Abstract
Phosphate homeostasis is critical for many physiological functions. Up to 85% of phosphate is stored in bone and teeth. The remaining 15% is distributed in cells. Phosphate absorption across the brush-border membrane (BBM) of enterocytes occurs mainly via a sodium-dependent pathway, which is mediated by type IIb sodium-phosphate cotransporters (NaPi-IIb). Patients of inflammatory bowel diseases (IBDs) suffer not only from diarrhea and nutrient malabsorption but also from bone loss. About 31–59% of patients with IBD develop bone disorders. Since the intestine is a primary location for dietary phosphate absorption, it is logical to postulate that there is an inverse relationship between gastrointestinal disorders and phosphate transport, which, in turn, contributes to bone disorders observed in patients with IBD. Phosphate absorption and NaPi-IIb expression was studied with BBM vesicles isolated from trinitrobenzene sulphonic acid (TNBS) animals as well as in Caco-2 cells. The mechanism of TNF-α downregulation of NaPi-IIb expression was investigated by luciferase assay, gel mobility shift assay (GMSA), and coimmunoprecipitation. Intestinal phosphate absorption mediated by NaPi-IIb was reduced both in TNBS colitis and in TNF-α-treated cells. Transient transfection indicated that TNF-α inhibits NaPi-IIb expression by reducing NaPi-IIb basal promoter activity. GMSAs identified NF1 protein as an important factor in TNF-α-mediated NaPi-IIb downregulation. Signaling transduction study and coimmunoprecipitation suggested that TNF-α interacts with EGF receptor to activate ERK1/2 pathway. Intestinal phosphate absorption mediated by NaPi-IIb protein is reduced in colitis. This inhibition is mediated by the proinflammatory cytokine TNF-α through a novel molecular mechanism involving TNF-α/EGF receptor interaction.
Keywords: trinitrobenzene sulphonic acid colitis, Caco-2 cells, type IIb sodium-phosphate cotransporters, EGF receptor
inflammatory bowel diseases (IBDs) are one of the most prevalent gastrointestinal disorders in the United States with its treatment costs of more than $1.7 billion (18). The two major categories of IBD are Crohn's disease and ulcerative colitis. Bone loss is a common outcome in patients of both subtypes of IBD (5, 13). The observed bone loss in patients with IBD is from either osteopenia or osteoporosis. It is estimated that 31–59% of adult patients with IBD are classified as osteopenic, whereas 18–24% are diagnosed with osteoporosis (1, 4, 13). The pathogenesis of IBDs is not fully understood, but the cytokine profiles of the patients hint at the possibility of cytokines playing a prominent role since these patients have an imbalance of proinflammatory cytokines (12, 26). Patients with IBD have increased levels in number of cytokines including IL-1, IL-6, interferon (IFN-γ), and tumor necrosis factor-α (TNF-α) (17). TNF-α has been named the mastermind of the inflammatory response since treatment of anti-TNF-α antibodies controls the progression of IBD and also increases bone mineral density (6, 16).
Phosphate is an important element for the body because it is essential for ATP synthesis, acid/base regulation, and the formation of nucleotides. Most importantly, it is a key component of bone. In fact, the bone matrix stores 85% of the body phosphate. Mammalian phosphate homeostasis is tightly regulated by controlling intestinal and renal epithelial transport mechanisms. In the renal proximal tubules, phosphate reabsorption is mediated by type IIa sodium-phosphate cotransporters (NaPi-IIa). Intestinal absorption of dietary phosphate is mediated by another subtype of type II NaPi cotransporters expressed on the apical membranes of enterocytes named NaPi-IIb (14, 19, 37). Enterocytes also express another NaPi cotransport protein on the basolateral membranes called NaPi-III, and this isoform most likely plays a role in cellular phosphate homeostasis (3, 9). For intestinal phosphate absorption, however, NaPi-IIb is the most important of these cotransporters to study. Phosphate transport in the intestine has already been shown to be regulated by age (2, 36, 38), vitamin D3 (8, 11, 36), and hormones such as glucocorticoid (2) and estrogen (42). Since metabolic bone diseases like osteopenia and osteoporosis are associated with IBD, it is necessary to understand the molecular mechanisms of aberrant phosphate homeostasis in patients with IBD. Although TNF-α has been found to affect bone density by inducing osteoclasts to erode the bone and by inhibiting osteoblasts to lay new bone matrix (35), how the state of inflammation affects the intestinal phosphate absorption is unknown. Because of the fact that phosphate absorption is segment specific (ileum in mouse and jejuna in rat), we chose both of the animals as our in vivo model in our present studies. We aim to study the effects of inflammation on intestinal phosphate transport and ultimately bone health.
MATERIALS AND METHODS
Animals.
Six-week-old male Balb/C mice or 3-wk-old male Sprague Dawley rats were administered trinitrobenzene sulphonic acid (TNBS) in 50% ethanol (2 mg/mouse or 1 mg/rat) by an enema into the colonic lumen. Six days after TNBS administration, animals were euthanized, and the small intestinal mucosa was harvested for brush-border membrane vesicle (BBMV) isolation and RNA purification. All animal works have been approved by the University of Arizona Institutional Animal Care and Use Committee. All experiments were repeated at least three times with different groups of animals (3–4 animals per group).
Cell culture.
Human intestinal epithelial (Caco-2) cells were purchased from American Type Culture Collection (ATCC, Manassas, VA) and cultured according to ATCC guidelines. In some experiments, cells were incubated with human recombinant TNF-α (Peprotech, Rock Hill, NJ) for 40 h.
RNA purification and PCR analysis to detect NaPi-IIb expression.
RNA was purified from Caco-2 cells or small intestinal mucosa. Real-time PCR was performed to detect NaPi-IIb expression. TATA binding protein (TBP) expression was used as an internal control to calculate the expression levels of NaPi-IIb. The primers used to detect NaPi-IIb and TBP were purchased from Applied Biosystems (Foster City, CA). Resulting data were analyzed by the comparative cycle threshold (Ct) method as a means of relative quantitation of gene expression, normalized to an endogenous reference (TBP) and relative to a calibrator (normalized Ct value obtained from control groups) and expressed as 2−Ct (Applied Biosystems User Bulletin no. 2: Rev B “Relative Quantitation of Gene Expression”).
Phosphate uptake analysis in BBMV protein and in Caco-2 cells.
Phosphate uptake with BBMV protein and in Caco-2 cells were performed as previously described methods (7, 40). The contribution of sodium-dependent uptake was calculated by subtracting the sodium-independent uptake values observed in the absence of sodium from the uptake values in the presence of sodium. The experiments were repeated in three to four different groups of animals or cells.
Protein purification and Western blot analysis.
BBMVs were prepared from intestinal mucosa as previously described (36). Total crude membrane protein was isolated from Caco-2 cells with RIPA buffer method (10). A 1:4,000 dilution of mouse NaPi-IIb antibody (42) or 1:1,000 dilution of human NaPi-IIb antibody (Alpha Diagnostic International, San Antonio, TX) was used to detect NaPi-IIb protein. A 1:5,000 dilution of the β-actin antiserum (Sigma Aldrich, St. Louis, MO) was used to detect β-actin protein. Western detection was performed with the BM Chemiluminescence Western Blotting Kit (Roche Diagnostics, Basel, Switzerland). For protein expression quantitation, a ratio of NaPi-IIb protein intensity over β-actin protein intensity was used. Western blotting experiments were done with proteins isolated from three different groups of animals or Caco-2 cells.
Transient transfection and functional promoter analysis.
Caco-2 cells were transfected with human NaPi-IIb (hNaPi-IIb) promoter constructs and control plasmids by Effectene-mediated transfection method (Qiagen, Valencia, CA). Promoter reporter assays were performed using the dual luciferase Assay kit (Promega, Madison, WI). Luciferase activities were measured with a luminometer (Femtomaster FB 12; Berthold Detection System, Pforzheim, Germany). Renilla luciferase activity driven by pRL-CMV (Promega) was used as an internal control to calculate the relative luciferase activity. For TNF-α treatment, 20 ng/ml human recombinant TNF-α was used. To determine the involvement of EGF receptor (EGFR) pathways, cells were treated with monoclonal anti-EGFR antibodies (50 ng/ml), AG1478 (1 μM, a specific receptor tyrosine kinase inhibitor), H7 (10 μM, an inhibitor of PKC), or PD98059 (25 μM, an inhibitor of ERK/MAPK) 2 h before TNF-α treatment. Anti-EGFR mouse monoclonal antibody was purchased from Calbiochem (La Jolla, CA), and inhibitors were purchased from Sigma-Aldrich.
Preparation of nuclear extracts for GMSA.
Nuclear extracts were prepared from Caco-2 cells and gel mobility shift assays (GMSAs) were performed by a previously described method (41). Synthetic DNA oligonucleotides covering NaPi-IIb promoter region −37 bp to −13 bp were end labeled with [-32P]ATP, and 5 μg of nuclear extract was incubated with 1 ng of labeled probe in GMSA binding buffer [10 mM HEPES, pH 7.5, 1 mM EDTA, 50 mM NaCl, 1 mM dithiothreitol, and 50 μg/ml poly(dI-dC)]. After incubation at room temperature for 20–30 min, the mixture was electrophoresed on a 6% polyacrylamide gel. For competition experiments, 100- to 500-fold molar excess of unlabeled oligos was added to the reaction mixture before adding labeled oligo probes. For supershift assays, 4 μg of anti-human NF1 antibody, rabbit IgG, or anti-human ELK antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was added to the reaction mixtures. The resulting products were separated on 6% polyacrylamide gel and exposed to X-ray film.
Coimmunoprecipitation.
Caco-2 cells were cultured in 100-mm plates and treated with normal or TNF-α-containing medium. Cells were then lysed in 0.5 ml RIPA buffer. Coimmunoprecipitation was performed according the protocol provided by the antibody manufacturer. Anti-EGFR mouse monoclonal antibody (Calbiochem) or anti-TNF-α goat antibody (Santa Cruz Biotechnology, Santa Cruz, CA) were used for coimmunoprecipitation. Rabbit anti-EGFR antibody and anti-TNF-α antibody (Santa Cruz Biotechnology) were used for Western detection.
Statistical analysis.
ANOVA post hoc tests (StatView 5.0.1; SAS Institute, Cary, NC) were used to compare values of the experimental data. P values <0.05 were considered significant.
RESULTS
Effect of TNBS colitis on phosphate absorption and NaPi-IIb expression in mouse small intestine.
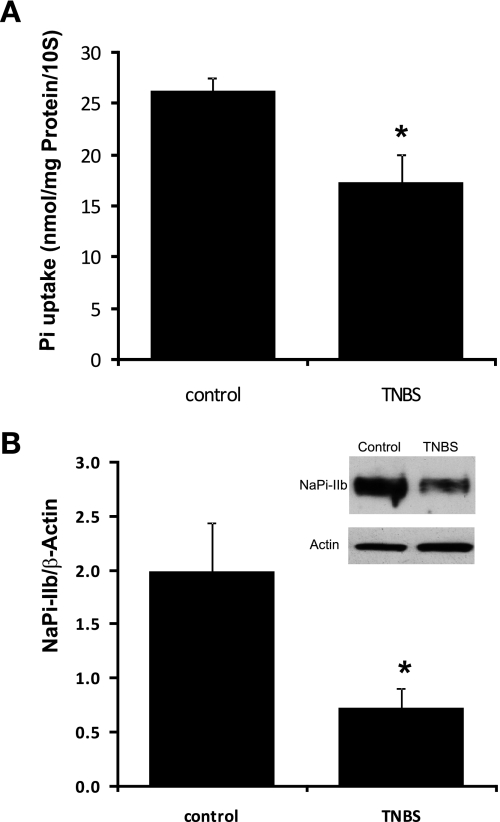
Male mice received TNBS (2 mg/mouse in 50% ethanol) or PBS buffer in a total volume of 100 μl by an enema into the colonic lumen. Six days after TNBS administration mice were killed, ileal mucosa was harvested and used for BBMV purification. Phosphate uptake and Western blot were then performed with purified BBMV proteins. Our data showed that TNBS administration significantly reduced intestinal sodium-dependent phosphate absorption in mice, from 26.3 ± 1.2 nmol/mg protein per 10 s in control mice to 17.3 ± 2.8 nmol/mg protein per 10 s in TNBS mice (n = 3; P = 0.028) (Fig. 1A). Western blot with BBMV protein showed that TNBS administration also decreased NaPi-IIb immunoreactive protein abundance (indicated by the ratio of optical densities of the NaPi-IIb band to that of the β-actin band) from 1.97 ± 0.45 in control mice to 0.73 ± 0.18 in TNBS mice (n = 3; P = 0.017) (Fig. 1B).
Fig. 1.
Effect of inflammation on intestinal phosphate absorption and type IIb sodium-phosphate cotransporter (NaPi-IIb) expression in trinitrobenzene sulfonic acid (TNBS) colitis mice. Six-week-old male mice were treated with TNBS (2 mg/mouse) and were harvested 6 days after TNBS administration. A: brush-border membrane (BBM) phosphate uptake in TNBS colitis mice. BBM vesicles (BBMVs) were isolated from the ileal mucosa of mice treated with PBS or TNBS, and BBM phosphate uptake was performed. The contribution of sodium-dependent uptake was calculated by subtracting the sodium-independent uptake values observed in the absence of sodium from the uptake values in the presence of sodium. Data presented are means ± SE in 3 or 4 different groups of animals. *P ≤ 0.028 for TNBS mice vs. control mice. B: NaPi-IIb expression in TNBS colitis mice. BBMVs were isolated from the ileal mucosa of mice treated with PBS or TNBS and used for Western detection. A 1:4,000 dilution of the mouse NaPi-IIb antibody was used. The expression of NaPi-IIb protein is calculated by the density of NaPi-IIb band over that of β-actin band. Bar chart shows NaPi-IIb protein expression indicated as means ± SE in the sum of 3 independent experiments. *P ≤ 0.017 for control groups vs. TNBS groups. Inset: typical immunoblot image.
Effect of TNBS colitis on NaPi-IIb expression in rat intestine.
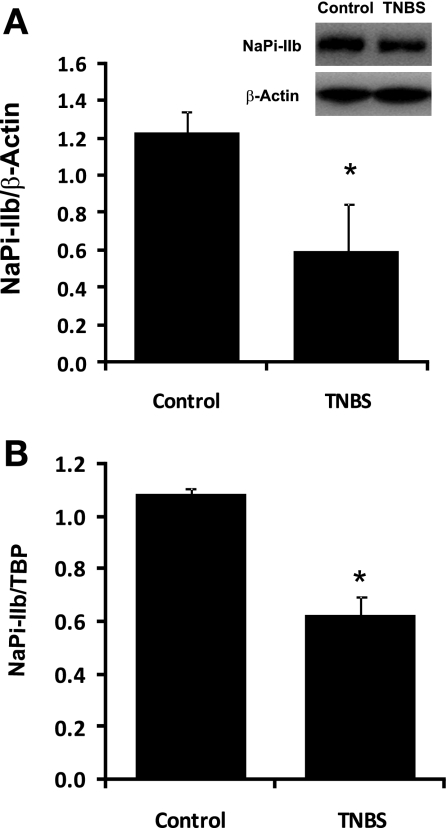
Male rats received TNBS (1 mg/rat in 50% ethanol) or PBS buffer in a total volume of 250 μl by an enema into the colonic lumen. Six days after TNBS administration, rats were killed and jejunal mucosa was harvested for BBMV and RNA purification. Western blot was used to determine NaPi-IIb protein abundance from BBMV protein. Real-time PCR was used to determine NaPi-IIb mRNA expression. Western blot results showed that NaPi-IIb immunoreactive protein abundance was reduced by ∼53% in TNBS colitis rats, from 1.23 ± 0.11 in control rats to 0.59 ± 0.26 in TNBS rats (n = 3; P = 0.04) (Fig. 2A). Real-time PCR data indicated that the expression of NaPi-IIb mRNA was decreased by ∼42% in TNBS colitis rats compared with control rats, from 1.09 ± 0.02 in control rats to 0.63 ± 0.07 in TNBS rats (n = 3; P = 0.004) (Fig. 2B).
Fig. 2.
Effect of inflammation on NaPi-IIb expression in TNBS colitis rats. Three-week-old rats were treated with TNBS (1 mg/rat) and were harvested 6 days after TNBS administration. A: BBMVs were isolated from the jejunal mucosa of rats treated with PBS or TNBS and used for Western detection. A 1:4,000 dilution of the mouse NaPi-IIb antibody was used. The expression of NaPi-IIb protein is calculated by the density of NaPi-IIb band over that of β-actin band. Bar chart shows NaPi-IIb protein expression indicated as means ± SE in the sum of 3 independent experiments. *P ≤ 0.04 for control groups vs. TNBS groups. Inset: a typical immunoblot image. B: RNAs were isolated from the jejunal mucosa of control or TNBS rats and used for real-time PCR. NaPi-IIb mRNA and TATA binding protein (TBP) mRNA were amplified with rat-specific NaPi-IIb and TBP primers. The changes in NaPi-IIb gene expression are analyzed by the comparative cycle threshold (Ct) method. Data are means ± SE from total 18 rats (9 for TNBS group, 9 for control group). *P ≤ 0.004 for control group vs. TNBS group.
Effect of TNF-α on phosphate uptake and NaPi-IIb expression in Caco-2 cells.
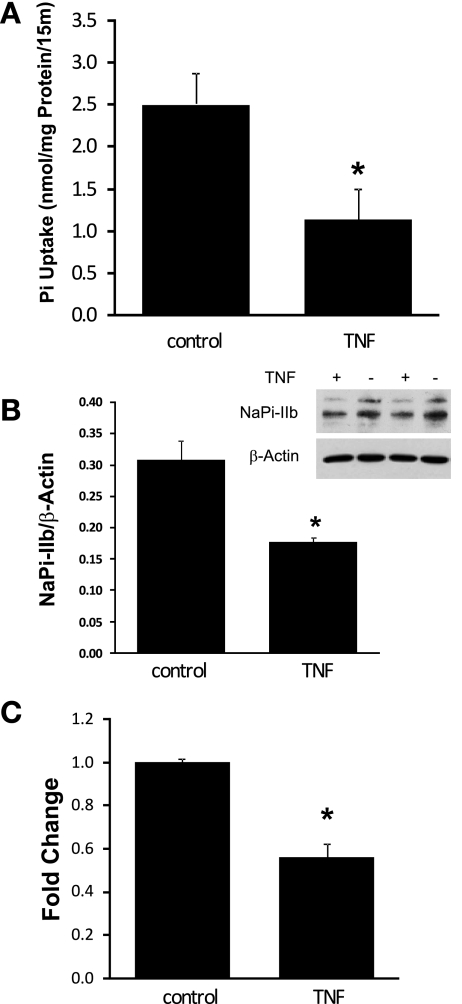
The endogenous NaPi-IIb expression in Caco-2 cells has been shown in our previous work (38). To study the effect of TNF-α on cellular phosphate absorption, Caco-2 cells were grown in 24-well plates and treated with normal or TNF-α-containing medium (20 ng/ml) for 40 h. Cellular phosphate uptake assays were performed to measure the rate of phosphate uptake in Caco-2 cells. Western blot detection was used to assess NaPi-IIb protein levels in Caco-2 cells. Real-time PCR was conducted to determine the expression of NaPi-IIb mRNA in Caco-2 cells. As shown in Fig. 3, sodium-dependent phosphate absorption was significantly decreased in Caco-2 cells after TNF-α treatment, from 2.51 ± 0.27 nmol/mg protein per 15 min in control cells to 1.14 ± 0.37 nmol/mg protein per 15 min in TNF-α-treated cells (n = 3; P = 0.013) (Fig. 3A). Western blot detection using human NaPi-IIb antibody indicated that NaPi-IIb immunoreactive protein abundance was reduced from 0.31 ± 0.03 in control cells to 0.18 ± 0.01 in TNF-α-treated cells (n = 3; P = 0.006) (Fig. 3B). Real-time PCR using human NaPi-IIb and TBP primers showed that TNF-α treatment inhibited NaPi-IIb gene expression by ∼44%, from 1.002 ± 0.016 in control cells to 0.56 ± 0.065 in TNF-α-treated cells (n = 4; P < 0.001) (Fig. 3C).
Fig. 3.
Effect of TNF-α on phosphate absorption and NaPi-IIb expression in Caco-2 cells. A: phosphate uptake in Caco-2 cells. Cellular phosphate uptake was performed from control and TNF-α-treated (20 ng/ml, 40 h) cells. The contribution of sodium-dependent uptake was calculated by subtracting the sodium-independent uptake values observed in the absence of sodium from the uptake values in the presence of sodium. Data presented are means ± SE from 3 separate experiments. *P ≤ 0.013 for TNF-α treatment vs. control. B: NaPi-IIb protein expression in Caco-2 cells. Crude membrane protein was prepared from control and TNF-α-treated (20 ng/ml, 40 h) cells. Western blot was performed, and human NaPi-IIb protein was detected by an anti-human sodium-phosphate cotransporter antibody. Data are means ± SE from 3 separate experiments. *P ≤ 0.006 for TNF-α treatment vs. control. Inset: typical immunoblot image. C: NaPi-IIb mRNA expression in Caco-2 cells. RNA was isolated from control and TNF-α-treated (20 ng/ml, 40 h) cells and used for first-strand cDNA synthesis. Real-time PCR was performed with human NaPi-IIb or TBP primers in separate reactions. Results are means ± SE from 4 separate experiments. *P ≤ 0.001 for control vs. TNF-α treatment.
Effect of TNF-α treatment on hNaPi-IIb gene promoter activity.
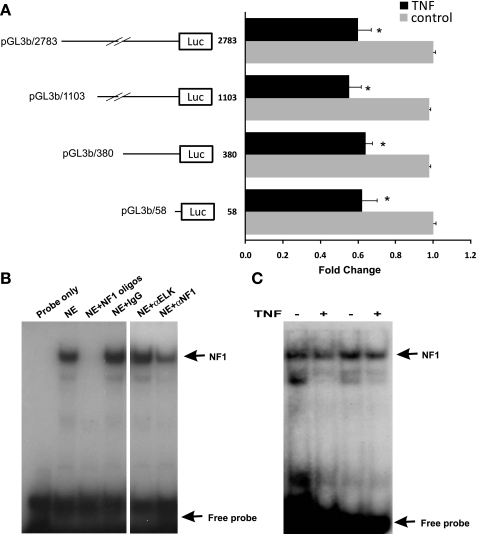
To explore whether the inhibition of TNF-α on human NaPi-IIb (hNaPi-IIb) gene expression is mediated by transcriptional mechanism, hNaPi-IIb promoter activity assays were conducted. Caco-2 cells were first transfected with hNaPi-IIb promoter constructs and then treated with TNF-α (20 ng/ml, 40 h) before analyzing promoter activity. As shown in Fig. 4A, hNaPi-IIb promoter activity was significantly decreased in TNF-α-treated Caco-2 cells (n = 7; P < 0.01). To locate the TNF-α responsive region, series of 5′ deletion constructs within the 2783 bp of the hNHE8 gene promoter region were used. Promoter reporter gene assays showed that promoter constructs pGL3b/2783, pGL3b/1103, pGL3b/380, and pGL3b/58 were all responsive to TNF-α treatment, suggesting that the TNF-α response element is located in the minimal promoter region of the hNaPi-IIb gene.
Fig. 4.
TNF-α response region on human NaPi-IIb gene promoter. A: Caco-2 cells were cotransfected with human NaPi-IIb promoter constructs (pGL3/-2783, pGL3/-1103, pGL3/-380, pGL3/-58) and pRL-CMV. TNF-α (20 ng/ml) was applied 40 h before measuring promoter activities. The degree of inhibition is shown as the ratio of luciferase activity in TNF-α-treated cells over luciferase activity in vehicle-treated cells. Results are means ± SE from 6 separate experiments. *P < 0.01 for control vs. TNF-α treatment. B: identification of nuclear protein bound on promoter region (−37 bp/−13 bp) by gel mobility shift assays (GMSAs). A 32P-labeled double-stranded oligonucleotide probe covering the proximal promoter region (−37 bp/−13 bp) was incubated with 5 μg of Caco-2 cell nuclear extract in the presence or absence of unlabeled 100 × excess NF1 consensus oligos (NF1 oligos), 4 μg rabbit IgG or anti-Elk antibody (αELK) or anti-NF1 antibody (αNF1). Image is representative of 3 independent experiments. C: identification of DNA region involving TNF-α regulation. Nuclear proteins were isolated from Caco-2 cells treated with normal medium or TNF-α medium. GMSAs were performed with DNA probe covering the basal promoter region (−37 bp/−13 bp). Results shown are representative of 4 separate experiments.
GMSA identification of DNA sequences involved in the TNF-α response of the hNaPi-IIb promoter.
Previous studies have identified that NF1 transcriptional factor is involved in activating hNaPi-IIb gene expression in Caco-2 cells (41). To identify whether the basal promoter activation region is also involved in the TNF-α response, DNA oligos homologous to the hNaPi-IIb promoter region from −37 bp to −13 bp were used as the probe for GMSAs. Nuclear protein was isolated from Caco-2 cells treated with normal or TNF-α-containing medium. As shown in Fig. 4, a strong DNA-protein interaction was detected with radiolabeled oligos probes, which could be inhibited in the presence of NF1 consensus oligos (TTTGGATTGAAGCCAATATGATAA; underline indicates core sequences for NF1 protein binding). NF1 antiserum (a blocking antibody that recognizes all NF1 family members) blocked the DNA-protein interaction, whereas control IgG or unrelated supershift antibody (Elk antibody) had no effect on the DNA-protein interaction (Fig. 4B). Furthermore, TNF-α treatment reduced NF1 binding at the hNaPi-IIb basal promoter region (Fig. 4C).
The inhibition of hNaPi-IIb expression by TNF-α is mediated through EGFR signaling transduction pathway.
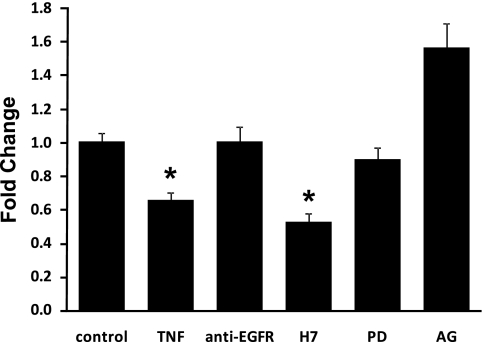
To further elucidate the signaling pathways involved in TNF-α-mediated hNaPi-IIb gene expression downregulation, various inhibitors were selected in our study. Caco-2 cells were transfected with promoter construct pGL3/−58 and pretreated with various inhibitors for 2 h before TNF-α was added. As shown in Fig. 5, hNaPi-IIb gene promoter activity was reduced 40% by TNF-α in Caco-2 cells. Administration of 50 nM mouse monoclonal anti-EGFR antibody blocked the response of hNaPi-IIb promoter to TNF-α. AG1478 (1 μM), a specific inhibitor of EGFR tyrosine kinase activity, also abolished the TNF-α effect on the hNaPi-IIb promoter activity. Furthermore, administration of ERK1/2 inhibitor PD098059 (25 μM) fully restored the hNaPi-IIb promoter activity, whereas administration of PKC inhibitor H7 (10 μM) had no effect on restoring hNaPi-IIb promoter activity. The inhibitors themselves used in this study had no effect on the hNaPi-IIb promoter activity.
Fig. 5.
Signaling pathways of TNF-α effect on NaPi-IIb expression. Caco-2 cells were cotransfected with human NaPi-IIb promoter construct (pGL3/−58) and pRL-CMV. Various inhibitors were applied 2 h before TNF-α treatment (20 ng/ml, 40 h). The degree of inhibition is shown as the ratio of luciferase activity in TNF-α-treated cells over luciferase activity in vehicle-treated cells. Results are means ± SE from 6 separate experiments. *P ≤ 0.01 for control vs. TNF-α treatment. EGFR, EGF receptor. PD, PD098059; AG, AG1478.
Complex formation between TNF-α and EGFR in Caco-2 cells.
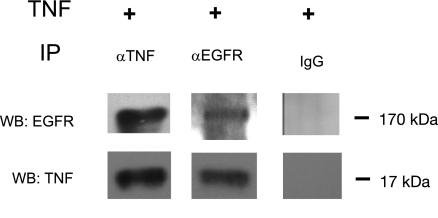
Since EGFR activation pathway is involved in the regulation of TNF-α on hNaPi-IIb expression, we thought to explore the interaction between TNF-α and EGFR in Caco-2 cells. To assess the physical interaction between TNF-α and EGFR, coimmunoprecipitation was performed. Coimmunoprecipitation results indicated that TNF-α/EGFR complexes were formed after TNF-α treatment in Caco-2 cells. TNF-α was detected by Western blot from samples coimmunoprecipitated with a mouse monoclonal anti-EGFR antibody, and EGFR was detected by Western blot from samples coimmunoprecipitated with a goat anti-TNF-α antibody. As control experiments, neither EGFR nor TNF-α could be detected from samples coimmunoprecipitated with mouse or goat IgG (Fig. 6).
Fig. 6.
TNF-α/EGFR complex detection. Caco-2 cells were treated with normal or TNF-α (20 ng/ml) medium for 40 h before harvest. Coimmunoprecipitation (IP) was performed with a mouse monoclonal anti-EGFR antibody or a goat anti-TNF-α antibody or regular IgG from goat or mouse. The resulting product was separated on 4–20% PAGE gels and detected with a rabbit polyclonal anti-EGFR antibody or a mouse monoclonal anti-TNF-α antibody. Results shown are representative of 3 separate experiments. WB, western blot detection.
DISCUSSION
Phosphate homeostasis is critical for many physiological functions. In bone and teeth, calcium apatite [Ca10(PO4)6(OH)2] serves as the inorganic filling in the organic network of collagen. Up to 85% of phosphate is stored in bone and teeth; the remaining 15% is distributed in cells as a key component for nucleic acids (DNA and RNA), energy molecules (e.g., ATP), and metabolic mediators. Phosphate absorption across the BBM of enterocytes occurs mainly via the sodium-dependent pathway, which is mediated by NaPi-IIb (14, 19, 37). Under normal physiological conditions, NaPi-IIb transporter is regulated by a number of factors, including dietary phosphate intake, vitamin D, estrogen, EGF, and glucocorticoids (2, 8, 27, 30, 36, 39, 42). However, essentially nothing is known regarding the regulation of phosphate transport in inflammatory pathophysiological conditions.
For patients of IBDs, decreased bone density is a common outcome of their disease (4). How IBD results in bone loss is not completely understood since many factors, like disease state, calcium and vitamin D3 deficiency, glucocorticoid treatment, estrogen levels, and overall nutrition, could result in unhealthy bones. Humans with inflammatory processes such as IBD result in elevated proinflammatory cytokine levels including IL-1β, TNF-α, and IL-6 (20, 24, 25, 31, 33). In TNBS colitis animal model, the levels of proinflammatory cytokines such as TNF-α, IFN-γ, and IL-1β are also increased (23). In particular, TNF-α is a key cytokine responsible for many of the symptoms of IBD, and anti-TNF-α antibodies reduce the severity of established colitis (29, 34). To explore whether intestinal phosphate absorption is impaired in IBDs, we used TNBS mouse and TNBS rat as our in vivo colitis models. The reason for utilizing the mouse and rat relates to the observations that phosphate absorption occurs in the ileum in the mouse, whereas the jejunum is the site of phosphate absorption in the rat. We found that the sodium-dependent phosphate absorption in the ileum was significantly reduced in mouse colitis. The reduction in intestinal phosphate absorption is correlated with the decrease of the intestinal NaPi-IIb expression. The similar results were also seen in colitis rats. These observations suggest that the intestinal phosphate absorption is impaired in colitis, and the involved protein is most likely the intestinal sodium-phosphate cotransporter (NaPi-IIb).
Since TNF-α is the main culprit in pathogenesis of colitis, we tested whether TNF-α is the main player in the reduction of the intestinal phosphate absorption in colitis. We treated Caco-2 cells with TNF-α (20 ng/ml for 40 h) and analyzed phosphate absorption and NaPi-IIb gene expression in these cells. Our data showed that TNF-α treatment not only reduced the phosphate transport rate in Caco-2 cells, but also reduced NaPi-IIb protein and mRNA expression. All these reductions in phosphate absorption and NaPi-IIb expression in Caco-2 cells are similar to what was observed in TNBS colitis animals. These results suggest that TNF-α is indeed an important factor that contributes to the abnormal phosphate metabolism in patients with IBD.
To understand the mechanism of TNF-α regulation on intestinal NaPi-IIb expression, we transfected Caco-2 cells with hNaPi-IIb promoter constructs and exposed these cells to TNF-α. Our results showed that TNF-α treatment (20 ng/ml, 40 h) reduced hNaPi-IIb gene promoter activity by ∼40%, a level that agrees with the observed NaPi-IIb mRNA reduction. This observation suggests that a transcriptional inhibition mechanism is likely involved in TNF-α-mediated NaPi-IIb downregulation. Further transfection with shortened hNaPi-IIb gene promoter constructs (pGL3b/2783, pGL3b/1103, pGL3b/380, pGL3b/58) showed that even the shortest promoter constructs were responsive to TNF-α treatment, suggesting that the TNF-α response region is located at the minimal promoter region of the hNaPi-IIb gene. GMSAs indicated that the DNA-protein interaction at the basal promoter activation region of the hNaPi-IIb gene was reduced by TNF-α treatment, and the nuclear factor involved in this interaction is the NF1 protein.
Our earlier study showed that EGF could inhibit NaPi-IIb expression in rat intestine and in Caco-2 cells (38, 39). Therefore, we considered the possibility of TNF-α utilizing EGFR activation pathway to downregulate NaPi-IIb gene expression. Caco-2 cells were transfected with hNaPi-IIb promoter construct pGL3b/58 and were treated with various inhibitors 2 h before 40 h of TNF-α treatment. Administration of PKC inhibitor H7 (10 μM) had no effect on restoring hNaPi-IIb promoter activity, suggesting that PKC pathway is not involved in TNF-α-mediated NaPi-IIb downregulation. Pretreatment with ERK1/2 inhibitor PD098059 (25 μM) completely restored the hNaPi-IIb promoter activity, implying the involvement of MAPK activation by TNF-α. Our data also showed that a specific EGFR tyrosine kinase inhibitor, AG 1478 (1 μM), blocked the inhibitory effect of TNF-α on NaPi-IIb promoter activity, which suggests the involvement of EGFR tyrosine kinase activation after TNF-α administration. Furthermore, a mouse monoclonal anti-EGFR antibody abolished the effect of TNF-α on NaPi-IIb promoter activity, indicating the participation of EGFR in this TNF-α-mediated NaPi-IIb downregulation. All these observations imply that a ligand-mediated EGFR activation is required in TNF-α-induced NaPi-IIb expression downregulation.
TNF-α has been shown to transactivate EGFR in human pancreatic cancer cells (32). In Caco-2 cells, TNF-α was also found to activate EGFR by nonligand-mediated means (28). Furthermore, EGFR transactivation by TNF-α happens as a result of TNF-α converting enzyme cleaving the membrane-bound form of the cytokine (15). Our data indicated that TNF-α affects NaPi-IIb expression through EGFR-MAPK cascade in Caco-2 cells, and this effect requires ligand binding-induced EGFR activation. The requirement of both EGFR activation and the following MAPK activation indicates that TNF-α-mediated NaPi-IIb gene regulation is unlikely a result of the crosstalk between the TNFR pathway and the EGFR pathway (21, 22) because EGFR activation is not required for MAPK activation in this case. Moreover, TNF-α effect on NaPi-IIb gene expression requires prolonged treatment time (40 h). This excludes the possibility of EGFR transactivation since EGFR transactivation by TNF-α is usually transient (28) and requires TNF-α converting enzyme-cleaved membrane-bound TNF-α (15).
To test the possibility of a direct interaction between TNF-α and EGFR, we performed coimmunoprecipitation with different antibodies and followed by Western blot detection. Immunoprecipitation with a mouse monoclonal anti-EGFR antibody resulted in the detection of the soluble form of TNF-α at 17 kDa by Western blot with the use of a rabbit anti-TNF-α antibody, whereas immunoprecipitation with a goat anti-TNF-α antibody resulted in the detection of the EGFR at ∼170 kDa by Western blot with the use of a rabbit anti-EGFR antibody. These observations suggest that there might be a direct interaction between soluble TNF-α and EGFR in TNF-α-mediated NaPi-IIb gene regulation in Caco-2 cells. Further studies will need to be conducted to identify the detail interaction between TNF-α and EGFR in regulating NaPi-IIb expression in Caco-2 cells.
In conclusion, we have shown that the intestinal phosphate absorption is decreased in TNBS colitis through reduced NaPi-IIb expression, and proinflammatory cytokine TNF-α is a main player in this regulation. TNF-α-mediated NaPi-IIb expression inhibition involves a novel pathway that requires direct TNF-α/EGFR interaction and EGFR/MAPK activation. With TNF-α considered the main perpetrator of inflammation in numerous inflammatory diseases and the fact that EGFR is expressed prominently in epithelial cells, the existence of this kind of interaction would significantly further our understanding of the pathogenesis and consequences of inflammatory disorders ranging from IBD to rheumatoid arthritis.
GRANTS
This study was supported by NIH Grant R01-DK033209 to F. K. Ghishan and by AGA Student Research Fellowship (2006 and 2007) to H. Chen.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, Chen S, Zehnder D, Lin YC, Yang H, Hewison M, Adams JS. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn's disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut 53: 1129–1136, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arima K, Hines ER, Kiela PR, Drees JB, Collins JF, Ghishan FK. Glucocorticoid regulation and glycosylation of mouse intestinal type IIb Na-P(i) cotransporter during ontogeny. Am J Physiol Gastrointest Liver Physiol 283: G426–G434, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bai L, Collins JF, Ghishan FK. Cloning and characterization of a type III Na-dependent phosphate cotransporter from mouse intestine. Am J Physiol Cell Physiol 279: C1135–C1143, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein CN, Leslie WD. Osteoporosis and inflammatory bowel disease (Review). Aliment Pharmacol Ther 19: 941–952, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein CN, Leslie WD. Therapy insight: osteoporosis in inflammatory bowel disease—advances and retreats. Nat Clin Pract Gastroenterol Hepatol 2: 232–239, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein M, Irwin S, Greenberg GR. Maintenance infliximab treatment is associated with improved bone mineral density in Crohn's disease. Am J Gastroenterol 100: 2031–2035, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Borowitz SM, Ghishan FK. Maturation of jejunal phosphate transport by rat brush border membrane vesicles. Pediatr Res 19: 1308–1312, 1985. [DOI] [PubMed] [Google Scholar]
- 8.Capuano P, Radanovic T, Wagner CA, Bacic D, Kato S, Uchiyama Y, St-Arnoud R, Murer H, Biber J. Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1αOHase-deficient mice. Am J Physiol Cell Physiol 288: C429–C434, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Collins JF, Bai L, Ghishan FK. The SLC20 family of proteins: dual functions as sodium-phosphate cotransporters and viral receptors. Pflügers Arch 447: 647–652, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Cell Physiol 273: C1937–C1946, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Cross HS, Debiec H, Peterlik M. Mechanism and regulation of intestinal phosphate absorption. Miner Electrolyte Metab 16: 115–124, 1990. [PubMed] [Google Scholar]
- 12.Dionne S, Hiscott J, D'Agata I, Duhaime A, Seidman EG. Quantitative PCR analysis of TNF-alpha and IL-1 beta mRNA levels in pediatric IBD mucosal biopsies. Dig Dis Sci 42: 1557–1566, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Dresner-Pollak R, Gelb N, Rachmilewitz D, Karmeli F, Weinreb M. Interleukin 10-deficient mice develop osteopenia, decreased bone formation, and mechanical fragility of long bones. Gastroenterology 127: 792–801, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Feild JA, Zhang L, Brun KA, Brooks DP, Edwards RM. Cloning and functional characterization of a sodium-dependent phosphate transporter expressed in human lung and small intestine. Biochem Biophys Res Commun 258: 578–582, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans 31: 1203–1208, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Franchimont D, Vermeire S, El Housni H, Pierik M, Van Steen K, Gustot T, Quertinmont E, Abramowicz M, Van Gossum A, Deviere J, Rutgeerts P. Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn's disease and ulcerative colitis. Gut 53: 987–992, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanauer SB Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 12, Suppl 1: S3–S9, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Heyman MB, Kirschner BS, Gold BD, Ferry G, Baldassano R, Cohen SA, Winter HS, Fain P, King C, Smith T, El-Serag HB. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr 146: 35–40, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc Natl Acad Sci USA 95: 14564–14569, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito R, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Kita M, Ueda Y, Iwakura Y, Kataoka K, Okanoue T, Mazda O. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol 146: 330–338, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser GC, Yan F, Polk DB. Conversion of TNF alpha from antiproliferative to proliferative ligand in mouse intestinal epithelial cells by regulating mitogen-activated protein kinase. Exp Cell Res 249: 349–358, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser GC, Yan F, Polk DB. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor kappaB activation in mouse colonocytes. Gastroenterology 116: 602–609, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamrani A, Tulliez M, Chauvelot-Moachon L, Chaussade S, Mauprivez C, Hagnere AM, Vidon N. Effects of octreotide treatment on early TNF-alpha production and localization in experimental chronic colitis. Aliment Pharmacol Ther 13: 583–594, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Latinne D, Fiasse R. New insights into the cellular immunology of the intestine in relation to the pathophysiology of inflammatory bowel diseases. Acta Gastroenterol Belg 69: 393–405, 2006. [PubMed] [Google Scholar]
- 25.Ludwiczek O, Kaser A, Novick D, Dinarello CA, Rubinstein M, Tilg H. Elevated systemic levels of free interleukin-18 (IL-18) in patients with Crohn's disease. Eur Cytokine Netw 16: 27–33, 2005. [PubMed] [Google Scholar]
- 26.MacRae VE, Wong SC, Farquharson C, Ahmed SF. Cytokine actions in growth disorders associated with pediatric chronic inflammatory diseases (Review). Int J Mol Med 18: 1011–1018, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Marks J, Srai SK, Biber J, Murer H, Unwin RJ, Debnam ES. Intestinal phosphate absorption and the effect of vitamin D: a comparison of rats with mice. Exp Physiol 91: 531–537, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Murthy S, Mathur SN, Field FJ. Tumor necrosis factor-alpha and interleukin-1beta inhibit apolipoprotein B secretion in CaCo-2 cells via the epidermal growth factor receptor signaling pathway. J Biol Chem 275: 9222–9229, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Buschenfelde KH, Strober W, Kollias G. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur J Immunol 27: 1743–1750, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Radanovic T, Wagner CA, Murer H, Biber J. Regulation of intestinal phosphate transport. I. Segmental expression and adaptation to low-P(i) diet of the type IIb Na(+)-P(i) cotransporter in mouse small intestine. Am J Physiol Gastrointest Liver Physiol 288: G496–G500, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Rijnierse A, Koster AS, Nijkamp FP, Kraneveld AD. TNF-α is crucial for the development of mast cell-dependent colitis in mice. Am J Physiol Gastrointest Liver Physiol 291: G969–G976, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Schmiegel W, Roeder C, Schmielau J, Rodeck U, Kalthoff H. Tumor necrosis factor alpha induces the expression of transforming growth factor alpha and the epidermal growth factor receptor in human pancreatic cancer cells. Proc Natl Acad Sci USA 90: 863–867, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spoettl T, Hausmann M, Klebl F, Dirmeier A, Klump B, Hoffmann J, Herfarth H, Timmer A, Rogler G. Serum soluble TNF receptor I and II levels correlate with disease activity in IBD patients. Inflamm Bowel Dis 13: 727–732, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol Today 18: 61–64, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Uno JK, Kolek OI, Hines ER, Xu H, Timmermann BN, Kiela PR, Ghishan FK. The role of tumor necrosis factor alpha in down-regulation of osteoblast Phex gene expression in experimental murine colitis. Gastroenterology 131: 497–509, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Bai L, Collins JF, Ghishan FK. Age-dependent regulation of rat intestinal type IIb sodium-phosphate cotransporter by 1,25-(OH)(2) vitamin D(3). Am J Physiol Cell Physiol 282: C487–C493, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Xu H, Bai L, Collins JF, Ghishan FK. Molecular cloning, functional characterization, tissue distribution, and chromosomal localization of a human, small intestinal sodium-phosphate (Na+-Pi) transporter (SLC34A2). Genomics 62: 281–284, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Xu H, Collins JF, Bai L, Kiela PR, Ghishan FK. Regulation of the human sodium-phosphate cotransporter NaP(i)-IIb gene promoter by epidermal growth factor. Am J Physiol Cell Physiol 280: C628–C636, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Xu H, Inouye M, Hines ER, Collins JF, Ghishan FK. Transcriptional regulation of the human NaPi-IIb cotransporter by EGF in Caco-2 cells involves c-myb. Am J Physiol Cell Physiol 284: C1262–C1271, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Inouye M, Missey T, Collins JF, Ghishan FK. Functional characterization of the human intestinal NaPi-IIb cotransporter in hamster fibroblasts and Xenopus oocytes. Biochim Biophys Acta 1567: 97–105, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, Uno JK, Inouye M, Collins JF, Ghishan FK. NF1 transcriptional factor(s) is required for basal promoter activation of the human intestinal NaPi-IIb cotransporter gene. Am J Physiol Gastrointest Liver Physiol 288: G175–G181, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Uno JK, Inouye M, Xu L, Drees JB, Collins JF, Ghishan FK. Regulation of intestinal NaPi-IIb cotransporter gene expression by estrogen. Am J Physiol Gastrointest Liver Physiol 285: G1317–G1324, 2003. [DOI] [PubMed] [Google Scholar]