Abstract
Divalent metal transporter-1 (DMT1) is a divalent cation transporter that plays a key role in iron metabolism by mediating ferrous iron uptake across the small intestine. We have previously identified several small molecule inhibitors of iron uptake (4). Using a cell line that stably overexpresses DMT1, we screened the ability of these inhibitors to specifically block this transporter's activity. One compound, NSC306711, inhibited DMT1-mediated iron uptake in a reversible and competitive manner. This inhibitor is a polysulfonated dye containing two copper centers. Although one of these two sites could be chelated by Triethylenetetramine copper chelation did not perturb NSC306711 inhibition of DMT1 activity. Several other polysulfonated dyes with structural features similar to NSC306711 were identified as potential DMT1 transport inhibitors. This study characterizes important pharmacological tools that can be used to probe DMT1's mechanism of iron transport and its role in iron metabolism.
Keywords: iron, iron transport inhibitors
divalent metal transporter-1 (DMT1) mediates the absorption of dietary iron across the duodenal brush border membrane (6, 8). It has been established that iron transport by DMT1 is pH dependent and that ferrous iron is the preferred form of the metal for uptake. DMT1 is also known to transport several other divalent metal cations, including manganese, cadmium, nickel, and cobalt (7, 13, 14). However, iron appears to be the most functionally important transport substrate, particularly since DMT1 is also thought to play a role in its delivery to peripheral tissues after intestinal absorption. Iron released from duodenal enterocytes enters circulation bound to the plasma protein transferrin (Tf). Tf-bound iron is subsequently delivered to cells by receptor-mediated endocytosis of the Tf receptor (TfR), which delivers this ligand to acidic endosomal compartments wherein iron is discharged from Tf to be transported across the intracellular endosomal membrane by DMT1 (5). Despite many molecular and genetic studies characterizing the central role of this transporter in iron metabolism, relatively little is known about mechanistic aspects of DMT1's transport activity.
We have taken advantage of small molecule libraries to initiate the development of pharmacological tools to study the mechanistic basis of iron transport. In particular, we have screened the National Cancer Institute's (NCI) Diversity Set for inhibitors of non-transferrin-bound iron (NTBI) uptake using a cell-based fluorescent assay (4). From this effort, we identified 10 compounds that blocked ferric iron uptake; two of these compounds were found to also inhibit Tf-mediated iron delivery (4). Although this observation suggested that these latter compounds might be plausible candidates to inhibit DMT1, the ability of the inhibitors to specifically act on this transporter was not tested. More recently, we have established a HEK293T cell line that stably expresses DMT1 at the cell surface for use in transport inhibition studies (16). Using this model system, we have now evaluated the ability of the NCI Diversity Set ferric iron uptake inhibitors to block DMT1 function in ferrous iron transport. Only two of the inhibitors, NSC306711 and NSC75600, were found to affect DMT1-mediated iron uptake. This report further characterizes the activity of NSC306711 in mechanistic detail.
EXPERIMENTAL PROCEDURES
HEK293T cell culture and 55Fe transport studies.
HEK293T(DMT1) cells were grown in alpha minimal essential medium supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin, and 10% fetal bovine serum. Cells were grown to 60–80% confluence in 10-cm plates, washed three times with phosphate-buffered saline (PBS), and lifted by trituration and counted. For most transport studies, 0.5–1.5 × 106 cells/transport reaction were incubated at 37°C for 20 min in assay buffer (25 mM Tris, 25 mM MES, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 5 mM glucose, pH 6.75) containing 1 μM 55Fe and 50 μM ascorbic acid. Inhibitors were added at the concentrations indicated immediately prior to the start of uptake unless otherwise noted. Cells were chilled on ice, collected onto nitrocellulose filters by vacuum filtration, then washed three times with PBS to remove any unbound 55Fe. Cell-associated radioactivity was determined by scintillation counting and normalized to control (vehicle alone). For initial rate analysis, cells were grown to 60–80% confluence on poly-l-lysine coated six-well plates, and transport assays were carried out at 37°C as described above with 0.25–2 μM 55Fe and the appropriate addition of 50-fold molar excess ascorbic acid. NSC306711 was added at the start of the reaction, and transport assays were stopped by addition of ice-cold PBS, and cells were washed three times with ice-cold PBS. Cells were lysed with solubilization buffer (0.1% Triton X-100/0.1% NaOH), and cell-associated radioactivity was counted and normalized to cell protein measured by Bradford assay. Similar reactions were carried out at 4°C and this nonspecific background was subtracted.
Western blotting.
Cell lysates were prepared from HEK293T cells stably transfected with pMT2 plasmid containing DMT1 cDNA in the sense or antisense direction in 10 mM Tris, pH 7.4, 150 mM NaCl, 5 mM EDTA, and 1% Triton with protease inhibitors added (Inhibitor Cocktail Set III, Calbiochem). Protein (10 μg) was prepared in Laemmli buffer, incubated at 37°C for 30 min, then electrophoresed on a 10% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. After being blocked in 5% nonfat milk in Tris-buffered saline, the blot was incubated in 2% nonfat milk in TBS containing 0.1% Tween-20 and a 1:1,000 dilution of primary rabbit anti-DMT1 serum (a kind gift of Dr. Phillippe Gros, McGill University). After washing, the blot was incubated with a 1:10,000 dilution of purified secondary donkey anti-rabbit IR 800 (Li-Cor Biosciences) and scanned on a Li-Cor Odessey imaging system. Similar blotting conditions were used to detect the TfR, except that blots were incubated with a 1:2,000 dilution of primary mouse anti-TfR (Zymed) and a 1:10,000 dilution of secondary donkey anti-mouse IR 800 (Li-Cor Biosciences). As a loading control, blots were stripped and reprobed for actin immunoreactivity by use of a 1:10,000 dilution of primary mouse anti-actin (ICN Biochemicals) and a 1:10,000 dilution of secondary donkey anti-mouse IR 800 or IR 680, respectively.
Calcein fluorescence quenching assay for inhibition of DMT1 activity in the presence of TRIEN.
Cells were cultured in 24-well plates as described above and then incubated for 1 h with 0.25 μM calcein-AM in serum-free and phenol red-free DMEM. After washing, a baseline fluorescence reading was taken. Cells were then incubated for 30 min at 37°C either with DMSO (vehicle control) or 50 μM NSC306711 in uptake buffer (pH 6.75) that had been preincubated for 2 h with 0–30 μM triethylenetetramine (TRIEN). After a second fluorescence measurement (Fd), 1 μM Fe and 50 μM ascorbic acid were added and a final fluorescence reading was taken after a 20-min iron uptake period (Ff). Values are expressed as the percentage of fluorescence quenching after incubation with iron, calculated as (Fd−Ff)×100/Fd. Incubation with TRIEN or ascorbic acid alone did not affect the baseline fluorescence readings.
Statistical analyses.
Values reported are means ± SE. Statistical significance was evaluated using two-tailed Student's t-test (unpaired assuming unequal variance) comparing nontreated vs. treated samples. Dose-response curves were analyzed by a four-parameter sigmoidal model. Nonlinear regression analysis to calculate Kmapp, Vmax, and Ki values was performed using WiNonlin software version 4.0 (Pharsight).
RESULTS
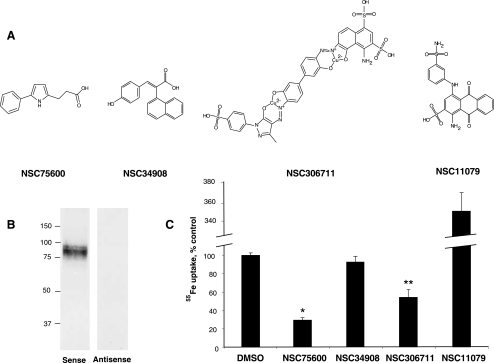
A previous chemical genetic screen of NCI's Diversity Set identified 10 inhibitors of non-transferrin-bound ferric iron uptake. Six of these inhibitors (NSC48874, NSC48010, NSC331973, NSC27236, NSC124808, and NSC13831) had IC50 values ≥ 20 μM and were toxic to cells at higher concentrations (4). Because the pharmacological utility of these inhibitors appeared doubtful, their effects on DMT1 were not studied. Instead, we restricted our focus to two known inhibitors of transferrin-mediated iron uptake, NSC11079 and NSC306711, as well as two other compounds, NSC34908 and NSC75600. The structures of the compounds studied in this investigation are shown in Fig. 1A. Although their chemical features are unique, each of the compounds was previously found to inhibit NTBI uptake with IC50 values that range from 5 to 20 μM (4). To determine their influence on DMT1-mediated iron transport, we took advantage of HEK293T(DMT1) cells, which stably overexpress DMT1 (16). Figure 1B shows a Western blot confirming the stable expression of DMT1 in HEK293T cells transfected with plasmid bearing the cDNA for DMT1 oriented in the sense, but not antisense, direction. Previous studies from our laboratory have shown ∼25-fold greater ferrous iron uptake activity with the sense HEK293T(DMT1) cells and have shown that the transporter is expressed at the surface of these cells (16). To measure DMT1 activity, we employed an assay previously established by others (5) wherein cells are incubated at pH 6.75 in the presence of 1 μM 55Fe reduced by a 50-fold molar excess of ascorbate. Nonspecific cell-associated radioactivity measured at 4°C was less than 5% of cell uptake measured at 37°C (data not shown). To assay inhibition of DMT1-mediated ferrous iron uptake, transport assays were performed with 50 μM of each inhibitor or vehicle control (DMSO). Two of the compounds, NSC75600 and NSC306711, significantly reduced the extent of 55Fe uptake relative to control (Fig. 1C). NSC34908 did not block DMT1-mediated transport activity, indicating that although this drug influences ferric iron uptake (16) it does not affect DMT1-mediated ferrous iron uptake. NSC11079 appeared to greatly stimulate ferrous iron uptake, but this effect was caused by precipitation of radioactivity captured in the filter assay owing to interactions with ferrous iron (data not shown). Since the net effect of this compound would be to inhibit cellular acquisition of iron by limiting its availability for transport and its use in radioisotope tracer studies would be compromised by these interactions with iron, further characterization of NSC11079 was not pursued.
Fig. 1.
Effect of inhibitors on divalent metal transporter-1 (DMT1)-mediated iron uptake. Four compounds previously identified to inhibit NTBI uptake were studied. A: structures of the 4 compounds. B: Western blot detecting DMT1 immunoreactivity in HEK293T(DMT1) cells stably transfected with pMT2 containing DMT1 cDNA in the sense and antisense (noncoding) orientation. C: effects of inhibitors on DMT1-mediated 55Fe uptake. Briefly, HEK293T(DMT1) cells were incubated either with 50 μM of the indicated compounds or DMSO (vehicle control) in uptake buffer (pH 6.75) containing 1 μM 55Fe and 50 μM ascorbic acid for 20 min. Cells were chilled on ice, collected on nitrocellulose filters, and washed with PBS. Cell-associated radioactivity was determined by dissolving the filters in scintillation fluid and counting. All cpm were normalized to vehicle control (112.7 ± 4.4 pmol 55Fe/million cells). Shown are mean values ± SE for inhibition (n = 5 or 6). *P = 6.98 × 10−08; **P = 0.00476.
In earlier studies of inhibitors of DMT1-mediated transport, the antioxidants ebselen and pyrrolidine dithiocarbamate were found to block iron uptake due to their influence on cellular redox status (16). To examine whether any of the inhibitors might exert similar effects, cellular levels of reduced vs. oxidized glutathione were determined (Table 1). None of the tested compounds significantly affected the GSH-to-GSSG ratio, suggesting that indirect effects of cellular redox on iron uptake do not account for the ability of these compounds to affect iron uptake.
Table 1.
Effects of inhibitors on GSH/GSSG ratio
| GSSG | GSH + GSSG | GSH/GSSG | |
|---|---|---|---|
| DMSO | 0.058±0.002 | 25.322±0.334 | 46.335±1.347 |
| NSC306711 | 0.061±0.001 | 25.040±0.345 | 43.379±0.825 |
| NSC75600 | 0.054±0.002 | 25.627±0.791 | 50.094±2.258 |
| NSC34908 | 0.058±0.002 | 26.137±0.528 | 47.724±2.321 |
Values are means ± SE (n = 9). HEK293T(DMT1) cells were incubated with 50 μM of either the indicated compounds or DMSO (vehicle control) in uptake buffer (pH 6.75) containing 1 μM Fe and 50 μM ascorbic acid for 20 min. After chilling on ice, cells were washed with PBS and GSSG equivalents (nmol/million cells) were determined by use of the GSH/GSSG-412 kit (OxisResearch, Portland, OR).
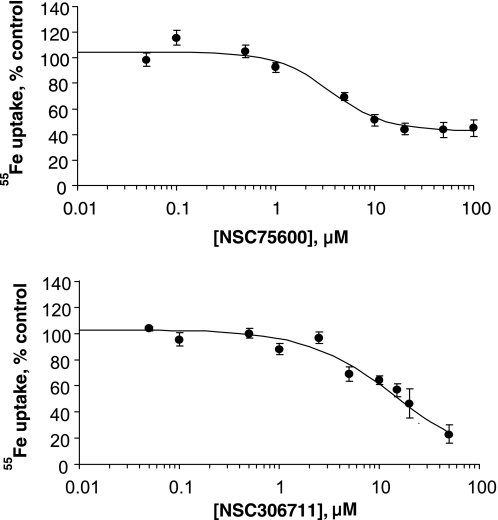
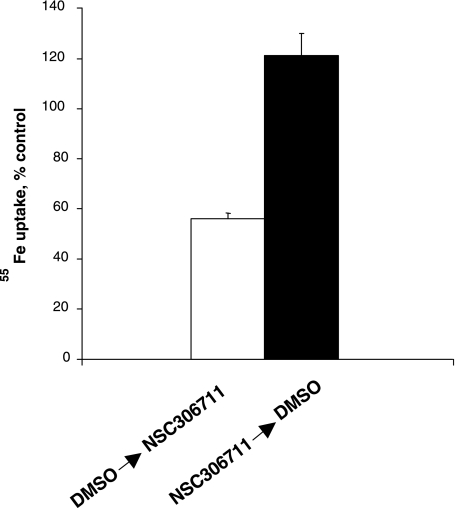
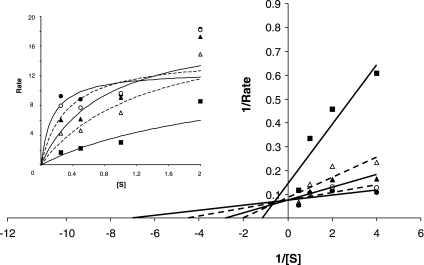
Dose-response experiments revealed that NSC75600 maximally blocked only ∼50% of DMT1-mediated transport activity at concentrations above 10 μM (Fig. 2A). Since concentrations of up to 100 μM only partially blocked uptake, use of this particular compound appears to be of limited value as a candidate inhibitor and further characterization was not pursued. In contrast, NSC306711 was observed to completely block DMT1 transport activity in a dose-responsive manner with an IC50 ∼14.7 ± 1.5 μM (Fig. 2B) and therefore our efforts focused on characterizing this compound's mechanism of action. To study the reversibility of NSC306711 effects, 55Fe uptake was measured after a 15-min pretreatment followed by 30-min recovery period. The inhibition observed when HEK293T(DMT1) cells were incubated with NSC306711 was not observed after the recovery period and in the absence of the compound, indicating that the compound acted in a reversible manner (Fig. 3). Initial rate analysis was also carried out to determine the mechanism of NSC306711 inhibition (Fig. 4). Qualitatively, these data suggest that NSC306711 acts as a competitive inhibitor. Nonlinear regression analysis with equal weighting confirmed that higher inhibitor concentrations increased Km values with Vmax unchanged (15.76 ± 1.56 μM; 95% confidence interval of 11.42–20.10 μM). The results of this analysis are provided in Supplemental Table S1, which can be found online at the American Journal of Physiology Gastrointestinal and Liver Physiology website. A Ki value of 6.90 ± 1.21 μM (95% confidence interval of 3.06–10.74 μM) was calculated from this analysis.
Fig. 2.
Dose response of DMT1 inhibition by NSC75600 and NSC306711. A: HEK293T(DMT1) cells were incubated with up to 100 μM NSC75600 in uptake buffer (pH 6.75) containing 1 μM 55Fe and 50 μM ascorbic acid for 20 min. Cells were chilled on ice, collected on nitrocellulose filters, and washed with PBS. Cell-associated radioactivity was determined and normalized to vehicle control (171.6 ± 11.2 pmol 55Fe/million cells). Shown are mean values ± SE (n = 6 to 9) fit to a 4-parameter sigmoidal curve (r2 = 0.967). B: similar experiments were performed with HEK293T(DMT1) cells incubated with up to 100 μM NSC306711 under the same conditions. Vehicle control was determined to be 191.7 ± 29.4 pmol 55Fe/million cells; shown are normalized mean values ± SE (n = 4 to 6) fit to a 4-parameter sigmoidal curve (r2 = 0.967).
Fig. 3.
Reversibility of DMT1-mediated iron uptake inhibition by NSC306711. HEK293T(DMT1) cells were incubated either with 20 μM NSC306711 or DMSO (vehicle control) in PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBS++) for 20 min, followed by a 30-min recovery period in the same buffer containing 5 mM dextrose (PBSG++). DMSO or NSC306711 were next added in uptake buffer (pH 6.75) and the cells were incubated with 55Fe for 20 min. Uptake was normalized to controls both preincubated and assayed in the presence of DMSO (42.5 ± 5.5 pmol 55Fe/million cells). Shown are mean values ± SE (n = 6).
Fig. 4.
DMT1 is inhibited by NSC306711 in a competitive manner. Initial rate analysis was carried out as described under experimental procedures to determine the effects of NSC306711 at the following concentrations: no inhibitor (•), 12.5 μM (○), 25 μM (▴), 50 μM (▵), and 100 μM (▪). Shown are Lineweaver-Burk plots of reciprocal values and Michaelis-Menton curves obtained for the rate of transport (pmol·min−1·mg cell protein−1) measured at the indicated 55Fe concentrations (0.25–2 μM). Michaelis-Menton curves were fit by nonlinear regression analysis (solid symbols with solid lines; open symbols with dashed lines). Ki, Kmapp, and Vmax values for each curve determined by nonlinear regression are provided in Supplemental Table S1.
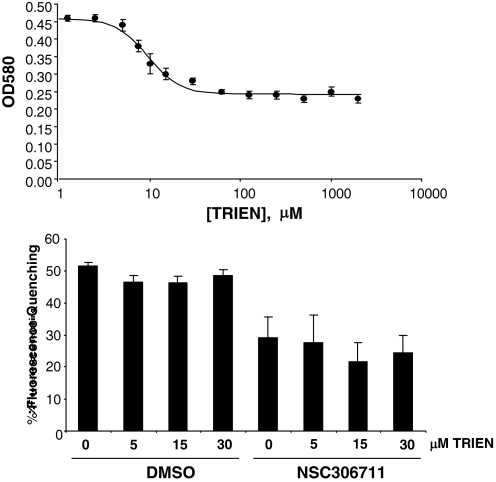
Owing to its two copper centers, NSC306711 has an intense blue color displaying λmax at 580 nm. Incubation with TRIEN, a copper chelator, reduced NSC306711 absorption at 580 nm in a dose-dependent manner (Fig. 5A). Although ∼50% the compound's absorbance was eliminated by 30 μM TRIEN, the copper chelator did not further reduce OD580. These observations are consistent with the idea that one of the two metal centers of NSC306711 is labile to copper chelation. To study how copper chelation affected the compound's activity, transport assays were carried out using a well-established calcein fluorescence quenching assay to monitor DMT1-mediated iron uptake in the presence of low concentrations of TRIEN. Incubation with up to 30 μM TRIEN did not affect DMT1-mediated iron uptake measured by the extent of fluorescence quenching of intracellular calcein in the absence of the drug (Fig. 5B). The presence of 50 μM NSC306711 alone significantly reduced the amount of calcein quenching, indicative of its inhibition of iron uptake. However, coincubation with up to 30 μM TRIEN did not affect inhibition, indicating that at least one of the copper sites is not required for the drug's activity.
Fig. 5.
Effect of copper chelation on DMT1-mediated iron uptake and NSC306711 inhibition. A: assay mixtures containing 100 μM NSC306711 with the indicated concentrations of the copper chelator TRIEN were incubated for 2 h at 37°C. Optical density was then measured at 580 nM. Shown are mean values ± SE (n = 3–4). B: HEK293T(DMT1) cells were loaded with 0.25 μM calcein for 1 h, washed, and then incubated for 30 min at 37°C either with DMSO or 50 μM NSC306711 in uptake buffer (pH 6.75) preincubated for 2 h with the indicated concentrations of TRIEN. Calcein quenching was measured as described under experimental procedures. Shown are means ± SE (n = 6) fit to a 4-parameter sigmoidal curve (r2 = 0.982).
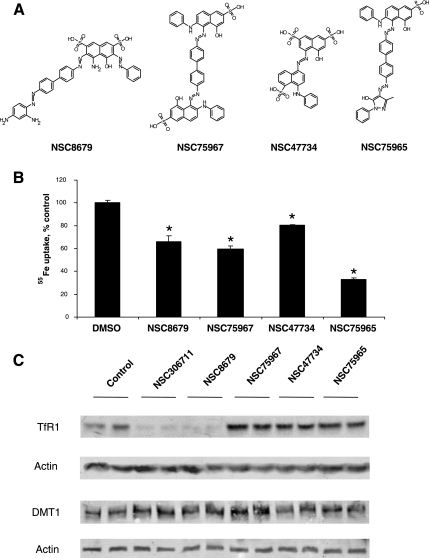
Structural derivatives of NSC306711 were sought from NCI's small molecule database. Four polysulfonic dyes were available as shown in Fig. 6A. Figure 6B shows results of inhibition studies comparing the effects of these compounds on DMT1-mediated 55Fe uptake activity. All four compounds interfered with iron transport, and none contained copper centers, supporting the notion that other structural features of NSC306711 impart its inhibitory effects. Other studies from our laboratory have revealed that NSC306711 can induce degradation of TfR (9). Therefore, we tested the ability of NSC306711 and its structural analogs to perturb the stability of DMT1. Like NSC306711, NSC8679 reduced cellular levels of TfR (Fig. 6C). However, none of these compounds affected DMT1 levels as assessed by Western blot analysis.
Fig. 6.
Inhibition of DMT1-mediated iron uptake by polysulfonated dyes. Four compounds with structural relationships to NSC306711 were tested for their ability to inhibit DMT1. A: compounds studied. B: effects of inhibitors (50 μM) on DMT1-mediated 55Fe uptake normalized to vehicle control (129.0 ± 1.5 pmol 55Fe/million cells). Shown are mean values ± SE (n = 3). *NSC8679, P = 0.02785; NSC75967, P = 0.00029; NSC47734, P = 0.004501; NSC75965, P = 0.000752. C: Western blot analysis to detect transferrin receptor (TfR) and DMT1 expression levels in HEK293T(DMT1) cells treated with 50 μM of the indicated compounds or DMSO (vehicle control) for 4 h. Cell lysates were prepared and protein samples were electrophoresed on a 7.5% SDS-polyacrylamide gel (TfR) or a 5–15% SDS-polyacrylamide gradient gel (DMT1). Immunoblotting was carried out as detailed under experimental procedures.
DISCUSSION
The capacity to differentiate between multiple pathways of iron uptake by mammalian cells has been primarily limited by a lack of pharmacological tools that help to define mechanistic elements. For example, although DMT1 has an established role in the absorption of dietary iron across the duodenal brush border membrane (6, 8) and iron delivery to erythroid cells via Tf (5), relatively little is known about how it may be involved in other pathways such as the hepatic uptake of NTBI during iron overload (2). Taking advantage of small molecules identified from the NCI Diversity Set as inhibitors of NTBI uptake, we identified two inhibitors, NSC306711 and NSC75600, that block DMT1-mediated ferrous iron transport. Importantly, our study also indicates that another compound, NSC34908, does not block DMT1 activity although it is an NTBI inhibitor. Although its mechanism of action to block NTBI uptake remains to be further defined, this compound also helps to provide a pharmacological profile for DMT1, which we can now define as sensitive to inhibition by NSC306711 and NSC75600 and resistant to inhibition by NSC34908. None of the compounds altered cellular GSH-to-GSSG ratio, supporting the idea that they do not affect redox regulation, which is known to influence iron transport (16).
One of the DMT1 inhibitors, NSC306711, was characterized in further detail. NSC306711 acts as a reversible competitive inhibitor to block DMT1-mediated iron uptake (Ki ∼7 μM). The reversibility and efficacy of DMT1 inhibition by NSC306711 suggests this drug and structurally related derivatives are amenable for in vitro cell-based studies of iron transport. Several polyaromatic sulfonated dyes similar to NSC306711 were identified from the NCI small molecule database and also found to perturb DMT1 function, providing some clues about inhibitor's structure-activity relationships. None of these compounds contained copper, and our study shows that at least one of the coppers of NSC306711 is not required for this compound to inhibit DMT1. Combined, these observations suggest that the metal centers are not involved in NSC306711's mechanism of inhibition.
NSC306711 is also known to inhibit Tf-mediated iron uptake (4). Recent studies from our laboratory have revealed that this compound induces degradation of unoccupied Tf receptors, suggesting that this iron uptake pathway is blocked because of destabilization of receptor by NSC306711 (9). NSC306711 effects on the Tf receptor are particularly surprising because degradation occurs through a clathrin-independent, nystatin-sensitive endocytic pathway, suggesting the name “ferristatin” for its cholesterol-dependent effects (9). The molecular basis for Tf receptor degradation remains to be more fully understood, but the fact that this compound inhibits DMT1 may provide some important clues. DMT1 functions in endocytic uptake of iron to erythroid cells delivered by Tf and is found in endocytic compartments (5). It is thought that DMT1 translocates iron released from Tf-TfR complexes across the endosomal membrane. It is possible that inhibition of DMT1 activity by NSC306711 turns on a cellular feedback mechanism to affect TfR stability, thereby protecting the cell against entry of excess iron by downregulating protein levels of the receptor. Our data show that DMT1 itself is not destabilized by NSC306711 or the other polysulfonated dyes tested, whereas both NSC306711 and NSC8679 reduced TfR levels. Further work is necessary to define whether NSC8679 or any of the other structural derivatives block Tf-mediated iron uptake and to elucidate the relationship, if any, between NSC306711 inhibition of DMT1 activity and NSC306711-induced Tf receptor degradation.
Additional studies to explore the use of polysulfonated dyes are also warranted as potential pharmacological tools for treating iron overload. Hereditary hemochromatosis and other forms of secondary iron overload frequently promote iron deposition in the liver and other tissues, leading to cirrhosis, increased incidence of hepatoma, cardiomyopathies, and other organ failures (1, 2, 10). Current treatments for iron overload disorders are limited to phlebotomy or chelation therapies (1, 10). Recently, Ludwiczek et al. (11) reported that the calcium channel blocker nifedipine reverses iron overload in mouse models of these disorders. These authors suggested nifedipine's effects arise through activation of DMT1 function, thereby mobilizing excess liver iron and enhancing urinary iron excretion. However, photodegradation products of nifedipine are also known to stimulate iron uptake (15) and direct effects of this drug on DMT1 remain controversial (12). NSC306711 and its structural derivatives may provide an alternate pharmacological mechanism to reduce iron loading by blocking dietary iron absorption. Antitumor testing screens conducted by the NCI have indicated that NSC306711 is nontoxic at concentrations up to 100 μM; therefore, it is likely that the compound could be administered without side effect to block intestinal DMT1 activity at the concentrations observed to block ferrous iron uptake in our studies. In addition, primary and secondary forms of hemochromatosis are associated with plasma iron levels in excess of Tf saturation that promote increased concentration of circulating NTBI (3). Since NSC306711 can also inhibit NTBI uptake, this drug may also be of particular utility in examining the role of DMT1 in NTBI transport and iron metabolism under overload conditions. Ultimately, the availability of a number of different small molecule inhibitors should help to begin to define the molecular basis for iron uptake by DMT1 and the mechanistic distinctions between other membrane transport pathways.
GRANTS
Support for this research was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (DK064750).
Supplementary Material
Acknowledgments
We thank the Harvard Institute of Chemistry and Biology (ICCB), who assisted with the initial screen, and the National Cancer Institute's Initiative for Chemical Genetics, who provided support for the ICCB. We also appreciate the help of Dr. Robert Schultz and Dr. Ven L. Narayanan at the Drug Synthesis & Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute in procuring test compounds used in this study. We thank Dr. Jonghan Kim for help with kinetic analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams PC, Barton JC. Haemochromatosis. Lancet 370: 1855–1860, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GJ, Powell LW. HFE and non-HFE hemochromatosis. Int J Hematol 76: 203–207, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Breuer W, Hershko C, Cabantchik ZI. The importance of non-transferrin bound iron in disorders of iron metabolism. Transfus Sci 23: 185–192, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Brown JX, Buckett PD, Wessling-Resnick M. Identification of small molecule inhibitors that distinguish between non-transferrin bound iron uptake and transferrin-mediated iron transport. Chem Biol 11: 407–416, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA 95: 1148–1153, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming MD, Trenor CC 3rd, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet 16: 383–386, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Garrick MD, Singleton ST, Vargas F, Kuo HC, Zhao L, Knopfel M, Davidson T, Costa M, Paradkar P, Roth JA, Garrick LM. DMT1: which metals does it transport? Biol Res 39: 79–85, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388: 482–488, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Horonchik L, Wessling-Resnick M. The small-molecule iron transport inhibitor ferristatin/NSC306711 promotes degradation of the transferrin receptor. Chem Biol 15: 647–653, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushner JP, Porter JP, Olivieri NF. Secondary iron overload. Hematology Am Soc Hematol Educ Program: 47–61, 2001. [DOI] [PubMed]
- 11.Ludwiczek S, Theurl I, Muckenthaler MU, Jakab M, Mair SM, Theurl M, Kiss J, Paulmichl M, Hentze MW, Ritter M, Weiss G. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter-1. Nat Med 13: 448–454, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Mackenzie B, Shawki A, Ahio AJ, Stonehuerner JD, Zhao L, Ghadersohl S, Garrick LM, Garrick MD. A role for the devalent metal-ion transporter (DMT1) is doubtful in the mechanism by which calcium-channel blockers reverse iron overload. FASEB J 22: 1192, 2008. [Google Scholar]
- 13.Mackenzie B, Takanaga H, Hubert N, Rolfs A, Hediger MA. Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1). Biochem J 403: 59–69, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picard V, Govoni G, Jabado N, Gros P. Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. J Biol Chem 275: 35738–35745, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Savigni DL, Wege D, Cliff GS, Meesters ML, Morgan EH. Iron and transition metal transport into erythrocytes mediated by nifedipine degradation products and related compounds. Biochem Pharmacol 65: 1215–1226, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Wetli HA, Buckett PD, Wessling-Resnick M. Small-molecule screening identifies the selanazal drug ebselen as a potent inhibitor of DMT1-mediated iron uptake. Chem Biol 13: 965–972, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.