, Brown JC, Pederson RA. Gastric inhibitory polypeptide release from a tumor-derived cell line. Am J Physiol Endocrinol Metab 269: E316–E322, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Lavin JH, Wittert GA, Andrews J, Yeap B, Wishart JM, Morris HA, Morley JE, Horowitz M, Read NW. Interaction of insulin, glucagon-like peptide 1, gastric inhibitory polypeptide, and appetite in response to intraduodenal carbohydrate. Am J Clin Nutr 68: 591–598, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Lin HC, Doty JE, Reedy TJ, Meyer JH. Inhibition of gastric emptying by glucose depends on length of intestine exposed to nutrient. Am J Physiol Gastrointest Liver Physiol 256: G404–G411, 1989. [DOI] [PubMed] [Google Scholar]
- 25.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 582: 379–392, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacIntosh CG, Andrews JM, Jones KL, Wishart JM, Morris HA, Jansen JB, Morley JE, Horowitz M, Chapman IM. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide 1, and peptide YY and their relation to appetite and pyloric motility. Am J Clin Nutr 69: 999–1006, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Maes BD, Ghoos YF, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, Vantrappen G. Combined carbon-13-glycine/carbon-14-octanoic acid breath test to monitor gastric emptying rates of liquids and solids. J Nucl Med 35: 824–831, 1994. [PubMed] [Google Scholar]
- 28.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104: 15075–15080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mezitis NH, Maggio CA, Koch P, Quddoos A, Allison DB, Pi-Sunyer FX. Glycemic effect of a single high oral dose of the novel sweetener sucralose in patients with diabetes. Diabetes Care 19: 1004–1005, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91: 301–307, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nauck MA, Meier JJ. Glucagon-like peptide 1 and its derivatives in the treatment of diabetes. Regul Pept 128: 135–148, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell 106: 381–390, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol 15: 1948–1952, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Olsson C, Chen BN, Jones S, Chataway TK, Costa M, Brookes SJ. Comparison of extrinsic efferent innervation of guinea pig distal colon and rectum. J Comp Neurol 496: 787–801, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Pilichiewicz AN, Chaikomin R, Brennan IM, Wishart JM, Rayner CK, Jones KL, Smout AJ, Horowitz M, Feinle-Bisset C. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab 293: E743–E753, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Rayner CK, Park HS, Wishart JM, Kong M, Doran SM, Horowitz M. Effects of intraduodenal glucose and fructose on antropyloric motility and appetite in healthy humans. Am J Physiol Regul Integr Comp Physiol 278: R360–R366, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes 51: 2757–2763, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Ritzel U, Fromme A, Ottleben M, Leonhardt U, Ramadori G. Release of glucagon-like peptide-1 (GLP-1) by carbohydrates in the perfused rat ileum. Acta Diabetol 34: 18–21, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz-Avila L, Wong GT, Damak S, Margolskee RF. Dominant loss of responsiveness to sweet and bitter compounds caused by a single mutation in alpha-gustducin. Proc Natl Acad Sci USA 98: 8868–8873, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Goke B. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 55: 243–251, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shima K, Suda T, Nishimoto K, Yoshimoto S. Relationship between molecular structures of sugars and their ability to stimulate the release of glucagon-like peptide-1 from canine ileal loops. Acta Endocrinol 123: 464–470, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Shuster LT, Go VL, Rizza RA, O'Brien PC, Service FJ. Incretin effect due to increased secretion and decreased clearance of insulin in normal humans. Diabetes 37: 200–203, 1988. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland K, Brierley SM, Horowitz M, Rayner CK, Blackshaw LA, Young RL. Sweet taste transduction molecules are expressed in the upper gastrointestinal tract in humans (Abstract). Gastroenterology 132: A587, 2007. [Google Scholar]
- 44.Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA. Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol 292: G1420–G1428, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Wachters-Hagedoorn RE, Priebe MG, Heimweg JA, Heiner AM, Englyst KN, Holst JJ, Stellaard F, Vonk RJ. The rate of intestinal glucose absorption is correlated with plasma glucose-dependent insulinotropic polypeptide concentrations in healthy men. J Nutr 136: 1511–1516, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Welch I, Saunders K, Read NW. Effect of ileal and intravenous infusions of fat emulsions on feeding and satiety in human volunteers. Gastroenterology 89: 1293–1297, 1985. [DOI] [PubMed] [Google Scholar]
- 47.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003. [DOI] [PubMed] [Google Scholar]