Abstract
Vascular aging is characterized by increased oxidative stress and proinflammatory phenotypic alterations. Metabolic stress, such as hyperglycemia in diabetes, is known to increase the production of ROS and promote inflammatory gene expression, accelerating vascular aging. The oxidative stress hypothesis of aging predicts that vascular cells of long-lived species exhibit lower steady-state production of ROS and/or superior resistance to the prooxidant effects of metabolic stress. We tested this hypothesis using two taxonomically related rodents, the white-footed mouse (Peromyscus leucopus) and the house mouse (Mus musculus), which show a more than twofold difference in maximum lifespan potential (8.2 and 3.5 yr, respectively). We compared interspecies differences in steady-state and high glucose (HG; 30 mmol/l)-induced production of O2•− and H2O2, endothelial function, mitochondrial ROS generation, and inflammatory gene expression in cultured aortic segments. In P. leucopus aortas, steady-state endothelial O2•− and H2O2 production and ROS generation by mitochondria were less than in M. musculus vessels. Furthermore, vessels of P. leucopus were more resistant to the prooxidant effects of HG. Primary fibroblasts from P. leucopus also exhibited less steady-state and HG-induced ROS production than M. musculus cells. In M. musculus arteries, HG elicited significant upregulation of inflammatory markers (TNF-α, IL-6, ICAM-1, VCAM, and monocyte chemoattractant protein-1). In contrast, the proinflammatory effects of HG were blunted in P. leucopus vessels. Thus, increased life span potential in P. leucopus is associated with decreased cellular ROS generation and increased resistance to prooxidant and proinflammatory effects of metabolic stress, which accord with predictions of the oxidative stress hypothesis of aging.
Keywords: senescence, comparative biology, vascular disease, atherosclerosis
age is a major risk factor for cardiovascular disease, which remains the leading cause of morbidity and mortality of older Americans. Despite recent advances in the biology of aging, the factors determining successful cardiovascular aging are still not completely understood (15, 53, 54). Mammalian life span ranges 100-fold, and comparative studies (53, 54) on long-lived, successfully aging animals can elucidate key cellular mechanisms that may contribute importantly to successful cardiovascular aging. We initiated a series of studies to compare mechanisms related to oxidative stress, oxidative stress resistance, and redox signaling between long-living species and shorter-living ones to test predictions of the oxidative stress theory of aging and to elucidate key mechanisms for delaying cardiovascular aging (12, 14, 30, 53, 54).
Recently, we introduced Peromyscus leucopus (white-footed mouse) as a study organism in a comparative approach to cardiovascular aging research (14, 53, 54). The P. leucopus and Mus musculus (house mouse) longevity contrast pair, originally proposed by Sacher and Hart (48), has several advantages that can be exploited to elucidate mechanisms responsible for phylogenetic differences in mammalian longevity and health span (14, 51, 53, 54). Both species belong to the superfamily of mouselike rodents (Muroidea) and share a common ancestor 20–25 million years ago (52). However, despite its close physical resemblance to M. musculus, P. leucopus has an unusually long life span for its size [maximal life span potential (MLSP): ∼8.2 yr (48)], which is approximately twice as long as the MLSP of M. musculus.
The oxidative stress theory of aging predicts that if oxidative stress plays a central role in cardiovascular aging, then vascular cells of longer living species should 1) produce less ROS under steady-state conditions (Fig. 1A), 2) be more protected from environmental or metabolic stress-induced ROS overproduction (14, 53, 54) (Fig. 1B), 3) exhibit superior resistance to the adverse effects of oxidative stress (Fig. 1C), and/or 4) exhibit a slower rate of age-related increases in ROS generation (Fig. 1D) than cells of shorter-living species. Our previous results on P. leucopus vessels thus far agreed with the predictions of the oxidative stress theory of aging. Accordingly, we demonstrated that endothelial cells of P. leucopus exhibit a significantly lower steady-state ROS generation by NAD(P)H oxidases than M. musculus cells (14). P. leucopus arteries also exhibit superior antioxidant systems and increased nitric oxide (NO) production compared with M. musculus vessels (14). Endothelial cells of P. leucopus have also been shown to resist oxidized LDL- and/or H2O2 -induced DNA damage and apoptosis (14, 54).
Fig. 1.
Schematic representation of potential strategies by which long-lived, successfully aging animals can delay/limit oxidative stress-induced damage in the cardiovascular system and in other organs. A: lower initial ROS generation at young ages under steady-state conditions, so that it takes longer to reach the critical threshold even at the same rate of aging. B: lower ROS generation in response to metabolic stress (and other stressors). C: increased tolerance for increases in ROS production. D: slower rate of age-related increases in ROS generation (increasing the time to reach a threshold beyond which oxidative damage significantly impairs cellular function). Long-lived animals may use a combination of all of these strategies. Nonlinear/exponential characteristics of age-related ROS increases are based on O2•− production in rodent blood vessels (12) as well as on DNA oxidation in the liver, brain, kidney, heart, and skeletal muscle of the same strain (25).
The present study was specifically designed to test the hypothesis that cells of longer-living P. leucopus are protected from metabolic stress-induced oxidative stress. To test this hypothesis, the effects of high glucose treatment (46) [a condition mimicking diabetic conditions (62)] on endothelial function, O2•− and H2O2 production, and inflammatory gene expression were compared in cultured arteries and cells of longer-living P. leucopus and shorter-living M. musculus.
METHODS
Animals and tissue collection.
All animal use protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas (San Antonio, TX) and New York Medical College (Valhalla, NY). C57BL/6 laboratory mice (purchased from Charles River Laboratories, Austin, TX; age: 3 mo old, n = 30) and P. leucopus (Peromyscus Genetic Stock Center, Department of Biological Sciences, University of South Carolina, Columbia, SC; age: 3 mo old, n = 30) were used. We compared young adults because that is the age at which health is maximal and degenerative changes due to aging will not yet have occurred. Both 3-mo-old P. leucopus and M. musculus are fully sexually mature young adults and have not endured aging-related changes. In a separate set of experiments to analyze age-dependent changes in endothelial function and vascular ROS generation in P. leucopus, animals at 3, 6, 18, and 24 mo of age were compared (n = 5 animals/group). Species characteristics are shown in Table 1. Longevity quotients were calculated from the ratio of maximum longevity to the predicted MLSP [based on the allometric equation of Austad and Fisher (5) for nonflying eutherian mammals (predicted longevity = 10.67 × M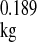 )]. Animals were killed, and aortas were carefully exposed and isolated from the surrounding tissues. Vessels were cleaned from the adventitial adipose tissue using an operating microscope and microsurgery instruments.
)]. Animals were killed, and aortas were carefully exposed and isolated from the surrounding tissues. Vessels were cleaned from the adventitial adipose tissue using an operating microscope and microsurgery instruments.
Table 1.
Body mass, maximum reported life span, and longevity quotient of the species used in this study
| Species | Common Name/Strain | Mass, g | Age, mo | Maximum Life Span, yr | Longevity Quotient |
|---|---|---|---|---|---|
| Mus musculus | House mouse (C57BL/6) | 28 | 3 | ∼3.5 | 0.6 |
| Peromyscus leucopus | White-footed mouse | 21 | 3 | ∼8.2 | 1.5 |
The longevity quotient is the ratio of reported life span to that expected based on body mass predictions using the equation of Austad and Fisher (5) for nonvolant eutherian mammals (maximum life span = 10.67 Mkg0.189). The maximum life span for male Peromyscus leucopus is based on the published data of Sacher and Hart (48).
Organoid culture.
Aortic segments of M. musculus and P. leucopus were isolated and maintained in organoid culture as previously described (14, 19, 30, 55, 57). Vessel segments were treated with high glucose (30 mmol/l) for 24 h (at 37°C). Mannitol was used for osmolarity control. After the culture period, vessels were subjected to subsequent functional experiments or frozen in liquid nitrogen.
Cell culture experiments.
Primary M. musculus and P. leucopus fibroblast cell lines were established using the methods of Villegas et al. (61) with modifications. In brief, skin samples were digested with collagenase (at 37°C and 5% CO2 for 30 min), washed twice with MEM, and then supplemented with 10% FBS (Hyclone). Cells were then plated onto 100-mm dishes with MEM media supplemented with 10% FBS plus penicillin-streptomycin-Fungizone (at 5% CO2 and 3% O2, balanced with air, at 37°C). After 18 h, media were changed to discard unattached cells. Fibroblasts were subsequently cultured as previously described (14). To compare oxidative stress in the two cell types, glucose (final concentration: 30 mmol/l, for 24 h) was added to the culture medium. Mannitol was used for osmolarity control.
Measurement of cellular O2•− production.
Hydroethidine, an oxidative fluorescent dye, was used to assess O2•− production in situ as we have previously reported (12, 14). In brief, high glucose-treated and untreated control aortic segments from M. musculus and P. leucopus were incubated with hydroethidine (3 × 10−6 mol/l at 37°C for 30 min). Arteries were washed three times, and optical sections of the endothelial layer of en face aortic preparations (n = 5 segments from 5 animals/group) were then obtained using an inverted confocal laser scanning microscope (Zeiss Pascal LSM 5) at ×40 magnification with the same settings. Images were analyzed using Zeiss Axionvision imaging software as previously described (14). Unstained aortas and vessels preincubated with polyethylene glycol (PEG)-SOD were used for background correction and negative control, respectively. In separate experiments for quantitative comparison of vascular O2•− generation, the time course of the build up of ethidium fluorescence in en face preparations of the aorta was recorded for 30 min by a Tecan Infinite M200 plate reader. The slope factor was calculated and normalized to Hoechst 33258 fluorescence representing DNA content per cell mass.
Functional experiments.
Endothelial function was assessed as previously described (12, 14, 30). In brief, aortas were cut into ring segments of 2 mm in length and were maintained in organoid culture with or without high glucose treatment (for 24 h). Segments were then mounted on 40-μm stainless steel wires in the myograph chambers (Danish Myo Technology) for measurements of isometric tension. Vessels were superfused with Krebs buffer solution (118 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 25 mM NaHCO3, 1.1 mM MgSO4, 1.2 mM KH2PO4, and 5.6 mM glucose; at 37°C and gassed with 95% air-5% CO2). Relaxations of precontracted (10−6 mol/l phenylephrine) vessels to ACh (from 10−9 to 10−4 mol/l), the NO donor S-nitroso-N-acetylpenicillamine (SNAP; from 10−9 to 3 × 10−5 mol/l) and the stable, cell permeable cGMP analog 8-bromo-cGMP (8-Br-cGMP; from 10−5 to 10−4 mol/l) were obtained. The effects of the O2− scavenger PEG-SOD (200 U/ml) on ACh-induced vascular responses of high glucose-treated aortas were also tested.
Measurement of cellular H2O2 production.
The cell-permeant oxidative fluorescent indicator dye 5 (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (C-H2DCFDA; Invitrogen, Carlsbad, CA) was used to assess H2O2 production (34) in high glucose-treated and untreated control aortas as we have previously reported (12, 14, 30). Unstained aortas and vessels preincubated with PEG-catalase were used for background correction and negative control, respectively. Mannitol was used for osmotic control. The endothelial surface of en face aortic preparations was imaged using fluorescent microscopy as previously reported (14, 56).
In separate experiments, the time course of the build up of C-H2DCFDA fluorescence in en face preparations of the aorta was recorded for 30 min by a Tecan Infinite M200 plate reader. The multi-spot detection/bottom reading feature of the plate reader was used to collect the fluorescence signal from the entire well. The slope factor was calculated and normalized to Hoechst 33258 fluorescence representing DNA content per cell mass.
In separate experiments, H2O2 production was measured fluorometrically in aortic segments using the Amplex red/horseradish peroxidase (HRP) assay as previously described (14). The H2O2 generation rate was compared by measuring the time course of the build up of resorufin fluorescence for 60 min by a Tecan Infinite M200 plate reader. Controls included measurements of tissue autofluorescence, time course measurements of dye-only controls, PEG-catalase controls, and obtaining calibration curves with exogenous H2O2.
In other experiments, primary fibroblasts (after passage 4) were cultured as previously described (14) in the presence or absence of high glucose (30 mM for 24 h; mannitol was used for osmotic control). After the culture period, cells were washed three times, and cellular ROS production was assessed using dihydroethidine (DHE) and C-H2DCFDA fluorescent methods, respectively (55). The time course of the build up in dichlorofluorescein (DCF) fluorescence was assessed by a Tecan Infinite M200 plate reader. The slope factor was calculated and normalized to Hoechst 33258 fluorescence representing DNA content (number of cells).
Measurement of mitochondrial ROS production.
MitoSox (Invitrogen), a mitochondrion-specific hydroethidine-derivative fluorescent dye (14, 39), was used to assess mitochondrial O2•− production in situ using en face aortic preparations as previously reported (14, 57). In brief, high glucose-treated and untreated control aortic segments from M. musculus and P. leucopus were incubated with MitoSox (10−6 mol/l at 37°C), and the time course of the build up of the fluorescent signal was recorded by a Tecan Infinite M200 plate reader. The slope factor was calculated and normalized to Hoechst 33258 fluorescence representing DNA content per cell mass. FCCP (10−6 mol/l) was used to uncouple mitochondria (57) to eliminate the MitoSox signal. Mannitol was used for osmotic control.
In further experiments, mitochondrial O2•− production was assessed in high glucose (15 and 30 mmol/l)-treated and untreated control cultured M. musculus and P. leucopus fibroblasts by flow cytometry (FAScalibur, BD Bioscience, San Jose, CA) using MitoSOX red as previously reported (38, 39). Cell debris (low forward and side scatter), dead cells (Sytox green and annexin V positive), and apoptotic cells (annexin V positive) were gated out for analysis (38, 39). Data are presented as fold changes in the mean intensity of MitoSOX fluorescence compared with the untreated control. Confocal images of MitoSox-loaded cells were obtained to show the perinuclear localization of the stained mitochondria.
Quantitative real-time PCR.
The effect of high glucose treatment (30 mmol/l for 24 h) on the expression of TNF-α, IL-6, ICAM-1, VCAM, and monocyte chemoattractant protein (MCP)-1 was also analyzed in cultured aortic segments of M. musculus and P. leucopus as previously reported (16, 17). In brief, total RNA from aortic segments was isolated with the Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III reverse transcriptase (Invitrogen) as previously described (16, 17). Real-time RT-PCR techniques were used to analyze mRNA expression using the Strategen MX3000 as previously reported (16). We used VISTA (http://www-gsd.lbl.gov/vista), a tool for comparative sequence analysis (23), to identify evolutionarily conserved regions of the mouse target genes for the design of the primers. The efficiency of the PCR was determined using dilution series of a standard vascular sample. The fidelity of the PCR was determined by melting temperature analysis and visualization of products on a 2% agarose gel. Quantification was performed using the ΔΔCT method, where CT is the threshold cycle. The housekeeping gene hypoxanthine phosphoribosyl transferase and β-actin were used for internal normalizations.
Data analysis.
Data were normalized to the respective control mean values and are expressed as means ± SD or SE. Statistical analyses of data were performed by Student's t-test or by two-way ANOVA followed by the Tukey post hoc test, as appropriate. P < 0.05 was considered statistically significant.
RESULTS
Resistance to high glucose-induced O2•− production in P. leucopus arteries.
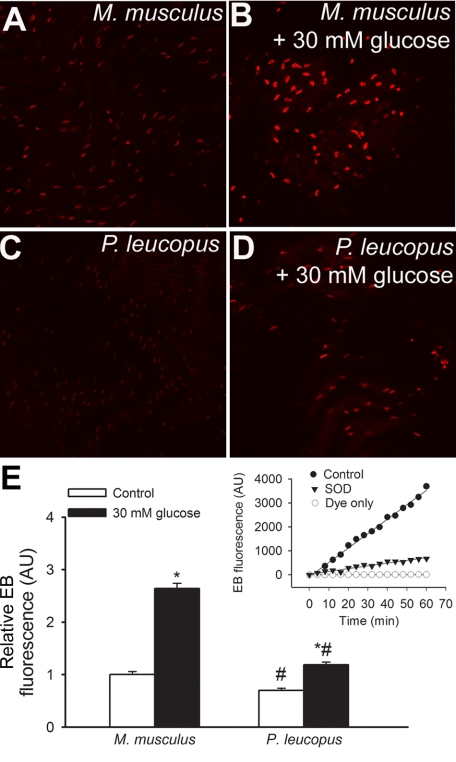
DHE staining experiments in en face aortic preparations were performed to measure endothelial O2•− production. Representative confocal images showing ethidium bromide staining in endothelial cells of aortas from M. musculus and P. leucopus are shown in Fig. 2, A–D, whereas summary data for cellular ethidium fluorescence intensities are shown in Fig. 2E. We found that after overnight culture, M. musculus endothelial cells exhibited more pronounced steady-state O2•− generation compared with P. leucopus cells (Fig. 2E). High glucose treatment (30 mM for 24 h) substantially increased ethidium staining in M. musculus endothelial cells (Fig. 2, B and E), whereas P. leucopus cells were more resistant to high glucose-induced oxidative stress (Fig. 2, D and E). The DHE signal was attenuated by pretreatment with PEG-SOD, showing the relative specificity of the assays for O2•− (Fig. 2F).
Fig. 2.
Results from dihydroethidine (DHE) staining experiments in en face aortic preparations to measure vascular O2•− production [DHE reacts with O2•− to form the fluorescent product ethidium (EB)]. A–D: representative confocal images showing EB staining (red fluorescence) in endothelial cells of aortas from Mus musculus (A) and Peromyscus leucopus (C; original magnification: ×20). High glucose treatment (30 mM for 24 h) substantially increased EB staining in M. musculus endothelial cells (B), whereas P. leucopus cells were more resistant to high glucose-induced oxidative stress (D). E: time course of the build up of EB fluorescence in aortic segments of mice. Bar graphs are summary data for slope factors normalized to cell number (i.e., nuclear DNA content, as assessed by Hoechst fluorescence intensity), representing tissue O2•− production. Data are means ± SE [in arbitrary units (AU) of ΔEB fluorescence/dt/DNA content]; n = 5 animals/group. *P < 0.05 vs. untreated animals; #P < 0.05 vs. M. musculus. Inset, control experiments showing increases in EB fluorescence in the presence of a vascular sample (control) treated with or without polyethylene glycol (PEG)-SOD or the assay buffer (DHE dye) only.
Resistance to high glucose-induced endothelial dysfunction in P. leucopus vessels.
We found that high glucose treatment elicited significant vasodilator dysfunction in M. musculus aortas, as shown by the impaired vasorelaxation response to ACh (Fig. 3A). In contrast, endothelium-dependent responses were preserved in high glucose-treated vessels of P. leucopus (Fig. 3B). Incubation with PEG-SOD significantly increased ACh-induced relaxation in high glucose-treated M. musculus aortas, whereas it had only minor effects on responses of high glucose-treated P. leucopus vessels. Endothelium-independent relaxations to the NO donor SNAP (Fig. 3C) and to 8-Br-cGMP (data not shown) were unaffected by high glucose treatment in both species.
Fig. 3.
A: relaxation to ACh was significantly impaired in high glucose (30 mM for 24 h)-treated aortic segments isolated from M. musculus compared with untreated controls. Preincubation with PEG-SOD restored ACh-induced relaxations in high glucose-treated aortic segments. Data are means ± SE; n = 5 vessels/group. *P < 0.05. B: relaxation to ACh was preserved in high glucose (30 mM for 24 h)-treated aortic segments isolated from P. leucopus. Data are means ± SE; n = 5 vessels/group. C: high glucose treatment did not affect relaxations to the nitric oxide (NO) donor S-nitroso-N-acetylpenicillamine (SNAP) in either group (n = 4 vessels/group) (not significant).
Resistance to high glucose-induced H2O2 production in P. leucopus arteries.
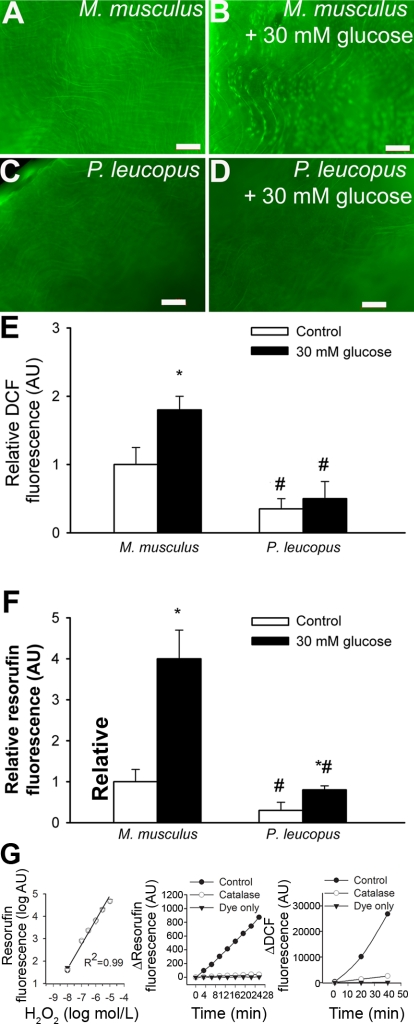
Vascular H2O2 generation was first measured by the DCF fluorescence method. We found that endothelial cells in vessels of M. musculus produced more H2O2 than vessels of P. leucopus (Fig. 4, A, C, and E) under steady state-conditions. We also found that high glucose treatment substantially increased DCF staining in M. musculus vessels (Fig. 4, B and E), whereas P. leucopus cells were more resistant to high glucose-induced oxidative stress (Fig. 4, D and E). Results similar to these were obtained using the Amplex red/HRP assay (Fig. 4F). DCF and Amplex red/HRP signals were attenuated by pretreatment with PEG-catalase and substantially increased by the administration of H2O2, showing the relative specificity of the assays for H2O2 (Fig. 4G).
Fig. 4.
A–D: representative fluorescent images showing H2O2 production [measured by the 5 (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (C-H2DCFDA) fluorescence method] in endothelial cells of en face preparations of aortas of M. musculus (A and B) and P. leucopus (C and D). High glucose treatment (30 mM for 24 h) substantially increased dichlorofluorescein (DCF) staining in M. musculus vessels (B), whereas P. leucopus cells were more resistant to high glucose-induced oxidative stress (D). Green fluorescence shows DCF (original magnification: ×10). E: time course of the build up of DCF fluorescence in aortic segments of mice. Bar graphs are summary data for slope factors normalized to cell number (i.e., nuclear DNA content, as assessed by Hoechst fluorescence intensity), representing tissue H2O2 production. Bar graphs are summary data of DCF fluorescent intensities in endothelial cells of M. musculus and P. leucopus aortas. Data are means ± SE (in AU of ΔDCF fluorescence/dt/DNA content); n = 5 animals/group. *P < 0.05 vs. untreated animals; #P < 0.05 vs. M. musculus. F: results from Amplex red/horseradish peroxidas assays. Shown is the time course of the build up of resorufin (the fluorescence product formed from Amplex red) fluorescence by aortic segments of M. musculus and P. leucopus. M. musculus aortas showed a significantly steeper slope than P. leucopus vessels both under steady-state conditions and after high glucose treatment. Data are means ± SE; n = 5 animals/group. *P < 0.05 vs. untreated animals; #P < 0.05 vs. M. musculus. G, left: increases in resorufin fluorescence in response to exogenous H2O2. Also shown are the time-dependent increases in resorufin (middle) and DCF (right) fluorescence in the presence of a vascular sample (control) treated with or without PEG-catalase or the assay buffer (Amplex red or C-H2DCFDA, respectively) only.
Resistance to high glucose-induced ROS production in P. leucopus fibroblasts.
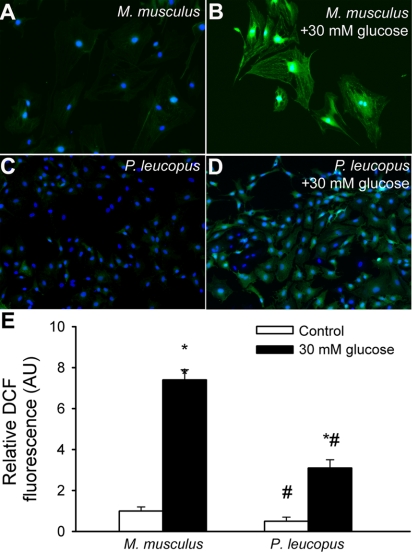
We also compared H2O2 production in cultured primary fibroblasts using the DCF fluorescence method. We found that M. musculus fibroblasts produced more H2O2 than vessels of P. leucopus (Fig. 5, A and E) under steady state-conditions. Treatment with high glucose also significantly increased H2O2 production (as indicated by the increased DCF fluorescence; Fig. 5, B and E) in M. musculus fibroblasts. In contrast, high glucose treatment elicited significantly less oxidative stress in P. leucopus cells (Fig. 5, D and E).
Fig. 5.
Representative fluorescent images showing stronger cellular C-H2DCFDA staining (green fluorescence; an indicator of production of ROS, including H2O2) in primary M. musculus fibroblasts (A) than in P. leucopus fibroblasts (C). High glucose treatment elicited substantial increases in DCF fluorescence in M. musculus fibroblasts (B), whereas high glucose-induced increases in mitochondrial ROS generation were significantly lower in P. leucopus fibroblasts (D). Hoechst 33258 (blue fluorescence) was used for nuclear staining. E: bar graphs are summary data of DCF fluorescent intensities in control and high glucose-treated M. musculus and P. leucopus fibroblasts. Data are means ± SE. *P < 0.05. untreated animals; #P < 0.05 vs. M. musculus.
Resistance to high glucose-induced mitochondrial ROS production in P. leucopus cells.
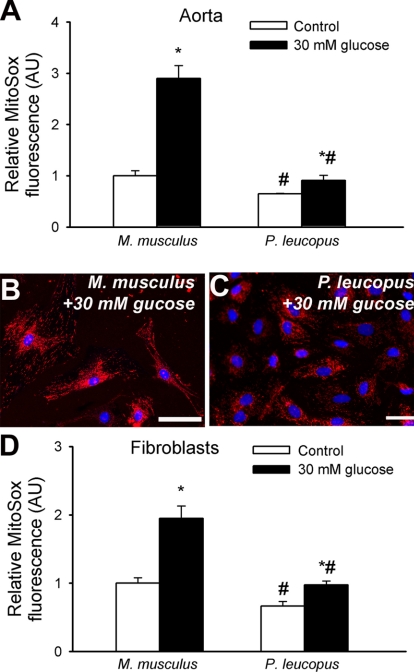
A series of experiments (Fig. 6) was conducted to examine the production of ROS by mitochondria. In en face preparations of M. musculus aortas, there was greater steady-state mitochondrial ROS production than in P. leucopus aortas (Fig. 6A). Compared with untreated controls, high glucose treatment elicited substantial increases in MitoSox fluorescence in M. musculus aortas, whereas high glucose-induced increases in mitochondrial ROS generation were significantly lower in P. leucopus vessels (Fig. 6A).
Fig. 6.
A: time course of the build up of MitoSox fluorescence in en face preparations of M. musculus and P. leucopus aortas. Bar graphs are summary data for the slope factor obtained from the time course experiments normalized to tissue mass, representing mitochondrial ROS production in the tissues. Shown are the effects of high glucose treatment (30 mM for 24 h) on mitochondrial ROS production in both species. Data are means ± SE; n = 5 animals/group. *P < 0.05 vs. untreated animals; #P < 0.05 vs. M. musculus. B and C: representative fluorescent images showing stronger perinuclear MitoSox staining (red fluorescence) in high glucose-treated primary M. musculus fibroblasts (B) than in P. leucopus fibroblasts (C). Hoechst 33258 (blue fluorescence) was used for nuclear staining. Scale bars = 50 μm. D: cellular MitoSox fluorescence intensities were assessed using flow cytometry as described in methods. Bar graphs are summary data for mean MitoSox fluorescent intensities. Data are means ± SE. Experiments were performed in quadruplicate with identical results. *P < 0.05 vs. untreated animals; #P < 0.05 vs. M. musculus.
Similar results were obtained when MitoSox fluorescence was compared in primary fibroblasts (Fig. 6, B–D). Representative fluorescent images are shown in Fig. 6, B and C. Cellular MitoSox fluorescence intensities were compared using flow cytometry (Fig. 6D). We found that primary M. musculus fibroblasts exhibited stronger perinuclear MitoSox staining (Fig. 6D) than P. leucopus fibroblasts (Fig. 6D). Compared with untreated controls, high glucose treatment elicited substantial increases in MitoSox fluorescence in M. musculus fibroblasts, whereas high glucose-induced increases in mitochondrial ROS generation were significantly lower in P. leucopus fibroblasts (Fig. 6, B–D). We also found that M. musculus cells exhibited significantly greater changes in MitoSox fluorescence in response to both 30 mM glucose (M. musculus: 0.95 ± 0.18 and P. leucopus: 0.31 ± 0.06, P < 0.05) and 15 mM glucose (M. musculus: 0.35 ± 0.07 and P. leucopus: 0.11 ± 0.05, P < 0.05) than P. leucopus cells.
Resistance to high glucose-induced inflammatory gene expression in P. leucopus vessels.
We assessed high glucose-induced inflammatory gene expression in M. musculus and P. leucopus aortas by analyzing mRNA expression of TNF-α, IL-6, ICAM-1, VCAM, and MCP-1. In cultured aortic segments isolated from M. musculus (Fig. 7A), high glucose treatment (30 mM for 24 h) elicited significant increases in the mRNA expression of TNF-α, IL-6, ICAM-1, VCAM, and MCP-1. In contrast, high glucose treatment marginally induced these inflammatory markers in cultured aortas of P. leucopus (Fig. 7B), but the trends were not statistically significant.
Fig. 7.
High glucose-induced inflammatory gene expression in M. musculus and P. leucopus aortas. A: in cultured aortic segments isolated from M. musculus, high glucose treatment (30 mM for 24 h) elicited significant increases in the mRNA expression of TNF-α, IL-6, ICAM-1, VCAM, and monocyte chemoattractant protein (MCP)-1. B: in contrast, 24 h of high glucose treatment did not result in the significant induction of these inflammatory markers in aortas of P. leucopus. Analysis of mRNA expression was performed by real-time quantitative RT-PCR. β-Actin was used for normalization. Data are means ± SE; n = 5 animals/group. *P < 0.05. vs. untreated animals.
Age-related changes in endothelial function and ROS production in P. leucopus vessels.
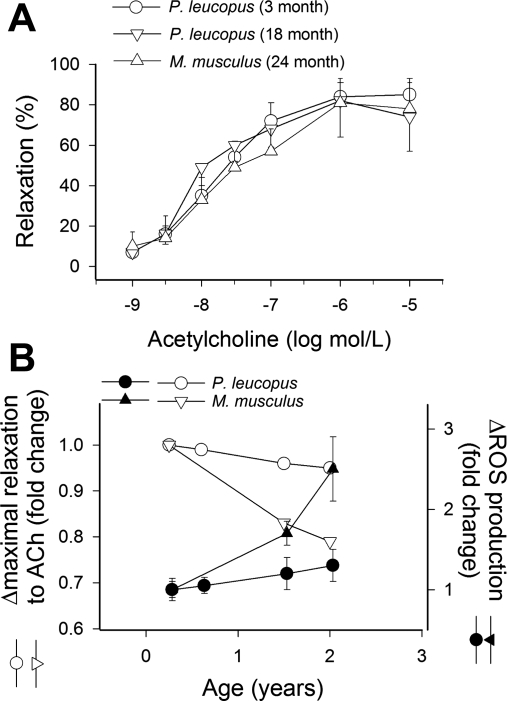
Vessel relaxation responses induced by ACh (Fig. 8, A and B) and vascular production of ROS (as determined by DHE staining; Fig. 8B) were similar in 3-, 6-, 18-, and 24-mo-old P. leucopus. Figure 8B shows the maximal relaxations to ACh in P. leucopus and M. musculus as a function of chronological age. M. musculus arteries showed a marked decline in induced maximal vessel relaxation and a significant increase in ROS production during the same time period.
Fig. 8.
A: relaxation to ACh in aortic ring preparations from 3-, 18-, and 24-mo-old P. leucopus. Data are means ± SE; n = 5 animals/group. B: age-dependent decline in the maximal relaxation responses induced by ACh (open symbols) in aortas of M. musculus and P. leucopus as a function of chronological age. M. musculus data were replotted from previous publications (12, 43). Age-dependent increases in vascular O2•− production (as assessed by DHE staining) in arteries of M. musculus and P. leucopus (filled symbols) are superimposed.
DISCUSSION
Three following key observations were made in this study, namely, in arteries of P. leucopus compared with those of M. musculus: 1) ROS production occurs at a lower steady-state rate and is independent of interspecies differences in circulating factors, 2) metabolic stress-induced total cellular ROS production and mitochondrial oxidative stress are significantly attenuated, and 3) high glucose treatment elicits a blunted inflammatory gene expression profile.
There is increasing evidence supporting an important role for oxidative stress in the aging process (8). According to the free radical theory of aging, the age-related progressive decline in cellular function is caused by the adverse effects of oxygen free radicals (8, 26). Indeed, in nonvertebrate model organisms under laboratory conditions, overexpression of antioxidant enzymes and/or treatment with antioxidants can extend life span (49). There is also solid evidence that aging in mammals is associated with significant oxidative stress and oxidative macromolecular damage in virtually every tissue studied (8, 25, 58, 60). Yet, the role of oxidative stress in determining the mammalian life span is not completely understood (8, 36, 44, 50, 59). Although there are reports suggesting that the overexpression of catalase increases life span in mice (63), in other studies, transgenic mice overexpressing antioxidant enzymes involved in the scavenging of ROS do not exhibit a longevity phenotype (37). In contrast, the concept that oxidative stress is involved in age-related cardiovascular diseases (including atherosclerosis and hypertension) appears robust. Previous studies by these and other laboratories have shown that vascular aging is characterized by increased ROS production in endothelial and smooth muscle cells (for recent reviews, see Refs. 15 and 53), which decreases the bioavailability of the vasodilator and antiapoptotic NO, increases cardiac oxygen demand (1), and promotes vascular inflammation (13, 17–19), at least in part, by inducing NF-κB (57). Oxidative stress-induced vascular inflammation is considered a critical initial step in the development of atherosclerosis in aging.
The oxidative stress theory of aging predicts that vascular cells of longer-living species produce less ROS than those of shorter-living ones. Using the M. musculus-P. leucopus longevity contrast pair, we demonstrated that freshly isolated vessels from longer-living P. leucopus exhibit lower steady-state levels of ROS production than vessels of shorter-living M. musculus (14). However, the question remained as to whether this difference is intrinsic to the cellular mechanisms of ROS homeostasis or due to differences in plasma levels of small-molecular-weight antioxidants and/or hormones that are known to regulate vascular ROS generation (e.g., IGF-1 or insulin). To address this question, we first compared cellular ROS production in isolated aortic segments maintained in organoid culture. The present study clearly demonstrated that differences in O2•− and H2O2 generation in P. leucopus and M. musculus vessels are retained in organoid culture conditions (Figs. 2 and 4, respectively). To further substantiate our findings and exclude the possibility that serum factors result in phenotypic changes that are not readily reversible in relatively short-term organoid culture, we also compared ROS homeostasis in primary fibroblast cultures. The finding that significantly lower steady-state ROS generation was observed in primary P. leucopus fibroblasts compared with M. musculus cells (Fig. 5) provided strong evidence that serum factors (e.g., differences in IGF-1 or insulin levels) do not likely contribute significantly to the attenuation of steady-state cellular ROS production in P. leucopus. It is possible that some of the mechanisms of cellular ROS homeostasis may differ among fibroblasts and vascular endothelial and smooth muscle cells (e.g., the expression profile of NADPH oxidases); thus, there is a clear need for conducting future mechanistic studies on primary endothelial and smooth muscle cell lines from both species.
To test the hypothesis that tissues of longer-living species are more resistant to metabolic stress-related oxidative stress, we exposed isolated arteries and cultured cells of P. leucopus to high glucose concentrations. We chose high glucose treatment as a metabolic stressor because hyperglycemia plays a central role in accelerated vascular aging in diabetes mellitus, as shown by the prevention or retardation of the development of vascular diseases by strict metabolic control (40). The harmful vascular effects of hyperglycemia have been attributed to, at least in part, increased oxidative stress (41). Our second major observation is that cellular O2•− levels and H2O2 generation induced by high glucose are blunted in arteries of P. leucopus (Figs. 2E and 4E, respectively) compared with those of M. musculus. In this regard, it is also noteworthy that endothelial function in high glucose-treated P. leucopus arteries is preserved (Fig. 3B), whereas metabolic stress caused significant endothelial dysfunction in M. musculus vessels (Fig. 3A). This marked resistance to metabolic stress-induced ROS generation is not characteristic to vascular endothelial and smooth muscle cells, because experiments in cultured P. leucopus fibroblasts yielded identical results (Fig. 5). Previous studies (42, 47) have suggested that adventitial fibroblasts play important roles in vascular oxidative stress. Thus, the attenuated ROS generation in fibroblasts of long-lived species may have pathophysiological relevance.
Enhanced glucose flux through glycolysis results in the increased generation of pyruvate, which shuttles into the mitochondria for subsequent oxidation through the tricarboxylic acid cycle, generating NADH (9, 21). This, in turn, causes accelerated electron flow through the respiratory chain, which may increase mitochondrial O2•− production (9, 21, 38, 39, 46). The mitochondrial hypothesis of aging predicts that if mitochondrial ROS production is a determinant in the rate of aging, then mitochondria of long-lived animals should produce less ROS. Our data support this premise in that steady-state mitochondrial ROS generation was significantly lower in intact P. leucopus arteries (Fig. 6A) and fibroblasts (Fig. 6, D and F) than in those of M. musculus (Fig. 6, A, B, and F), extending previous findings (14, 54). Moreover, we and others have demonstrated that the rate of ROS production in cardiac mitochondria isolated from P. leucopus is substantially less than those isolated from M. musculus (10, 14, 51, 54). Conclusions similar to these were reached by studies demonstrating lower ROS production in mitochondria isolated from the heart of other long-lived small mammals, including the small brown bat [Myotis lucifugus, life span: 34 yr (10, 31)] and the naked mole rat [Heterocephalus glaber, life span: >28 yr (31)], as well as avian species [canary, life span: 24 yr (27); parakeet, life span: 21 yr (27); and pigeon, life span: 35 yr (7, 28, 29)]. The present study clearly demonstrates that in M. musculus arteries (Fig. 6A) and fibroblasts (Fig. 6, C and D), high glucose treatment substantial increases mitochondrial ROS generation, whereas P. leucopus arteries (Fig. 6A) and fibroblasts (Fig. 6, C and D) exhibited a marked resistance against the prooxidant effects of metabolic stress. A previous study (14) has showm that vascular endothelial cells of P. leucopus are also resistant to the proapoptotic effects of high glucose. These studies also corroborate with our findings that isolated P. leucopus mitochondria exhibit superior resistance to oxidative stress compared with M. musculus mitochondria (54). Thus, it is reasonable to hypothesize that cellular resistance to metabolic stress-induced mitochondrial oxidative stress will protect cellular viability in longer-living species.
At present, the mechanisms underlying the differences in mitochondrial ROS production between shorter-living and long-lived species are not well understood (31–33) and may include, among others, differences in the efficiency of the electron transport chain, uncoupling proteins, mitochondrial membrane composition, mitochondrial thiol redox state, amounts of coenzyme Q associated with mitochondrial membrane proteins, and differential regulation of the entry of electrons into the cytochrome chain, especially at the level of the dehydrogenase site of complex I. Current evidence also supports that mitochondrial ROS production is determined by the number of mitochondria within the cell (6). Mitochondrial biogenesis is a tightly controlled process (56), and we have recently provided evidence that mitochondrial content declines with age in vascular endothelial and smooth muscle cells (56). Importantly, the induction of mitochondrial biogenesis in calorie-restricted animals is also associated with decreases in mitochondrial ROS production (35). Factors that are known to regulate mitochondrial biogenesis and thus mitochondrial ROS include sirtuin 1, peroxisome proliferator-activated receptor-γ coactivator-1α, and NO (56). To date, it is unclear how cellular processes involved in mitochondrial biogenesis differ between short-lived and long-lived species. We know that NO bioavailability is higher in P. leucopus than in M. musculus (14); however, whether this results in a higher number of mitochondria in P. leucopus cells is unknown. Interestingly, tissue oxygen consumption in the cardiac muscle is significantly higher in P. leucopus than in M. musculus (14), which may be an indicator of increased mitochondrial mass.
Although the interspecies differences in metabolic stress-induced mitochondrial ROS production likely contribute to the observed differences in total cellular O2•− and H2O2 production, we cannot completely eliminate other mechanisms as well. Importantly, activation of NAD(P)H oxidase has also been suggested to be involved in high glucose-induced oxidative stress (11). Previously, we (14) found that P. leucopus vessels exhibit less expression of NAD(P)H oxidase and lower basal NADPH oxidase activity than vessels of M. musculus. This difference may also contribute to the lower level of total cellular ROS production in P. leucopus tissues. In addition, P. leucopus vessels have been shown to exhibit higher expression of antioxidant enzymes (including glutathione peroxidase and catalase) (14), which likely also limit oxidative stress in P. leucopus cells.
According to the original formulation of the free radical theory of aging by Harman (26), the progressive decline in cellular function with age results from the accumulation of oxidative damage to cellular constituents. However, in addition to causing macromolecular “damage,” ROS play important signaling roles as well. Accordingly, hypotheses have recently been put forward linking oxidative stress-induced inflammatory signaling processes with the regulation of longevity and development of age-related diseases (for a review, see Ref. 22). Accordingly, age-related oxidative stress promotes vascular inflammation in aged laboratory rodents (15, 17–19, 53, 57). Relevant to our study, high glucose-induced oxidative stress has also been reported to promote proinflammatory phenotypic alterations in cultured endothelial cells (46). As a corollary of the free radical theory of aging, we predict that if inflammation is detrimental in the vascular aging process, then metabolic stress-induced inflammatory changes should be less pronounced in longer-living species.
Finally, our study shows that arteries of M. musculus overexpress several inflammatory markers (TNF-α, IL-6, ICAM-1, VCAM, and MCP-1; all of which are known to play a role in accelerated vascular aging in metabolic diseases) in response to high glucose treatment (Fig. 7). In contrast, arteries of P. leucopus were refractory to the proinflammatory effects of metabolic stress (Fig. 7). Although protein expression for the inflammatory markers was not assessed, future studies need to determine whether metabolic stress-induced upregulation of protein expression of adhesion molecules and monocyte adhesiveness to the endothelium are similarly attenuated in successfully aging species. Because high glucose treatment elicited less oxidative stress in P. leucopus cells than in M. musculus cells (Figs. 2, 4, 5, and 7), we would predict that the observed differences in the expression of vascular inflammatory markers are secondary to interspecies differences in ROS homeostasis. This view is supported by a previous study (46) showing that antioxidants can attenuate high glucose-induced vascular inflammatory gene expression. Yet, it is also possible that the same level of oxidative stress would still elicit a blunted inflammatory response in P. leucopus due to the differential activity of anti-inflammatory pathways. This possibility will be tested in future studies. Additional in vivo studies are needed to determine whether P. leucopus arteries are also refractory to the adverse effects of chronic hyperglycemia in diabetes mellitus than M. musculus vessels. It is also important to note that inflammatory cytokines (especially TNF-α) may also contribute to vascular oxidative stress under diabetic conditions (24, 45). Whether this positive feedback loop is attenuated in longer-living species is presently unknown.
On the basis of our previous studies (12, 53) on naked mole rats, we hypothesized that increased cellular resistance to oxidative stress may correlate with a slower age-related decline of endothelial function in P. leucopus. To test this hypothesis, we assessed age-related alterations in endothelial function and ROS production in P. leucopus vessels. Endothelial function and O2•− production did not significantly differ between arteries from 3- and 24-mo-old P. leucopus (Fig. 8), whereas a substantial decline in endothelial function and increases in O2•− production in M. musculus arteries were evident over a similar time interval (Fig. 8B). These findings may suggest a possible link between mechanisms regulating increased metabolic stress resistance and the slower rate of aging in longer-living species.
Limitations of the study.
It is important to stress that comparative biological approaches are widely used by investigators to exploit the 100-fold differences in chronological life span of mammalian species. A variety of cellular studies have been proposed as a means to study long-lived species (4, 20), e.g., those using cells from extremely long-lived Odontocetes and bats. These investigations have concluded that the assessment of differences in redox homeostasis between successful agers and nonsuccessful agers is best determined by comparing cells of the young adult of each species. This approach can clearly differentiate cellular responses to oxidative challenges when cell health is maximal and degenerative changes due to cellular aging have not become evident (4). Although investigating animals at different points in their life cycle may also be highly informative, these studies are often difficult to conduct in many of the long-lived species due to the lack of availability of aging colonies. We would like to point out that due to the lack of availability of very old P. leucopus, it is impossible at present to obtain functional data on senescent P. leucopus approaching their maximal life span.
Measurement of cellular responses of animals at the same chronological age has also met with criticism in that some investigators believe that differences in cellular responses should be measured in animals of the same relative age (i.e., 3 and 6 mo of age would represent 7% of the maximal chronological life span of M. musculus and P. leucopus, respectively). The key issue addressed in our study is whether cells of M. musculus and P. leucopus, with all other parameters being equal, exhibit differences in resistance to metabolic and oxidative stresses. Therefore, we believe that it is important to compare animals of the same chronological ages. Furthermore, there were no meaningful differences between responses of 3- and 6-mo-old P. leucopus (Fig. 8B).
Conclusions.
Collectively, our findings in the P. leucopus-M. musculus longevity contrast pair corroborate with the predictions of the oxidative stress theory and the mitochondrial theory of aging, suggesting that longevity is associated with increased cellular resistance to metabolic stress-induced ROS production and inflammatory phenotypic changes. One may wonder whether there is an association between genetically inherited factors that promote cardiovascular health and can predict longevity in humans as well. Results from studies by Arking et al. (2) and Atzmon et al. (3) that screened for longevity-associated genes in centenarians appear to support this view.
GRANTS
This work was supported by American Heart Association Grants 0430108N (to Z. Ungvari) and 0435140N (to A. Csiszar), the American Diabetes Association (to Z. Ungvari), the American Federation for Aging Research (to A. Csiszar), National Institutes of Health (NIH) Grants HL-077256 and HL-43023 (to Z. Ungvari), Philip Morris U.S.A. Incorporated and Philip Morris International (to Z. Ungvari), Hungarian Scientific Research Fund OTKA-K68758 (to G. Losonczy), the Intramural Research Program of NIH (to P. Pacher), and the Children's Hospital Foundation (to P. Ballabh).
Acknowledgments
Some of the experiments took place during the 2007 Molecular Biology of Aging Course at the Marine Biological Laboratory (Woods Hole, MA) (organized by S. N. Austad), for which we thank The Ellison Medical Foundation. Apologies are extended to all those whose findings or opinions pertinent to this topic were not referenced or discussed due to limitations of space or inadvertent omissions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer-344 rats. Am J Physiol Heart Circ Physiol 285: H1015–H1022, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res 96: 412–418, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Atzmon G, Rincon M, Schechter CB, Shuldiner AR, Lipton RB, Bergman A, Barzilai N. Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol 4: e113, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austad SN An experimental paradigm for the study of slowly aging organisms. Exp Gerontol 36: 599–605, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Austad SN, Fischer KE. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J Gerontol 46: B47–B53, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Barja G, Herrero A. Localization at complex I and mechanism of the higher free radical production of brain nonsynaptic mitochondria in the short-lived rat than in the longevous pigeon. J Bioenerg Biomembr 30: 235–243, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev 78: 547–581, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Brunet-Rossinni AK Reduced free-radical production and extreme longevity in the little brown bat (Myotis lucifugus) versus two non-flying mammals. Mech Ageing Dev 125: 11–20, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H, Volpe M, Luscher TF. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation 107: 1017–1023, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Heart Circ Physiol 293: H919–H927, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-α treatment in aging. Am J Pathol 170: 388–698, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell 6: 783–797, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Csiszar A, Pacher P, Kaley G, Ungvari Z. Role of oxidative and nitrosative stress, longevity genes and poly(ADP-ribose) polymerase in cardiovascular dysfunction associated with aging. Curr Vasc Pharmacol 3: 285–291, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: role of NF-κB inhibition. Am J Physiol Heart Circ Physiol 291: H1694–H1699, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. FASEB J 17: 1183–1185., 2003. [DOI] [PubMed] [Google Scholar]
- 19.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21–30, 2004. [DOI] [PubMed] [Google Scholar]
- 20.de Magalhaes JP Species selection in comparative studies of aging and antiaging research. In: Handbook of Models for Human Aging, edited by Conn PM. Burlington, MA: Academic, 2006, p. 9–20.
- 21.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA 97: 12222–12226, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finch CE The Biology of Human Longevity. Burlington, MA: Academic, 2007.
- 23.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res 32: W273–W279, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation 115: 245–254, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci USA 98: 10469–10474, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harman D Aging: a theory based on free radical and radiation chemistry. J Gerontol: 298–300, 1956. [DOI] [PubMed]
- 27.Herrero A, Barja G. H2O2 production of heart mitochondria and aging rate are slower in canaries and parakeets than in mice: sites of free radical generation and mechanisms involved. Mech Ageing Dev 103: 133–146, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Ku HH, Brunk UT, Sohal RS. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic Biol Med 15: 621–627, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Ku HH, Sohal RS. Comparison of mitochondrial pro-oxidant generation and anti-oxidant defenses between rat and pigeon: possible basis of variation in longevity and metabolic potential. Mech Ageing Dev 72: 67–76, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, Ungvari Z. Comparison of endothelial function, O2•− and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol 291: H2698–H2704, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R, Brand MD. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell 6: 607–618, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Lass A, Agarwal S, Sohal RS. Mitochondrial ubiquinone homologues, superoxide radical generation, and longevity in different mammalian species. J Biol Chem 272: 19199–19204, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lass A, Sohal RS. Comparisons of coenzyme Q bound to mitochondrial membrane proteins among different mammalian species. Free Radic Biol Med 27: 220–226, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA 103: 1768–1773, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van Remmen H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev 127: 298–306, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Mele J, Van Remmen H, Vijg J, Richardson A. Characterization of transgenic mice that overexpress both copper zinc superoxide dismutase and catalase. Antioxid Redox Signal 8: 628–638, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Prot 2: 2295–2301, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun 358: 203–208, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, O'Leary DH, Genuth S. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 348: 2294–2303, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagano PJ, Gutterman DD. The adventitia: the outs and ins of vascular disease. Cardiovasc Res 75: 636–639, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, Kensler TW, Yamamoto M, Egan JM, Longo DL, Ingram DK, Navas P, de Cabo R. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci USA 105: 2325–2330, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res 99: 69–77, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR, Obrosova IG, Pacher P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol 293: H610–H619, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rey FE, Li XC, Carretero OA, Garvin JL, Pagano PJ. Perivascular superoxide anion contributes to impairment of endothelium-dependent relaxation: role of gp91phox. Circulation 106: 2497–2502, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Sacher GA, Hart RW. Longevity, aging and comparative cellular and molecular biology of the house mouse, Mus musculus, and the white-footed mouse, Peromyscus leucopus. Birth Defects Orig Artic Ser 14: 71–96, 1978. [PubMed] [Google Scholar]
- 49.Sampayo JN, Olsen A, Lithgow GJ. Oxidative stress in Caenorhabditis elegans: protective effects of superoxide dismutase/catalase mimetics. Aging Cell 2: 319–326, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Sentman ML, Granstrom M, Jakobson H, Reaume A, Basu S, Marklund SL. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem 281: 6904–6909, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Sohal RS, Ku HH, Agarwal S. Biochemical correlates of longevity in two closely related rodent species. Biochem Biophys Res Commun 196: 7–11, 1993. [DOI] [PubMed] [Google Scholar]
- 52.Steppan S, Adkins R, Anderson J. Phylogeny and divergence-date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Syst Biol 53: 533–553, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci 13: 5056–5070, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Ungvari Z, Krasnikov BF, Csiszar A, Labinskyy N, Mukhopadhyay P, Pacher P, Cooper AJL, Podlutskaya N, Austad SN, Podlutsky A. Testing hypotheses of aging in long-lived mice of the genus Peromyscus: association between longevity and mitochondrial stress resistance, ROS detoxification pathways and DNA repair efficiency. AGE. In press. [DOI] [PMC free article] [PubMed]
- 55.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol 292: H2417–H2424, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Van Remmen H, Hamilton ML, Richardson A. Oxidative damage to DNA and aging. Exerc Sport Sci Rev 31: 149–153, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics 16: 29–37, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Van Remmen H, Richardson A. Oxidative damage to mitochondria and aging. Exp Gerontol 36: 957–968, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Villegas J, McPhaul M. Establishment and culture of human skin fibroblasts. Curr Protoc Mol Biol Chapter 28: unit 28 23, 2005. [DOI] [PubMed]
- 62.Weihrauch D, Lohr NL, Mraovic B, Ludwig LM, Chilian WM, Pagel PS, Warltier DC, Kersten JR. Chronic hyperglycemia attenuates coronary collateral development and impairs proliferative properties of myocardial interstitial fluid by production of angiostatin. Circulation 109: 2343–2348, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Wu S, Li Q, Du M, Li SY, Ren J. Cardiac-specific overexpression of catalase prolongs lifespan and attenuates ageing-induced cardiomyocyte contractile dysfunction and protein damage. Clin Exp Pharmacol Physiol 34: 81–87, 2007. [DOI] [PubMed] [Google Scholar]