Abstract
Protein kinase C (PKC) stimulation of NAD(P)H oxidases (Nox) is an important component of multiple vascular disease processes; however, the relationship between oxidase activation and the regulation of vascular smooth muscle contraction by PKC remains poorly understood. Therefore, we examined the signaling cascade of PKC-elicited Nox activation and the role of superoxide and hydrogen peroxide in mediating PKC-induced vascular contraction. Endothelium-denuded bovine coronary arteries showed a PKC-dependent basal production of lucigenin (5 μM)-detected Nox oxidase-derived superoxide, which was stimulated fourfold by PKC activation with 10 μM phorbol 12,13-dibutyrate (PDBu). PDBu appeared to increase superoxide generation by Nox2 through both p47phox and peroxide-dependent Src activation mechanisms based on the actions of inhibitors, properties of Src phosphorylation, and the loss of responses in aorta from mice deficient in Nox2 and p47phox. The actions of inhibitors of contractile regulating mechanisms, scavengers of superoxide and peroxide, and responses in knockout mouse aortas suggest that a major component of the contraction elicited by PDBu appeared to be mediated through peroxide derived from Nox2 activation stimulating force generation through Rho kinase and calmodulin kinase-II mechanisms. Superoxide generated by PDBu also attenuated relaxation to nitroglycerin. Peroxide-derived from Nox2 activation by PKC appeared to be a major contributor to the thromboxane A2 receptor agonist U46619 (100 nM)-elicited contraction of coronary arteries. Thus a p47phox and Src kinase activation of peroxide production by Nox2 appears to be an important contributor to vascular contractile mechanisms mediated through activation of PKC.
Keywords: hydrogen peroxide, NADPH oxidase, protein kinase C, Src kinases, coronary artery
previous studies have identified important roles for protein kinase C (PKC) in the actions of receptors mediating smooth muscle contraction (3, 5, 18, 23) and in the activation of NAD(P)H or Nox oxidases (4, 13, 14, 22). However, only minimal attention has been given to defining the role of Nox oxidase-derived reactive oxygen species (ROS) in smooth muscle contraction regulated through activation of PKC systems. Studies on the ROS-dependent growth-promoting actions of the angiotensin II type 1 receptor by Griendling and colleagues (22) in cultured vascular smooth muscle cells (VSMCs) have identified an initial PKC-stimulated activation of Nox-derived superoxide production by a mechanism involving phosphorylation and membrane binding of its p47phox subunit. Peroxide derived from the initial increase in Nox activity appears to cause the subsequent stimulation of a Src kinase-elicited EGF receptor transactivation mechanism that promotes rac1 binding to the membrane-associated Nox subunit and a greater level of more sustained oxidase activation. Although this previous study on Nox activation was conducted in vascular smooth muscle cells known to contain Nox1 (22), other forms of Nox, such as Nox2 found in the bovine coronary arteries (BCA) used in the present study (8), have similar mechanisms of oxidase activation initiated by PKC (4, 13, 14). Recent studies examining the role of PKC in promoting vascular smooth muscle contraction have generally focused on comparing its importance or interactions with other systems such as Rho kinase (5, 11, 18, 23), and a role for oxidase activation by PKC is generally not considered in the mechanisms discussed.
ROS have been linked to the activation of several different signaling systems that can promote vascular contraction and modulate relaxing mechanisms (1, 14, 17, 25–27). For example, in a recent study, evidence was provided suggesting that peroxide could promote contraction in rat vascular segments through mechanisms involving extracellular signal-regulated kinase 1 and 2 (ERK), p38 mitogen-activated protein kinase (MAPK), Rho kinase, and Src kinase systems (24). Although it is known that multiple forms of protein phosphatases are inhibited by ROS (6, 26), the actual ways ROS stimulate each of these contraction-associated signaling systems remains poorly understood. There also appears to be multiple ways peroxide can promote contraction through increasing intracellular calcium (1, 17, 25, 27). Superoxide-mediated inhibition of nitric oxide stimulation of cGMP-mediated relaxation is also a key target of the vasoactive actions of superoxide. Although mechanisms of contraction by ROS involving the Src, ERK, p38 MAPK, and Rho kinase systems have been the subject of in-depth studies (1, 10, 19, 24), the importance of a role of PKC in regulating these processes is not well defined.
The objective of the present study is to determine whether superoxide and/or hydrogen peroxide resulting from the activation of Nox oxidases in vascular smooth muscle is an important contributing factor to mediating PKC-induced contraction. Since PKC signaling contributes to the actions of many of the G-coupled receptors, an additional objective was to examine whether Nox activation and ROS were significant components of the mechanism of contractile stimuli such as the thromboxane A2 receptor agonist U46619, which typically (18, 20) uses PKC signaling in promoting force generation in vascular smooth muscle.
MATERIALS AND METHODS
Many of the reagents used in the present study were obtained from Sigma Chemical (St. Louis, MO), as previously described (8, 9, 19); phorbol-12,13-dibutyrate (PDBu), staurosporine, chelerythrine, calphostin C, allopurinol, diphenyleneiodonium, tiron, peg-superoxide dismutase, peg-catalase, BAPTA, EGTA, and phenylenephrine were purchased from Sigma Chemical; U-46619 was purchased from Cayman Chemical (Ann Arbor, MI); and PP2 was purchased from Calbiochem (San Diego, CA). All salts were purchased from J. T. Baker & Co. (Phillipsburg, NJ). Wild-type (C57BL) and knockout (p47phox and gp91phox) mice were purchased from Jackson Laboratories. The Institutional Animal Use Committee approved all protocols and surgical procedures, which were in accordance with National Institutes of Health and American Physiological Society guidelines.
Measurement of changes of force generation in BCA and mouse aorta.
Isolated, endothelium-rubbed left anterior descending coronary arterial rings (BCA) were prepared from slaughterhouse-derived bovine hearts and studied for changes in isometric force, as previously described (8, 9). The rings were incubated in individually thermostated (37°C) 10-ml baths (Metro Scientific) for 2 h at an optimal passive tension of 5 g in Krebs bicarbonate buffer (pH 7.4) containing the following (in mM): 118 NaCl, 4.7 KCl, 1.5 CaCl2, 25 NaHCO3, 1.1 MgSO4, 1.2 KH2PO4, and 5.6 glucose, gassed with 21% O2-5% CO2-balance N2. After a 2-h equilibration and a brief depolarization with123 mM KCl, because it increases the reproducibility of subsequent contractions, BCA were re-equilibrated in Krebs buffer for 30 min before the experiments were conducted with 30 mM KCl, 100 nM U46619, and 10 μM PDBu. The isometric force development in mice aorta was studied as described above with slight modifications. Briefly, endothelium-denuded mouse aortic rings were incubated for 2 h at an optimal passive tension of 1 g in Krebs buffer and after equilibration period changes in isometric force were measured. In studies examining the actions of mechanistic inhibitors on the response to PDBu and U46619, probes were usually added at least 15 min before exposure to the contractile agent, employing doses of probes selected to be most effective and selective for the action investigated. In some experiments, contracted arteries were exposed to increasing cumulative concentrations of relaxing agents. None of the probes or treatments, except those mentioned in results, had a statistically significant effect on force generation.
Measurement of superoxide levels in BCA and mouse aorta.
Employing previously described methods (8), we prepared BCA or aortic rings as described for force measurements and then incubated the arterial rings in tissue baths for subsequent placement in plastic scintillation minivials containing 5 μM lucigenin for the detection of superoxide. These measurements were made in the absence or presence of probes in a final volume of 1 ml of air-equilibrated Krebs solution buffered with 10 mM HEPES-NaOH (pH 7.4). Chemiluminescence was measured in a liquid scintillation counter (LS6000IC, Beckman Instruments) at 37°C, and data are reported as chemiluminescence unit (counts/min) per gram wet weight of BCA or mouse aorta after subtraction of the background chemiluminescence measured in samples that did not contain vascular tissue.
Immunoprecipitation and immunoblotting.
Vascular smooth muscle cells or tissue were lysed in lysis buffer containing 50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 0.5% NP-40, 100 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 200 mM pepstatin, and immunoprecipitation was carried out as described by Gupte et al. (7). The precleared lysates were incubated with anti-Src (total) or (phospho-416) antibody, and the immunocomplexes were then analyzed by standard electrophoresis technique using 8% SDS-PAGE. Western blot procedure was performed with specific antibodies followed by incubation with horseradish peroxidase-labeled appropriate secondary antibodies (Pierce, IL). Specific proteins were detected by chemiluminescence (Pierce).
Isolation of cytoplasm and microsomes.
To separate cytoplasm and microsomes, tissue was homogenized in MOPS (50 mM)-sucrose (250 mM) buffer (pH 7.4) in a 1:3 ratio as previously described (16). Briefly, homogenates was centrifuged at 900 g, and the supernatant was further centrifuged at 27,000 g in a Sorval RC2-B centrifuge, after which the post-mitochondrial fraction was subjected for centrifugation at 100,000 g in a Beckman L8-M ultra-centrifuge. After differential centrifugation, the supernatant was used as the cytoplasm and the pellet as reconstituted in MOPS-sucrose buffer (pH 7.4) after three washes and used as the microsomal fraction.
Statistical analysis.
ANOVA statistical analysis employing a post hoc Fisher's protected t-test was used for all studies on vascular contractile function. All chemiluminescence data were analyzed by Student's t-tests employing a Bonferroni correction for multiple comparisons. The acceptable level of significance was P < 0.05. The number of experimental determinations (n) in all cases is equal to the animals from which an arterial ring was employed for a treatment or a control group in all studies. Values are reported as means ± SE.
RESULTS
PKC regulates superoxide generation from NAD(P)H oxidase in coronary artery.
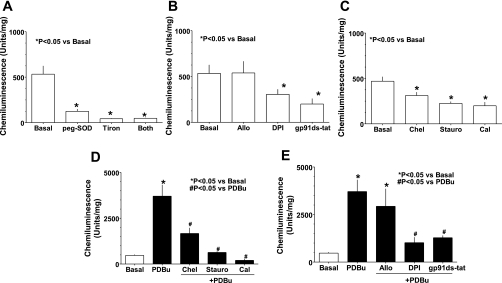
ROS were detected in BCA by lucigenin (5 μM) chemiluminesence (8) under basal conditions. Treatment of BCA ring with cell permeable superoxide scavengers, peg-SOD (300 U/ml) and tiron (10 mM), for 30 min decreased chemiluminescence signals by 80–90% (Fig. 1A), consistent with lucigenin signals originating primarily from superoxide generation. Data in Fig. 1B show that basal superoxide significantly decreased by pretreatment with diphenyliodonium (DPI; 10 μM), a nonspecific flavoprotein inhibitor, and gp91ds-tat (50 μM), a NAD(P)H oxidase inhibitor (a gift from Dr. Pagano, Henry Ford Health System, Detroit, MI), and not by the xanthine oxidase inhibitor allopurinol (100 μM). Furthermore, the PKC inhibitors staurosporine (10 nM), chelerythrine (10 μM), and calphostin C (100 nM) also decreased superoxide generation in untreated BCA (Fig. 1C). In contrast, superoxide generation was increased by 7.5-fold in BCA treated with PDBu, a cell permeable PKC activator, in a manner that was inhibited by pretreatment with PKC inhibitors (Fig. 1D). Inhibitors NAD(P)H oxidase, DPI, and gp91ds-tat attenuated the elevation in superoxide generation in BCA due to activation of PKC by PDBu (Fig. 1E).
Fig. 1.
Basal and protein kinase C (PKC)-stimulated increases in superoxide are derived from NADPH oxidase in bovine coronary artery. Basal superoxide production in bovine coronary arteries (BCA) rings (n = 15–20) detected by lucigenin (5 μM) chemiluminescence is decreased by (A) superoxide scavengers peg-superoxide dismutase (peg-SOD; 300 U/ml), tiron (10 M), and a combination of both (A); NADPH oxidase inhibitors diphenyleneiodonium (DPI; 10 μM) and gp91ds-tat (50 μM), but not by the xanthine oxidase inhibitor allopurinol (Allo; 100 μM) (B); and PKC inhibitors chelerythrine (Chel; 10 μM), staurosporine (Stauro; 100 nM), and calphostin C (Cal; 1 μM) (C). Conversely, superoxide generation is increased in coronary artery by cell permeable PKC activator phorbol 12,13-dibutyrate (PDBu; 10 μM), and this increase in superoxide generation is inhibited by pretreatment of coronary artery with protein kinase C inhibitors (Chel, Stauro and Cal) (D) and NADPH oxidase inhibitors (DPI and gp91ds-tat) (E). Scrambled sequence of gp91ds-tat did not affect lucigenin chemicluminesce in basal (497 ± 69 U/mg) and PDBu (3,247 ± 201 U/mg) treated samples.
PKC-dependent microsomal translocation of p47phox and p67phox in coronary artery smooth muscle.
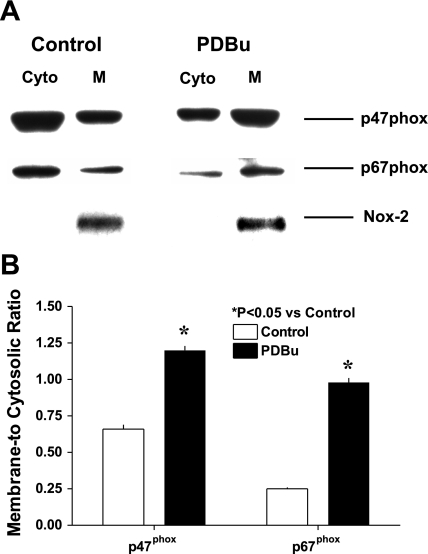
Western blot analysis of cytosolic and membrane fractions for p47phox and p67phox are shown in Fig. 2A. In resting BCA, p47phox and p67phox appear to be predominantly in cytosolic compared with membrane fractions. However, on pretreating a portion of the same BCA with PDBu (10 μM) for 30 min at 37°C, more p47phox and p67phox were detected in the membrane fraction compared with the cytosolic fraction.
Fig. 2.
PDBu-elicits changes in PKC-mediated membrane binding of Nox oxidase subunits in BCA smooth muscle cells. A: a representative blot from four separate experiments shows that a 20-min treatment with 10 μM PDBu increases (P < 0.05) p47phox in the membrane fraction. Nox-2 is detected in membranes but not in the cytosolic fraction. B: summary data of increase in membrane-to-cytosolic ratio of p47phox and p67phox.
Hydrogen peroxide derived from Nox oxidase contributes to the development of coronary artery contraction evoked by activation of PKC.
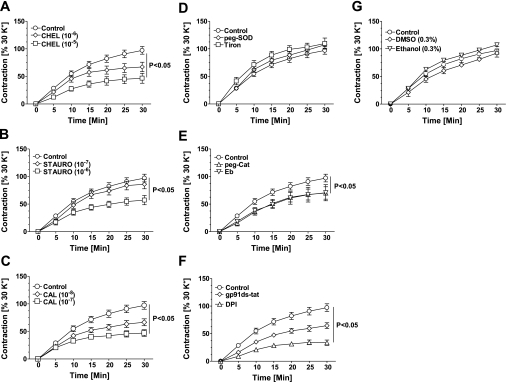
To determine the role of elevated superoxide generation by PKC activation in evoking PDBu-induced BCA contraction, we performed isometric tension studies (Fig. 3). Consistent with previous studies (9), PDBu elicited 90–110% contraction compared with 123 mM KCl in a time-dependent manner. The PDBu-induced contraction was inhibited by pretreating the BCA for 30 min with three different PKC inhibitors in a dose-dependent manner (Fig. 3, A–C). In addition, pretreatment of BCA with peroxide scavengers peg-catalase and ebselen, but not superoxide scavengers peg-SOD or tiron, also significantly inhibited PDBu-evoked contraction (Fig. 3, D and E). Consistent with these findings, we found that inhibition of NAD(P)H oxidase, a major source of superoxide, with DPI, a flavoprotein inhibitor that has other nonspecific effects on the cell, and gp91ds-tat, a Nox oxidase inhibitor that inhibits Nox-1 or -2 by blocking the p47phox binding site (12), decreased PDBu-induced contraction by 60 and 50%, respectively, compared with untreated control (Fig. 3F), whereas the DMSO (0.1–0.3%) and ethanol (0.1–0.3%) vehicles utilized to dissolve drugs did not affect PDBu-induced contractions (Fig. 3G).
Fig. 3.
Phorbol 12,13-dibutyrate-induced contraction of coronary artery is inhibited by PKC and NADPH oxidase inhibitors. PKC activator phorbol 12,13-dibutyrate (PDBu; 10 μM) increased force generation in isolated BCA (n = 25–30) in a time-dependent manner. Pretreatment of BCA with PKC inhibitors chelerythrine (Chel; A), staurosporine (Stauro; B), and calphostin C (Cal; C) decreased the force generation evoked by PDBu. Contraction evoked by PDBu is not attenuated by superoxide scavengers tiron or peg-superoxide dismutase (300 U/ml SOD; D) but decreased significantly by hydrogen peroxide scavengers ebselen (10 μM Eb; E) and peg-catalase (150 U/ml peg-Cat; E), and by NADPH oxidase inhibitors diphenyleneiodonium (10 μM DPI; F) and gp91ds-tat (50 μM; F). Vehicle control has no effect on PDBu-induced contraction of coronary artery (G).
PKC enhances superoxide in a Ca2+-independent manner.
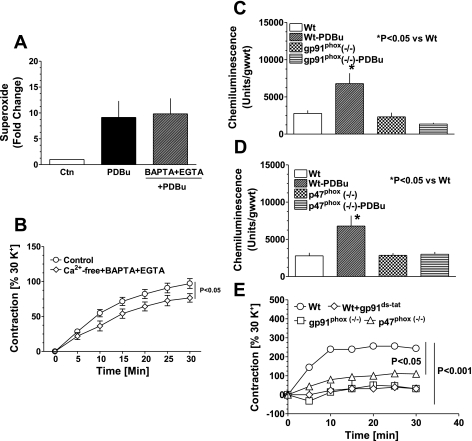
To investigate whether PDBu-induced increase in NAD(P)H oxidase-derived superoxide is dependent on Ca2+, we incubated BCA with PDBu in Ca2+-free Krebs buffer containing EGTA + BAPTA to scavenge extra- and intracellular Ca2+ for 30 min as described previously (9) before estimating superoxide. The data in Fig. 4A demonstrate that PKC-stimulated generation of superoxide by NAD(P)H oxidase did not require Ca2+. In contrast, PDBu-induced contraction of BCA is partly dependent on Ca2+ (Fig. 4B).
Fig. 4.
PDBu-elicited increases in force generation, and superoxide are involved in a p47phox stimulation of Nox2, which is not dependent on extracellular calcium. Effect of calcium on increase in superoxide generation (A) and coronary artery contraction (B) by PDBu is shown. Increase in Nox-derived superoxide generation induced by PDBu is not affected by removing and chelating extracellular calcium with 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-AM (200 μM BAPTA-AM) and ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid tetrasodium salt (100 μM EGTA). Effect of PDBu on superoxide generation (C and D) and contraction (E) of aorta from gp91phox and p47phox knockout mice is demonstrated. Superoxide generation and contraction evoked by PDBu were completely inhibited in endothelium-denuded gp91phox and p47phox mouse aorta (n = 6) and by acute treatment with gp91ds-tat (n = 6). Note that error bars are overlapping with the symbols.
Essential role of Nox2 activation by p47phox in PKC-elicited increases in mouse aortic superoxide and force.
Endothelium removed aorta from mice deficient in Nox2 and p47phox were utilized to determine whether the increase in superoxide by PDBu was dependent on these pathways of Nox oxidase activation. As observed in BCA, superoxide levels were increased (by ∼2.5-fold) in aorta from wild-type mice by PDBu (Fig. 4C). In contrast, PDBu did not increase superoxide production from aorta of Nox2- and p47phox-deficient animals (Fig. 4, C and D). Correspondingly, PDBu-induced contractions of aortic rings were markedly attenuated in Nox2- and p47phox-deficient mice and by the inhibitor of Nox2 activation, gp91ds-tat, in wild-type animals (Fig. 4E).
Src kinase mediates PKC-induced activation of Nox2.
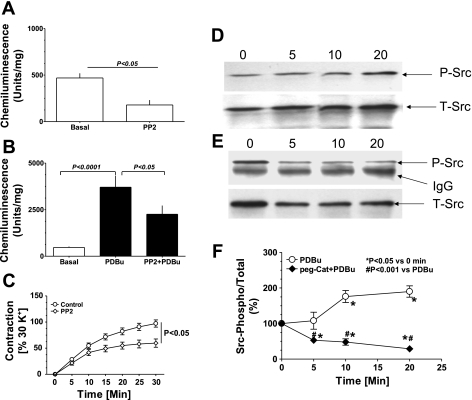
To evaluate whether a previously described (22) Src-dependent mechanism of activating Nox oxidases was contributing to the actions of PDBu, a 30-min pretreatment with the Src kinase inhibitor PP2 (10 μM) was examined for its effects on superoxide and force in BCA. This pretreatment decreased basal superoxide by ∼50% and markedly attenuated the increase elicited by PDBu treatment (Fig. 5, A and B). Moreover, PP2 also partly decreased PDBu-induced contraction of BCA at 20–30 min by ∼40% (Fig. 5C). Western blot analysis of Src phosphorylation, an indicator of enzyme activation, detected a time-dependent increase in PDBu-treated BCA (Fig. 5D), whereas pretreatment of BCA with peg-cat prevented phosphorylation of Src by PKC, decreasing phosphorylated Src to below basal levels (Fig. 5, E and F). The data summarized in Fig. 5F demonstrate that PDBu causes a time-dependent increase in phospho-Src by 80–90% of the baseline, and peg-cat decreases phospho-Src in the presence of PDBu by 90% in a time-dependent manner to ∼25% of the basal levels of phosphorylation. Consistent with these findings, we also found that U46619-induced elevation in superoxide levels was decreased by 35–40% (P < 0.05; n = 5) with PP2 treatment compared with untreated controls.
Fig. 5.
Activation of Src kinase modulates superoxide generation and contraction of coronary artery evoked by PDBu. Inhibition of Src kinase by 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (10 μM PP2) decreased basal (A; n = 10) and PDBu-induced superoxide generation (B; n = 10) and contraction (C; n = 10). Inactive analog of PP2, PP3 (10 μM) did not decrease superoxide generation (basal: 475 ± 25; and PDBu: 3,046 ± 538 U/mg). Src kinase activation by PDBu was determined by changes in phospho-Src416-to-total-Src ratios after immunoprecipitation. Stimulation of coronary arterial rings with PDBu (10 μM) increased Src phosphorylation in a time (0, 5, 10, and 20 min)-dependent manner (D). Pretreatment of coronary artery with peg-catalase (150 U/ml) in the presence of PDBu decreased Src kinase phosphorylation below basal levels in the absence of PDBu (E). F: summary data for the effect of peg-catalase on the PDBu-elicited increase in phospho-Src-to-total-Src ratios.
Activation of PKC signaling by stimulation of thromboxane receptors increases superoxide and elicits coronary artery contraction.
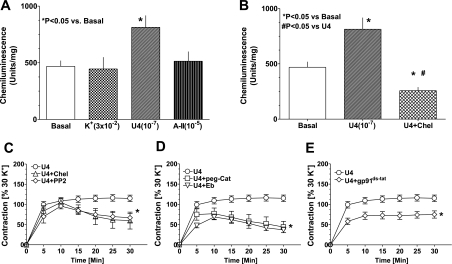
Exposure of BCA with U46619 (100 nM), a thromboxane A2 receptor agonist, increased superoxide generation by 60–70%, whereas angiotensin II (10 μM) and KCl (30 mM) did not elevate superoxide (Fig. 6A). The PKC inhibitor chelerythrine (Fig. 6B) and rotterlin (10 μM), a PKCδ inhibitor, attenuated the increase in superoxide production evoked by U46619. As shown in Fig. 6C, the PKC inhibitor, chelerythrine, and the Src inhibitor, PP2, significantly decreased by ∼30–40% the sustained contraction to U46619, whereas the initial contraction did not appear to be altered by these agents. Rotterlin inhibited the contraction of BCA evoked with U46619 and PDBu by 60 and 40%, respectively. The sustained phase of U46619-induced BCA contraction was inhibited 40–50% by scavenging peroxide with peg-cat and with ebselen. Pretreatment of BCA with the inhibitor of p47phox activation of Nox oxidase, gp91ds-tat, decreased both the initial and sustained phase of BCA contraction evoked by U46619 by ∼40%.
Fig. 6.
A PKC and Src kinase stimulation of Nox oxidase generated increases in superoxide generation, and elevated hydrogen peroxide contributes to the contraction of coronary arteries by U46619. A: effect of 30 mM KCl (K+), 100 nM U46619 (U4), and 10 μM angiotensin II (A-II) on coronary arterial superoxide detected by 5 μM lucigenin. U46619 significantly elevated superoxide levels. B: treatment of coronary artery with chelerythrine (10 μM) for 30 min before the application of U46619 decreased superoxide generation. C: chelerythrine (10 μM) and PP2 (10 μM) partially blocked the sustained, but not the initial, contraction to U46619. D and E: hydrogen peroxide scavengers ebselen (Eb; 10 μM) and peg-catalase (peg-cat; 150 U/ml) (D) and the NADPH oxidase inhibitor gp91ds-tat (50 μM) (E) decreased both the initial and sustained contraction to PDBu. C and E: P < 0.05 vs. U4.
Calmodulin kinase II and rho kinase mediate hydrogen peroxide-induced coronary artery contraction resulting from PKC activation.
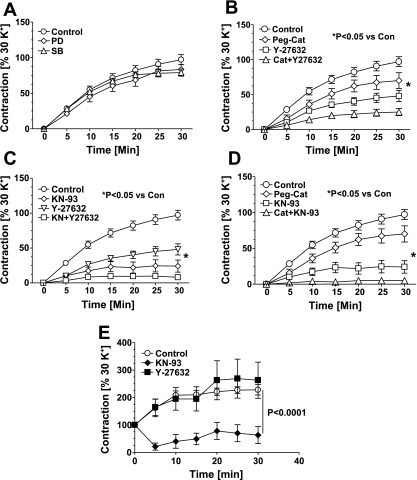
Based on potential processes through which peroxide can promote contraction, we examined the effects inhibitors of specific signaling mechanisms (19, 21, 24) that are potentially involved. An ERK1/2 inhibitor, PD98,059 (10 μM), and a p38 MAPK inhibitor, SB203,580 (10 μM), did not alter the contractions of BCA induced by PDBu (Fig. 7A). The PDBu-induced contraction was inhibited partly by Y27362 (10 μM), a Rho kinase inhibitor, and KN-93 (10 μM), a calmodulin kinase (CaMK) II inhibitor. A combination of both of these inhibitors completely inhibited the contraction evoked by PDBu (Fig. 7B). To elucidate whether peroxide regulates Rho kinase- or CaMK II-mediated contraction, we studied the effects of peg-cat and Y27362, separately and in combination, on the PDBu-induced contraction. As demonstrated in Fig. 7C, peg-cat + Y27362 had an additive inhibitory effect compared with peg-cat or Y27362 alone. Similarly, peg-cat + KN-93 almost completely abolished the contraction evoked by PDBu (Fig. 7D). Based on the actions of Y27362 and KN-93 on contractions to PDBu in mouse aorta (Fig. 7E), CaMK II, but not Rho kinase, appears to mediate the response observed in this vascular segment.
Fig. 7.
Roles for Rho kinase and CaM kinase II pathways in contractions elicited by PDBu. A: inhibition of p38 MAP kinase and ERK1 and 2 by SB202190 (SB; 10 μM) and PD98059 (PD; 10 μM), respectively, did not affect PDBu-induced coronary arterial contraction (n = 15). B: PDBu-induced contraction of coronary arteries was partially inhibited by the Rho kinase inhibitor Y-27632 (10 μM) and the CaM kinase II inhibitor KN-93 (10 μM) and completely by a combination of the two inhibitors (n = 20–25). C and D: hydrogen peroxide scavenging with peg-catalase (150 U/ml) had additive inhibitory effect in combination with Y-27632 (C; n = 8–10) or KN-93 (D; n = 8–10) on contractions evoked by PDBu. E: in mouse aorta, PDBu-induced contraction is completely inhibited by KN-93 (10 μM) but not by Y-27632 (10 μM).
PKC-elicited contraction causes a superoxide-dependent attenuation of coronary arterial relaxation to nitroglycerin.
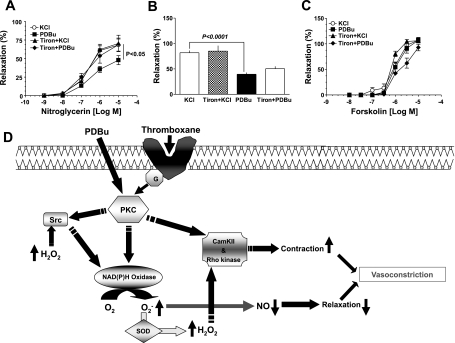
To examine the effect of superoxide on nitroglycerin-induced relaxation of the coronary artery, we compared the effects of nitroglycerin on BCA precontracted with either KCl (30 mM) or PDBu (10 μM). The data in Fig. 8A show that nitroglycerin-induced relaxation was shifted to the right in BCA precontracted with PDBu compared with the BCA precontracted with KCl, which does not increase superoxide (Fig. 6A). In the presence of tiron (10 mM), PDBu did not inhibit the nitroglycerin-induced relaxation. Relaxation of BCA to 8-bromo-cGMP, an activator of cGMP-dependent protein kinase (see Fig. 8B), or forskolin, an activator of adenylate cyclase (see Fig. 8C), was not altered by the superoxide scavenger tiron in BCA contracted with PDBu, suggesting that endogenous superoxide generation promoted by PDBu did not alter relaxation mechanisms linked to cAMP or cGMP.
Fig. 8.
Relaxation of coronary artery to nitroglycerin is attenuated by PDBu-evoked superoxide generation. A: nitroglycerin relaxed coronary arterial rings precontracted with KCl (30 mM) and PDBu (10 μM) in a dose-dependent manner (n = 20–25). The relaxation of coronary artery to nitroglycerin is significantly shifted to the right in arterial rings precontracted with PDBu compared with KCl, and this inhibition in relaxation is prevented by tiron (10 mM), a superoxide scavenger. B and C: in contrast, relaxation to 8-bromo-cGMP (B; n = 5–8) and forskolin (C; n = 5–8) is not affected by tiron. D: a model showing potential mechanisms involved in the induction of coronary artery contraction by the PKC activator PDBu through a Src kinase activation of Nox-2-derived hydrogen peroxide generation and inhibition of nitroglycerin-induced relaxation by Nox-2-derived superoxide.
DISCUSSION
The data in this study provide evidence detecting a major role for Nox2 activation in arterial smooth muscle responses associated with the activation of PKC. It appears that stimulation of PKC by a combination of p47phox and Src kinase mechanisms results in activation of a peroxide-mediated contractile mechanism. In BCA, the mechanism of this Nox-derived peroxide-mediated contraction to PDBu has both calcium-dependent and calcium-independent components involving systems including CaM and Rho kinases. The stimulation of PKC during contraction also seems to be associated with a superoxide-mediated inhibition of relaxation to nitroglycerin. However, based on the absence of an effect of superoxide scavengers that do not prevent the formation of peroxide (peg-SOD and tiron), superoxide-mediated inhibition of NO-associated relaxation did appear to be contributing to the contraction elicited by PDBu under the conditions examined in this study. Since the activation of PKC is known to be an important component of multiple receptors promoting vasoconstriction, PKC-elicited stimulation of Nox oxidases in vascular smooth muscle may have major roles in physiological processes controlling vascular force through ROS-linked mechanisms, which have not been previously recognized.
Activation of Nox2-derived peroxide generation by a p47phox-dependent mechanism appears to be a key component of the contraction elicited by PDBu promoting PKC activation in both BCA and mouse aorta. This observation is consistent with findings that Nox2 oxidase is present in large arteries from many species, including rat, rabbit, pig, cow, and mouse (8, 15). Inhibition of contraction and increases in superoxide in both of these vascular segments by gp91ds-tat supports a key role for a p47phox mechanism of Nox oxidase activation in these responses. Our previous work (8), investigating the oxidases present in BCA, detected Nox2 as the only known form of this oxidase present in endothelium-denuded BCA, which can be stimulated by p47phox. This interpretation is further supported by the observation of 1) an increased membrane p47phox, which is required for activation of Nox2 in PDBu-treated BCA and 2) inhibition of PDBu-elicited contraction of aorta from wild-type mice by gp91ds-tat, a putative Nox2 inhibitor that decreases activity of oxidases found in vascular tissue by binding to the p47phox docking site (12). Furthermore, the lack of our ability to detect contractions or increases in superoxide in aortas from mice deficient in either Nox2 (gp91phox) or p47phox on exposure to PDBu provides definitive evidence for the role of both of these oxidase components in the PKC-elicited responses being studied. Based on the absence of an effect of scavengers of superoxide (peg-SOD and tiron) and partial, but significant, inhibition of contraction by systems promoting peroxide metabolism (peg-catalase and ebselen), peroxide appears to be the key ROS produced by Nox2, which is participating in the contractile response elicited by PDBu. Thus data in this study implicate an essential role of peroxide derived from a PKC-elicited stimulation of Nox2 by a p47phox mechanism in the contraction to PDBu.
Several additional aspects of Nox oxidase regulation appear to be participating in the activation of superoxide production and the promoting of peroxide-mediated contraction by PDBu. Based on the inhibition of contraction and superoxide production elicited by PDBu in BCA by the Src kinase inhibitor PP2, it appears that a previously identified (22) Src kinase elicited rac1 stimulation of Nox oxidase is also a key component of the responses being studied. The data in Fig. 5, detecting an inhibition of PDBu-elicited increases in Src phosphorylation by peg-catalase, support the previously reported (22) crucial role of endogenous oxidase-derived peroxide in this component of the mechanism of Nox activation. Interestingly, our control experiments in the absence of PDBu also detected a basal level of Nox stimulation in BCA that appears to be dependent of mechanisms involving PKC, p47phox, and Src kinase. Although it has been difficult to identify a role for p67phox in vascular smooth muscle Nox oxidase regulation (1), our studies detected the expression of this subunit in BCA. Interestingly, the data in Fig. 2, suggest there is increased p67phox in membrane fraction in PDBu-treated BCA. However, the role of these changes in p67phox associated with PDBu-elicited Nox oxidase activation remains to be defined. Thus a Src kinase-mediated mechanism of Nox oxidase activation appears to be a major essential component of basal superoxide production and PKC-stimulated contractile responses in BCA.
Hydrogen peroxide appears to be able to regulate force through multiple mechanisms (1, 14, 17, 19, 24–27). Since recent studies suggest peroxide can promote contraction as a result of releasing intracellular calcium (1, 17, 25, 27) or through stimulation of p38 MAPK, ERK, Rho kinase, and Src systems (19, 24), these mechanisms were investigated for their role in the contraction elicited by PDBu. Although our experiments in BCA did not detect a role for p38 MAPK and ERK, the Src and Rho kinase systems appeared to be important contributors to the contraction elicited by PDBu. A system potentially sensitive to regulation by elevated intracellular Ca2+, CaM kinase II, also appeared to contribute to contraction elicited by PDBu. The absence of a role for ERK in the peroxide-mediated contraction to PDBu was surprising because our previous work detected a key role for a force-enhancing effect mediated by peroxide activation of ERK when BCA were exposed to hypertensive levels of stretch or extracellular peroxide (19). Since Nox appeared to be activated by a p47phox- but not a Src kinase-dependent mechanism in this previously studied response to stretch, it appears that activation of Nox by a Src pathway may be a key factor in determining the signaling mechanisms that contribute to the control of force. Our observations on the importance of Rho kinase in the peroxide-dependent contractile response to PDBu is consistent with previous evidence that it is a major contributor to a peroxide-dependent sensitization of force generation by intracellular Ca2+ observed when rat aorta were exposed to xanthine oxidase-derived ROS (10). The observed partial dependence of the contraction to PDBu on the availability extracellular Ca2+ and on CaM kinase II in BCA suggests that peroxide derived from Nox oxidase activation is promoting contraction through increasing intracellular Ca2+ and stimulating CaM kinase II-dependent pathway. An additive inhibition of PDBu-induced contraction of BCA by peg-catalase and Rho kinase or CaM kinase II inhibitors suggests that either both these pathways are simultaneously turned on by peroxide or alternately peroxide mediates contraction through pathways in addition to activation of Rho and CaM kinase. Although the contraction of BCA to PDBu did not appear to be completely dependent on Nox oxidase and ROS, there is evidence for sites where protein phosphorylation by PKC can promote contraction through mechanisms that are likely to be independent of ROS. For example, PKC directly phosphorylates CPI-17, generating a very potent myosin light chain phosphatase inhibitor, which is thought to promote force generation through sensitizing the contractile apparatus to the actions of Ca2+ (23). Interestingly, Rho kinase also phosphorylates the same site on CPI-17 as PKC. Thus our data support a model shown in Fig. 8D involving a PKC activation by PDBu promoting Src kinase-elicited stimulation of Nox2, which generates peroxide in a manner that appears to increase force generation in BCA through stimulation of both Rho kinase and CaM kinase II mechanisms.
Nox oxidase activation by PKC is potentially an important factor in the mechanism of receptors for contractile agents, which promote force generation through PKC signaling. Our studies confirm previous evidence that PKC is an important participant in the contraction elicited by the thromboxane receptor agonist U46619 and provide novel evidence for the role of a Src kinase-dependent activation of Nox2 and hydrogen peroxide in the contraction that is observed in BCA. Interestingly, previous studies have documented an attenuation of NO-mediated relaxation and guanylate cyclase activation in arteries precontracted with U46619 (2, 20). These data suggest that when thromboxane receptor agonists promote a PKC-mediated activation of Nox oxidases, the increase in ROS such as superoxide in vascular smooth muscle may also function to attenuate NO-mediated relaxation. Thus Nox oxidase activation and ROS signaling are likely to be important contributors to vascular smooth muscle force regulatory mechanisms of receptors that stimulate PKC.
Overall, this study provides novel evidence for a key role of a p47phox-Src kinase mechanism of Nox oxidase activation and ROS in vascular smooth muscle contraction resulting from the stimulation of PKC. The properties of this mechanism suggest that the extent of PKC activation by receptors promoting vascular smooth muscle contraction may be a key factor in determining the role of ROS in the responses observed and perhaps the expression of pathophysiological changes that are often associated with increased oxidant production.
GRANTS
This study was supported by an American Heart Association Scientist Development Grant 0435070N (S. A. Gupte) and by National Heart, Lung, and Blood Institute Grants HL-085352 (S. A. Gupte), HL-31069, HL-43023, and HL-66331 (M. S. Wolin).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 231: 237–251, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Arshad M, Vijay V, Floyd BC, Marks B, Sarabu MR, Wolin MS, Gupte SA. Thromboxane receptor stimulation suppresses guanylate cyclase-mediated relaxation of radial arteries. Ann Thorac Surg 81: 2147–2154, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Barman SA Vasoconstrictor effect of endothelin-1 on hypertensive pulmonary arterial smooth muscle involves Rho-kinase and protein kinase C. Am J Physiol Lung Cell Mol Physiol 293: L472–L479, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bedard K, Lardy B, Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie 89: 1107–1112, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Budzyn K, Paull M, Marley PD, Sobey CG. Segmental differences in the roles of rho-kinase and protein kinase C in mediating vasoconstriction. J Pharmacol Exp Ther 317: 791–796, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Forman HJ, Fukuto JM, Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287: C246–C256, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Gupte RS, Pozarowski P, Grabarek J, Traganos F, Darzynkiewicz Z, Lee MY. RIalpha influences cellular proliferation in cancer cells by transporting RFC40 into the nucleus. Cancer Biol Ther 4: 429–437, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Gupte SA, Kaminski PM, Floyd B, Agarwal R, Ali N, Ahmad M, Edwards J, Wolin MS. Cytosolic NADPH may regulate differences in basal Nox oxidase-derived superoxide generation in bovine coronary and pulmonary arteries. Am J Physiol Heart Circ Physiol 288: H13–H21, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Iesaki T, Wolin MS. Thiol oxidation activates a novel redox-regulated coronary vasodilator mechanism involving inhibition of Ca2+ influx. Arterioscler Thromb Vasc Biol 20: 2359–2365, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol 287: H1495–H1500, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, Eto Y, Morishige K, Matsumoto Y, Obara K, Nakayama K, Takahashi S, Takeshita A. Evidence for protein kinase C-mediated activation of Rho-kinase in a porcine model of coronary artery spasm. Arterioscler Thromb Vasc Biol 23: 2209–2214, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Lambeth JD NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–R297, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology 21: 269–280, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Miller AA, Drummond GR, De Silva TM, Mast AE, Hickey H, Williams JP, Broughton BR, Sobey CG. NADPH oxidase activity is higher in cerebral versus systemic arteries of four animal species: role of Nox2. Am J Physiol Heart Circ Physiol 296: H220–H225, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Mohazzab-H KM, Wolin MS. Sites of superoxide anion production detected by lucigenin in calf pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol 267: L815–L822, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol 98: 390–403, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Nobe K, Paul RJ. Distinct pathways of Ca2+ sensitization in porcine coronary artery: effects of Rho-related kinase and protein kinase C inhibition on force and intracellular Ca2+. Circ Res 88: 1283–1290, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res 92: 23–31, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Vizcaino F, Villamor E, Duarte J, Tamargo J. Involvement of protein kinase C in reduced relaxant responses to the NO/cyclic GMP pathway in piglet pulmonary arteries contracted by the thromboxane A2-mimetic U46619. Br J Pharmacol 121: 1323–1333, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rokolya A, Singer HA. Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am J Physiol Cell Physiol 278: C537–C545, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Thakali K, Davenport L, Fink GD, Watts SW. Cyclooxygenase, p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase MAPK, Rho kinase, and Src mediate hydrogen peroxide-induced contraction of rat thoracic aorta and vena cava. J Pharmacol Exp Ther 320: 236–243, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Waypa GB, Schumacker PT. Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. J Appl Physiol 98: 404–414, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Wolin MS Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol 20: 1430–1442, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Wolin MS, Ahmad M, Gupte SA. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol 289: L159–L173, 2005. [DOI] [PubMed] [Google Scholar]