Abstract
We have previously reported that postresuscitation myocardial dysfunction is accompanied by the release of cytochrome c and caspase-3 activation. We now investigated the role of caspase-3 activation by examining whether such process prompts apoptotic DNA fragmentation, whether caspase-3 inhibition attenuates myocardial dysfunction, and whether myocardial protective effects of sodium-hydrogen exchanger isoform-1 (NHE-1) inhibition involve caspase-3 inhibition using a rat model of ventricular fibrillation (VF) of closed-chest resuscitation. Resuscitation after 4 or 8 min of untreated VF caused significant reductions in left ventricular stroke work index averaging 23% of sham control rats at 4 h postresuscitation. Left ventricular dysfunction was accompanied by increases in cytosolic cytochrome c, decreases in pro- and cleaved caspase-9 fragments, increases in 17-kDa caspase-3 fragments, and increases in caspase-3 activity indicating the activation of the mitochondrial apoptotic pathway but without evidence of apoptotic DNA fragmentation. In addition, levels of heat shock protein 70 were increased and levels of X-linked inhibitor of apoptosis protein and αβ-crystallin were preserved, all of which can exert antiapoptotic effects. In a separate series, the caspase-3 inhibitor z-Asp-Glu-Val-Asp chloromethyl ketone given before the induction of VF failed to prevent postresuscitation myocardial dysfunction despite reductions in caspase-3 activity (2.3 ± 0.5 vs. 1.3 ± 0.5 pmol fluorophore AFC released·mg protein−1·min−1; P < 0.03). Treatment with the NHE-1 inhibitor cariporide had no effect on caspase-3 activity. Accordingly, in this rat model of VF and severe postresuscitation myocardial dysfunction, activation of caspase-3 did not lead to DNA fragmentation or contribute to myocardial dysfunction. Concomitant activation of intrinsic antiapoptotic mechanisms could play a protective role downstream to caspase-3 activation.
Keywords: ventricular fibrillation, cardiopulmonary resuscitation, apoptosis, ventricular function
we have recently reported in a rat model of ventricular fibrillation (VF) and closed-chest resuscitation that postresuscitation myocardial dysfunction is accompanied by mitochondrial release of cytochrome c and cleavage of procaspase-3 with release of its 17-kDa fragment (35). Caspase-3 is one of the executioner caspases and is responsible for apoptotic cell death, the hallmark of which is internucleosomal DNA fragmentation (2, 45). Activated caspase-3 also cleaves cardiac sarcomeric proteins such as troponin I, troponin T, actin, and ventricular essential myosin light chain-1, leading to contractile dysfunction (10, 27, 37). We therefore hypothesized that caspase-3 activation could be mechanistically linked to postresuscitation myocardial dysfunction by promoting apoptotic cell death and/or by compromising sarcomeric function.
To test this hypothesis we used the same rat model of VF and closed-chest resuscitation and examined whether caspase-3 activation leads to apoptotic DNA fragmentation using a ligation-mediated (LM)-PCR. We also sought additional understanding of the apoptotic response to cardiac arrest and examined whether caspase-3 activation resulted from activation of the intrinsic and/or extrinsic apoptotic pathway and whether antiapoptotic proteins of the heat shock protein (HSP) family and inhibitor of apoptosis protein (IAP) family could play a role. We then examined whether selective inhibition of caspase-3 using z-Asp-Glu-Val-Asp chloromethyl ketone (z-DEVD-cmk) could attenuate postresuscitation myocardial dysfunction and whether the known myocardial protective effects of sodium-hydrogen exchanger isoform-1 (NHE-1) (4, 5, 19, 44) involve attenuation of caspase-3 activation.
MATERIALS AND METHODS
The studies were approved by our Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
Rat Models
Two rat models were used: a model of VF and closed-chest resuscitation for the main experiments, emcompassing three series of experiments, and a model of coronary occlusion and reperfusion for assessing caspase-3 activation after a longer interval of ischemia (positive control).
VF and closed-chest resuscitation model.
ANIMAL PREPARATION.
Adult male Sprague-Dawley rats (455–545 g) were anesthetized using pentobarbital sodium (45 mg/kg ip for induction and 10 mg/kg iv every 30 min for maintenance). A 5-Fr cannula was advanced into the trachea for positive pressure ventilation during and after cardiac resuscitation. Proper endotracheal tube placement was verified by infrared CO2 analysis. A lead II ECG was recorded through subcutaneous needles. Polyethylene (PE)50 catheters were advanced into the right atrium, the left ventricle, and the abdominal aorta for pressure measurement and blood sampling. A thermocouple microprobe (IT-18; Physitemp) was advanced into the thoracic aorta for measuring cardiac output. A PE50 catheter was advanced into the right atrium and used for injection of thermal tracer. A 3-Fr catheter (C-PUM-301J; Cook) was advanced into the right atrium, and through its lumen a precurved guide wire was fed into the right ventricle for electrical induction of VF. Core temperature was maintained between 36.5°C and 37.5°C using an infrared heating lamp.
VF AND RESUSCITATION PROTOCOL.
VF was induced by delivering a 60-Hz alternating current (0.1 to 0.6 mA) to the right ventricle for 3 min after which it was turned off and VF was allowed to continue untreated for a predetermined interval (see below). Chest compression was performed using an electronically controlled and pneumatically driven chest compressor set to deliver 200 compressions/min with a 50% duty cycle. Compression depth was adjusted to attain an aortic diastolic pressure between 26 and 28 mmHg to ensure a coronary perfusion pressure above the resuscitability threshold of 20 mmHg in rats (43). Positive pressure ventilation was provided using an electronically controlled solenoid valve set to deliver 0.39 ml/100 g body wt of 100% oxygen every two compressions. After 8 min of chest compression, a maximum of two 2-J monophasic transthoracic shocks (Lifepak 9P; Physio-Control) were delivered. If VF persisted or an organized rhythm with a mean aortic pressure ≤25 mmHg ensued, chest compression was resumed for 30 s. The defibrillation-compression cycle was repeated up to three times, increasing the shock energy if VF persisted to 4-J and then 8-J. This resuscitation protocol was used in the first half of series 1 and then changed incorporating recent advances in resuscitation, applying these changes to the second half of series 1 and to series 2. These changes included use of a volume-controlled ventilator (model 683; Harvard Apparatus) delivering 25 unsynchronized breaths/min with a tidal volume of 6 ml/kg body wt and defibrillation attempts using a biphasic waveform defibrillator (Smart Biphasic Heartstream XL M4735A; Agilent Technologies) delivering 3-, 5-, and 7-J biphasic shocks in lieu of the monophasic shocks. For series 3 the initial resuscitation protocol was used because the experiments were completed before incorporating the recent changes in resuscitation. In this series defibrillation was attempted after 5 or 6 min of chest compression to match in time spontaneous reversal of VF that occurs with cariporide (13). Successful resuscitation in all series was defined as an organized rhythm with a mean aortic pressure ≥60 mmHg for ≥5 min. Resuscitated rats were ventilated with 100% O2 for 15 min followed by 50% O2–50% N2.
Coronary occlusion and reperfusion model.
ANIMAL PREPARATION.
Male Sprague-Dawley rats (533–543 g) were anesthetized with pentobarbital sodium (45 mg/kg ip for induction and 10 mg/kg iv every 30 min for maintenance). PE50 catheters were advanced into the right atrium and abdominal aorta for pressure measurements. Over the left hemithorax, a 3-cm skin incision was made and the pectoral muscles were retracted to expose the ribs. A thoracotomy was performed at the level of the fifth intercostal space. A 4-0 silk suture was passed around the left anterior descending coronary artery (LAD) at 3 mm from its origin. Core temperature was maintained between 36.5°C and 37.5°C using an infrared heating lamp.
CORONARY OCCLUSION AND REPERFUSION.
The LAD was ligated using a slipknot with one end brought out through the chest wall to permit subsequent reperfusion. LAD occlusion was confirmed by the development of myocardial cyanosis, bulging, and dyskinesis. The chest wall, muscles, and skin were then closed, and the LAD occlusion was maintained for 30 min after which the exteriorized end of the ligature was pulled free allowing reperfusion for 4 h. The LAD was then reoccluded, and Evan's blue (1% in PBS, 5 ml) was injected into the right atrium to stain the area that was not subject to LAD occlusion enabling to define the area at risk. The heart was then excised, and the left ventricle was processed as described below under Measurements.
Experiments
Series 1.
Experiments were conducted to determine 1) which apoptotic pathways are involved in caspase-3 activation, 2) whether activation of caspase-3 leads to apoptotic DNA fragmentation, and 3) to assess changes in antiapoptotic proteins. Three groups of eight rats each were investigated. Two groups were subjected to either 4 or 8 min of untreated VF before attempting resuscitation. Another group served as sham control.
Series 2.
Experiments were conducted to determine whether caspase-3 inhibition could reduce postresuscitation myocardial dysfunction. Two groups of four rats each were randomized to receive a bolus of the irreversible caspase-3 inhibitor z-Asp-Glu-Val-Asp chloromethyl ketone (z-DEVD-cmk; Bachem) (42) or vehicle control into the right atrium 10 min before inducing VF. z-DEVD-cmk was dissolved in 100% DMSO to a concentration of 20 mg/ml and given in a dose of 3 mg/kg body wt in a total DMSO volume of 100 μl. Control rats received only DMSO. Rats were subjected to 8 min of untreated VF before attempting resuscitation.
Series 3.
Experiments were conducted to determine whether NHE-1 inhibition, an intervention shown by our group to consistently attenuate postresuscitation myocardial dysfunction (4, 5, 19, 44), is associated with amelioration of caspase-3 activation. We used hearts that were available from another experimental series in which two groups of four rats each were randomized to receive a 3-mg/kg bolus of cariporide (4-isopropyl-methylsulfonylbenzoyl-guanidine methanesulfonate; Aventis Pharma Deutschland) or an equal amount of 0.9% NaCl (control) into the right atrium immediately before starting chest compression. Rats were subjected to 10 min of untreated VF before attempting resuscitation.
Materials
CaCl2, 3-[(3-cholamidopropyl)dimethyl ammonio]-1-propane sulfonate (CHAPS), DTT, EDTA, EGTA, glucose, HEPES, KCl, mannitol, MOPS, NaCl, paraformaldehyde, PMSF, proteinase K, protease inhibitor cocktail, phosphatase inhibitor cocktail, rat heart cytochrome c, RNase A (DNase free), SDS, NaF, Na3VO4, sucrose, Triton X-100, and triphenyltetrazolium chloride (TTC) were purchased from Sigma; N-acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin (Ac-DEVD-AFC; caspase-3 substrate) and DEVD-CHO (reversible caspase-3 inhibitor) were from Biomol; 1-mm-thick 12% and 14% Novex tris-glycine polyacrylamide gels were from Invitrogen; complete protease inhibitor cocktail tablets and polyvinylidene difluoride (PVDF) membrane were from Roche Applied Science; West femto maximum sensitivity chemiluminescent detection kit was from Pierce Biotechnology; protein concentration assay BCA kit based on the Bradford method (7) was from Bio-Rad; APO-DNA1 PCR kit for DNA ladder assay was from Maxim Biotech; T4-DNA ligase was from New England Biolabs; phase lock gel tubes were from Eppendorf; and bromophenol blue, ethidium bromide, Evan's blue, glycerol, isopropanol, phenol/chloroform/isoamyl alcohol, 2-mercaptoethanol, and Tris were from Fisher Biotech.
Antibodies.
Mouse monoclonal anti-β-actin antibody was from Sigma; rabbit polyclonal anti-cytochrome c, rabbit polyclonal anti-caspase-3, rabbit polyclonal anti-caspase-9, and rabbit monoclonal anti-glyceraldehyde 3-phosphate dehydrogenase antibodies were purchased from Cell Signaling Technology; mouse monoclonal anti-prohibitin antibody was from Calbiochem; mouse-monoclonal anti-cytochrome c antibody (clone 7H8.2C12) was from BD Biosciences PharMingen; mouse monoclonal anti-HSP70, rat monoclonal anti-heat shock congnate (HSC)70, mouse monoclonal anti-αβ-crystallin, rabbit polyclonal anti-phospho serine 59 (S59)-αβ-crystallin, and mouse monoclonal anti-X-linked inhibitor of apoptosis protein (XIAP) antibodies were from Stressgen; goat polyclonal anti-rabbit IgG and goat polyclonal anti-mouse IgG horseradish peroxidase (HRP) conjugated antibodies were from Pierce Biotechnology; and goat polyclonal anti-rat IgG HRP conjugated antibody was from KPL protein research products.
Measurements
Hemodynamic and metabolic measurements.
Vascular and ventricular pressures were measured using pressure-transducers (Maxxim Medical) zeroed to midchest level. The transduced pressure and ECG signals were conditioned (Biopac Systems) and digitized at 250 Hz using a 16-bit data acquisition board (AT-MIO-16XE-50; National Instruments). Cardiac output was determined by thermodilution after a right atrial bolus injection of 200 μl of 0.9% NaCl at room temperature, and the curve was analyzed using custom-developed LabView-based software. Cardiac output was normalized to body weight in kilograms (cardiac index). Left ventricular stroke work index (LVSWI) was calculated multiplying stroke volume index by the left ventricular systolic-to-diastolic pressure difference. The maximum rate of left ventricular pressure rise (+dP/dtmax) was obtained from the first derivative of the left ventricular pressure. Arterial lactate and bicarbonate levels were measured using Nova Stat Profile pHOx Plus L.
Left ventricular tissue measurements from the VF and closed-chest resuscitation model.
In series 1 and 2, hearts were harvested at 240 min postresuscitation or earlier if the mean aortic pressure decreased below 40 mmHg. Hearts were rinsed in ice-cold PBS supplemented with protease inhibitors. The left ventricle was isolated, immersed in liquid N2, and stored at −80°C. In series 3, hearts were harvested at 60 min postresuscitation. After excision, hearts were immersed in liquid N2, freeze-dried, and stored at −80°C. The left ventricular tissue from series 1 and 2 was used to measure cytosolic and mitochondrial cytochrome c, and cytosolic procaspases and cleaved caspases-9, -3, and -8 along with XIAP, HSP70, HSC70, phospho/total αβ-crystallin, and total homogenate caspase-3 activity. The left ventricular tissue from series 3 was used to measure cytosolic HSP70 and total homogenate caspase-3 activity.
IMMUNOBLOTTING.
Processing of the left ventricular tissue for measurements of cytochrome c and caspases was carried out as previously described (35). For other measurements (XIAP, HSP70, HSC70, and phospho/total αβ-crystallin), a 100-mg sample of frozen tissue was homogenized in 2-ml ice-cold buffer composed of (in mM) 50 Tris·HCl (pH 7.5), 5 EDTA, 10 EGTA, 1 DTT, 1 Na3VO4, 1 NaF, and 0.2 PMSF and complete protease inhibitor tablet and phosphatase inhibitor cocktail using a Dounce homogenizer. The homogenate was centrifuged at 100 g for 10 min at 4°C, and its supernatant was centrifuged at 45,000 g for 30 min at 4°C to separate the cytosolic fraction. Protein concentration was determined, and the fractions (≈400 μl) were stored at −80°C. All proteins were resolved in 1-mm-thick 12% gels except for XIAP, HSP70, and HSC70, which were resolved in 14% gels, and transferred to PVDF membrane using a wet electroblotting apparatus. Blots were blocked and incubated at 4°C overnight with the specific primary antibody and for 1 h at room temperature with the appropriate HRP-conjugated secondary antibody. All primary antibodies were used at 1:1,000 dilution unless otherwise specified. Secondary antibodies were used at 1:2,000–2,500 dilution. Chemiluminescence was documented in X-ray film. Blots were stripped using a stripping buffer containing 100 mM 2-mercaptoethanol, 2% SDS, and 62.5 mM Tris·Cl (pH 6.7) at 70°C for 1 h and then probed with antibodies specific for loading controls.
CASPASE-3 ACTIVITY (12, 20).
Frozen left ventricular tissue from series 1, 2, and 3 was homogenized using a Dounce homogenizer in ice-cold lysis buffer (2 ml/100 mg tissue) composed of 50 mM HEPES (pH 7.4), 0.1% CHAPS, 5 mM DTT, 2 mM EDTA, 2 mM EGTA, 0.1% Triton X-100, and complete protease inhibitor tablet. Homogenates were centrifuged at 14,000 g for 10 min, and the supernatants were stored at −80°C.
Estimation of total protein in the homogenates was performed by Bradford method before running the caspase-3 activity assay. For series 1 and 2, 200 μg of protein were diluted in assay buffer containing 50 mM HEPES (pH 7.4), 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 2 mM EDTA, 2 mM EGTA, 0.1% Triton X-100, 1 mM PMSF, and protease inhibitor cocktail to a final reaction volume of 200 μl and incubated at 37°C with the fluorimetric substrate Ac-DEVD-AFC (50 μM final concentration) in 96-well microtiter plates covered with aluminum foil in a humidified chamber for 18 h. Ac-DEVD-AFC cleavage was determined by measuring AFC fluorescence at 400 nm excitation and 505 nm emission with a microplate reader (SpectraMax GeminiXS; Molecular Devices). Results were calibrated with known concentrations of AFC and expressed in picomoles of substrate cleaved per minute per milligram protein at 37°C.
For series 3, the SpectraMax GeminiXS plate reader was unavailable, and a plate reader at excitation 360/40 nm and emission 528/20 nm wavelengths (BioTek, VT) had to be used. This required a modification in the technique to compensate for auto fluorescence from homogenate and substrate. Accordingly, the standards were prepared in a reaction mixture in which the tissue homogenate, the assay buffer, the caspase-3 substrate (DEVD-AFC), and escalating known concentrations of AFC were added. The blank was prepared without AFC. Standard curves were prepared with and without caspase-3 inhibitor DEVD-CHO, which enabled calculations of activity in the samples treated with and without inhibitor. The assay reactions of the samples were carried out in duplicates with and without inhibitor. Respective standard curves were used for calculations.
LM-PCR.
Apoptotic DNA fragmentation was determined by LM-PCR enabling amplification of 5′ phosphorylated blunt-ended DNA fragments, 180 to 200 bp or multiple thereof, generated by endonucleolytic cleavage specific of apoptotic cell death (39). The reaction involves ligation of dephosphorylated adaptors to blunt-ended DNA fragments followed by PCR amplification using the same adaptors as primers conferring specificity and sensitivity to the reaction (39).
DNA was extracted from 100 mg of left ventricular tissue as previously reported (3, 6). Briefly, frozen tissue (≈100 mg) was minced in ice and digested in 800 μl of buffer composed of 50 mM Tris·Cl (pH 8), 25 mM EDTA (pH 8), 100 mM NaCl, 0.5% SDS, and 0.1 mg/ml proteinase K for 16 h at 55°C. DNase-free RNase A (1 μg/ml) was then added and incubated for 1 h at 37°C. NaCl (4 M) was added to a final concentration of 1.48 M, incubated for another hour at 4°C, and centrifuged at 10,000 g for 30 min at 4°C to remove precipitated proteins. The supernatant was extracted twice, first with an equal volume of phenol/chloroform/isoamyl alcohol (25:24:1) and then with chloroform/isoamyl alcohol (24:1) using phase lock gel tubes, and centrifuged at 12,000 g for 5 min. An equal volume of isopropanol was added to the aqueous phase and was centrifuged at 10,000 g for 10 min to precipitate DNA. The pellet was rinsed with 1 ml of 70% ethanol, air dried, and suspended in 100 μl of 1× TE buffer containing 10 mM Tris·Cl (pH 8) and 1 mM EDTA (pH 8). The DNA solution was then scanned between A225 and A325, and purity was documented by visualizing the characteristic DNA spectrum and documenting an A260-to-A280 ratio between 1.6 and 1.9.
LM-PCR was performed according to the APO-DNA1 PCR kit instruction with modifications. Blunt-ended DNA fragments were ligated to dephosphorylated adaptors in a reaction mixture containing 5 μl of ligation buffer (10×), DNA (0.5 μg), and adaptors (10×) to a final volume of 50 μl. The mixture was incubated at 55°C for 10 min (to denature adaptors) and cooled down to 10°C over 1 h (to anneal adaptors). One microliter of T4-DNA ligase (400 U/μl) was added and incubated at 16°C for 16 h. The adaptor-ligated DNA was stored at −20°C until PCR.
PCR was performed in a reaction mixture containing 25 μl of LM-PCR buffer (2×), 5 μl of adaptor primer (10×), 10 μl of adaptor-ligated DNA (150 ng), and 1 μl of Taq DNA polymerase (5 U/μl) to a final volume of 50 μl, using a hot-start and three-step cycles as follows: 72°C for 8 min with Taq polymerase added at the minute 3 followed by 40 cycles (94°C for 1 min, 58°C for 1 min, and 72°C for 2 min) and extension at 72°C for 15 min. After amplification, 25 μl of the PCR reaction along with controls were resolved in 1.2% agarose gel electrophoresis at 6-V/cm of gel for 2 h. The gel was stained with ethidium bromide, and the bands detected by one-dimensional image analysis software (Gel Logic 200 imaging system; Kodak) after UV light exposure.
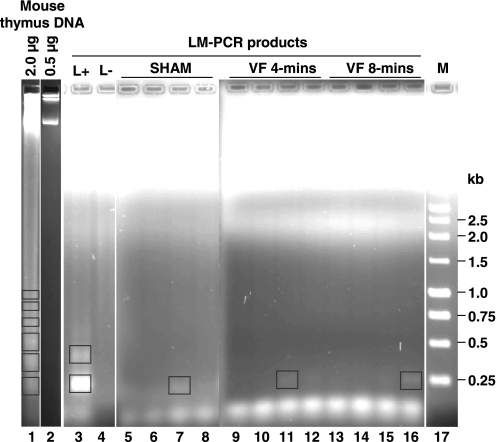
For positive control, DNA was isolated from mouse thymus 8 h after an intraperitoneal injection of dexamethasone (5 mg/kg) to induce apoptosis. A characteristic apoptotic DNA laddering was observed by gel electrophoresis after loading 2 μg of DNA but not after loading 0.5 μg of DNA. DNA laddering, however, was again observed after subjecting 0.15 μg of apoptotic thymus DNA to LM-PCR (Fig. 3).
Left ventricular tissue measurements from the coronary occlusion and reperfusion model.
The left ventricle of the excised heart was sliced perpendicular to the major axis into six 1-mm-thick sections using a stainless steel rat heart slicer (HSMS001.1; Zivic Instruments) starting at the level and moving distally toward the apex. Sections 2 and 4 were snap-frozen in liquid N2 and stored at −80°C until analysis for caspase-3 activity. Sections 1, 3, and 5 were stained with TTC (0.5% solution in PBS) at 37°C for 20 min. After TTC staining, sections were fixed in 4% paraformaldehyde for 20 min and stored at 4°C. The tetrazolium dye forms a red formazan complex in the presence of coenzyme and dehydrogenases in the viable myocardium, whereas areas of infarcted tissue remain unstained. About 50 mg of tissue from the myocardial region above the occlusion were snap-frozen in liquid N2 and stored at −80°C for later analysis of control caspase-3 activity.
INFARCT SIZE CALCULATION.
Infarct size was calculated to assess the severity of injury. Photographs of the TTC-stained sections were taken using a digital camera, and the images were analyzed by planimetry to identify the area at risk and the area of infarct. The tissue sections that were used for measuring caspase-3 activity included both normally perfused area and area at risk. The activity in the area at risk was calculated according to the following equation: caspase-3 activity in area at risk = {total tissue caspase-3 activity − [activity in the normally perfused tissue × percentage of normally perfused tissue (x)]} / [% of area at risk (1 − x)], in which x equals the percentage of normally perfused tissue.
Statistical Analysis
For continuous variables, differences among groups were analyzed using one-way ANOVA applying the Holm-Sidak's for multiple comparisons, and differences between groups were analyzed using Student's t-test. Alternative nonparametric tests were used if the data failed tests for normality or equal variance. The data were reported as means ± SD unless otherwise stated. A two-tail value of P < 0.05 was considered significant.
RESULTS
Series 1
Rats subjected to VF developed profound postresuscitation myocardial dysfunction with reductions in LVSWI to ≈23% of sham rats accompanied by marked reductions in +dP/dtmax and cardiac index. There were marked increases in arterial lactate with proportional decreases in arterial bicarbonate, which typically occur consequent to cardiac arrest. These metabolic changes partially normalized by minute 240 postresuscitation and were virtually identical in VF 4-min and VF 8-min groups, suggesting that the duration on untreated VF was not a determinant of the severity of postresuscitation myocardial and hemodynamic dysfunction (Table 1). This concept extended to the various left ventricular tissue measurements. Three rats in each VF groups died between minutes 120 and 240 following progressive myocardial dysfunction.
Table 1.
Hemodynamic and metabolic measurements: series 1
| Baseline, min −10 |
Postresuscitation, min |
||||
|---|---|---|---|---|---|
| 30 | 60 | 120 | 240 | ||
| MAP, mmHg | |||||
| Sham | 134±10 | 128±13 | 135±16 | 124±20 | 119±18 |
| VF 4-min | 141±7 | 94±32‡ | 99±21§ | 94±22‡ | 94±16* |
| VF 8-min | 142±8 | 94±9‡ | 97±8§ | 97±17† | 85±17‡ |
| CI, ml·min−1·kg−1 | |||||
| Sham | 134±24 | 120±33 | 117±27 | 114±26 | 131±39 |
| VF 4-min | 130±10 | 86±22† | 71±12§ | 51±9§ | 42±11§ |
| VF 8-min | 134±18 | 83±13‡ | 71±7§ | 53±13§ | 42±12§ |
| LVSWI, mmHg·ml/kg | |||||
| Sham | 0.80±0.16 | 0.69±0.23 | 0.71±0.21 | 0.64±0.18 | 0.74±0.27 |
| VF 4-min | 0.77±0.08 | 0.46±0.08† | 0.34±0.04§ | 0.22±0.05§ | 0.17±0.07§ |
| VF 8-min | 0.87±0.24 | 0.36±0.17‡ | 0.34±0.05§ | 0.26±0.09§ | 0.17±0.09§ |
| +dP/dtmax, mmHg/s·103 | |||||
| Sham | 4.82±1.50 | 4.52±1.58 | 5.00±2.03 | 4.61±2.40 | 4.48±1.90 |
| VF 4-min | 4.10±0.29 | 2.53±0.78‡ | 2.81±0.51‡ | 2.71±0.39† | 2.58±0.49* |
| VF 8-min | 3.80±0.38 | 2.61±0.23‡ | 2.99±0.22‡ | 2.94±0.37* | 2.60±0.25* |
| Lactate (Ao), mmol/l | |||||
| Sham | 1.0±0.2 | 1.5±0.4 | 1.6±0.4 | 2.0±0.9 | 1.6±0.4 |
| VF 4-min | 1.1±0.3 | 12.6±2.5§ | 7.2±2.8§ | 5.0±2.7‡ | 4.5±1.4‡ |
| VF 8-min | 1.1±0.3 | 12.0±3.5§ | 6.0±1.9§ | 4.5±1.8† | 4.5±2.1‡ |
| HCO3− (Ao), mmol/l | |||||
| Sham | 26±4 | 23±2 | 23±2 | 22±3 | 24±2 |
| VF 4-min | 24±3 | 10±3§ | 13±3§ | 12±4§ | 12±4§ |
| VF 8-min | 25±5 | 9±2§ | 13±2§ | 12±3§ | 11±3§ |
Values are means ± SD. Each group included eight rats; because some rats died before completing the maximum 240-min postresuscitation observation interval, only 5 rats in each of the VF groups remained at 240 min postresuscitation. Ventricular fibrillation (VF) 4-min and VF 8-min denote groups that were successfully resuscitated after 4 or 8 min of untreated VF. MAP, mean aortic pressure; CI, cardiac index; LVSWI, left ventricular stroke work index; +dP/dtmax, maximal rate of left ventricular pressure rise.
P < 0.05;
P < 0.02;
P < 0.01;
P < 0.001 vs. sham by one-way ANOVA and Holm-Sidak's test for multiple comparisons.
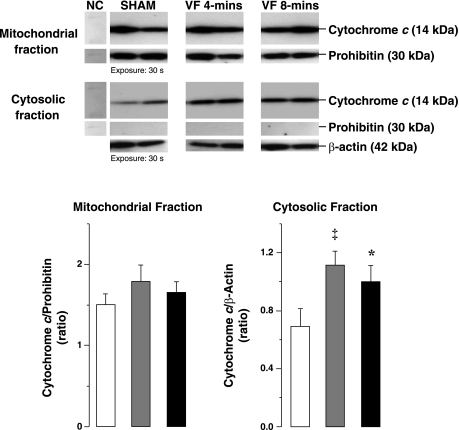
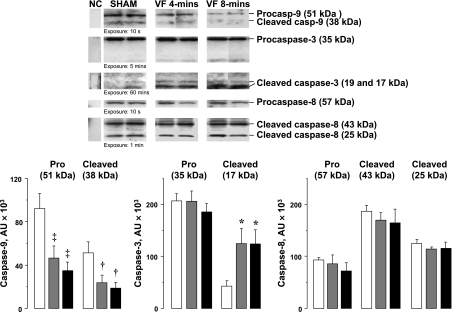
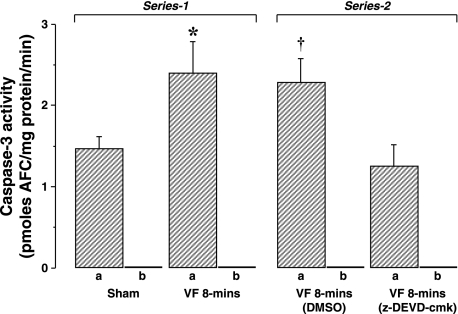
Left ventricular dysfunction was accompanied by prominent increases in cytosolic cytochrome c to ≈1.4-fold of sham hearts but without changes mitochondrial cytochrome c (Fig. 1). Procaspase-9 and cleaved caspase-9 were concomitantly reduced along with prominent increases in 17-kDa cleaved caspase-3 fragments to 2.9-fold of sham hearts (Fig. 2). Caspase-3 activity in VF group was increased by 1.6-fold of sham hearts (Fig. 5). In contrast, no changes in procaspase-8 and cleaved caspase-8 levels were noted (Fig. 2).
Fig. 1.
Representative immunoblots of left ventricular tissue from 2 sham, 2 ventricular fibrillation (VF) 4-min, and 2 VF 8-min groups showing cytochrome c and prohibitin in cytosolic and mitochondrial fractions and β-actin in cytosolic fraction. The corresponding densitometries for the entire group are shown in graphs depicting cytochrome c in mitochondrial fraction indexed to prohibitin (left) and cytochrome c in cytosolic fraction indexed to β-actin (right). White columns denote sham (n = 8), gray columns denote VF 4-min (n = 8), and black columns denote VF 8-min (n = 8). NC, negative control. Means are ± SE. *P < 0.05, ‡P < 0.01 vs. sham by 1-way ANOVA and Holm-Sidak's test for multiple comparisons.
Fig. 2.
Representative immunoblots of left ventricular tissue from 2 sham, 2 VF 4-min, and 2 VF 8-min groups showing procaspases and cleaved caspases-9, -3, and -8 in cytosolic fractions. The corresponding densitometries in arbitrary units (AU) are shown in graphs depicting procaspase and cleaved caspase-9 (n = 4), procaspase and cleaved caspase-3 (n = 8), and procaspase and cleaved caspase-8 (n = 4) in the cytosolic fraction. White columns denote sham, gray columns denote VF 4-min, and black columns denote VF 8-min. Means are ± SE. ‡P < 0.01; †P < 0.02; *P < 0.05 vs. sham by 1-way ANOVA and Holm-Sidak's test for multiple comparisons.
The data on apoptotic DNA fragmentation are shown in Figure 3 comparing the four sham hearts with the four hearts from each of the VF groups along with the proper LM-PCR controls. Noted were bands of ≈0.25 kb in one sham heart and in the heart of each VF group indicating that the level of DNA fragmentation in VF hearts was not different than the level in sham hearts despite profound left ventricular dysfunction and activation of caspase-3.
Fig. 3.
Gel electrophoresis of genomic DNA and products of a ligation-mediated (LM)-PCR. Genomic DNA, isolated from mouse thymus after treatment with dexamethasone (5 mg/kg), shows the characteristic apoptotic laddering when 2 μg but not when 0.5 μg of DNA were loaded (lanes 1 and 2). Yet, 0.15 μg of dexamethasone-treated mouse thymus DNA subjected to LM-PCR yielded bands starting at ≈0.20 kb only in the presence of ligase (L+) representing a positive control for the LM-PCR reaction (lanes 3 and 4). Left ventricular genomic DNA (0.15 μg) amplified by LM-PCR is shown for 4 sham (lanes 5-8), 4 VF 4-min (lanes 9-12), and 4 VF 8-min (lanes 13-16). Molecular weight markers (M) are shown on lane 17. Primer dimer products are seen below the 0.25-kb marker. Boxes represent bands identified by densitometry using 1-dimensional image analysis software (Kodak).
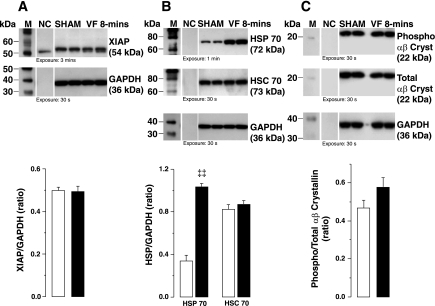
The levels of XIAP and S59 phosphorylated fraction of the small HSP αβ-crystallin were similar in VF and sham groups (Fig. 4, A and C). In contrast, HSP70, an inducible HSP, was 3.1-fold higher in the VF group. However, no differences were noted in the constitutive HSC70 (Fig. 4B).
Fig. 4.
Representative immunoblots of left ventricular tissue from 2 sham and 2 VF 8-min groups showing X-linked inhibitor of apoptosis protein (XIAP; A), heat shock protein (HSP)70 and heat shock congnate (HSC)70 (B), and phospho [serine 59 (S59)] and total αβ-crystallin (Cryst; C). GAPDH in cytosolic fractions was used as internal control. The corresponding densitometries for the entire series are shown in graphs depicting XIAP indexed to GAPDH (left), HSP70 and HSC70 indexed to GAPDH (middle), and phospho (S59) αβ-crystallin indexed to total αβ-crystallin (right). White columns denote sham (n = 4), and black columns denote VF 8-min (n = 4). Means are ± SE. ‡‡P < 0.001 vs. sham by Student's t-test.
Series 2
Rats treated with the caspase-3 inhibitor z-DEVD-cmk and rats treated with vehicle control (DMSO) developed the characteristic postresuscitation myocardial dysfunction without measurable differences between groups (Table 2). The absence of differences occurred despite reductions in caspase-3 activity to 45% of control, a value that was similar to the value in sham hearts (Fig. 5). Accordingly, inhibition of caspase-3 activity failed to prevent or ameliorate postresuscitation left ventricular dysfunction.
Table 2.
Hemodynamic measurements: series 2
| Baseline, min −10 |
Postresuscitation, min |
||||
|---|---|---|---|---|---|
| 30 | 60 | 120 | 240 | ||
| MAP, mmHg | |||||
| DMSO | 145±8 | 109±19 | 99±17 | 101±22 | 95±42 |
| z-DEVD-cmk | 144±6 | 99±10 | 96±12 | 91±14 | 99±11 |
| CI, ml·min−1·kg−1 | |||||
| DMSO | 151±8 | 82±27 | 76±18 | 55±15 | 65±20 |
| z-DEVD-cmk | 148±13 | 89±22 | 77±12 | 56±5 | 46±15 |
| LVSWI, mmHg·ml/kg | |||||
| DMSO | 1.03±0.14 | 0.49±0.22 | 0.38±0.11 | 0.27±0.10 | 0.32±0.21 |
| z-DEVD-cmk | 0.94±0.15 | 0.50±0.15 | 0.40±0.06 | 0.27±0.04 | 0.24±0.08 |
| +dP/dtmax, mmHg/s·103 | |||||
| DMSO | 3.96±0.27 | 3.05±0.65 | 2.89±0.49 | 2.70±0.77 | 2.85±1.03 |
| z-DEVD-cmk | 3.58±0.67 | 2.84±0.35 | 2.98±0.13 | 2.91±0.32 | 2.93±0.66 |
Values are means ± SD. Each group included four rats, each pretreated with DMSO or the caspase-3 inhibitor z-Asp-Glu-Val-Asp chloromethyl ketone (z-DEVD-cmk); because some rats died before completing the maximum 240-min postresuscitation observation interval, only 3 rats per group remained at 240 min postresuscitation. Rats underwent 8 min of untreated VF and were successfully defibrillated after 8 min of closed-chest resuscitation.
Fig. 5.
Caspase-3 activity in left ventricular tissue. In series 1, activity in sham rats (n = 4) was compared with activity in VF 8-min group (n = 4). In series 2, activity in rats pretreated with DMSO (n = 4) was compared with activity in rats pretreated with the caspase-3 inhibitor z-Asp-Glu-Val-Asp chloromethyl ketone (z-DEVD-cmk; n = 4). For each group, samples were treated in the absence (a) and presence (b) of the caspase-3 inhibitor DEVD-CHO to control for the specificity of the reaction. Means are ± SE. *P < 0.05 vs. sham; †P < 0.03 vs. z-DEVD-cmk by Student's t-test. AFC, 7-amino-4-trifluoromethyl coumarin.
Series 3
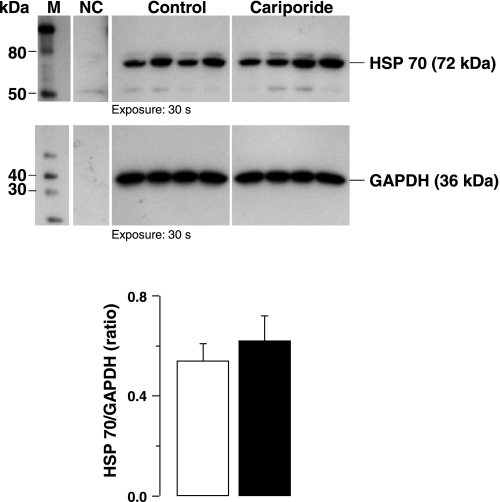
Baseline hemodynamic measurements were comparable between control and cariporide groups (Table 3). Postresuscitation, there were numerical differences consistent with less postresuscitation myocardial dysfunction in rats treated with cariporide, as previously reported using larger sample sizes (5, 13), but the differences were statistically insignificant. Caspase-3 activity was not statistically different between groups; however, numerically caspase-3 activity was higher in cariporide-treated rats. Accordingly, there was no evidence to suggest that amelioration of postresuscitation myocardial dysfunction by NHE-1 inhibition involved the attenuation of caspase-3 activation. Likewise, there were no differences in HSP70 levels (Fig. 6).
Table 3.
Hemodynamic and caspase-3 activity measurements: series 3
| Baseline, min −10 |
Postresuscitation, min |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 15 | 30 | 60 | ||||||
| MAP, mmHg | |||||||||
| Control | 130±14 | 64±33 | 63±23 | 80±17 | 80±20 | ||||
| Cariporide | 138±14 | 91±31 | 92±23 | 96±13 | 99±15 | ||||
| CI, ml·min−1·kg−1 | |||||||||
| Control | 130±24 | 48±29 | 75±43 | 86±22 | 61±23 | ||||
| Cariporide | 132±28 | 50±16 | 87±7 | 90±11 | 102±29 | ||||
| LVSWI, mmHg·ml/kg | |||||||||
| Control | 0.74±0.15 | 0.27±0.17 | 0.34±0.23 | 0.40±0.07 | 0.27±0.10 | ||||
| Cariporide | 0.76±0.16 | 0.34±0.20 | 0.47±0.15 | 0.44±0.11 | 0.46±0.16 | ||||
| +dP/dtmax, mmHg/s·103 | |||||||||
| Control | 3.57±0.11 | 1.83±0.88 | 1.74±0.68 | 2.31±0.59 | 2.49±0.58 | ||||
| Cariporide | 3.64±0.62 | 2.43±0.88 | 2.28±0.54 | 2.65±0.32 | 2.82±0.39 | ||||
| Caspase-3 activity, pmol AFC·min−1·mg protein−1 | |||||||||
| Control | 1.57±0.17 | ||||||||
| Cariporide | 1.91±0.30 | ||||||||
Values are means ± SD. Each group included four rats, each treated with saline or the sodium-hydrogen exchanger isoform-1 inhibitor cariporide given immediately before initiating chest compression. Rats underwent 10 min of untreated VF and were successfully defibrillated after 5 to 6 min of closed-chest resuscitation. Differences between groups were not statistically significant. AFC, 7-amino-4-trifluoromethyl coumarin.
Fig. 6.
Representative immunoblots of left ventricular tissue from 4 control and 4 cariporide rats (series 3) showing HSP70 levels. GAPDH in cytosolic fractions was used as internal control. The corresponding densitometries for the entire series are shown in graphs. White columns denote control (n = 4), and black columns denote cariporide group (n = 4). Means are ± SE.
DISCUSSION
We have demonstrated in a rat model of postresuscitation myocardial dysfunction selective activation of the mitochondrial apoptotic pathway accompanied by activation of caspase-3 at 240 min postresuscitation coincident with severe myocardial dysfunction. However, despite caspase-3 activation and myocardial dysfunction, levels of apoptotic DNA fragmentation were not increased and caspase-3 inhibition failed to attenuate postresuscitation myocardial dysfunction. Moreover, the administration of the NHE-1 inhibitor cariporide, an intervention previously shown to attenuate postresuscitation myocardial dysfunction, had no effects on caspase-3 activity. Thus caspase-3 activation could not be implicated in the pathogenic mechanism leading to the severe myocardial dysfunction that develops early after resuscitation from cardiac arrest. Yet, delayed effects of caspase-3 playing a role during the recovery phase of myocardial dysfunction were not studied and cannot be excluded. In addition, further work examining the potential antiapoptotic roles of HSP70 and XIAP during the early postresuscitation phase is warranted.
Postresuscitation Myocardial Dysfunction
Postresuscitation myocardial dysfunction is a well-documented phenomenon in animal models and in human victims of cardiac arrest, with myocardial function returning to baseline within days or weeks (14, 21). Reversibility suggests that the principal mechanism of dysfunction is myocardial stunning. However, it is not clear whether a similar mechanism develops in subjects who do not survive and succumb to severe myocardial dysfunction. We documented marked reductions in LVSWI to 23% of sham controls with 38% mortality. Postresuscitation myocardial dysfunction at the cell level is primarily a consequence of abnormalities precipitated by ischemia and reperfusion and includes oxidative injury (46), Ca2+ overload (44), and disruption of energy metabolism (4). In the present study, we documented myocardial dysfunction associated with selective activation of the mitochondrial apoptotic pathway leading to the activation of caspase-3. In the first series in which we varied the duration of untreated VF, and presumably the severity of injury, comparable postresuscitation defects at the functional and molecular levels were documented. This is an intriguing observation that would support the concept that injury occurs consequent to reperfusion with the duration of the preceding ischemic interval being less important.
Mitochondrial Apoptotic Pathway
Left ventricular tissue analysis demonstrated increased levels of cytochrome c in the cytosol, decreased levels of procaspase-9 and cleaved caspase-9, and activation of caspase-3 evidenced by the release of 17-kDa fragments and increased enzymatic activity. However, there were no changes in procaspase-8 and cleaved caspase-8. These findings support a selective activation of the mitochondrial apoptotic pathway with apoptosome formation leading to caspase-3 activation (23). The apoptosome is a cytosolic complex composed of multimers of the apoptotic protease activating factor-1, cytochrome c, dATP or ATP, and procaspase-9. In the apoptosome, procaspase-9 undergoes conformational changes and cleavage with the release of caspase-9, which in turn cleaves and activates caspase-3. Caspase-3 activation is an important step in the “executioner” path, leading to internucleosomal DNA fragmentation (11, 25).
The decreased level of procaspase-9 is consistent with cleavage via apoptosome complex. The concomitant decrease of cleaved caspase-9 could reflect nuclear translocation as reported in a mouse model of middle cerebral artery occlusion and reperfusion (32) or possibly proteasomal degradation (15).
DNA Fragmentation
DNA fragmentation is a terminal event mediated through executioner caspases, including caspase-3 activation and nuclear translocation of a DNase known as caspase-3-activated DNase (CAD) (11, 25), which cleaves genomic DNA into 180 to 200 bp fragments or multiples thereof (38). We used a highly sensitive and specific LM-PCR technique after failing to document apoptotic fragmentation of left ventricular DNA by direct gel electrophoresis (data not shown). With LM-PCR, DNA fragments that have blunt ends, characteristic of endonucleolytic cleavage (11), are first ligated to dephosphorylated adaptors using T4-DNA ligase and then amplified by PCR (39). No differences were detected between VF and sham rats. It is worth noting that the positive control for LM-PCR (thymus DNA from dexamethasone-treated mouse) demonstrated detection of apoptotic DNA fragmentation at levels not detectable by direct gel electrophoresis (Fig. 3, lanes 1-4). DNA fragmentation after ischemia and reperfusion has been reported in other models after longer intervals of ischemia before reperfusion. For example, DNA fragmentation was reported in rat hearts after 30 min of global ischemia followed by 30 min (37) or 240 min (29) of reperfusion and after 30 min of coronary occlusion followed by 6 h of reperfusion (33). It is possible that longer duration of ischemia could promote the distinct pathophysiological process yielding predominantly cell death, with shorter ischemic intervals promoting predominantly cell dysfunction.
Accordingly, the lack of detection of apoptotic DNA fragments using the highly sensitive LM-PCR technique at 4 h postresuscitation despite caspase-3 activation and severe myocardial dysfunction support the concept that postresuscitation myocardial dysfunction in our rat model is not the result of apoptotic cell death. Caspase-3 activation without DNA fragmentation has also been documented after β-adrenergic stimulation (10) and in patients with heart failure caused by ischemic cardiomyopathy (31).
Caspase-3 Effects on Contractile Function
Besides promoting cell death, caspase-3 activation can cleave structural and regulatory sarcomeric proteins leading to contractile dysfunction (10, 27). This effect can be prevented by the administration of caspase-3 inhibitors (10, 27). Thus the possibility remained that caspase-3 activation still played a pathogenic role in postresuscitation myocardial dysfunction by altering contractile function rather than promoting cell death. However, the administration of the irreversible caspase-3 inhibitor z-DEVD-cmk failed to prevent postresuscitation myocardial dysfunction despite reductions in caspase-3 activity to levels comparable with those in sham hearts (Fig. 5). Accordingly, there was no evidence that caspase-3 activation played a role on early postresuscitation myocardial dysfunction.
We further examined whether an intervention previously shown to ameliorate postresuscitation myocardial dysfunction in our model, namely NHE-1 inhibition using cariporide (5, 13), could be associated with reductions in caspase-3 activation. However, caspase-3 activity was not reduced by the administration of cariporide. In fact, caspase-3 activity was numerically higher when indexes of myocardial function were also higher in hearts from cariporide-treated rats.
Intensity of Caspase-3 Activation
We cannot exclude that caspase-3 activation in our model was modest and that more intense caspase-3 activation could have played a more prominent role. To evaluate whether a more severe injury could lead to higher levels of caspase-3 activity, we assessed myocardial caspase-3 activity in a rat model of coronary occlusion (30 min) and reperfusion (4 h). The model resulted in a left ventricular area at risk below LAD occlusion of 48% with a percent infarct of 25%. Caspase-3 activity in the area at risk was 3.9 compared with 0.3 pmol AFC·min−1·mg protein−1 in the normally perfused area representing a 13-fold increase in activity (Table 4). With the use of the same technique, caspase-3 activity in our VF model was ≈1.7 pmol AFC·min−1·mg protein−1 (Table 3) representing ≈40% of the activity of the coronary occlusion model. Caspase-3 inhibitors have been shown to reduce infarct size in models of coronary occlusion and reperfusion (26, 37). Taken together, the data suggest that the injury precipitated by VF and resuscitation fails to maximally activate caspase-3 (relative to coronary occlusion and reperfusion) and that such level of activity may not be sufficient to induce cell death as in a coronary occlusion and reperfusion model.
Table 4.
Infarct size measurements and caspase-3 activity
| Area at risk, % | Border Zone Over Area at Risk, % | Infarct Zone Over Area at Risk, % |
Caspase-3 Activity, pmol AFC·min−1·mg protein−1 |
||
|---|---|---|---|---|---|
| Total Tissue | Perfused Tissue | Area at Risk | |||
| 48±2 | 75±17 | 25±17 | 2.0±1.2 | 0.3±0.3 | 3.9±2.1 |
Values are means ± SD. Two rats were subjected to a myocardial infarction protocol of 30 min of occlusion and 4 h of reperfusion. Infarct size was calculated using planimetry. Caspase-3 activity at the area of risk was measured as described in materials and methods. Total tissue activity included normally perfused tissue and tissue of area at risk.
Extrinsic Apoptotic Pathway
Apoptosis can also be initiated through the extrinsic pathway, which involves activation via death receptors with recruitment of procaspase-8 (9, 30). In our model, levels of procaspase-8 and cleaved caspase-8 were unchanged, suggesting that the extrinsic apoptotic pathway was not activated. Several extracellular mediators of inflammation can activate the extrinsic apoptotic pathway (22). This is relevant to resuscitation because a prominent systemic inflammatory response develops after the return of spontaneous circulation characterized by increased plasma levels of TNF-α, various interleukins, and endotoxin (1). However, this response leads to a hyperdynamic state, which was not observed in our model. Yet, we cannot exclude that activation of the extrinsic apoptotic pathway could play a role late in the postresuscitation phase.
Intrinsic Antiapoptotic Mechanisms
We also examined responses to apoptotic signaling by measuring HSP70, HSC70, αβ-crystallin, and XIAP. HSP70, which does not prevent caspase-3 activation but acts downstream and inhibits DNA fragmentation (16), was found to be significantly upregulated at 240 min postresuscitation. Upregulation of HSP70 has also been reported in a rabbit model of coronary artery ligation and reperfusion (18). Overexpression of HSP70 can protect the myocardium from ischemia and reperfusion injury (17, 34, 40). Levels of the constitutively expressed HSC70 were unchanged, consistent with reports by other investigators (34). We also observed a statistically insignificant increase in S59 phosphorylated small HSP αβ-crystallin, which has been shown to confer the protection of neonatal rat cardiomyocytes against hypoxia through inhibition of caspase-3 activation and DNA fragmentation (28). Of the IAP family, XIAP is the best characterized and most potent in degrading activated caspase-3 (8, 36, 41). Levels of XIAP were preserved in our model. In other settings of regional ischemia, XIAP has been shown to be degraded and associated with DNA fragmentation (24). Accordingly, these intrinsic antiapoptotic mechanisms could have limited potential detrimental effects of caspase-3 activation in myocardial tissue.
Limitations of the Study
The tissue measurements were limited to 240 min postresuscitation. Although we found no evidence that caspase-3 activation played a role, we cannot exclude that DNA fragmentation, and caspase-3-dependent sarcomeric disruption could develop at a later postresuscitation time. Examining this possibility, however, would be challenging requiring hemodynamic intervention to avert death and enable measurements after a much longer postresuscitation interval. Lowering the level of injury (i.e., shorter VF duration and shorter resuscitation time) would not be an option because such approach would most likely reduce the intensity of the injury we wanted to examine.
The antiapoptotic processes examined here were limited to measuring XIAP, HSP70, HSC70, and αβ-crystallin. There is a myriad of mechanisms and pathways that are known to contribute to postischemic dysfunction. Hence, there exists a possibility that many other protective mechanisms could have played roles. Moreover, these observations apply to healthy rats anesthetized with pentobarbital sodium, which can also exert myocardial protective effects.
Conclusions
We confirm activation of the mitochondrial apoptotic pathway in our rat model of VF and severe postresuscitation myocardial dysfunction. Yet, contrary to our hypothesis and observations made by others after more prolonged ischemic intervals, there was no evidence that caspase-3 activation played a role in postresuscitation myocardial dysfunction, at least within the postresuscitation time frame that we were interested in. Yet, antiapoptotic mechanisms could have played a role. These observations suggest that myocardial dysfunction resulting from cardiac arrest and resuscitation might have pathophysiological mechanisms different than those associated with more prolonged intervals of ischemia and reperfusion. Intrinsic antiapoptotic mechanisms might play a role worth further research.
GRANTS
This work is supported by National Heart, Lung, and Blood Institute Grant R01-HL71728-01 and a Veterans Affairs Merit Review Grant (both to R. J. Gazmuri).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C, Dhainaut JF, Cavaillon JM. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation 106: 562–568, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Arends MJ, Morris RG, Wyllie AH. Apoptosis. The role of the endonuclease. Am J Pathol 136: 593–608, 1990. [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Analysis of proteins. In: Short Protocols in Molecular Biology. John Wiley & Sons, 2004.
- 4.Ayoub IM, Kolarova J, Kantola R, Radhakrishnan J, Gazmuri RJ. Zoniporide preserves left ventricular compliance during ventricular fibrillation and minimizes post-resuscitation myocardial dysfunction through benefits on energy metabolism. Crit Care Med 35: 2329–2336, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Ayoub IM, Kolarova JD, Yi Z, Trevedi A, Deshmukh H, Lubell DL, Franz MR, Maldonado FA, Gazmuri RJ. Sodium-hydrogen exchange inhibition during ventricular fibrillation: beneficial effects on ischemic contracture, action potential duration, reperfusion arrhythmias, myocardial function, and resuscitability. Circulation 107: 1804–1809, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bialik S, Cryns VL, Drincic A, Miyata S, Wollowick AL, Srinivasan A, Kitsis RN. The mitochondrial apoptotic pathway is activated by serum and glucose deprivation in cardiac myocytes. Circ Res 85: 403–414, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Bradford MM A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 8.Cheung HH, LaCasse EC, Korneluk RG. X-linked inhibitor of apoptosis antagonism: strategies in cancer treatment. Clin Cancer Res 12: 3238–3242, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81: 505–512, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci USA 99: 6252–6256, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391: 43–50, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Fauvel H, Marchetti P, Chopin C, Formstecher P, Neviere R. Differential effects of caspase inhibitors on endotoxin-induced myocardial dysfunction and heart apoptosis. Am J Physiol Heart Circ Physiol 280: H1608–H1614, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Gazmuri RJ, Ayoub IM, Hoffner E, Kolarova JD. Successful ventricular defibrillation by the selective sodium-hydrogen exchanger isoform-1 inhibitor cariporide. Circulation 104: 234–239, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Gazmuri RJ, Weil MH, Bisera J, Tang W, Fukui M, McKee D. Myocardial dysfunction after successful resuscitation from cardiac arrest. Crit Care Med 24: 992–1000, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Hao Y, Sekine K, Kawabata A, Nakamura H, Ishioka T, Ohata H, Katayama R, Hashimoto C, Zhang X, Noda T, Tsuruo T, Naito M. Apollon ubiquitinates SMAC and caspase-9, and has an essential cytoprotection function. Nat Cell Biol 6: 849–860, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J 17: 6124–6134, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayakumar J, Suzuki K, Sammut IA, Smolenski RT, Khan M, Latif N, Abunasra H, Murtuza B, Amrani M, Yacoub MH. Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation 104: I303–I307, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Knowlton AA, Brecher P, Apstein CS. Rapid expression of heat shock protein in the rabbit after brief cardiac ischemia. J Clin Invest 87: 139–147, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolarova JD, Ayoub IM, Gazmuri RJ. Cariporide enables hemodynamically more effective chest compression by leftward shift of its flow-depth relationship. Am J Physiol Heart Circ Physiol 288: H2904–H2911, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Lancel S, Joulin O, Favory R, Goossens JF, Kluza J, Chopin C, Formstecher P, Marchetti P, Neviere R. Ventricular myocyte caspases are directly responsible for endotoxin-induced cardiac dysfunction. Circulation 111: 2596–2604, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut JF. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol 40: 2110–2116, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci 118: 265–267, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Liu HR, Gao E, Hu A, Tao L, Qu Y, Most P, Koch WJ, Christopher TA, Lopez BL, Alnemri ES, Zervos AS, Ma XL. Role of Omi/HtrA2 in apoptotic cell death after myocardial ischemia and reperfusion. Circulation 111: 90–96, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89: 175–184, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Mocanu MM, Baxter GF, Yellon DM. Caspase inhibition and limitation of myocardial infarct size: protection against lethal reperfusion injury. Br J Pharmacol 130: 197–200, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moretti A, Weig HJ, Ott T, Seyfarth M, Holthoff HP, Grewe D, Gillitzer A, Bott-Flugel L, Schomig A, Ungerer M, Laugwitz KL. Essential myosin light chain as a target for caspase-3 in failing myocardium. Proc Natl Acad Sci USA 99: 11860–11865, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison LE, Hoover HE, Thuerauf DJ, Glembotski CC. Mimicking phosphorylation of alphaB-crystallin on serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circ Res 92: 203–211, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem 279: 47985–47991, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. An induced proximity model for caspase-8 activation. J Biol Chem 273: 2926–2930, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Narula J, Haider N, Arbustini E, Chandrashekhar Y. Mechanisms of disease: apoptosis in heart failure—seeing hope in death. Nat Clin Pract Cardiovasc Med 3: 681–688, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Noshita N, Sugawara T, Fujimura M, Morita-Fujimura Y, Chan PH. Manganese superoxide dismutase affects cytochrome c release and caspase-9 activation after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab 21: 557–567, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Okamura T, Miura T, Takemura G, Fujiwara H, Iwamoto H, Kawamura S, Kimura M, Ikeda Y, Iwatate M, Matsuzaki M. Effect of caspase inhibitors on myocardial infarct size and myocyte DNA fragmentation in the ischemia-reperfused rat heart. Cardiovasc Res 45: 642–650, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Okubo S, Wildner O, Shah MR, Chelliah JC, Hess ML, Kukreja RC. Gene transfer of heat-shock protein 70 reduces infarct size in vivo after ischemia/reperfusion in the rabbit heart. Circulation 103: 877–881, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Radhakrishnan J, Wang S, Ayoub IM, Kolarova JD, Levine RF, Gazmuri RJ. Circulating levels of cytochrome c after resuscitation from cardiac arrest: a marker of mitochondrial injury and predictor of survival. Am J Physiol Heart Circ Physiol 292: H767–H775, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richter BW, Duckett CS. The IAP proteins: caspase inhibitors and beyond. Sci STKE 2000: PE1–PE4, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Ruetten H, Badorff C, Ihling C, Zeiher AM, Dimmeler S. Inhibition of caspase-3 improves contractile recovery of stunned myocardium, independent of apoptosis-inhibitory effects. J Am Coll Cardiol 38: 2063–2070, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 391: 96–99, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Staley K, Blaschke AJ, Chun J. Apoptotic DNA fragmentation is detected by a semiquantitative ligation-mediated PCR of blunt DNA ends. Cell Death Differ 4: 66–75, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki K, Sawa Y, Kaneda Y, Ichikawa H, Shirakura R, Matsuda H. In vivo gene transfection with heat shock protein 70 enhances myocardial tolerance to ischemia-reperfusion injury in rat. J Clin Invest 99: 1645–1650, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci USA 98: 8662–8667, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thornberry NA Caspases: key mediators of apoptosis. Chem Biol 5: R97–R103, 1998. [DOI] [PubMed] [Google Scholar]
- 43.von Planta I, Weil MH, von Planta M, Bisera J, Bruno S, Gazmuri RJ, Rackow EC. Cardiopulmonary resuscitation in the rat. J Appl Physiol 65: 2641–2647, 1988. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Radhakrishnan J, Ayoub IM, Kolarova JD, Taglieri DM, Gazmuri RJ. Limiting sarcolemmal Na+ entry during resuscitation from VF prevents excess mitochondrial Ca2+ accumulation and attenuates myocardial injury. J Appl Physiol 103: 55–65, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Wyllie AH Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284: 555–556, 1980. [DOI] [PubMed] [Google Scholar]
- 46.Zweier JL, Kuppusamy P, Williams R, Rayburn BK, Smith D, Weisfeldt ML, Flaherty JT. Measurement and characterization of postischemic free radical generation in the isolated perfused heart. J Biol Chem 264: 18890–18895, 1989. [PubMed] [Google Scholar]