Abstract
Heart rate (HR), body temperature (Temp), locomotor activity (LA), and oxygen consumption (O2C) were studied in awake mice lacking one or both of the adenosine A1 or A2A receptors (A1R or A2AR, respectively) using telemetry and respirometry, before and after caffeine administration. All parameters were lower during day than night and higher in females than males. When compared with wild-type (WT) littermates, HR was higher in male A1R knockout (A1RKO) mice but lower in A2ARKO mice and intermediate in A1-A2AR double KO mice. A single dose of an unselective β-blocker (timolol; 1 mg/kg) abolished the HR differences between these genotypes. Deletion of A1Rs had little effect on Temp, whereas deletion of A2ARs increased it in females and decreased it in males. A1-A2ARKO mice had lower Temp than WT mice. LA was unaltered in A1RKO mice and lower in A2ARKO and A1-A2ARKO mice than in WT mice. Caffeine injection increased LA but only in mice expressing A2AR. Caffeine ingestion also increased LA in an A2AR-dependent manner in male mice. Caffeine ingestion significantly increased O2C in WT mice, but less in the different KO mice. Injection of 30 mg/kg caffeine decreased Temp, especially in KO mice, and hence in a manner unrelated to A1R or A2AR blockade. Selective A2B antagonism had little or no effect. Thus A1R and A2AR influence HR, Temp, LA, and O2C in mice in a sex-dependent manner, indicating effects of endogenous adenosine. The A2AR plays an important role in the modulation of O2C and LA by acute and chronic caffeine administration. There is also evidence for effects of higher doses of caffeine being independent of both A1R and A2AR.
Keywords: diurnal rhythm, metabolic rate, locomotor activity, adenosine, telemetry
caffeine (1,3,7-trimethylxanthine), the most widely consumed stimulant in the world, acts as a competitive antagonist of adenosine receptors (ARs) (18). It is much more potent on three of the known receptors, A1, A2A, and A2B (A1R, A2AR, and A2BR, respectively) than on the fourth, the A3R (17). Since a competitive antagonist will have effects only when and where receptors are activated by an agonist, it is relevant that endogenous agonist adenosine is more potent on A1R and A2AR than on A2BR. The potency of adenosine as an agonist in addition depends on the density of the receptors (16). The levels of adenosine are low under physiological condition but are sufficient to partially activate A1R, A2AR, and A3Rs where the receptors are abundantly expressed. For these reasons caffeine is generally believed to act on A1R and A2AR to exert most of its effects, at least when given in lower doses (18). However, a recent study showed that A2BR knockout (A2BRKO) mice have elevated cytokines, vascular adhesion molecules, and leukocyte adherence (49), indicating that A2BR is probably also activated by endogenous adenosine. This could indicate that at some sites local adenosine concentrations may be high enough to activate A2BR.
One way to determine the correctness of this assumption is to examine the effect of caffeine in mice that lack ARs. With the use of this approach, it has been shown that mice in which the A2AR has been deleted respond less or not at all to caffeine with regard to locomotion and wakefulness (13, 26, 31). Mice that lack A1Rs, however, show little alteration in locomotion and sleep responses to caffeine (23, 26). In the present study, we have examined caffeine effects in mice that lack either A1R or A2AR or both. We also examined effects of the selective A2BR antagonists, enprofylline (20, 39) and 1-propyl-8-p-sulfophenylxanthine (PSB1115) (11, 22, 48). The parameters we examined were heart rate (HR), body temperature (Temp), locomotor activity (LA), and oxygen consumption (O2C) since they could be examined in awake animals and for prolonged periods of time. Acute or single-dose caffeine treatment mainly showed a stimulating effect on HR but clearly biphasic effects on Temp and LA (18, 27, 36, 51). We examined not only acute effects of injecting caffeine but also the effect of adding caffeine to the drinking water for 7 days. We have also determined whether the genotype per se leads to changes, since that would indicate an important role of endogenous adenosine in the physiological regulation. Since a previous study (51) revealed major sex differences, we examined both male and female mice.
MATERIAL AND METHODS
Animals.
The study was performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH) and the ethical guidelines of the European Union and Sweden and was approved by the Animal Ethics Committee of Northern Stockholm.
The A1RKO mice were generated by inactivating the second protein coding exon of the mouse A1R gene as previously described (29). A2ARKO mice were generated by inactivating the exon 2 of mouse adenosine A2AR gene as described previously (8). The A1RKO mice were cross-bred to C57BL/6 mice for six generations by the Jackson Laboratory using a speed congenic approach, whereas A2ARKO mice were backcrossed to C57BL/6 mice for more than 10 generations. DNA marker analysis confirmed that >99% of microsatellite DNA markers are from C57BL/6 genetic background in both A1RKO and A2ARKO mice. These congenic A1RKO and A2ARKO mice were cross bred to generate double heterozygous A1R-A2ARKO mice (A1R+/−, A2AR+/−). The double heterozygous A1R-A2ARKO mice were cross bred to generate all four genotypes from the same littermates, i.e., WT (A1R+/+, A2AR+/+), A1RKO (A1R−/−, A2AR+/+), A2ARKO (A1R+/+, A2AR−/−) and A1R-A2AR double KO (A1R−/−. A2AR−/−) mice. Genotypes were identified by PCR analysis as described previously (8, 29, 44). Mice were kept in individual cages under normal conditions with a 12-h:12-h light-dark cycle during the experiments. All animals were given free access to both food and tap water as described in more detail below (see The telemetry and Oxymax study).
Implantation of telemetry transmitter.
To prevent the infection of mice from the operation, all the instruments and transmitters were sterilized before used in the surgery. The protocol of surgery was described previously (51). Briefly, under anesthesia induced and maintained by isoflurane, the transmitter (TA10ETA-F20) was implanted into the peritoneal cavity and the leads were sutured in a lead II position. Analgesia was ascertained by the local application of 5% xylocaine salve (AstraZeneca, Södertälje, Sweden) and subcutaneous injection of Temgesic (0.3 mg kg−1; Schering-Plough Europe, Brussels, Belgium) after the implantation. Each mouse was allowed at least 7 days of recovery before the start of registration. There was not clear evidence for the infection of mouse after the implantation.
The telemetry and Oxymax study.
We used a telemetry system (Data Sciences, St. Paul, MN) consisting of implantable transmitters (TA10ETA-F20), telemetry receivers (DSI PhysioTel Receivers-RPC-1 Model), and eight universal adapters (UA 10 PC) as previously described (51). A computer program (Data quest A.R.T gold acquisition) sampled calibrated values of HR and Temp as well as noncalibrated LA counts. HR and Temp are recorded as such, whereas LA was determined from recorded displacements of the telemetric device relative to the bottom plate of the cage. Minor movements are not recorded. The protocol is shown in Fig. 1G. After 5 days of baseline registration, the mice received saline, 7.5 mg/kg and 30 mg/kg caffeine (Sigma, St. Louis, MO; dissolved in saline) or enprofylline (kindly provided by Dr. C. G. A. Persson; a selective A2BR antagonist, in a separate experiment) intraperitoneally injected (bolus) to the mice at 2-h intervals. In yet another separate experiment [using only male wild-type (WT) mice], a single dose of 1-propyl-8-p-sulfophenylxanthine (PSB1115, a highly selective A2BR antagonist kindly provided by Dr. Christa E. Muller, University of Bonn) was given. After 2 days recovery from the injection, the animals were moved to Oxymax (v 5.11) system one day for the recording of the basal oxygen consumption (O2C) from changes in total oxygen tension in a closed box. Thereafter, the drinking water was replaced by tap water containing 0.3 g/l caffeine. Again, after 7 days of registration on telemetry, the animals were moved to Oxymax one day for recording of the O2C. After 8 days of caffeine drinking, the water was changed back to normal tap water. Two days later, the mice were administered a single dose of an unselective β-adrenergic blocker (timolol, 1 mg·kg−1; Sigma).
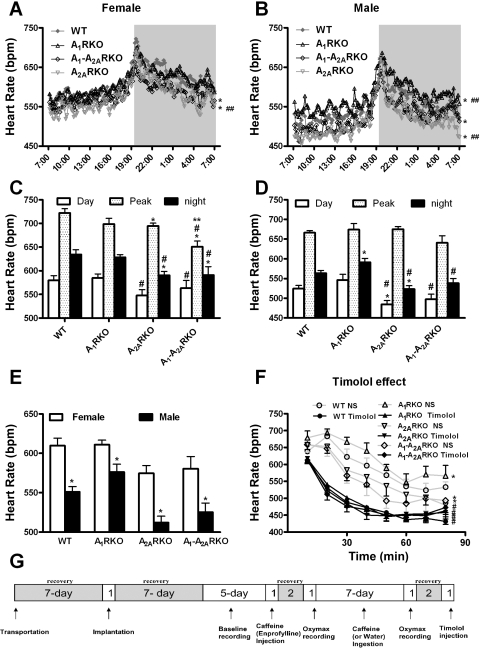
Fig. 1.
A1 and A2A adenosine receptors (A1R or A2AR, respectively) affect heart rate (HR) in sex-dependent manner. A and B: HR is recorded for consecutive 10-min periods in wild-type [WT; Female (F), n = 8; Male (M), n = 8], A1R-knockout (A1RKO; F, n = 8; M, n = 8), A2ARKO (F, n = 8; M, n = 10), and A1-A2ARKO (F, n = 8; M, n = 9) mice. The dark period is from 7 PM to 7 AM. C and D: summarizing the corresponding top panels. The value for Day shows the HR average between 9 AM and 5 PM; the value for Night shows the HR average between 9 PM and 5 AM; the value for Peak shows the HR average between 7:30 PM and 7:40 PM. In A–D, data are shown as a 5-day average; data are presented as mean values or mean values ± SE (*P < 0.05 compared with WT mice; #P < 0.05 compared with A1RKO mice; **P < 0.05 compared with A2ARKO mice; ##P < 0.05 compared with A1-A2ARKO mice at the corresponding time period). E: HR was measured telemetrically during 24 h. The data are shown as a 5-day average and presented as mean values or mean values ± SE (*P < 0.05 compared with corresponding female mice). F: effects of saline and timolol (1 mg/kg) on HR in male mice. The HR was measured at 10-min intervals until 80 min after injection. Data are presented as means ± SE (*compared with WT, P < 0.05; #compared with saline injection, P < 0.05). Abbreviations and mice number are the same as above. G shows the protocol of experiments. bpm, Beats/min; NS, saline.
Statistical analysis.
Two-way ANOVA or Student's t-test was employed to evaluate the differences between groups. Data are presented as means ± SE. Statistical significance was defined as P < 0.05.
RESULTS
ARs affect heart rate (HR) in a sex-dependent manner.
HR shows marked diurnal variation (Fig. 1, A and B). At nighttime genotypes (Fig. 1, A and B). Since the lights were progressively dimming, there was a major increase (about 100 beats/min) in HR in both sexes and in all genotypes. During nighttime, HR gradually returned to the daytime values. When the light was switched on, only a small farther fall in HR was observed. Sex difference was marked: HR in male WT mice was about 550 beats/min and was about 60 beats/min higher in female WT mice (Fig. 1E). A similar trend was found in all genotypes, but in A1RKO mice, the sex difference of HR was less pronounced than in the other mice (Fig. 1E).
There were also clear effects of genotype, but as noted before in the case of A1R (51), they were partly sex dependent (Fig. 1, A–E). Although HR was similar in female A1RKO and female WT mice (Fig. 1, A and C), HR was higher in male A1RKO mice than in male WT mice (Fig. 1, B and D). There was a highly significant decrease in HR (about 35 beats/min) in A2ARKO mice of both sexes, and HR in A1-A2ARKO was also (about 25 beats/min) lower than that in their WT controls of both sexes (Fig. 1, A–E). Again, HR in A1-A2ARKO was higher than in A2ARKO male mice. Thus A1R and A2AR appear to have independent and opposite effects on HR in mice.
To determine whether the differences in HR are due to intrinsic differences in the heart or to differences in the autonomic vasomotor control, we administered a single dose of an unselective β-blocker (timolol; 1 mg/kg). This decreased HR in all genotypes compared with a saline injection (Fig. 1F). After the saline injection, the HR was higher in A1RKO, whereas lower in A2ARKO and A1-A2ARKO than in WT mice. Thus the pattern was similar to that already described in the unstressed animals. Administration of timolol eliminated the HR difference between genotypes in male (Fig. 1F) and female mice (not shown). These data indicated that A1R and A2AR regulate HR via altering sympathetic tone.
Acute administration of caffeine (7.5 and 30 mg/kg) had little effect on HR (results not shown) as reported earlier (51). Injection of enprofylline (A2BR antagonist, at the doses of 7.5 and 30 mg/kg) increased HR in male WT mouse from 529 ± 23 to 590 ± 20 and 562 ± 20 after the low and high dose, respectively (means ± SE; n = 6), but not in females (data not shown). PSB1115 (10 mg/kg) had no effect (PSB1115 vs. saline: 468 ± 16 vs. 479 ± 24, n = 4) in male mice. Week-long oral intake of caffeine decreased HR in WT mice including both sexes, which is more pronounced in the female mouse (50), but there was no clear HR effect in the other genotypes mice (results not shown).
ARs affect body temperature (Temp) in a sex-dependent manner.
Essentially, Temp exhibited similar variations as HR (Fig. 2, A and B). Temp is about 1°C higher in nighttime than in daytime, and this difference was observed in all genotypes. When the lights were turning off, Temp rapidly rose more than 1°C, and then it decreased gradually but did not return to daytime values as long as lights were off; rather, it decreased sharply by 0.5–1°C when the lights were switched on. During daytime, Temp in male WT mice was about 36°C and was about 0.3–0.4°C higher in female mice. During nighttime, the sex difference remained even though the Temp was higher than that during daytime. The sex difference in Temp (Fig. 2E) was less pronounced in A1-A2ARKO than in WT mice (and A1RKO and A2ARKO mice).
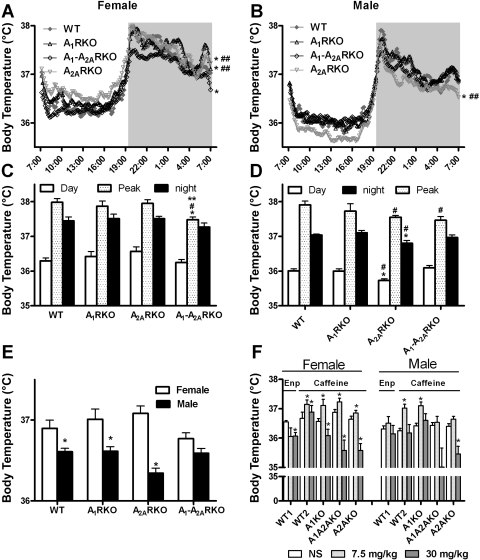
Fig. 2.
A1R and A2AR affect body temperature (Temp) in sex-dependent manner. A and B: abbreviations and mice number are the same as in legend to Fig. 1. Temp is recorded for consecutive 10-min periods in mice. The dark period is from 7 PM to 7 AM. C and D: summarizes the corresponding top panels and periods are as described in Fig. 1. The data are shown as a 5-day average and presented as mean values or mean values ± SE (*P < 0.05 compared with WT mice; #P < 0.05 compared with A1RKO mice; **P < 0.05 compared with A2ARKO mice; ##P < 0.05 compared with A1-A2ARKO mice at the corresponding time period). E: Temp was measured telemetrically during 24 h and recorded for every consecutive 30-min period. The data are shown as a 5-day average and presented as mean values or mean values ± SE (*P < 0.05 compared with corresponding female mice). F: injection of saline (NS), caffeine/enprofylline (Enp) 7.5 mg/kg or 30 mg/kg, was administered intraperitoneally at 2-h intervals in WT1 (F, n = 5; M, n = 6), WT2 (F, n = 8; M, n = 8), A1RKO (F, n = 8; M, n = 8), A1-A2ARKO (F, n = 8; M, n = 9), and A2ARKO mice (F, n = 8; M, n = 10). Temp is shown as the average from 70 to 80 min after the injection. Data are presented as means ± SE (*P < 0.05 compared with saline injection).
Deletion of the A1Rs had small effects on Temp (Fig. 2, A and B). By contrast, deletion of the A2ARs had a major effect, especially in the daytime and surprisingly the effect was qualitatively different in males and females (Fig. 2, A–D). Thus in females it was higher (Fig. 2A), whereas in males it was lower (Fig. 2, B and D). The Temp of the double KO mice tended to decrease in both sexes (Fig. 2, A–D). Female mice had higher Temps (about 0.2 to 0.7°C) than males (Fig. 2E) in all genotypes, and the difference was most pronounced in A2ARKO mice and least in A1-A2ARKO mice.
Acute administration of caffeine had a biphasic effect on Temp (Fig. 2F): a dose of 7.5 mg/kg caffeine increased Temp in all genotypes, whereas the administration of 30 mg/kg caffeine produced a smaller effect in WT mice and actually decreased Temp compared with saline injection in the other genotypes. A high dose of enprofylline (30 mg/kg) also decreased Temp compared with saline injection in female (but not in males) WT mice as studied in a separate experiment (Fig. 2F), but a low dose (7.5 mg/kg) enprofylline had little effect on mouse Temp. The selective antagonist PSB1115 did not change Temp relative to a saline injection (at 70 min after injection: saline gave a Temp of 36.09 ± 0.34°C and after PSB1115 injection the Temp was 36.23 ± 0.14°C; n = 4). As with HR, chronic oral intake of caffeine had little effect on Temp (data not shown).
ARs affect locomotor activity and O2C in a sex-dependent manner.
A pronounced diurnal pattern is also seen in LA of the mice (Fig. 3, A–D). LA in nighttime is about 2–5 times higher than in daytime, and this difference was observed in all genotypes. When the lights were turning off, the mice started moving around quickly (up to 16 counts/min in female WT mice); then their activity gradually declined (from 16 to 6 counts/min in female WT mice and from 10 to 5 in male mice). When the lights were turning on, the mice adopted the daytime activity pattern. The sex difference of LA was much more pronounced in the dark than that in the light (Fig. 3, A–D). The female mice were more active (2 to 3 counts/min higher) than the males (Fig. 3E).
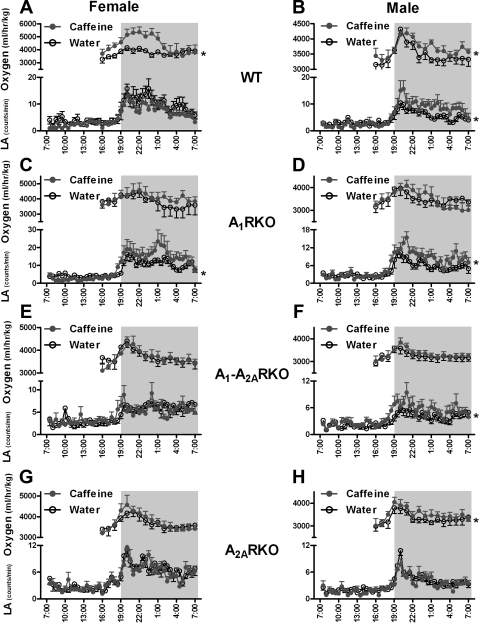
Fig. 3.
A1R and A2AR affect locomotor activity (LA) in sex-dependent manner. Graphs constructed essentially as described in legend to Fig 2. A and B: LA is recorded for consecutive 30-min periods in mice. The dark period is from 7 PM to 7 AM. C and D: summarizes the corresponding top panels. Mean values or mean values ± SE (*P < 0.05 compared with WT mice; #P < 0.05 compared with A1RKO mice; **P < 0.05 compared with A2ARKO mice; ##P < 0.05 compared with A1-A2ARKO mice at the corresponding time period) are given. E: LA were measured telemetrically during 24 h and recorded for every consecutive 30-min periods. The data are shown as a 5-day average and presented as mean values or mean values ± SE (*P < 0.05 compared with corresponding female mice). F: normal saline (NS), caffeine/enprofylline (Enp) 7.5 mg/kg or 30 mg/kg, was administered intraperitoneally at 2-h intervals in WT1 (F, n = 5; M, n = 6), WT2 (F, n = 8; M, n = 8), A1RKO (F, n = 8; M, n = 8), A1-A2ARKO (F, n = 8; M, n = 9), and A2ARKO mice (F, n = 8; M, n = 10). LA is shown as the average from 60 to 90 min after the injection. Data are presented as means ± SE (*P < 0.05 compared with saline injection).
Locomotor activity was essentially unaltered in A1RKO but lower in A2ARKO and A1-A2ARKO than in WT mice (Fig. 3, A and B). In A1-A2ARKO mice, the increase in activity normally seen when the lights are dimming was markedly reduced (Fig. 3, A–D).
As expected, caffeine caused a dose-dependent stimulation of locomotion in WT mice, which was markedly reduced in the other genotypes and even eliminated in mice lacking A2ARs (Fig. 3F). However, injection of the A2B antagonist enprofylline did not affect the LA in WT in another set of experiment (Fig. 3F). PSB1115 (10 mg/kg) was similarly ineffective (not shown).
Chronic caffeine ingestion did increase LA in A1RKO, mice and this was mainly observed in nighttime (Fig. 4, C and D). LA was also increased in WT and A1-A2ARKO male mice, but no significant change was observed in the corresponding females (Fig. 4, A, B, E, and F). There was no difference in LA in A2ARKO mice after caffeine ingestion compared with before caffeine ingestion.
Fig. 4.
Effects of chronic caffeine intake on LA and oxygen consumption (O2C) in mice. After the baseline recording (Water), mice drank tap water with 0.3 g/l caffeine for 7 days (Caffeine). The LAs in the water group are shown as 5-day averages in 30-min intervals, whereas LA data in caffeine group are shown as 3-day averages (days 5, 6, and 7 during chronic caffeine treatment). O2C is shown as 1-h average. The values are presented as means ± SE (*Compared with Water, P < 0.05). Abbreviations are the same as in legend Fig. 2 or otherwise indicated. A and B: chronic caffeine ingestion increased oxygen consumption in WT mice (Water: F, n = 8; M, n = 8; Caffeine: F, n = 7, M, n = 8). It also increased LA in male WT mice but not in female WT mice (Water: F, n = 7; M, n = 6; Caffeine: F, n = 9, M, n = 6). C and D: in A1RKO mice, chronic caffeine ingestion increased LA in both females and males (Water: F, n = 8; M, n = 8; Caffeine: F, n = 8, M, n = 8) but had no effect on O2C (Water: F, n = 7; M, n = 9; Caffeine: F, n = 9, M, n = 8). E and F: in A1-A2ARKO mice, chronic caffeine ingestion increased LA in males but not in females (Water: F, n = 8; M, n = 10; Caffeine: F, n = 8, M, n = 9). Chronic caffeine ingestion did not affect O2C (Water: F, n = 6; M, n = 7; Caffeine: F, n = 6, M, n = 7). G and H: in A2ARKO mice, chronic caffeine ingestion increased O2C in males but not in females (Water: F, n = 7; M, n = 6; Caffeine: F, n = 7, M, n = 4). LA was not affected in females or males (Water: F, n = 8; M, n = 10; Caffeine: F, n = 8, M, n = 8).
As for the other parameters studied, there was a velar diurnal pattern in oxygen consumption (O2C). This could be observed despite the fact that for technical reasons this was measured mainly during the nighttime (Fig. 5, A and B). Sex difference in O2C was also revealed (Fig. 5C), and the female mice consumed more oxygen than the male mice.
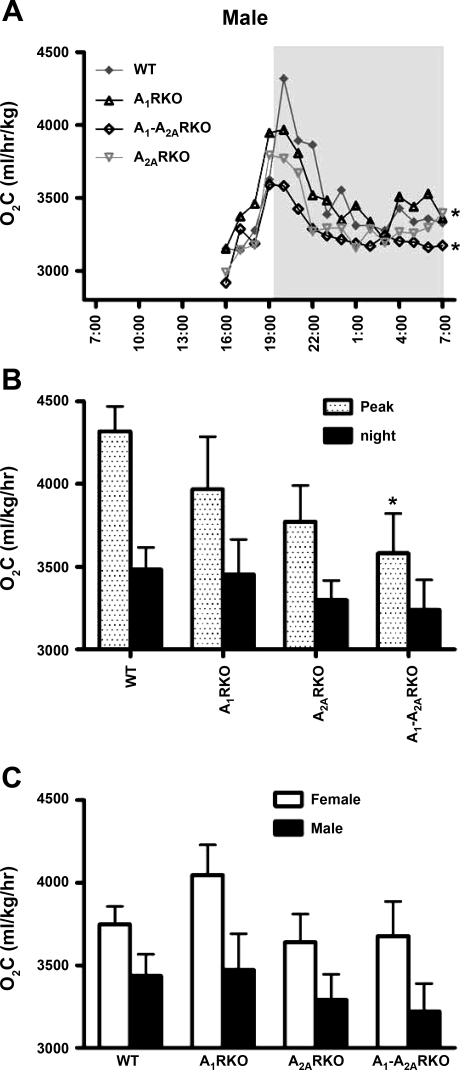
Fig. 5.
Genotype differences in O2C. Abbreviations are the same as in legend to Fig. 1. A: O2C was measured for 60-min periods in WT (F, n = 7; M, n = 6), A1RKO (F, n = 7; M, n = 9), A1-A2ARKO (F, n = 6; M, n = 7), and A2ARKO mice (F, n = 7; M, n = 4) mice. The dark period is from 7 PM to 7 AM. B summarizes the top panel. The black column shows the O2C average between 9 PM and 5 AM; the spotted column for Peak shows the O2C average between 7:30 PM and 8:30 PM. Data are presented as mean values or mean values ± SE (*P < 0.05 compared with WT mice). C: O2C (Oxygen) was measured for 16-h mice.
There was no major difference in O2C between genotypes in the females (data not shown). In male mice (Fig. 5, A and B), the O2C in A2ARKO and A1-A2ARKO male mice was lower than in the WT controls.
Chronic caffeine ingestion significantly increased O2C in WT mice (Fig. 4, A and B). In A2ARKO male, mice oxygen consumption tended to increase, but this was not the case in females (Fig. 4, G and H) or in the A1RKO and A1-A2ARKO mice. When comparing with the caffeine effects on LA, the increase of O2C was not always consistent with the increase of LA via chronic caffeine ingestion.
DISCUSSION
The present study provides some evidence that endogenous adenosine acting on A1R and/or A2ARs influences HR, Temp, locomotion and O2C under physiological conditions. We will briefly discuss these possible roles.
Regulation of HR by ARs.
All four ARs are present in the mouse heart (51). However, the mRNA levels of A1 and A2A are about 100-fold higher than those of A2BR and A3R (51), and the affinity of adenosine to A1R and A2AR is at least as high as to A2B and A3Rs (17). Since the potency of adenosine as an agonist depends on the density and affinity of the receptors (16, 17), the possibility exists that intracardiac A1R and A2AR could regulate HR. However, indirect effects are also possible. Our data indicate that both A1R and A2AR were involved in physiological HR regulation. A1RKO mice had higher HR than WT mice in males and A1-A2ARKO mice had higher HR than A2ARKO mice in both females and males, indicating that endogenous adenosine lowered HR via the A1R under physiological conditions. This confirms our previous study showing that isolated hearts from A1RKO mice had higher HR than those from WT controls (51). It is well known that the activation of the A1R decreases HR by slowing atrioventricular nodal conduction (4, 25), and adenosine is used clinically to alleviate supraventricular tachyarrhythmia. Surprisingly, although the HR of male A1RKO mice was higher than that of WT mice, the HR of female A1RKO mice was not increased. This indicates an interesting sex difference in A1R function of regulating HR. It may be related to the higher HR in females, but the exact reason for the sex difference remains to be explored in future studies.
In this study, we also found that A2ARKO mice had significantly lower HR than WT mice, and HR of A1-A2ARKO mice was much lower than that of A1RKO mice, suggesting that adenosine increases HR via activation of A2AR. This was surprising since a selective A2AR agonist does not influence HR of isolated hearts (33), indicating that the A2AR plays a minor role in local regulation of HR. However, systemic administration of selective A2AR agonists can increase HR, probably due to reflex activation secondary to reduced blood pressure (6, 31, 32, 34, 35). In addition, there is evidence that there are ARs in the brain stem regulating cardiovascular control centers (2–4).
Given that A2ARs apparently mediate a positive effect and A1Rs a negative effect on HR, it was interesting that the HR in A1-A2ARKO mice was lower than in WT controls. This suggests that under physiological conditions, the influence of the A2AR on HR is actually larger than that of the A1R, despite the fact that the latter effect is much more widely recognized.
We found that the administration of timolol (β-blocker) completely abolished the difference in HR between ARKO and WT mice. This argues that the endogenous adenosine that regulates HR under physiological conditions is acting not mainly on A1R and A2AR on cardiac cells but by regulating nervous input [see reviews (5, 15, 17)]. This could be achieved in many ways. Adenosine, through the activation of A1R, decreases the release of neurotransmitters including norepinephrine and acetylcholine from sympathetic and parasympathetic neurons (19) and via A1R and A2AR regulates firing of important cell groups in central nervous system and directly influences cardiovascular control centers (1, 2). Furthermore, adenosine acting at chemoreceptors (14) or on cells in the carotid body (30) could cause reflex activation of sympathetic nerves. Finally, the HR changes may be secondary to changes in blood pressure. However, the A2ARKO mice show no change in blood pressure (40) and A1RKO mice show either a small increase (7) or no change (42). Thus alterations in blood pressure cannot readily explain the observed HR differences between genotypes. Similarly a major increase in pressure after β-receptor blockade by timolol is unlikely as an explanation for the fall in HR.
Regulation of Temp by adenosine receptors.
Adenosine (47) and other AR agonists (10, 43) can induce profound hypothermia, indicating that AR are involved in the regulation of Temp. It has been shown that a selective A1R agonist (CPA) decreases Temp (43), and this effect is reduced in mice lacking A1Rs (25) and improves tolerance to cold temperature (47). We found the Temp of female A1RKO mice to be slightly higher than in WT mice, but this was not observed in male A1RKO mice. Thus, endogenous adenosine acting on A1Rs appears to have a modest influence on Temp in unstressed mice.
The role of A2AR in the regulation of Temp is controversial. It was reported that the administration of a selective A2R agonist did not influence Temp (43), but at the time agonists were not very selective. However, in a later study a selective A2AR agonist decreased Temp in the rat (9). In the present study, we found that A2ARs clearly appeared to play a role in regulating Temp, but this was strongly sex dependent. Although female A2ARKO mice had higher Temp than WT mice, the Temp of male A2ARKO mice was lower than that of WT mice. This difference was particularly obvious in daytime. We lack an explanation for this observation but can conclude that neither differences in activity nor differences in metabolic rate as judged by O2C appear to provide a complete explanation. We also do not ascribe the difference to the estrous cycle, since we averaged the temperature over 5 days and since variability was similar in males and females. The activation of A1R and especially A2AR by adenosine either in central never system (38) or locally (41) could induce a cutaneous vasodilatation, subsequently increased heat loss, and reduced Temp. It is, however, difficult to see how this could adequately explain the qualitative differences between males and females.
Regulation of locomotor activity and O2C by A1R or A2AR.
The roles of the different ARs in regulating activity state including LA are still incompletely understood. We found LA to be slightly higher in female (but not male) A1RKO mice than in their WT controls, but the effect is minor. This small effect agrees with several previous studies on A1KO mice (18, 25). A2ARKO mice had lower LA than their WT controls, implying A2AR stimulated LA. Although LA response to the light turning off in A1-A2ARKO mice was smaller than in A2ARKO mice, there was no difference in LA between A2ARKO and A1-A2ARKO mice. These findings suggest that A2ARs play much more important roles than A1Rs in regulating LA.
LA in A1-A2ARKO mice was much lower than in WT mice, which is in apparent contrast with the finding that pharmacological blockage of A1R and A2AR elevated locomotor activities in rats (28). Similarly, several studies have indicated a small stimulatory effect of A2AR antagonists, but A2ARKO mice have lowered activity. The difference could be partially related to the difference between acute blockade and the life-long elimination of a receptor and perhaps also to the difference between eliminating the receptor and partially blocking it with a competitive antagonist. The latter possibility is supported by our recent finding that double heterozygous mice show increased LA (50).
Male A2ARKO and A1-A2ARKO mice consumed less oxygen than male WT mice. This is consistent with their lower LA. However, we did not find clear differences in O2C among the females, suggesting that oxygen demand is not always covariant with spontaneous activity and that the regulation of oxygen demand via ARs is also sex dependent.
Caffeine effects.
The roles of the different ARs can be revealed also by the use of caffeine as an antagonist. As expected, the administration of caffeine, either as a single dose or repeatedly in the drinking water, increased the activity of mice as determined by measuring locomotor activity. The effect of the single dose was dose dependent. The effect of the oral caffeine intake was not observed in the female mice but only in the male WT mice, perhaps because they showed a lower basal activity than the females. The stimulating effect of single injections of caffeine was not clearly altered in the A1KO mice of either sex, but the nighttime activity appeared to be more strongly affected by oral caffeine in these mice than in WT mice. As also expected from previous data, effects of caffeine were largely eliminated in mice lacking A2AR. Caffeine, injected or ingested, had a statistically significant stimulatory effect in male mice, but it was small. These results suggested that single-dose caffeine injection and chronic caffeine intake increased LA via actions that depended more on A2AR than on A1R.
Caffeine blocks not only A1 and A2AR but also A2BR. We therefore compared the effect of caffeine with that of enprofylline, which is about equipotent with theophylline and hence more potent than caffeine as an A2B antagonist (18, 20, 39). It was chosen in preference to some more recent and selective antagonists because its pharmacokinetics is well known and direct in vivo comparisons with the parent compound have been repeatedly performed (37, 45). It essentially lacks activity at other ARs. Enprofylline and PSB1115 did not activate the mice, and we conclude that A1 and A2AR are the important targets for this action. Moreover, as discussed in detail elsewhere (18, 21), the basal gangla is an important target area.
HR was scarcely affected by caffeine essentially as described (51). Enprofylline had a minor effect in male but no effect in female mice. Enprofylline is not only an adenosine A2BR antagonist but also acts as a phosphodiesterase inhibitor (45) and could by this means increase HR (24). It is also virtually equipotent with caffeine on most studied isoforms of PDE (45) and less potent than other caffeine metabolites such as theophylline or paraxanthine. The more specific A2B antagonist PSB1115 (11, 12, 22, 48) had no effect on HR, suggesting that induced PDE inhibition may be important. We also did not observe any changes in Temp following long-term oral caffeine ingestion. By contrast, acute injection of the lowest dose (7.5 mg/kg) of caffeine caused a small temperature increase. A higher dose of caffeine (30 mg/kg) had little effect on Temp in WT mice but caused a clear-cut decrease in the other genotypes. The fall was quite dramatic. This clearly indicates a biphasic effect of caffeine on temperature and that this depressant effect does not require either A1 or A2AR. Since enprofylline caused at most a modest fall in body temperature, and PSB1115 (10 mg/kg) had no effect at all, we conclude that the temperature lowering effect of 30 mg/kg caffeine in the KO mice is most likely unrelated to AR blockade. The modest fall is compatible with some role of PDE inhibition, especially as we find that a very selective PDE inhibitor (rolipram) had a temperature lowering effect that was, as in the case of enprofylline, especially obvious in male mice (Yang and Fredholm, unpublished observations).
It is well recognized that caffeine can have biphasic effects on, e.g., locomotion and mood exhibiting stimulation at low doses and inhibitory effects at higher doses (18). In a study of A2ARKO mice, it was shown that the stimulatory effect on activity required A2ARs (13) as confirmed here, but the authors suggested that the depressant effect, which remained, was perhaps due to A1R blockade. In a follow-up study in our laboratory, the first conclusion was supported, but the second was not because in A1RKO mice the depressant effect also remained (23). The present finding of a major fall in Temp at doses where this depressant effect is commonly recorded provides an intriguing possible explanation and also indicates that we are dealing with an effect that is not dependent on either A1 or A2AR.
In conclusion, both A1 and A2AR, which are activated by endogenous adenosine under normal physiological conditions, are involved in the regulation of HR, Temp, locomotion activities, and oxygen demand in mice in a sex-dependent manner. The regulation of HR by adenosine through A1R and A2AR is mainly due to altering the sympathetic tone. The A2AR plays a very important role in the modulation of oxygen demand and locomotor activities by acute and chronic caffeine administration.
GRANTS
This study was supported by the Swedish Science Council Grant No. 2553, by NIH Grant RO1 NS048995, the Swedish Heart and Lung Foundation, Karolinska Institutet, and the European Commission (CT 2005-518189). The content of this paper is the responsibility of the authors, and the granting agencies take no responsibility therefore.
Acknowledgments
We thank Professor Guro Valen, Department of Physiology, Oslo, Lilian Sundberg, Eva Irenius, and Karin Lindström for important input. Much of the work was carried out at the Karolinska Institutet Core Facility for Genetic Physiology headed by Professor Anders Arner. The gift of PSB1115 by Dr. Christa Muller is gratefully acknowledged.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barraco RA, Campbell WR, Schoener EP, Shehin SE, Parizon M. Cardiovascular effects of microinjections of adenosine analogs into the fourth ventricle of rats. Brain Res 424: 17–25, 1987. [DOI] [PubMed] [Google Scholar]
- 2.Barraco RA, Janusz CJ, Polasek PM, Parizon M, Roberts PA. Cardiovascular effects of microinjection of adenosine into the nucleus tractus solitarius. Brain Res Bull 20: 129–132, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Barros RC, Branco LG, Carnio EC. Respiratory and body temperature modulation by adenosine A1 receptors in the anteroventral preoptic region during normoxia and hypoxia. Respir Physiol Neurobiol 153: 115–125, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Belardinelli L, Shryock JC, Song Y, Wang D, Srinivas M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J 9: 359–365, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Biaggioni I Contrasting excitatory and inhibitory effects of adenosine in blood pressure regulation. Hypertension 20: 457–465, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Bonizzoni E, Milani S, Ongini E, Casati C, Monopoli A. Modeling hemodynamic profiles by telemetry in the rat. A study with A1 and A2a adenosine agonists. Hypertension 25: 564–569, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Brown RD, Thoren P, Steege A, Mrowka R, Sallstrom J, Skott O, Fredholm BB, Persson AE. Influence of the adenosine A1 receptor on blood pressure regulation and renin release. Am J Physiol Regul Integr Comp Physiol 290: R1324–R1329, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 19: 9192–9200, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coupar IM, Tran BL. Effects of adenosine agonists on consumptive behaviour and body temperature. J Pharm Pharmacol 54: 289–294, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Dunwiddie TV, Worth T. Sedative and anticonvulsant effects of adenosine analogs in mouse and rat. J Pharmacol Exp Ther 220: 70–76, 1982. [PubMed] [Google Scholar]
- 11.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111: 2024–2035, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115: 1581–1590, 2007. [DOI] [PubMed] [Google Scholar]
- 13.El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. Caffeine reduces hypnotic effects of alcohol through adenosine A2A receptor blockade. Neuropharmacology 45: 977–985, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Engelstein ED, Lerman BB, Somers VK, Rea RF. Role of arterial chemoreceptors in mediating the effects of endogenous adenosine on sympathetic nerve activity. Circulation 90: 2919–2926, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Fredholm BB Adenosine receptors as targets for drug development. Drug News Perspect 16: 283–289, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Fredholm BB Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14: 1315–1323, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53: 527–552, 2001. [PMC free article] [PubMed] [Google Scholar]
- 18.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51: 83–133, 1999. [PubMed] [Google Scholar]
- 19.Fredholm BB, Hedqvist P. Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol 29: 1635–1643, 1980. [DOI] [PubMed] [Google Scholar]
- 20.Fredholm BB, Persson CG. Xanthine derivatives as adenosine receptor antagonists. Eur J Pharmacol 81: 673–676, 1982. [DOI] [PubMed] [Google Scholar]
- 21.Fredholm BB, Svenningsson P. Adenosine-dopamine interactions: development of a concept and some comments on therapeutic possibilities. Neurology 61: S5–S9, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Godfrey L, Yan L, Clarke GD, Ledent C, Kitchen I, Hourani SM. Modulation of paracetamol antinociception by caffeine and by selective adenosine A2 receptor antagonists in mice. Eur J Pharmacol 531: 80–86, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Halldner L, Aden U, Dahlberg V, Johansson B, Ledent C, Fredholm BB. The adenosine A1 receptor contributes to the stimulatory, but not the inhibitory effect of caffeine on locomotion: a study in mice lacking adenosine A1 and/or A2A receptors. Neuropharmacology 46: 1008–1017, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto H, Ishii K, Satoh K, Taira N. Comparison of vasodilator effects of DN-9693, a selective inhibitor of cyclic AMP phosphodiesterase, and isobutylmethylxanthine, a non-selective one, in dogs. Jpn J Pharmacol 44: 405–412, 1987. [DOI] [PubMed] [Google Scholar]
- 25.Heller LJ, Olsson RA. Inhibition of rat ventricular automaticity by adenosine. Am J Physiol Heart Circ Physiol 248: H907–H913, 1985. [DOI] [PubMed] [Google Scholar]
- 26.Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci 8: 858–859, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Ilback NG, Siller M, Stalhandske T. Evaluation of cardiovascular effects of caffeine using telemetric monitoring in the conscious rat. Food Chem Toxicol 45: 834–842, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson KA, Nikodijevic O, Padgett WL, Gallo-Rodriguez C, Maillard M, Daly JW. 8-(3-Chlorostyryl)caffeine (CSC) is a selective A2-adenosine antagonist in vitro and in vivo. FEBS Lett 323: 141–144, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hardemark A, Betsholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA 98: 9407–9412, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koos BJ, Mason BA, Ducsay CA. Cardiovascular responses to adenosine in fetal sheep: autonomic blockade. Am J Physiol Heart Circ Physiol 264: H526–H532, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388: 674–678, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Lieu HD, Shryock JC, von Mering GO, Gordi T, Blackburn B, Olmsted AW, Belardinelli L, Kerensky RA. Regadenoson, a selective A2A adenosine receptor agonist, causes dose-dependent increases in coronary blood flow velocity in humans. J Nucl Cardiol 14: 514–520, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Maddock HL, Broadley KJ, Bril A, Khandoudi N. Effects of adenosine receptor agonists on guinea-pig isolated working hearts and the role of endothelium and NO. J Pharm Pharmacol 54: 859–867, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Mathot RA, Cleton A, Soudijn W, IJzerman AP, Danhof M. Pharmacokinetic modelling of the haemodynamic effects of the A2a adenosine receptor agonist CGS 21680C in conscious normotensive rats. Br J Pharmacol 114: 761–768, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nekooeian AA, Tabrizchi R. Effects of adenosine A2A receptor agonist, CGS 21680, on blood pressure, cardiac index and arterial conductance in anaesthetized rats. Eur J Pharmacol 307: 163–169, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Pelissier AL, Gantenbein M, Bruguerolle B. Caffeine-induced modifications of heart rate, temperature, and motor activity circadian rhythms in rats. Physiol Behav 67: 81–88, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Persson CG, Erjefalt I. Seizure activity in animals given enprofylline and theophylline, two xanthines with partly different mechanisms of action. Arch Int Pharmacodyn Ther 258: 267–282, 1982. [PubMed] [Google Scholar]
- 38.Proctor KG, Stojanov I, Bealer SL. Activation of brain adenosine receptors evokes vasodilation in skin arterioles. Am J Physiol Heart Circ Physiol 261: H457–H462, 1991. [DOI] [PubMed] [Google Scholar]
- 39.Robeva AS, Woodard RL, Jin X, Gao Z, Bhattacharya S, Taylor HE, Rosin DL, Linden J. Molecular characterization of recombinant human adenosine receptors. Drug Dev Res 39: 243–252, 1996. [Google Scholar]
- 40.Sakata M, Sei H, Eguchi N, Morita Y, Urade Y. Arterial pressure and heart rate increase during REM sleep in adenosine A2A-receptor knockout mice, but not in wild-type mice. Neuropsychopharmacology 30: 1856–1860, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Stojanov I, Proctor KG. Pharmacological evidence for A1 and A2 adenosine receptors in the skin microcirculation. Circ Res 65: 176–184, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA 98: 9983–9988, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ticho SR, Radulovacki M. Role of adenosine in sleep and temperature regulation in the preoptic area of rats. Pharmacol Biochem Behav 40: 33–40, 1991. [DOI] [PubMed] [Google Scholar]
- 44.Turner CP, Seli M, Ment L, Stewart W, Yan H, Johansson B, Fredholm BB, Blackburn M, Rivkees SA. A1 adenosine receptors mediate hypoxia-induced ventriculomegaly. Proc Natl Acad Sci USA 100: 11718–11722, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ukena D, Schudt C, Sybrecht GW. Adenosine receptor-blocking xanthines as inhibitors of phosphodiesterase isozymes. Biochem Pharmacol 45: 847–851, 1993. [DOI] [PubMed] [Google Scholar]
- 47.Wang LC, Lee TF. Enhancement of maximal thermogenesis by reducing endogenous adenosine activity in the rat. J Appl Physiol 68: 580–585, 1990. [DOI] [PubMed] [Google Scholar]
- 48.Yan L, Muller CE. Preparation, properties, reactions, and adenosine receptor affinities of sulfophenylxanthine nitrophenyl esters: toward the development of sulfonic acid prodrugs with peroral bioavailability. J Med Chem 47: 1031–1043, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest 116: 1913–1923, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang JN, Bjorklund O, Lindstrom-Tornqvist K, Lindgren E, Eriksson TM, Kahlstrom J, Chen JF, Schwarzschild MA, Tobler I, Fredholm BB. Mice heterozygous for both A1 and A2A adenosine receptor genes show similarities to mice given long-term caffeine. J Appl Physiol 106: 631–639, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang JN, Tiselius C, Dare E, Johansson B, Valen G, Fredholm BB. Sex differences in mouse heart rate and body temperature and in their regulation by adenosine A1 receptors. Acta Physiol (Oxf) 190: 63–75, 2007. [DOI] [PubMed] [Google Scholar]