Abstract
Opioids/opiates are commonly administered to alleviate pain, unload the heart, or decrease breathlessness in patients with advanced heart failure. As such, it is important to evaluate whether the myocardial opioidergic system is altered in cardiac disease. A hamster model of spontaneous hypertension was investigated before the development of hypertension (1 mo of age) and in the hypertensive state (10 mo of age) to evaluate the effect of prolonged hypertension on myocardial opioidergic activity. Plasma β-endorphin was decreased before the development of hypertension and in the hypertensive state (P < 0.05). There was no change in cardiac β-endorphin content at either time point. No differences were detected in cardiac or plasma dynorphin A, Met-enkephalin, or Leu-enkephalin, or in cardiac peptide expression of κ- or δ-opioid receptors. μ-Opioid receptor was not detected in either model. To determine how hypertension affects myocardial opioid signaling, the ex vivo work-performing heart was used to assess the cardiac response to opioid administration in healthy hearts and those subjected to chronic hypertension. Agonists selective for the κ- and δ-opioid receptors, but not μ-opioid receptors, induced a concentration-dependent decrease in cardiac function. The decrease in left ventricular systolic pressure on administration of the κ-opioid receptor-selective agonist, U50488H, was attenuated in hearts from hamsters subjected to chronic, untreated hypertension (P < 0.05) compared with control. These results show that peripheral and myocardial opioid expression and signaling are altered in hypertension.
Keywords: hypertension, hamsters, cardiac physiology, cardiac function, β-endorphin, cardiac opioid receptors
hypertension is one of many etiologies that, if left uncontrolled, can lead to heart failure. According to the National Health and Nutrition Examination Survey 1999–2004, there are greater than 72 million Americans over the age of 20 living with high blood pressure, and in ∼95% of the cases, the cause is unknown (1). Hypertension is often characterized by increased sympathetic activity and production of ANG II (16), which along with decreased vasodilator production (21) results in increased cardiac work and myocardial oxygen demand. The resulting hemodynamic stress can lead to left ventricular hypertrophy and/or dilation (16, 21), and if left untreated can progress to heart failure. Studies in the spontaneously hypertensive rat (SHR) (9) and humans (15) have shown increased cardiac function after the onset of hypertension, independent of the development of hypertrophy. Investigation of SHR ventricular cardiomyocytes at a time point after the onset of hypertension, but before the development of hypertrophy, showed increased fractional shortening, resulting from prolonged action potential duration and increased Ca2+-induced Ca2+ release (9). Thus the hypertensive phenotype, independent of hypertrophy, is sufficient to trigger molecular changes in the myocardium.
Opioidergic signaling has been linked to numerous physiological responses, including modulation of cardiac function (3, 19). The central localization of opioid peptides and receptors to known cardiovascular control centers enables opioid peptides to modulate the rate and force of contraction as well as vascular tone (3, 19). Opioids primarily signal through the inhibitory G protein, Gi, and thus inhibit the cAMP/protein kinase A (PKA) pathway (12, 34), and through this pathway opioids have cardiac depressant effects. Alterations in central opioidergic expression, as well as functional changes on exogenous opioid agonist or antagonist administration, have been well documented in hypertension (14, 19, 42). Evidence suggests that the peripheral opioidergic system may also be altered in the hypertensive state (6, 18, 30, 60). These findings suggest a role for the opioidergic system in either the development or maintenance of hypertension. Despite evidence demonstrating alterations in the expression of opioid receptors and peptides in hypertension, whether hypertension influences the ability of cardiac opioid receptors to modulate myocardial function is still unknown.
A hamster model of spontaneous hypertension was used to elucidate whether opioid-induced negative inotropy and lusitropy were altered in hearts subjected to prolonged, uncontrolled hypertension. These studies demonstrated decreased circulating β-endorphin in the hypertensive hamster, and this deficit could be detected before the onset of hypertension. Furthermore, shown here for the first time is an attenuated decrease in left ventricular systolic pressure following opioid agonist administration in the hypertensive heart, independent of alterations in opioid receptor number. These results indicate the opioidergic system is altered in the hypertensive state and may play a role in the development or maintenance of myocardial functional changes observed in hypertension.
METHODS
Hamsters were housed in a pathogen-free environment and handled in accordance with standard use protocols, animal welfare regulations, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee. Spontaneously hypertensive hamsters (H4) and normotensive controls (CHF148) were originally obtained from Ohio University. All hamsters used were male and were evaluated at 1 mo (prehypertensive) or 10 mo (hypertensive) of age.
Blood pressure measurements.
Hamsters were anesthetized with pentobarbital sodium (137 mg/kg ip), placed supine on a heat pad, and a heparinized, fluid-filled catheter (PE-10) was inserted into the right carotid artery. The catheter was connected to a pressure transducer enabling heart rate and diastolic and systolic blood pressures to be recorded using DigiMed Blood Pressure Analyzer and DigiMed System Integrator software.
Cardiac function evaluation.
The perfused, work-performing heart preparation was utilized to directly evaluate the effects of opioid agonist administration on the myocardium. The work-performing heart is a powerful diagnostic tool as it allows evaluation of heart function, and its response to pharmacological or physiological stimuli, independent of neuronal or humoral influences. Briefly, hamsters were anesthetized, the thoracic cavity was opened, and the heart rapidly removed and placed in a bath of cold Krebs-Henseleit solution containing heparin. The aorta was cannulated and the heart was perfused with a modified Krebs-Henseleit solution under constant infusion of 95% O2-5% CO2 in a retrograde capacity. A catheter was fed through the pulmonary vein, past the mitral valve, and into the left ventricle of the heart to measure left intraventricular pressure. Subsequently, the pulmonary vein was cannulated, and the direction of perfusion was changed to anterograde/work-performing mode. The placement of the catheter in the left ventricle along with cannulation of the aorta and pulmonary vein allowed for measurement of systolic and diastolic intraventricular pressures, rates of contraction (+dP/dt) and relaxation (−dP/dt), aortic pressure, and left atrial pressure, respectively. Venous return (preload) was maintained at 10 ml/min in juvenile (1 mo) and 15 ml/min in adult (10 mo) hamsters, and aortic pressure (afterload) was maintained at 50 mmHg at both ages. Hearts were paced (10–20 beats/min above their intrinsic rate), and the temperature of perfusate was maintained at 37.7°C.
Opioid receptor agonist concentration-response curves.
Opioid receptor-selective agonists [U50488H for the κ-opioid receptor (KOR), SNC80 for the δ-opioid receptor (DOR), and fentanyl for the μ-opioid receptor (MOR)] were infused at a constant rate for 10 min per concentration in a cumulative concentration-response curve. The KOR agonist, U50488H, was perfused at a range between 10 nM and 2 μM. The DOR agonist, SNC80, was perfused at a range from 1 nM to 3 μM. The concentrations of the MOR agonist, fentanyl, were between 100 pM and 10 μM. Agonist concentrations were based on known agonist affinity (38, 40) to ensure drug concentrations used were within the range of agonist selectivity for the desired receptor. Based on these values, concentration-response curves were performed to ensure an effective range of concentrations. Following agonist concentration-response curves, the heart was perfused in the absence of drug for 20 min, and cardiac functional parameters were recorded during the washout period. To ascertain whether opioid-induced cardiac depression was receptor mediated, antagonist studies were performed. The κ-antagonist nor-binaltorphimine (nor-BNI, 5 μM) was administered for 20 min before the initiation of the κ-agonist U50488H (500 nM) for 10 min. The δ-antagonist naltrindole (Nal, 500 nM) was administered for 15 min before administration of the δ-agonist SNC80 (500 nM) for 10 min.
Cardiomyocyte isolation.
H4 and CHF148 hamsters at 10 mo were anesthetized, the thoracic cavity was opened, and the heart was rapidly removed and placed in a bath of cold 2.5 mM Ca2+-Tyrode (135 mM NaCl, 4 mM KCl, 1 mM MgCl2, 10 mM glucose, 0.33 mM NaH2PO4, 10 mM HEPES, pH 7.3) solution containing heparin. The aorta was cannulated, and the heart was flushed with 2.5 mM Ca2+-Tyrode to remove blood; the heart was subsequently perfused with 25 μM Ca2+-Tyrode for 5 min to remove additional blood. The perfusate was then switched to Ca2+-free Tyrode, and the heart was perfused a further 5 min. At this time, collagenase (15 U/ml Tyrode, Worthington type II) and protease (0.004 mg/ml Tyrode, Sigma type XIV) were added to the Ca2+-free Tyrode, and the heart was perfused until sufficiently digested (∼25–30 min). The atria and right ventricle were removed and the left ventricle was placed in 10 ml of Ca2+-free Tyrode with collagenase and protease, to which 50 mg of BSA was added. The heart was minced in this solution, and the cells were further dissociated by pipetting up and down with a 10-ml serological pipet. The cell solution was then filtered through 250-μm nylon mesh. The cells were allowed to settle for 5 min, the supernatant was removed, and the cells were resuspended in fresh Ca2+-free Tyrode.
Measurement of cAMP.
The ability of opioid agonists to inhibit isoproterenol-induced cAMP production was tested in cardiomyocytes isolated from the left ventricle of H4 and CHF148 hamsters at 10 mo of age. Briefly, hearts were digested with collagenase as described above and maintained in Ca2+-free Tyrode with 0.1 mg/ml 3-isobutyl-1-methylxanthine (IBMX), a phosphodiesterase inhibitor, to prevent cAMP degradation. Left ventricular myocytes were pretreated with opioid agonists in the absence or the presence of selective antagonists for 15 min at 37°C before a 5-min stimulation of adenylyl cyclase with 10 μM isoproterenol at 37°C. Opioids used were U50488H (100 μM), nor-BNI (KOR antagonist, 100 μM), SNC80 (100 μM), and naloxone (nonselective opioid receptor antagonist, 100 μM). Following isoproterenol stimulation, cardiomyocytes were briefly spun down, Tyrode was removed, and the cells were lysed by addition of 100 μl ice-cold Tris-EDTA buffer. The samples were then boiled for 8 min and put on ice for 5 min before centrifugation to pellet out cellular debris. To measure cAMP content, 50 μl of the corresponding samples were placed in an Eppendorff tube. To this sample, 1.89 nmol of [3H]cAMP was added before addition of 6 μg of PKA. This mixture was allowed to incubate for 1 h at room temperature. Following incubation, activated charcoal was added, and samples were centrifuged to pellet out the activated charcoal, which bound the free (non-PKA bound) cAMP. Two-hundred microliters of supernatant was placed in scintillation fluid and measured. The effect of opioid agonist or agonist plus antagonist coadministration on isoproterenol-induced cAMP accumulation was evaluated as a percentage of the effect of isoproterenol alone.
Measurement of opioid peptide concentrations.
Hearts and plasma were collected from hamsters at the indicated time points (1 and 10 mo of age) and lyophilized to concentrate samples. Cardiac tissue preparation was via the method of Bhargava and colleagues (6), and plasma preparation was via the method of Wintzen (56). Briefly, hearts were submerged in 1 M acetic acid and heated at 95°C for 15 min, then homogenized at 4°C with Polytron, and centrifuged at 4°C for 20 min at 10,000 g. The resulting supernatant was lyophilized and stored at −80°C until use. Blood was collected and EDTA added to prevent coagulation. Blood was then centrifuged at 1,200 g for 15 min at 4°C. The resulting supernatant was lyophilized and stored at −80°C. Lyophilized tissues and plasma were reconstituted in assay buffer, and dynorphin A (Dyn A), β-endorphin (BE), Met-enkephalin (ME), and Leu-enkephalin (LE) were measured by a competitive binding assay in which a labeled standard peptide competed with endogenous opioid peptides (EOPs) in the samples for peptide-specific antibodies as described (Peninsula Laboratories). Concentrations of EOPs were determined based on a standard curve on a per-assay basis normalized to total protein concentration (ng of peptide/mg total protein).
Opioid receptor Western blots.
Left ventricles from 10-mo hypertensive and normotensive hamster hearts were pulverized using mortar and pestle chilled by liquid nitrogen and homogenized using a glass-teflon homogenizer. Protein concentration was determined by Lowry assay, and samples were stored at −80°C until used for Western blot analysis. Samples were separated on a 10% acrylamide gel and transferred to 0.2-μm nitrocellulose membrane. Membranes were blocked with 5% milk in 0.1% PBS-Tween. Membranes were then probed with selective opioid receptor antibodies [KOR and MOR (1:1,000), Santa Cruz Biotech; DOR (1:5,000 dilution), Millipore]. Horseradish peroxidase-conjugated goat anti-rabbit secondary [(1:5,000 dilution), Santa Cruz Biotech] was used with ECL Western blotting detection reagent (Amersham) to visualize opioid receptors. Densitometry was determined using Alpha Innotech FlourChem8800 Imager and FlourChem 8900 software. Opioid receptor expression was normalized to GAPDH content.
Myocyte size.
Hearts from 1-mo and 10-mo hamsters were fixed in paraformaldehyde (4%), embedded in paraffin, and sectioned serially (5 μm). Cardiac sections were deparaffinized, rehydrated, and incubated for 1 h at room temperature with fluorescent TRITC-labeled red wheat germ agglutinin (1:4 dilution). Wheat germ agglutinin-stained slides were imaged using a Nikon Diaphot microscope with a 40× fluorescent objective and a Diagnostics Instruments 7.3 three-shot color camera. Images were captured using Spot software, and myocyte size was determined morphometrically on a minimum of 60 cardiomyocytes per heart from four hearts per group using Image Pro software.
Drugs and chemicals.
Fentanyl, U50488H, SNC80, nor-BNI, naltrindole, naloxone, isoproterenol, PKA, cAMP, and wheat germ agglutinin were purchased from Sigma-Aldrich. [3H]cAMP was purchased from Perkin-Elmer and provided in solution. Fentanyl, U50488H, naloxone, isoproterenol, PKA, cAMP, and naltrindole were dissolved in milli-Q water. SNC80 was dissolved in ethanol. Nor-BNI was dissolved in DMSO. Final concentrations of ethanol and DMSO were <0.1%, and these concentrations had no effect alone.
Data analysis.
All values are shown as means ± SE. Cardiac functional data were analyzed using two-way ANOVA with Bonferroni's posttest. Blood pressure measurements, cAMP content, heart and plasma opioid peptide concentrations, receptor peptide expression, and parameters of cardiac size were analyzed by Student's t-test. Statistical differences were considered significant at P < 0.05.
RESULTS
Cardiac characterization of normotensive and hypertensive hamsters.
There was no difference in body weight, heart weight, heart weight-to-body weight ratio, wet lung weight-to-dry weight ratio, cardiomyocyte area, or cardiomyocyte length between H4 and control hamsters at the prehypertensive or hypertensive stage (data not shown). There was also no significant difference in basal cardiac function or basal cardiomyocyte cAMP content between the two lines at either of the time points studied (data not shown).
Blood pressure measurements.
No difference in blood pressure was detected between H4 and control hamsters at 1 mo. However, 10-mo hypertensive H4 hamsters demonstrated a significant increase in systolic and diastolic blood pressures, mean arterial pressure, and pulse pressure compared with 10-mo control hamsters and 1-mo H4 hamsters (P < 0.05, Table 1). There was also a slight, but significant, increase in heart rate in the hypertensive hamsters at 10 mo (P < 0.05).
Table 1.
Blood pressure and heart rate in H4 and control hamsters
| Mean Arterial Pressure, mmHg | Diastolic Blood Pressure, mmHg | Systolic Blood Pressure, mmHg | Heart Rate, beats/min | |
|---|---|---|---|---|
| 1-mo Control | 111±6 | 93±6 | 129±6 | 422±19 |
| 1-mo H4 | 109±9 | 94±9 | 124±10 | 418±6 |
| 10-mo Control | 91±6* | 79±4 | 103±9* | 313±34* |
| 10-mo H4 | 137±4*† | 111±4*† | 167±6*† | 387±18† |
Values are means ± SE; n = 5–8 animals. H4, spontaneously hypertensive hamsters.
P < 0.05 compared with 1-mo cohort;
P < 0.05 compared with 10-mo control.
Opioid receptor agonist concentration-response curves.
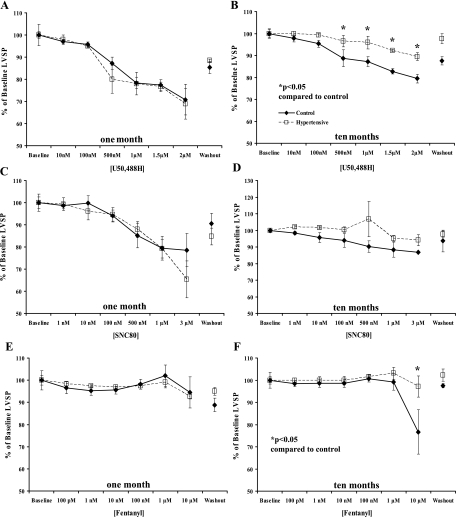
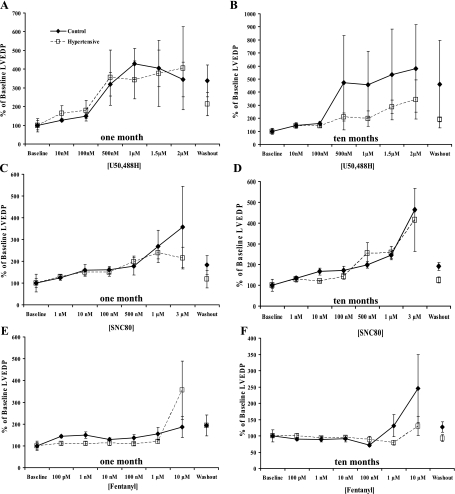
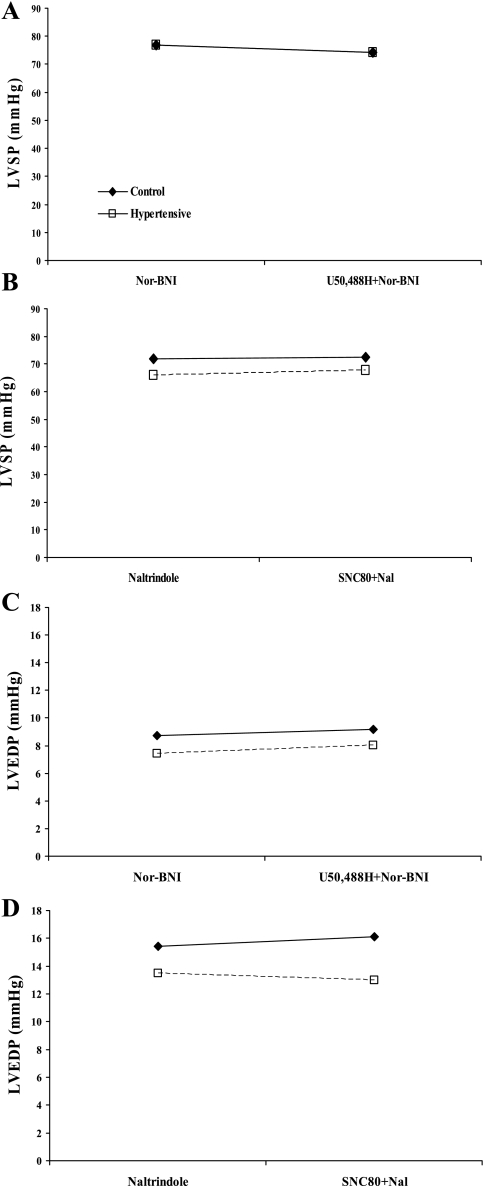
Administration of KOR-selective (U50488H, 10 nM–2 μM) and DOR-selective (SNC80, 1 nM–3 μM) agonists caused concentration-dependent decreases in cardiac systolic (Fig. 1) and diastolic parameters (Fig. 2) in 1- and 10-mo normotensive and hypertensive hamster hearts. Moreover, KOR agonist administration resulted in a markedly attenuated decrease in left ventricular systolic pressure (LVSP), a measure of inotropy (4, 5, 26, 46, 58), in hearts from 10-mo hypertensive hamsters compared with normotensive hamster hearts of the same age (P < 0.05, Fig. 1B), although the KOR effect on left ventricular end-diastolic pressure (LVEDP) was unaltered (Fig. 2). The DOR agonist induced a concentration-dependent decrease in cardiac function that was similar in both lines at both time points (Figs. 1 and 2). Since the MOR has been previously shown to be expressed in the fetal and neonatal heart but not in the adult heart (31, 39, 50, 52, 60), it was hypothesized the MOR may be recapitulated under conditions of prolonged cardiac stress similar to recapitulation of other fetal genes such as β-myosin heavy chain (β-MHC) and α-smooth muscle actin in heart failure (24). However, administration of a MOR-selective agonist, fentanyl (100 pM–10 μM), failed to elicit a change in cardiac function in either model at either time point (Figs. 1 and 2) at concentrations that are selective for the MOR. Prior administration of the KOR-selective antagonist nor-BNI (5 μM) or the DOR-selective antagonist naltrindole (500 nM) blocked the effects of U50488H and SNC80, respectively (Fig. 3), indicating opioid receptor-mediated effects on cardiac function.
Fig. 1.
Systolic effect of opioid receptor-selective agonist administration. Increasing concentrations of receptor-selective agonists decreased left ventricular systolic pressure (LVSP) in a concentration-dependent manner in spontaneously hypertensive (H4; □ and dashed lines) and control (⧫ and solid lines) hamster hearts at 1 and 10 mo. Agonists were administered for 10 min each in a cumulative concentration-response curve, and then drugs were washed out for 20 min. Agonists used were U50488H (10 nM–2 μM; A and B) for κ-opioid receptor (KOR); SNC80 (1 nM–3 μM; C and D) for δ-opioid receptor (DOR), and fentanyl (100 pM–10 μM; E and F) for μ-opioid receptor (MOR). Data are means ± SE; n = 4 hamster hearts. *P < 0.05 compared with control hamster heart.
Fig. 2.
Negative diastolic effect of opioid receptor-selective agonist administration. Increasing concentrations of receptor-selective agonists decreased left ventricular end-diastolic pressure (LVEDP) in a concentration-dependent manner in H4 (□ and dashed lines) and control (⧫ and solid lines) hamster hearts at 1 and 10 mo. Agonists were administered for 10 min each in a cumulative concentration-response curve; drugs were then washed out for 20 min. Agonists used were U50488H (10 nM–2 μM; A and B) for KOR, SNC80 (1 nM-3-μM; C and D) for DOR, and fentanyl (100 pM–10 μM; E and F) for MOR. Data are means ± SE; n = 4 hamster hearts.
Fig. 3.
Receptor-selective antagonists inhibit opioid agonist effects. The KOR-selective agonist nor-binaltorphimine (nor-BNI; 5 μM) was administered for 15 min before 10-min infusion of U50488H (500 nM) in H4 (□ and dashed lines) and control (⧫ and solid lines) hamster hearts at 10 mo, and LVSP (A) and LVEDP (C) were measured. The DOR-selective antagonist naltrindole (Nal; 500 nM) was administered for 10 min before 10 min SNC80 (500 nM) infusion in H4 and control hamsters hearts at 10 mo, and LVSP (B) and LVEDP (D) were measured. Data are means ± SE; n = 3 hamster hearts.
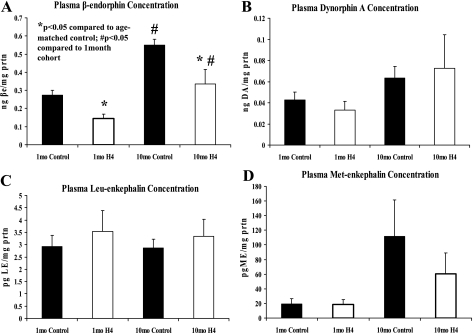
Plasma β-endorphin concentration decreased in hypertensive hamster.
A significant decrease in circulating β-endorphin (BE) was detected in hamsters predisposed to hypertension (1 mo of age) and at 10 mo, with chronic uncontrolled hypertension (P < 0.05, Fig. 4). Both lines showed a significant increase in plasma BE at 10 mo compared with 1 mo. There were no alterations in cardiac BE content detected at either time point between H4 and control hamsters (data not shown). There were no differences detected in ME, LE, or Dyn A in heart tissue (data not shown) or plasma (Fig. 4) at either time point between H4 and control hamsters.
Fig. 4.
Plasma concentrations of endogenous opioid peptides. Plasma concentration of β-endorphin (βe; A), dynorphin A (DA; B), Leu-enkephalin (LE; C), and Met-enkephalin (ME; D) in H4 (open bars) and control (black bars) hamsters at 1 and 10 mo. Peptide concentrations were determined by a competitive immunoassay and normalized on a per-assay basis to a standard control and to mg protein. Data are means ± SE; n = 10–15 hamsters. *P < 0.05 compared with age-matched control; #P < 0.05 compared with 1-mo cohort.
Opioid receptor peptide expression unchanged in hypertension.
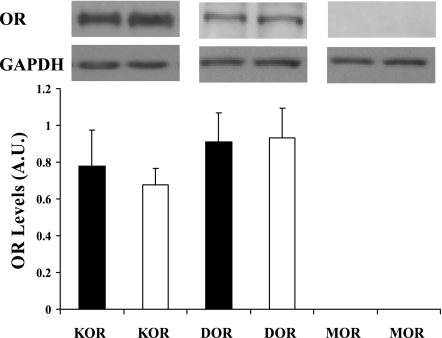
No difference in protein expression was observed in cardiac DOR or KOR from normotensive or hypertensive hamsters (Fig. 5). The MOR was not detected in hearts from either model (Fig. 5).
Fig. 5.
Cardiac opioid receptor (OR) levels. ORs were detected by Western blot using antibodies for the KOR, DOR, and MOR in left ventricles from H4 (open bars) and control (black bars) hamsters following prolonged hypertension. Opioid receptor levels were normalized to GAPDH levels on the same membrane. Top: representative Western blots for each receptor. AU, arbitrary units; n = 5 hamster hearts.
Inhibition of cAMP accumulation induced by opioid receptor stimulation unaltered in hypertension.
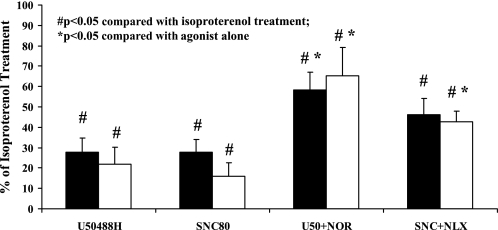
The κ-agonist, U50488H (100 μM), and the δ-agonist, SNC80 (100 μM), were able to inhibit isoproterenol-induced cAMP accumulation in left ventricular cardiomyocytes from hamsters subjected to prolonged hypertension as well as age-matched control hamsters (P < 0.05, Fig. 6). Opioid-dependent inhibition of cAMP accumulation was similar in cardiomyocytes from hypertensive and control hamsters despite hearts from hypertensive hamsters exhibiting an attenuated capacity of κ-agonist to decrease LVSP. Furthermore, inhibition of cAMP accumulation by U50488H and SNC80 was receptor-mediated as it was attenuated by administration of the antagonists nor-BNI (100 μM) and naloxone (100 μM), respectively (P < 0.05, Fig. 6). There was no difference in basal cAMP levels or the response to isoproterenol stimulation in cardiomyocytes from hypertensive or control hamsters (data not shown).
Fig. 6.
Inhibition of cAMP accumulation by opioid receptor-selective agonists. The KOR agonist U50488H (100 μM) and DOR agonist SNC80 (100 μM) inhibited the isoproterenol-induced accumulation of cAMP in left ventricular cardiomyocytes from normotensive control (black bars) and hypertensive (open bars) hamsters. Agonist effects were blocked by the antagonists nor-BNI (NOR; 100 μM, KOR inhibitor) and naloxone (NLX; 100 μM, nonselective OR blocker). Data are represented as percentage of isoproterenol alone. Data are means ± SE; n = 7 hamsters. *P < 0.05 compared with agonist treatment alone. #P < 0.05 compared with isoproterenol treatment alone.
DISCUSSION
Endogenous and exogenous opioids/opiates acting centrally or systemically have been shown to modulate cardiac function. Stimulation of DOR or KOR decreased cardiac inotropy and chronotropy in Langendorff hearts (13, 53) and decreased contractility in isolated cardiomyocytes (3, 51, 55, 57) from healthy rats. Yet, the importance of the opioidergic system in cardiovascular pathophysiology remains to be determined. The purpose of this investigation was to elucidate functional alterations in the myocardial response to opioid agonist administration following sustained hypertension, a common etiology leading to heart failure. Studies reported here showed a concentration-dependent decrease in cardiac systolic and diastolic parameters on administration of κ-and δ-agonists, with no response to a μ-agonist. It has been previously shown that the MOR is expressed in the fetal and neonatal heart but not in the juvenile or adult heart (31, 39, 50, 52, 60). Whether the MOR is recapitulated in hypertension, similar to the recapitulation of other fetal genes in heart failure (24), was investigated here. Receptor selective concentrations of the μ-agonist fentanyl did not modulate cardiac function, and this was taken as an indication that the MOR is not expressed during hypertension. This was confirmed by Western blot analysis as the MOR was not detected in hearts from 10-mo control or hypertensive hamsters. The depression in cardiac function observed on administration of 10 μM fentanyl was likely produced by either a non-receptor-mediated mechanism or via another opioid receptor. Fentanyl has been previously shown to exhibit significant binding to the KOR at nanomolar concentrations (40). Furthermore, there appeared to be a decrease in the cardiac response following KOR stimulation in 10-mo hypertensive hamster hearts compared with 1-mo cohorts and 10-mo normotensive hearts. The mechanism for this is not clear, but previous studies have shown increased ME and LE concentrations in cardiac tissue from aged animals, which could result in receptor downregulation (8). Opioid agonists have also been shown to protect the heart from ischemia-reperfusion injury (37, 41, 44), and studies indicate the ability of opioids to protect the heart is decreased in the aged animal (36), indicating a decrease in cardiac opioidergic function in the aged myocardium. Quantification of opioid peptides in this hamster model of hypertension showed decreased plasma concentrations of the endogenous opioid peptide BE (P < 0.05, Fig. 4), a ligand for the DOR and MOR (40). There was no difference in cardiac BE content. No changes were detected in the plasma or cardiac concentrations of the other EOPs investigated, ME, LE, and Dyn A. Still, opioidergic regulation of the diseased myocardium is poorly defined. This study showed, for the first time, a diminished whole heart response (measured in the isolated work-performing heart) to opioid receptor agonist administration in a rodent model of hypertension. The isolated work-performing heart is a powerful analytic tool and is particularly useful to study the direct effects of the opioidergic system on the myocardium as it allows for evaluation of the response of the myocardium to either physiological or pharmacological stimuli in the absence of central or humoral influences. Therefore, the attenuated response to the KOR-selective agonist, U50488H, observed here results from alterations in opioidergic function in the myocardium and not centrally mediated mechanisms. The endogenous KOR agonist dynorphin 1–9 has been previously shown to act prejunctionally to decrease norepinephrine release from sympathetic nerve terminals (22). This action could be responsible for the decrease in cardiac function seen on opioid agonist administration as norepinephrine has been shown to be released from sympathetic nerve endings in the isolated heart (10, 11, 27, 45, 61). Furthermore, the decreased effect of KOR agonism on systolic function is independent of changes in KOR protein expression, cardiac or plasma concentrations of the endogenous ligand Dyn A, or the ability of KOR function to inhibit cAMP accumulation in cardiomyocytes. These findings suggest attenuated cardiac KOR function is not due to changes in receptor number or coupling to the stimulatory G protein, Gs, or inhibitory G protein, Gi, but results from alterations in downstream function or coupling to an alternative G protein. Along these lines, it has been previously shown that κ-opioid receptors can couple to and signal through Gq (47). As this model exhibits altered KOR function without hypertrophy, it demonstrates that KOR function is altered in the hypertensive state independently of cellular remodeling.
The spontaneously hypertensive hamster represents a novel and highly relevant model of hypertension. The model was generated when hypertension arose spontaneously in a colony of Golden Syrian hamsters, and elevated blood pressure was observed at 8 wk of age in this model. Hypertensive phenotype was maintained by inbreeding (49), resulting in a hypertensive line that is highly similar to the ancestral strain. Previous characterizations of this model showed, in addition to a significant elevation in mean arterial pressure, increased plasma renin activity (49), elevated endothelin-1 content in the heart and kidney (23), and increased sympathetic tone in the vasculature (33). Findings reported here also show elevated mean arterial pressure, as well as systolic and diastolic blood pressure, in the hypertensive H4 hamster compared with the normotensive control at 10 mo of age (Table 1).
Similar to reports in hypertensive patients (29, 30, 59) and the SHR (7), findings of this study also show decreased plasma β-endorphin (P < 0.05, Fig. 4). Cardiac Met-enkephalin was unaltered in the present study, employing the spontaneously hypertensive hamster, similar to previous reports from the SHR (6, 7, 18). However, contrary to findings in the SHR (6, 7, 18), there was no change in cardiac or plasma LE or Dyn A or cardiac BE in the spontaneously hypertensive hamster. There was also no change in cardiac KOR or DOR in the present study, which is in contrast to findings in the SHR (18, 60). These discrepancies may result from a species difference or could be the product of studies being performed at different time points in pathological progression, as previous evaluations in the SHR were performed at postnatal day 90 and at 4 mo for the KOR and DOR, respectively, and were studied here in the spontaneously hypertensive hamster at 10 mo.
The mechanism by which BE concentrations and KOR-induced antagonism of systolic function become deficient in hypertension is unclear. As BE was already low before the development of hypertension, decreased plasma BE was not a response to hemodynamic stress but rather due to an intrinsic difference in these animals. Along these lines, other studies of hypertension, including human, have shown altered circulating endogenous opioid peptide expression in those predisposed to and exhibiting high blood pressure (20, 29, 32, 59). Angiotensin-converting enzyme (ACE), which creates the potent vasoconstrictor ANG II, has been shown to degrade several EOPs (25, 35, 54), and ACE inhibition has been shown to potentiate opioid effects (17, 48). However, whether ACE has an effect on BE degradation is still unclear. In addition, certain EOPs, including BE, can inhibit ACE activity (28), and a decrease in this tonic inhibition could be an important component in the induction and maintenance of hypertension. These changes in circulating opioid peptide concentrations could have significant effects on both vascular and cardiac opioid receptor activation. Plasma BE could activate both cardiac DOR (31, 50, 52) as well as both MOR and DOR in the vasculature (43). However, results of this study did not show altered cardiac DOR function between normotensive and hypertensive groups, suggesting that chronic decreased BE did not have a significant effect on the expression or coupling of the cardiac DOR and ultimately may not have an influence on the functional outcome of the heart subjected to untreated, prolonged hypertension.
Attenuated KOR agonist-induced depression of systolic function in the hypertensive hamster heart is independent of alterations in KOR-induced inhibition of cAMP accumulation in cardiomyocytes and occurs despite the lack of hypertrophy or cardiac remodeling. KOR signaling did inhibit cAMP accumulation in cardiomyocytes from hypertensive hamsters; however, the degree of this effect was unaltered in hearts from hypertensive and control animals. This indicates the KOR does couple to Gi per the traditional paradigm and that this coupling is not modified by prolonged hypertension. Whether attenuated KOR-induced depression of systolic function results from signaling alterations downstream of adenylate cyclase activity or via coupling to another G protein, such as Gq, remains to be determined. Furthermore, the time point and/or mechanism by which diminished cardiac KOR signaling occurs in the progression to hypertension has yet to be elucidated. However, previous experiments have shown altered cardiac function and molecular signaling in cardiac pathology and hypertension, independent of hypertrophy or cardiac remodeling (9, 15). An intrinsic deficiency in opioid signaling would represent a distinct problem in the hypertension phenotype. As hypertension is commonly associated with sympathetic hyperactivity and increased cardiac contractility, presumably the cause for subsequent hypertrophy and heart failure, a decreased ability of the opioidergic system to act as an endogenous check on sympathetic function could exacerbate heart failure progression. Increased sympathetic tone in the vasculature has been previously reported in the spontaneously hypertensive hamster (33); however, there was no difference in basal cAMP content or the ability of isoproterenol to induce cAMP accumulation in left ventricular cardiomyocytes from control hamsters and those subjected to prolonged hypertension. This suggests there is likely not increased sympathetic tone in hypertensive hamster cardiomyocytes, although the potential for altered prejunctional opioidergic function persists as cardiac opioids can act prejunctionally to inhibit catecholamine release from sympathetic nerves (22). Furthermore, the opioidergic system appears to have a bimodal effect on the vagal tone of the cardiac pacemaker in the normal heart (2). Whether this dynamic is maintained in the diseased heart has not been investigated.
In summary, this study has shown altered opioidergic expression and function in a hypertensive model of heart failure progression. The endogenous opioid β-endorphin is decreased in the spontaneously hypertensive hamster at both the prehypertensive stage as well as in hypertension. Furthermore, the decreased cardiac systolic function of the KOR agonist U50488H was diminished in the hypertensive heart at a time point that precedes the development of hypertrophy. This indicates that prolonged hypertension is a sufficient stimulus to alter myocardial opioidergic response without overt cellular remodeling.
GRANTS
This work was supported by grants from the American Heart Association (SDG 23004N), the Pharmaceutical Research and Manufacturers of America (Research Starter Grant), the National Heart, Lung, and Blood Institute (R01-HL-075633), and the National Institute on Drug Abuse (R21-DA-0804) to J. E. J. Schultz.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.American Heart Association/American Stroke Association. Know the Facts, Get the Stats, 2007. [http://www.americanheart.org/downloadable/heart/116861545709855–1041%20KnowTheFactsStats07_loRes.pdf].
- 2.Barlow MA, Deo S, Johnson S, Caffrey JL. Vagotonic effects of enkephalin are not mediated by sympatholytic mechanisms. Exp Biol Med (Maywood) 231: 387–395, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Barron BA Cardiac opioids. Proc Soc Exp Biol Med 224: 1–7, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Beyer ME, Slesak G, Brehm BR, Hoffmeister HM. Hemodynamic and inotropic effects of the endothelin A antagonist BQ-610 in vivo. J Cardiovasc Pharmacol 31, Suppl 1: S258–S261, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Beyer ME, Slesak G, Hovelborn T, Kazmaier S, Nerz S, Hoffmeister HM. Inotropic effects of endothelin-1: interaction with molsidomine and with BQ 610. Hypertension 33: 145–152, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava HN, Matwyshyn GA, Hanissian S, Tejwani GA. Opioid peptides in pituitary gland, brain regions and peripheral tissues of spontaneously hypertensive and Wistar-Kyoto normotensive rats. Brain Res 440: 333–340, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Braas KM, Hendley ED. Anterior pituitary proopiomelanocortin expression is decreased in hypertensive rat strains. Endocrinology 134: 196–205, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Caffrey JL, Boluyt MO, Younes A, Barron BA, O'Neill L, Crow MT, Lakatta EG. Aging, cardiac proenkephalin mRNA and enkephalin peptides in the Fisher 344 rat. J Mol Cell Cardiol 26: 701–711, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Chen-Izu Y, Chen L, Banyasz T, McCulle SL, Norton B, Scharf SM, Agarwal A, Patwardhan A, Izu LT, Balke CW. Hypertension-induced remodeling of cardiac excitation-contraction coupling in ventricular myocytes occurs prior to hypertrophy development. Am J Physiol Heart Circ Physiol 293: H3301–H3310, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Azuma M, Maeda K, Kajimoto N, Higashino H. Impaired heart function and noradrenaline release after ischaemia in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 27: 664–670, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Chen LB, Liu T, Wu JX, Chen XF, Wang L, Fan CL, Gao PJ, Higashino H, Lee WH, Yuan WJ, Chen H. Hypertonic perfusion reduced myocardial injury during subsequent ischemia and reperfusion in normal and hypertensive rats. Acta Pharmacol Sin 24: 1077–1082, 2003. [PubMed] [Google Scholar]
- 12.Clark MJ, Harrison C, Zhong H, Neubig RR, Traynor JR. Endogenous RGS protein action modulates mu-opioid signaling through Gαo. Effects on adenylyl cyclase, extracellular signal-regulated kinases, and intracellular calcium pathways. J Biol Chem 278: 9418–9425, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Clo C, Muscari C, Tantini B, Pignatti C, Bernardi P, Ventura C. Reduced mechanical activity of perfused rat heart following morphine or enkephalin peptides administration. Life Sci 37: 1327–1333, 1985. [DOI] [PubMed] [Google Scholar]
- 14.Cozzolino D, Sasso FC, Cataldo D, Gruosso D, Giammarco A, Cavalli A, Di Maggio C, Renzo G, Salvatore T, Giugliano D, Torella R. Acute pressor and hormonal effects of beta-endorphin at high doses in healthy and hypertensive subjects: role of opioid receptor agonism. J Clin Endocrinol Metab 90: 5167–5174, 2005. [DOI] [PubMed] [Google Scholar]
- 15.de Simone G, Di Lorenzo L, Costantino G, Moccia D, Buonissimo S, de Divitiis O. Supernormal contractility in primary hypertension without left ventricular hypertrophy. Hypertension 11: 457–463, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Deedwania PC The progression from hypertension to heart failure. Am J Hypertens 10: 280S-288S, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Douglas H, Kitchen I. Mechanisms involved in the cardiovascular responses to opioid products of proenkephalin in the anaesthetised rat. Gen Pharmacol 23: 269–277, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Dumont M, Lemaire S. Increased content of immunoreactive Leu-enkephalin and alteration of delta-opioid receptor in hearts of spontaneously hypertensive rats. Neurosci Lett 94: 114–118, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Feuerstein G, Siren AL. The opioid system in cardiac and vascular regulation of normal and hypertensive states. Circulation 75: I125–I129, 1987. [PubMed] [Google Scholar]
- 20.Fontana F, Bernardi P, Merlo Pich E, Boschi S, De Iasio R, Capelli M, Carboni L, Spampinato S. Endogenous opioid system and atrial natriuretic factor in normotensive offspring of hypertensive parents at rest and during exercise test. J Hypertens 12: 1285–1290, 1994. [PubMed] [Google Scholar]
- 21.Frohlich ED State of the Art lecture. Risk mechanisms in hypertensive heart disease. Hypertension 34: 782–789, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Gu H, Barron BA, Gaugl JF, Caffrey JL. Dynorphin, naloxone, and overflow of norepinephrine during cardiac nerve stimulation in dogs. Am J Physiol Heart Circ Physiol 263: H153–H161, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Gulati A, Artwohl JE, Kumar A, Gao XP, Rubinstein I. Endothelin-1-like immunoreactivity in a new rodent model of spontaneous hypertension. Am J Hypertens 11: 866–869, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Hefti MA, Harder BA, Eppenberger HM, Schaub MC. Signaling pathways in cardiac myocyte hypertrophy. J Mol Cell Cardiol 29: 2873–2892, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Hiranuma T, Iwao K, Kitamura K, Matsumiya T, Oka T. Almost complete protection from [Met5]-enkephalin-Arg6-Gly7-Leu8 (Met-enk-RGL) hydrolysis in membrane preparations by the combination of amastatin, captopril and phosphoramidon. J Pharmacol Exp Ther 281: 769–774, 1997. [PubMed] [Google Scholar]
- 26.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg 135: 799–808, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Isaka M, Imamura M, Sakuma I, Shiiya N, Ishizuka T, Ogata Y, Yasuda K. Experimental study of pegylated liposomal hemoglobin on norepinephrine release and reperfusion arrhythmias in isolated guinea pig hearts. Ann Thorac Cardiovasc Surg 13: 391–395, 2007. [PubMed] [Google Scholar]
- 28.Koyuncuoglu H, Enginar N, Hatipoglu I. The in vitro and in vivo effects of enkephalins and beta-endorphin on ACE activity in mice. Pharmacol Res Commun 18: 301–309, 1986. [DOI] [PubMed] [Google Scholar]
- 29.Kraft K, Theobald R, Kolloch R, Stumpe KO. Normalization of blood pressure and plasma concentrations of beta-endorphin and leucine-enkephalin in patients with primary hypertension after treatment with clonidine. J Cardiovasc Pharmacol 10, Suppl 12: S147–S151, 1987. [PubMed] [Google Scholar]
- 30.Kraft KS, Behrendt M, Kolloch R, Stumpe KO. Reduced beta-endorphin secretion in young patients with mild essential hypertension at rest and during exercise. J Hypertens Suppl 6: S381–S383, 1988. [DOI] [PubMed] [Google Scholar]
- 31.Krumins SA, Faden AI, Feuerstein G. Opiate binding in rat hearts: modulation of binding after hemorrhagic shock. Biochem Biophys Res Commun 127: 120–128, 1985. [DOI] [PubMed] [Google Scholar]
- 32.Ku YH Role of limbic peptidergic circuits in regulation of arterial pressure, relevant to development of essential hypertension. Neuropeptides 40: 299–308, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Kurjiaka DT The conduction of dilation along an arteriole is diminished in the cremaster muscle of hypertensive hamsters. J Vasc Res 41: 517–524, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol 40: 389–430, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Oudit GY, Crackower MA, Backx PH, Penninger JM. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med 13: 93–101, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Peart JN, Gross ER, Headrick JP, Gross GJ. Impaired p38 MAPK/HSP27 signaling underlies aging-related failure in opioid-mediated cardioprotection. J Mol Cell Cardiol 42: 972–980, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pepe S, van den Brink OW, Lakatta EG, Xiao RP. Cross-talk of opioid peptide receptor and beta-adrenergic receptor signalling in the heart. Cardiovasc Res 63: 414–422, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Plobeck N, Delorme D, Wei ZY, Yang H, Zhou F, Schwarz P, Gawell L, Gagnon H, Pelcman B, Schmidt R, Yue SY, Walpole C, Brown W, Zhou E, Labarre M, Payza K, St-Onge S, Kamassah A, Morin PE, Projean D, Ducharme J, Roberts E. New diarylmethylpiperazines as potent and selective nonpeptidic delta opioid receptor agonists with increased In vitro metabolic stability. J Med Chem 43: 3878–3894, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Pugsley MK The diverse molecular mechanisms responsible for the actions of opioids on the cardiovascular system. Pharmacol Ther 93: 51–75, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol 45: 330–334, 1994. [PubMed] [Google Scholar]
- 41.Romano MA, McNish R, Seymour EM, Traynor JR, Bolling SF. Differential effects of opioid peptides on myocardial ischemic tolerance. J Surg Res 119: 46–50, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Rossi F, Lampa E, Berrino L, Di Giaimo A, Marmo E. Central and peripheral cardiovascular effects of beta-endorphin in normotensive and spontaneously hypertensive rats and in rats rendered hypertensive by deoxycorticosterone acetate administration. Res Commun Chem Pathol Pharmacol 55: 181–192, 1987. [PubMed] [Google Scholar]
- 43.Saeed RW, Stefano GB, Murga JD, Short TW, Qi F, Bilfinger TV, Magazine HI. Expression of functional delta opioid receptors in vascular smooth muscle. Int J Mol Med 6: 673–677, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Schultz JE, Gross GJ. Opioids and cardioprotection. Pharmacol Ther 89: 123–137, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Schutte F, Burgdorf C, Richardt G, Kurz T. Adenosine A1 receptor-mediated inhibition of myocardial norepinephrine release involves neither phospholipase C nor protein kinase C but does involve adenylyl cyclase. Can J Physiol Pharmacol 84: 573–577, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Silva CO, Monteiro-Filho WO, Duarte GP, Lahlou S. Effects of long-term pretreatment with isoproterenol on inotropic responsiveness to alpha-adrenoceptor stimulation: study in isolated perfused rat hearts. J Pharm Pharmacol 53: 233–242, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Standifer KM, Pasternak GW. G proteins and opioid receptor-mediated signalling. Cell Signal 9: 237–248, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Takai S, Song K, Tanaka T, Okunishi H, Miyazaki M. Antinociceptive effects of angiotensin-converting enzyme inhibitors and an angiotensin II receptor antagonist in mice. Life Sci 59: PL331–PL336, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Thomas CL, Artwohl JE, Suzuki H, Gao X, White E, Saroli A, Bunte RM, Rubinstein I. Initial characterization of hamsters with spontaneous hypertension. Hypertension 30: 301–304, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Ventura C, Bastagli L, Bernardi P, Caldarera CM, Guarnieri C. Opioid receptors in rat cardiac sarcolemma: effect of phenylephrine and isoproterenol. Biochim Biophys Acta 987: 69–74, 1989. [DOI] [PubMed] [Google Scholar]
- 51.Ventura C, Pintus G, Tadolini B. Opioid peptide gene expression in the myocardial cell. TCM 8: 102–110, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Ventura C, Guarnieri C, Bastagli L, Bernardi P, Puddu P, Caldarera CM. Calcium stimulates opioid receptor agonism in rat cardiac sarcolemma. Cardioscience 1: 151–154, 1990. [PubMed] [Google Scholar]
- 53.Ventura C, Muscari C, Spampinato S, Bernardi P, Caldarera CM. Effects of naloxone on the mechanical activity of isolated rat hearts perfused with morphine or opioid peptides. Peptides 8: 695–699, 1987. [DOI] [PubMed] [Google Scholar]
- 54.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277: 14838–14843, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Wenzlaff H, Stein B, Teschemacher H. Diminution of contractile response by kappa-opioid receptor agonists in isolated rat ventricular cardiomyocytes is mediated via a pertussis toxin-sensitive G protein. Naunyn Schmiedebergs Arch Pharmacol 358: 360–366, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Wintzen M, Ostijn DM, Polderman MC, le Cessie S, Burbach JP, Vermeer BJ. Total body exposure to ultraviolet radiation does not influence plasma levels of immunoreactive beta-endorphin in man. Photodermatol Photoimmunol Photomed 17: 256–260, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Xiao RP, Spurgeon HA, Capogrossi MC, Lakatta EG. Stimulation of opioid receptors on cardiac ventricular myocytes reduces L type Ca2+ channel current. J Mol Cell Cardiol 25: 661–666, 1993. [DOI] [PubMed] [Google Scholar]
- 58.Zakrzeska A, Schlicker E, Kwolek G, Kozlowska H, Malinowska B. Positive inotropic and lusitropic effects mediated via the low-affinity state of beta1-adrenoceptors in pithed rats. Br J Pharmacol 146: 760–768, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng X, Zhang T, Ding H, Wang C. Plasma levels of beta-endorphin, leucine enkephalin and arginine vasopressin in patients with essential hypertension and the effects of clonidine. Int J Cardiol 51: 233–244, 1995. [DOI] [PubMed] [Google Scholar]
- 60.Zimlichman R, Gefel D, Eliahou H, Matas Z, Rosen B, Gass S, Ela C, Eilam Y, Vogel Z, Barg J. Expression of opioid receptors during heart ontogeny in normotensive and hypertensive rats. Circulation 93: 1020–1025, 1996. [DOI] [PubMed] [Google Scholar]
- 61.Zugck C, Lossnitzer D, Backs J, Kristen A, Kinscherf R, Haass M. Increased cardiac norepinephrine release in spontaneously hypertensive rats: role of presynaptic alpha-2A adrenoceptors. J Hypertens 21: 1363–1369, 2003. [DOI] [PubMed] [Google Scholar]