Abstract
Oxidant injury occurs when an organ is severed from its native blood supply and then reperfused and continues during subsequent periods of immune attack. Experiments here test the hypothesis that an antioxidant given only in the peri-reperfusion period protects against not only oxidative but also nitrosative stress, leading to reduced vasculopathy long after cardiac allotransplantation. Experiments were performed using a murine heterotopic cardiac transplantation model. An antioxidant, in the form of intraperitoneal high-dose riboflavin, was given to recipients during the initial 3 days after transplantation. Antioxidant-treated mice showed significantly longer graft survival than control mice. At 4 h after transplantation, antioxidant treatment significantly reduced graft lipid peroxidation and oxidized DNA and preserved antioxidant enzyme activity. At day 6 posttransplantation, the redox-sensitive transcription factor nuclear factor-κB and inducible nitric oxide synthase were significantly reduced following antioxidant treatment, with concomitant reduction of nitrotyrosine. Despite the limited duration of antioxidant treatment, both acute and chronic rejection were significantly suppressed. In vitro experiments confirmed suppression of nitrosative and oxidative stress and cardiomyocyte damage in antioxidant-treated cardiac allografts. Collectively, antioxidant administration during the initial 3 days after transplantation significantly reduces nitrosative and oxidative stress in cardiac allografts, modulates immune responses, and protects against vasculopathy.
Keywords: antioxidant, ischemia-reperfusion injury, inducible nitric oxide synthase, nuclear factor-κB, riboflavin
cardiac allograft vasculopathy (CAV) is an irreversible pathological hallmark of chronic rejection after cardiac transplantation. CAV is a rapidly progressing form of atherosclerosis characterized by diffuse concentric narrowing of the lumen in allograft vessels, leading to reduced blood flow and ischemia of distal tissues. Although much attention has focused on CAV development, driving mechanisms are incompletely understood, although they are very likely multifactorial, including both immunological and nonimmunological triggers (19).
Graft oxidative stress has been implicated as one of the multiple potential nonimmunological adjuvants driving allograft rejection and vasculopathy. A net increase in reactive oxygen species (ROS) generation by cells within or recruited to the graft, particularly when associated with a decline of antioxidant enzymes, can lead to covalent oxidative modifications of lipids, proteins, and DNA. Additional chemically reactive species, particularly reactive nitrogen species (RNS), are also extant in the allograft reperfusion milieu, where they can contribute to tissue modification and destruction. These RNS are derived from nitric oxide (NO) produced by graft cells or recruited leukocytes via upregulation of inducible NO synthase (iNOS) (16, 26, 31), with excess NO reacting with superoxide anion to form RNS, such as peroxynitrite (5). Peroxynitrite's nitrating and oxidizing properties produce significant cellular toxicity in the context of cardiac allograft rejection (1). The formation of both ROS and RNS can be intrinsically linked as well through the induction of a ROS-sensitive transcription factor, nuclear factor-κB (NF-κB), which can itself drive iNOS upregulation (11).
The importance of ROS generation, both as an intrinsically deleterious consequence of allograft transplantation, as well as a potential tripwire for the formation of other damaging RNS, led us to study whether an antioxidant given early during transplantation could potentially abort both oxidative graft damage as well as NF-κB activation, iNOS induction, and RNS generation. Prior work has shown that an antioxidant such as riboflavin could protect cardiac allografts, but the mechanisms underlying this protection have not been fully defined (15). Riboflavin, as a naturally occurring antioxidant, which can repeatedly cycle through biological redox cycles to continually eliminate oxidative species (14, 18), could be an outstanding agent to test the effect of oxidative stress, as well as its mitigation in the setting of cardiac transplantation. Accordingly, the present study was designed to evaluate the following hypothesis: antioxidant treatment during the initial reperfusion period following cardiac allotransplantation can mitigate not only graft oxidant stress, but also graft nitrosative stress and molecular mechanisms leading to nitrosative stress (such as NF-κB activation and iNOS expression), and consequently reduce CAV and improve allograft survival.
MATERIALS AND METHODS
Animals.
Male mice of C57BL/6J (H-2b), CBA/J (H-2k), and B10A (H-2a) strains, 8–12 wk of age, were obtained from Jackson Laboratories (Bar Harbor, ME). All experiments were performed according to the protocols approved by the University of Michigan Committee on Use and Care of Animals.
Antioxidant treatment protocol.
Riboflavin (Sigma-Aldrich, St. Louis, MO) was dissolved in normal saline. An Alzet osmotic minipump (model 1003D; DURECT, Cupertino, CA) was primed with the riboflavin solution or vehicle (normal saline), according to the manufacturer's instruction. The pumps provided a continuous infusion of solution for a period of up to 3 days. Alzet pumps containing either riboflavin (n = 6) or vehicle (control, n = 6) were placed in the recipient peritoneal cavity just after cardiac transplantation.
Heterotopic cardiac transplantation.
Completely allomismatched murine heterotopic cardiac transplantation was performed using two rejection models, acute and chronic, as described previously (12, 15). The aorta and pulmonary artery of the donor heart were anastomosed to the recipient's abdominal aorta and inferior vena cava, respectively. Before skin closure, an Alzet osmotic minipump was placed in the peritoneal cavity of each mouse. In the acute rejection model, C57BL/6J mice were used as donors and CBA/J mice as recipients. Recipient mice received no immunosuppressive agents. In the chronic rejection model, C57BL/6J mice were used as donors and B10A mice as recipients. Preoperatively, recipient mice received transient immunosuppression using a brief course of anti-murine CD4 (clone GK1.5) and anti-murine CD8 (clone 2.43) antibodies (Harlan Bioproducts for Science, Indianapolis, IN). No immunosuppressive treatment was given posttransplantation.
Graft survival and histomorphometric assessment.
Survival of cardiac allografts and histomorphometric quantification of parenchymal rejection (PR) and CAV area were assessed as previously described (12). Briefly, graft survival was evaluated by daily palpitation, and absence of pulsation was interpreted as rejection. Grafts were fixed in 10% formalin, paraffin embedded, and sectioned transversely at the maximal circumference of the ventricle. Sections were cut (5 μm thick), and the PR severity was analyzed by hematoxylin and eosin staining and graded using a modified form of the Working Formulation of the International Society for Heart and Lung Transplantation criteria: 0 = no rejection, 1 = interstitial and/or perivascular infiltration of mononuclear cells without myocyte damage, 2 = a single focal infiltration of mononuclear cells with associated myocyte damage, 3 = multifocal or diffuse infiltration of mononuclear cells with myocyte damage, and 4 = multifocal or diffuse infiltration of mononuclear and polymorphonuclear cells with extensive myocyte damage (6, 29). The CAV area was analyzed using elastica van Gieson staining to highlight the internal elastic lamina (IEL) and external elastic lamina (EEL). The cross-sectional area of luminal occlusion and the intima-to-media ratio (I/M) were calculated using the Image-Pro Plus version 4.5 software (Media Cybernetics, Silver Spring, MD) as follows:
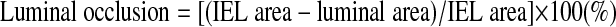 |
 |
Myeloperoxidase assay.
Myeloperoxidase activity was assessed as previously described (24). Data are standardized to the protein concentration of each sample, as determined by a micro BCA protein assay kit (Pierce, Rockford, IL).
Lipid peroxidation assay.
A lipid peroxidation assay kit (Oxford Biochemical Research, Oxford, MI) was used to measure malondialdehyde (MDA) in graft samples, according to the manufacturer's instructions. Data are standardized by the protein concentration.
Manganese superoxide dismutase activity.
To determine manganese superoxide dismutase (MnSOD) activity, a SOD assay kit-WST (Dojindo Molecular Technologies, Gaithersburg, MD) was used, according to manufacturer's instructions. Specific inhibition of Cu/ZnSOD activity was performed using potassium cyanide (Sigma-Aldrich). Data were standardized by protein concentration.
8-Hydroxy-2′-deoxyguanosine measurement.
The DNA of each graft sample was extracted using a DNeasy blood and tissue kit (QIAGEN, Valencia, CA), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) was measured using an oxidative DNA damage enzyme-linked immunosorbent assay (ELISA) kit (Cell Biolaboratories, San Diego, CA), according to the manufacturer's instructions. Data were standardized by DNA concentration.
Preparation of protein extractions.
Graft samples were homogenized in an extraction buffer containing 50 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.2% SDS, and 1 mM EDTA, supplemented with protease inhibitors (20 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride). After centrifugation, the supernatant was stored for protein analysis. For the cellular protein, nuclear and cytosolic extracts were prepared using a nuclear extraction kit (Panomics, Fremont, CA), according to the manufacturer's instructions.
NF-κB binding activity.
DNA binding activity of NF-κB was assayed using a transcription factor ELISA NF-κB p50 and p65 kits (Panomics) with nuclear extracts (10 μg of protein), according to the manufacturer's instructions.
Western blot analysis.
An equal amount of protein was separated by NuPAGE 4–12% Bis-Tris Gel (Invitrogen) and electrotransferred to a nitrocellulose membrane by using iBot Dry Blotting System (Invitrogen). After transfer, Western blot analysis was performed by an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE) per the manufacturer's instructions. The primary antibodies used were rabbit anti-iNOS and anti-NF-κB p65 (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-nitrotyrosine (Cayman Chemical, Ann Arbor, MI) and anti-β-actin (Sigma-Aldrich). The secondary antibodies used were IRDye 800CW goat anti-rabbit IgG and goat anti-mouse IgG (LI-COR Biosciences).
Quantitative real-time polymerase chain reaction analysis.
Total RNA was isolated from graft samples using an RNeasy fibrous tissue mini-kit (QIAGEN), and from cultured cells using an RNeasy mini-kit (QIAGEN), according to the manufacturer's instructions. Quantitative real-time polymerase chain reaction (PCR) analysis of mRNA for the genes of interest was performed using an ABI Prism 7000 sequence detector system (Applied Biosystems) with TaqMan universal PCR master mix and TaqMan real-time PCR primers (Applied Biosystems). The expression level of each mRNA was divided by the mRNA level of the housekeeping gene GAPDH.
Immunohistochemical staining.
Immunohistochemical staining was performed on frozen sections (5 μm thick) using anti-Ig HRP detection kit (BD Pharmingen, San Diego, CA) with primary antibodies, rat anti-mouse Ly6G (SouthernBiotech, Birmingham, AL), and rat anti-mouse CD4 and CD8 (BD Pharmingen). Diaminobenzidine substrate was used as a chromogen, and cell nuclei were identified by counterstaining with hematoxylin. Cells demonstrating positive immunoreactivity were quantified by counting immunoreactive cells in 10 non-overlapping high-power fields using the cell count plug-in of the ImageJ version 1.38 software (National Institute of Health, Bethesda, MD).
Immunofluorescent staining.
Immunofluorescent staining was performed on frozen sections with primary antibodies: rat anti-mouse CD68 (AbD Serotec, Raleigh, NC) and rabbit anti-iNOS (Santa Cruz Biotechnology). For detection of these antibodies, a tyramide signal amplification system (PerkinElmer LAS, Boston, MA) was utilized (tetramethylrhodamine for anti-CD68, fluorescein for anti-iNOS). Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (Pierce, Rockford, IL).
Donor-reactive alloantibodies.
Measurement of donor-reactive alloantibodies was performed as previously described (23). A two-parameter display of fluorescein isothiocyanate-conjugated, F(ab′)2, Fc fragment-specific, goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) and phycoerythrin-conjugated hamster anti-mouse CD3e monoclonal antibody (BD Pharmingen) was generated using the BD FACSCalibur flow cytometer (BD, Franklin Lakes, NJ), and data were analyzed using CellQuest version 3.3 software (BD Biosciences). Serum from recipient mice sensitized by subcutaneous injection of donor splenocytes was used as a positive control.
Mixed lymphocyte reaction.
Spleens were harvested and minced, and splenocyte suspensions were then passed through a 70-μm cell strainer and treated with a red blood cell lysis buffer (eBioscience, San Diego, CA). A one-way mixed lymphocyte reaction (MLR) was performed using responder splenocytes from recipient mice and mitomycin-C-inactivated stimulator splenocytes from native donors. A total of 5 × 105 responder cells and an equal number of stimulator cells were cocultured for 3 days. Cell proliferation was assessed with the Cell Counting Kit-8 (Dojindo Molecular Technologies, Gaithersburg, MD), according to manufacturer's instruction. Control wells contained responder cells in the absence of stimulator cells. Cell proliferation was expressed as the optical density of responder cells.
Cell isolation.
Peritoneal macrophages were obtained by peritoneal lavage from CBA/J mice that had received an intraperitoneal administration of 2 ml of 4% thioglycolate (Sigma-Aldrich) 4 days previously. T cells were purified from splenocytes of CBA/J mice using a Dynal mouse T-cell negative isolation kit (Invitrogen), and cardiomyocytes were isolated from the ventricles of C57BL/6J mice by using an adult cardiomyocyte isolation kit (Cellutron, Highland Park, NJ), according to the manufacturer's instructions.
Macrophage/myocyte/T cell/coculture.
Isolated cardiomyocytes were cultured for 24 h in either a standard cell culture incubator (37°C, 5% CO2) or a similar incubator within a hypoxia chamber (oxygen tension of 14–18 Torr; BioSpherix, Redfield, NY). For coculture experiments, cells were placed on HTS Transwell 24-well plates (0.4-μm pore size, 6.5-mm diameter, polycarbonate membrane; Corning Life Sciences, Lowell, MA). Transwell inserts containing the T cells (5 × 105/well) and 24-h cultured cardiomyocytes (1 × 105/well) were placed over the macrophage lawn (5 × 105/well). Cells were cocultured for 3 days (37°C, 5% CO2) in medium consisting of Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin/streptomycin (Invitrogen), and the indicated concentrations of riboflavin.
ELISA.
The concentrations of nitrotyrosine, interferon (IFN)-γ, and cardiac troponin I (TnI) in the coculture supernatants were measured with ELISA kits (nitrotyrosine, Cell Biolaboratories; IFN-γ, R&D Systems; TnI, Life Diagnostics, West Chester, PA) per the manufacturer's instructions. Plasma levels of riboflavin were measured by an ELISA with anti-conjugated riboflavin polyclonal antibody (Advanced Targeting Systems, San Diego, CA), according to the manufacturer's instructions.
Statistical analysis.
Database management and statistical analysis were performed with Statview version 5.0 software (SAS Institute, Cary, NC). All values are expressed as means ± SE for n number of mice or independent analyses. Kaplan-Meier analysis was performed to evaluate graft survival, and survival differences were compared by a log-rank test. Comparisons among groups were performed with an unpaired Student t-test or one-way ANOVA where appropriate. Post hoc analysis to establish significant differences between group means was performed using the Bonferroni test. Values of P < 0.05 were considered statistically significant.
RESULTS
Antioxidant dose-response testing in cardiac allotransplantation.
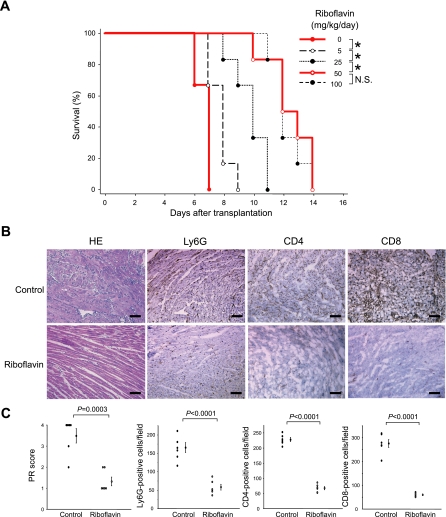
To determine the effective dosage for antioxidant treatment, experiments were performed in a preliminary study to assess the survival rate in an acute rejection model of cardiac allografts given different dosages of riboflavin in the peritransplant period (0, 5, 25, 50, or 100 mg·kg−1·day−1). Riboflavin significantly prolonged allograft survival in a dose-dependent manner (Fig. 1A). Of note, allografts treated with both 50 and 100 mg·kg−1·day−1 of riboflavin survived significantly longer than control allografts (12.5 ± 0.6 and 12.3 ± 0.4 vs. 6.7 ± 0.2 days, P < 0.0001 for both comparisons). Because there was no significant difference in the survival rate between these two treatments, we selected 50 mg·kg−1·day−1 of riboflavin as our antioxidant treatment dose for subsequent experiments. For riboflavin treatment of 50 mg·kg−1·day−1, plasma levels of riboflavin at 4 h after transplantation were 4.1 ± 2.1 μg/ml, whereas those at 6 days after transplantation were almost normalized (0.03 ± 0.01 μg/ml).
Fig. 1.
Graft survival and histological findings of cardiac allografts in acute rejection. A: survival rate at different dosages of riboflavin (0, 5, 25, 50, or 100 mg·kg−1·day−1). n = 6 in each group. *P < 0.01. B: hematoxylin and eosin (HE) staining and immunohistochemical staining of Ly6G, CD4, and CD8. Bar = 50 μm. C: parenchymal rejection (PR) score and quantitative analysis of Ly6G-, CD4-, and CD8-positive cells. All data are expressed as means ± SE for n = 6 mice per group. NS, nonsignificant.
Reduction of oxidative stress in early ischemia-reperfusion.
At 4 h after transplantation, graft neutrophil infiltration (Ly6G-positive cells; Fig. 1, B and C) and graft myeloperoxidase activity (Fig. 2A) in riboflavin-treated allografts were significantly lower than in controls. Tissue levels of oxidative damage were measured in cardiac allografts by quantifying MDA, as an indicator of lipid peroxidation, and 8-OHdG, as an indicator of oxidative DNA damage. Riboflavin treatment significantly reduced both MDA and 8-OHdG levels in cardiac allografts compared with control treatment (Fig. 2, B and C). Moreover, although activity of MnSOD, an antioxidant enzyme, was decreased in transplanted controls, the loss of MnSOD activity was prevented by riboflavin treatment (Fig. 2D).
Fig. 2.
Tissue oxidative damage in cardiac allografts by antioxidant treatment at 4 h after transplantation. A: myeloperoxidase (MPO) activity. ΔAbs, change in absorbance. B: malondialdehyde (MDA) levels as an indicator of lipid peroxidation. C: 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels as an indicator of oxidative DNA damage. D: antioxidant enzyme, manganese superoxide dismutase (MnSOD) activity. All data are expressed as means ± SE for n = 6 mice per group.
Suppression of acute allograft rejection.
Histologically, diffuse infiltration of mononuclear or polymorphonuclear cells with associated cardiomyocyte damage was present in most areas of control allografts at day 6 posttransplantation. Far less cellular infiltration and a lack of cardiomyocyte damage was present in antioxidant-treated allografts (Fig. 1B). PR scores and the numbers of infiltrating CD4- and CD8-positive cells were significantly lower in antioxidant-treated allografts than in controls (Fig. 1, B and C).
Reduction of nitrosative stress in acute rejection phase.
At day 6 posttransplantation, we evaluated the effect of antioxidant on iNOS expression in cardiac allografts. Both iNOS mRNA (Fig. 3A) and protein (Fig. 3B) levels were significantly decreased in riboflavin-treated allografts compared with controls. To investigate further the localization of iNOS expression and the extent of macrophage infiltration in cardiac allografts, double immunofluorescent staining for iNOS and CD68 was performed. The immunoreactivity of both iNOS and CD68 was decreased in riboflavin-treated allografts. Interestingly, iNOS expression was localized primarily in infiltrating macrophages, and the graft infiltration of iNOS-positive macrophages (colocalization of iNOS and CD68) was reduced by antioxidant treatment (Fig. 3C). The level of nitrotyrosine, a major product of peroxynitrite's reaction with proteins, was lower in antioxidant-treated cardiac grafts than in controls (Fig. 3B).
Fig. 3.
Nitrosative stress in cardiac allografts at day 6 posttransplantation. A: inducible nitric oxide synthase (iNOS) mRNA expression. B: protein expression of iNOS and nitrotyrosine by Western blotting. The expression of each band was normalized to its corresponding β-actin band. All data are expressed as means ± SE for n = 6 mice per group. C: double immunofluorescent staining for iNOS (green) and CD68 (red). Sites of colocalization of iNOS and CD68 are indicated as a yellow emission. Cell nuclei are counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Bar = 50 μm.
Suppression of NF-κB activation.
NF-κB is an important transcription factor that drives cytokine-induced iNOS gene transcription (4, 20). The heterodimer of p50 and p65 represents the most abundant and major form of NF-κB (27). At day 6 posttransplantation, both nuclear and cytosolic NF-κB p65 protein levels in cardiac allografts were significantly decreased by riboflavin treatment. In addition, the nuclear-to-cytosolic protein ratio was also reduced by riboflavin, which means that riboflavin treatment indeed reduced NF-κB p65 translocation into the nucleus (Fig. 4A). DNA binding activities of NF-κB p50 and p65 were significantly reduced in riboflavin-treated cardiac allografts compared with controls (Fig. 4B).
Fig. 4.
Nuclear factor-κB (NF-κB) expression and activity in cardiac allograft at day 6 posttransplantation. A: nuclear (N) and cytosolic protein (C) expression of NF-κB p65 by Western blotting. *P < 0.0001 vs. nuclear p65 protein levels in controls. **P < 0.0001 vs. cytosolic p65 levels in controls. B: DNA binding activities of NF-κB p50 and p65. OD, optical density. All data are expressed as means ± SE for n = 6 mice per group.
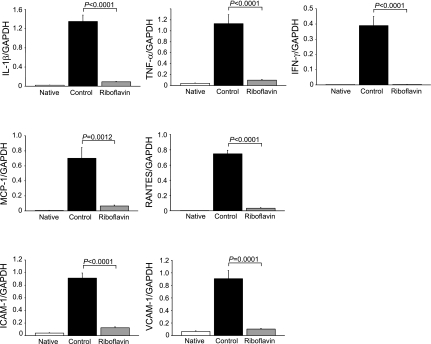
Downregulation of cytokine, chemokine, and adhesion molecule expression.
Proinflammatory cytokines (IL-1β, TNF-α, IFN-γ) are known to regulate iNOS expression (17), and the expression of IFN-γ (17), chemokines (monocyte chemoattractant protein-1, regulated on activation normal T cell expressed and secreted RANTES) (10, 13), and adhesion molecules (ICAM-1, VCAM-1) (2, 9) have been shown to be upregulated in models of CAV. Therefore, we examined whether antioxidant treatment could modulate the expression of mRNA in cardiac allografts at day 6 posttransplantation. Levels of mRNA for each of these cytokine and adhesion receptors were significantly downregulated in riboflavin-treated allografts compared with controls (Fig. 5).
Fig. 5.
Messenger RNA expression of cytokines (IL-1β, TNF-α, IFN-γ), chemokines [monocyte chemoattractant protein-1 (MCP-1), regulated on activation normal T cell expressed and secreted (RANTES)], and adhesion molecules (ICAM-1 and VCAM-1) in cardiac allograft at day 6 posttransplantation. All data are expressed as means ± SE for n = 6 mice per group.
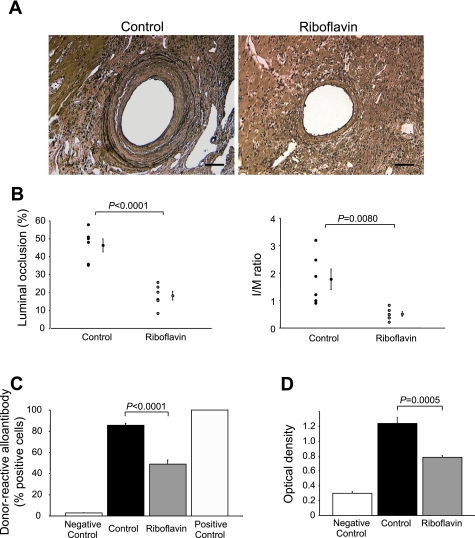
Protection against CAV.
Initial experiments examining the effect of antioxidant treatment on acute rejection were done in the absence of immunosuppression. To determine whether antioxidant treatment modulates vasculopathy, under chronic conditions more similar to those seen in human cardiac allotransplantation, immunosuppression was employed preoperatively in recipients. At day 60 posttransplantation, luminal occlusion and the I/M ratio of vessels in transplanted hearts were significantly decreased in antioxidant-treated allografts compared with controls (luminal occlusion, 18.1 ± 2.5 vs. 46.2 ± 3.7%, P < 0.0001; I/M, 0.5 ± 0.2 vs. 1.8 ± 0.4%, P = 0.008; Fig. 6, A and B). As histological rejection was also apparently reduced by antioxidant treatment, experiments were performed to investigate the impact of antioxidant treatment on humoral and cellular immunity in chronic rejection. Both donor-reactive alloantibody formation and MLR-driven T-cell proliferation were significantly suppressed in riboflavin-treated recipients compared with controls (Fig. 6, C and D).
Fig. 6.
Chronic allograft rejection at day 60 posttransplantation. A: cardiac allograft vasculopathy development in cardiac allografts (elastin staining). Bar = 50 μm. B: histomorphometrical quantification of luminal occlusion and intima-to-media (I/M) ratio. C: donor-reactive alloantibodies in recipient serum. D: T-cell proliferation in mixed lymphocyte reaction ex vivo. All data are expressed as means ± SE for n = 6 mice per group.
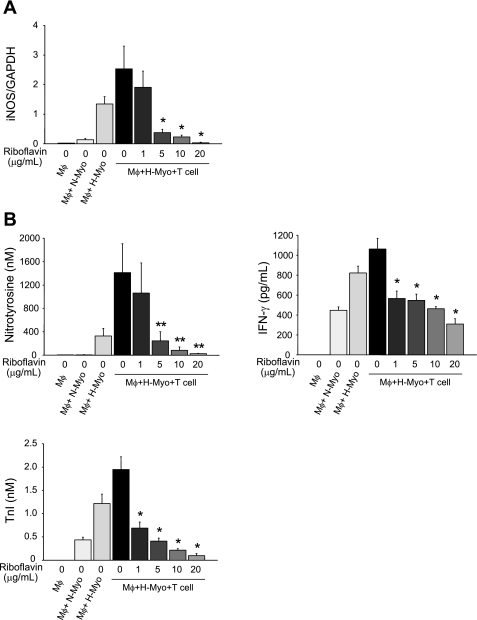
Riboflavin suppresses nitrosative and oxidative stress in vitro.
To further understand the effects of an antioxidant on the suppression of both nitrosative and oxidative stress seen in vivo, experiments were performed in vitro to simulate a microenvironment in which an ischemically injured heart is exposed to an alloimmune cellular milieu. For these experiments, recipient macrophages and T cells were cocultured with donor cardiomyocytes that had been exposed to hypoxia to simulate ischemia. Without riboflavin, expression of iNOS mRNA in macrophages and levels of nitrotyrosine, IFN-γ, and TnI were most increased in macrophage/hypoxic cardiomyocyte/T-cell coculture. However, riboflavin suppressed them dose dependently (Fig. 7, A and B).
Fig. 7.
Coculture of recipient macrophages (Mφ) and T cells with donor cardiomyocytes in vitro. A: expression of iNOS mRNA in Mφ. B: nitrotyrosine, IFN-γ, and troponin I (TnI) in coculture supernatants. All data are expressed as means ± SE for 6 independent analyses. *P < 0.0001 (by ANOVA) vs. Mφ + hypoxic cardiomyocytes (H-Myo) + T cells in 0 μg/ml of riboflavin. **P < 0.005 (by ANOVA) vs. Mφ + H-Myo + T cells in 0 μg/ml of riboflavin. N-Myo, normoxic cardiomyocytes.
DISCUSSION
It has long been suspected that oxidative stress contributes to injury of ischemic and reperfused tissues. In the setting of cardiac allotransplantation, not only are there ischemic and reperfusion periods obligated by the harvest, preservation, and implantation procedures, but placement of the heart into an immune milieu can act as an adjuvant for oxidative damage. The current studies evaluated the mechanisms by which one particularly potent antioxidant, riboflavin, can mitigate graft injury following cardiac allotransplantation. Riboflavin is a particularly intriguing antioxidant, as it is reduced to dihydroriboflavin by flavin reductase, a cytoplasmic NADPH-dependent reductase, which not only rapidly terminally quenches ROS as they are formed, but is regenerated cyclically as reducing equivalents are generated. Although there has been some previous work suggesting beneficial effects of riboflavin in various conditions of ischemia and reperfusion injury, including transplantation (14, 15, 18), the physiological protective mechanisms that are bolstered by riboflavin have been the subject of conjecture.
It is generally believed that ischemia-reperfusion injury occurs when ROS production exceeds the buffering capacity of native antioxidant defenses, and/or components of these defense systems are reduced (30). The present study demonstrates several potential mechanisms by which riboflavin treatment depresses ROS levels: by inhibiting the induction of NF-κB, cytokine and adhesion receptor expression, and graft leukosequestration, all of which can contribute to a diminution in the generalized ambient ROS milieu. In addition, a major endogenous antioxidant defense system (superoxide dismutase) is buttressed by an early period of riboflavin treatment. Consequently, it is not surprising that the biological footprints of tissue oxidative stress, lipid peroxidation, and DNA damage are diminished. These new data suggest that riboflavin can act not only directly as an antioxidant by scavenging ROS, but also as an indirect antioxidant by preserving endogenous antioxidant defenses. This duality of action is likely to amplify the protective effects of this agent in a biologically meaningful way.
Another major new finding of these experiments is that suppression of oxidative stress in the setting of cardiac allotransplantation leads to a substantial reduction in nitrosative stress. Our previous studies showed that NO produced via iNOS, either in macrophages or in cardiac myocytes themselves, acts as a lethal effector cytotoxin (25), and enhanced iNOS expression and NO production lead to promotion of the graft rejection process (20, 21). Previous studies by other groups have shown that peroxynitrite itself can mediate oxidizing tissue injury, resulting in a reciprocal cycling of superoxide and the amplification of oxidant stress (3, 22). In the present study, administration of riboflavin affects the concomitant inhibition of iNOS expression and peroxynitrite formation. These data suggest that antioxidant treatment during the ischemia-reperfusion phase suppresses not only nitrosative stress early on, but also diminishes oxidative stress in the acute rejection phase. These early salutary features are likely to be the underpinnings of the observed reduction in allograft rejection and CAV development at time points far later out than the initial treatment period. The data shown here, in which antioxidant treatment results in diminished iNOS expression and reduced evidence of tissue nitrosative stress, suggests that oxidative and nitrosative stress are inseparably intertwined in the setting of cardiac allograft transplantation. This is not entirely surprising, as the iNOS gene contains an important NF-κB regulatory element, and NF-κB activation is known to enhance induction of iNOS gene expression by cytokines (4, 20). In the present study, antioxidant treatment significantly reduced nuclear localization of NF-κB. One can conjecture that suppression of initial activation of the NF-κB transcription factor can quell the inflammatory maelstrom triggered by cardiac allotransplantation by reducing both oxidative and nitrosative stress.
Little is known about the effects antioxidants have on the immune system in the setting of cardiac allotransplantation. In this work, data indicate that antioxidant treatment significantly suppresses CD4- and CD8-positive T-cell graft infiltration, donor-reactive alloantibody formation, and T-cell proliferation, as measured by MLR. These findings indicate the possibility that an antioxidant given during the peri-implantation period can modulate both cellular and humoral immune responses and play an important role in allograft tolerance induction. In our previous study (15), there was no significant effect of riboflavin on the development of alloantibodies 4–8 wk after transplantation. We concluded there that humoral sensitization is not likely to be the dominant reason for the clear-cut suppression by riboflavin of CAV development. However, it is possible that design bias involving riboflavin dosages and method of administration caused the discrepancy between riboflavin's effects on the development of alloantibodies in models of acute rejection and chronic vasculopathy. Although the current studies do not prove a cause-effect relationship between antioxidant-mediated suppression of IFN-γ expression and subsequent reduced rejection, a link can be postulated as IFN-γ is known to enhance antigen presentation and promote cellular immunity by activated macrophages and T cells (7). A recent study has shown that IFN-γ is a central mediator of allograft dysfunction through dysregulation of iNOS expression, which links early dysfunction with late arteriosclerosis (17). In this study, intragraft IFN-γ mRNA expression was significantly downregulated by antioxidant treatment. Moreover, coculture experiments in vitro demonstrated that riboflavin suppresses both iNOS expression in activated macrophages, as well as IFN-γ production by activated T cells, which are associated with increased viability of cardiomyocytes. This finding strengthens the hypothesis that immunomodulation and allograft protection in cardiac transplantation by riboflavin may result from suppressive effects of both macrophage iNOS and T-cell IFN-γ production.
One of the most interesting facets of the current experiments is that riboflavin treatment, even provided as a short pulse in the several days surrounding the transplantation procedure, can elicit a striking protection against CAV long after the period of its administration. This suggests that a cascade of oxidizing events triggered (or inhibited) during the peritransplant period can have profound long-term consequences for a cardiac graft. Whether or not this might be of any therapeutic utility remains a subject of conjecture, but clinical studies have reported that high-dose riboflavin appears to be safe, without long-term toxicity, and has relatively few adverse effects (8, 28). These findings argue for the clinical study of a peritransplant antioxidant strategy as being one that is simple, safe, inexpensive, and potentially efficacious. The mechanisms by which such a strategy could confer benefit are myriad, but likely include downregulation of mediators of both oxidative and nitrosative stress.
GRANTS
This work was supported by a Taubman Institute Scholarship, The Ruth Professorship, the Scleroderma Research Foundation, and the National Heart, Lung, and Blood Institute Grants P01HL089407, R01HL055397, R01HL085149, and T32-HL-007853.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akizuki E, Akaike T, Okamoto S, Fujii S, Yamaguchi Y, Ogawa M, Maeda H. Role of nitric oxide and superoxide in acute cardiac allograft rejection in rats. Proc Soc Exp Biol Med 225: 151–159, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Ardehali A, Laks H, Drinkwater DC, Ziv E, Drake TA. Vascular cell adhesion molecule-1 is induced on vascular endothelia and medial smooth muscle cells in experimental cardiac allograft vasculopathy. Circulation 92: 450–456, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Arteel GE, Briviba K, Sies H. Protection against peroxynitrite. FEBS Lett 445: 226–230, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 12: 141–179, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87: 1620–1624, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, McCallister HA, Snovar DC, Winters GL, Zerbe A. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant 9: 587–593, 1990. [PubMed] [Google Scholar]
- 7.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol 15: 749–795, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Boehnke C, Reuter U, Flach U, Schuh-Hofer S, Einhaupl KM, Arnold G. High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur J Neurol 11: 475–477, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich H, Hu Y, Zou Y, Dirnhofer S, Kleindienst R, Wick G, Xu Q. Mouse model of transplant arteriosclerosis: role of intercellular adhesion molecule-1. Arterioscler Thromb Vasc Biol 20: 343–352, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Fleury S, Li J, Simeoni E, Fiorini E, von Segesser LK, Kappenberger L, Vassalli G. Gene transfer of RANTES and MCP-1 chemokine antagonists prolongs cardiac allograft survival. Gene Ther 13: 1104–1109, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Hall G, Hasday JD, Rogers TB. Regulating the regulator: NF-kappaB signaling in heart. J Mol Cell Cardiol 41: 580–591, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa T, Visovatti SH, Hyman MC, Hayasaki T, Pinsky DJ. Heterotopic vascularized murine cardiac transplantation to study graft arteriopathy. Nat Protoc 2: 471–480, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Horiguchi K, Kitagawa-Sakakida S, Sawa Y, Li ZZ, Fukushima N, Shirakura R, Matsuda H. Selective chemokine and receptor gene expressions in allografts that develop transplant vasculopathy. J Heart Lung Transplant 21: 1090–1100, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Hultquist DE, Xu F, Quandt KS, Shlafer M, Mack CP, Till GO, Seekamp A, Betz AL, Ennis SR. Evidence that NADPH-dependent methemoglobin reductase and administered riboflavin protect tissues from oxidative injury. Am J Hematol 42: 13–18, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Iwanaga K, Hasegawa T, Hultquist DE, Harada H, Yoshikawa Y, Yanamadala S, Liao H, Visovatti SH, Pinsky DJ. Riboflavin-mediated reduction of oxidant injury, rejection, and vasculopathy after cardiac allotransplantation. Transplantation 83: 747–753, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Koglin J, Granville DJ, Glysing-Jensen T, Mudgett JS, Carthy CM, McManus BM, Russell ME. Attenuated acute cardiac rejection in NOS2−/− recipients correlates with reduced apoptosis. Circulation 99: 836–842, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Koh KP, Wang Y, Yi T, Shiao SL, Lorber MI, Sessa WC, Tellides G, Pober JS. T cell-mediated vascular dysfunction of human allografts results from IFN-gamma dysregulation of NO synthase. J Clin Invest 114: 846–856, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mack CP, Hultquist DE, Shlafer M. Myocardial flavin reductase and riboflavin: a potential role in decreasing reoxygenation injury. Biochem Biophys Res Commun 212: 35–40, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Mazer SP, Pinsky DJ. Alive and kicking: endothelium at the geographic nexus of vascular rejection. Circ Res 91: 1085–1088, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Minamoto K, Harada H, Lama VN, Fedarau MA, Pinsky DJ. Reciprocal regulation of airway rejection by the inducible gas-forming enzymes heme oxygenase and nitric oxide synthase. J Exp Med 202: 283–294, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minamoto K, Pinsky DJ. Recipient iNOS but not eNOS deficiency reduces luminal narrowing in tracheal allografts. J Exp Med 196: 1321–1333, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilakantan V, Halligan NL, Nguyen TK, Hilton G, Khanna AK, Roza AM, Johnson CP, Adams MB, Griffith OW, Pieper GM. Posttranslational modification of manganese superoxide dismutase in acutely rejecting cardiac transplants: role of inducible nitric oxide synthase. J Heart Lung Transplant 24: 1591–1599, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Okada M, Wang CY, Hwang DW, Sakaguchi T, Olson KE, Yoshikawa Y, Minamoto K, Mazer SP, Yan SF, Pinsky DJ. Transcriptional control of cardiac allograft vasculopathy by early growth response gene-1 (Egr-1). Circ Res 91: 135–142, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Pinsky D, Oz M, Liao H, Morris S, Brett J, Sciacca R, Karakurum M, Van Lookeren CM, Platt J, Nowygrod R. Restoration of the cAMP second messenger pathway enhances cardiac preservation for transplantation in a heterotopic rat model. J Clin Invest 92: 2994–3002, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinsky DJ, Cai B, Yang X, Rodriguez C, Sciacca RR, Cannon PJ. The lethal effects of cytokine-induced nitric oxide on cardiac myocytes are blocked by nitric oxide synthase antagonism or transforming growth factor beta. J Clin Invest 95: 677–685, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell ME, Wallace AF, Wyner LR, Newell JB, Karnovsky MJ. Upregulation and modulation of inducible nitric oxide synthase in rat cardiac allografts with chronic rejection and transplant arteriosclerosis. Circulation 92: 457–464, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-kappa B. Chem Biol 2: 13–22, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology 50: 466–470, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 24: 1710–1720, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka M, Mokhtari GK, Terry RD, Balsam LB, Lee KH, Kofidis T, Tsao PS, Robbins RC. Overexpression of human copper/zinc superoxide dismutase (SOD1) suppresses ischemia-reperfusion injury and subsequent development of graft coronary artery disease in murine cardiac grafts. Circulation 110: II200–II206, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Worrall NK, Lazenby WD, Misko TP, Lin TS, Rodi CP, Manning PT, Tilton RG, Williamson JR, Ferguson TB Jr. Modulation of in vivo alloreactivity by inhibition of inducible nitric oxide synthase. J Exp Med 181: 63–70, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]