Abstract
The lysosomal storage disorder Fabry disease is characterized by excessive globotriaosylceramide (Gb3) accumulation in major organs such as the heart and kidney. Defective lysosomal α-galactosidase A (Gla) is responsible for excessive Gb3 accumulation, and one cell sensitive to the effects of Gb3 accumulation is vascular endothelium. Endothelial dysfunction is associated with Fabry disease and excessive cellular Gb3. We previously demonstrated that excessive vascular Gb3 in a mouse model of Fabry disease, the Gla-knockout (Gla−/0) mouse, results in abnormal vascular function, which includes abnormal endothelium-dependent contractions, a vascular phenomenon known to involve cyclooxygenase (COX). Therefore, we hypothesized that the vasculopathy in the Gla knockout mouse may be due to a vasoactive COX-derived product. To test this hypothesis, vascular reactivity experiments were performed in aortic rings from wild-type (Gla+/0) and Gla−/0 mice in the presence and absence of specific and nonspecific COX inhibitors. Specific inhibition of COX1 or COX2 in endothelium-intact rings from Gla−/0 mice decreased overall phenylephrine contractility compared with untreated Gla−/0 rings, whereas COX inhibitors had no effect on contractility in endothelium-denuded rings. Nonspecific inhibition of COX with indomethacin (10 μmol/l) or COX1 inhibition with valeryl salicylate (3 mmol/l) improved endothelial function in rings from Gla−/0 mice, but COX2 inhibition with NS-398 (1 μmol/l) further increased endothelial dysfunction in rings from Gla−/0 mice. These results suggest that, in the Gla−/0 mice, COX1 and COX2 activity are increased and localized in the endothelium, producing vasopressor and vasorelaxant products, which contribute to the Fabry-related vasculopathy.
Keywords: endothelium, globotriaosylceramide, cyclooxygenase
the lysosomal disorder Fabry disease is characterized by the unusually high accumulation of the glycosphingolipid globotriaosylceramide (Gb3) in many organs, including the kidney and heart (10). Gb3 accumulation is seen not only in the lysosomes, but also in the plasma membranes and, more specifically, the caveolae of vascular endothelium (32). Although this rare disease is lysosomal in origin, the increased mortality in patients with Fabry disease is due to strokes and myocardial infarctions (10, 28). The excessive vascular endothelial accumulation of Gb3 is associated with endothelial dysfunction in humans with Fabry disease (1, 12, 20, 21, 24) as well as the α-galactosidase A-knockout (Gla−/0) mouse model of Fabry disease (15, 27). The endothelial dysfunction observed in the Gla−/0 mouse correlates with a markedly decreased time to thrombosis (11), as well as increased plaque formation in atherosclerosis (4). We (27) and others (15) previously demonstrated endothelial dysfunction in Gla−/0 mice.
We also demonstrated that receptor-induced endothelium-dependent contractions were impaired in the aortas of Gla−/0 mice (27). Receptor-mediated endothelium-dependent contractions, especially to the muscarinic receptor agonist ACh, are cyclooxygenase (COX) dependent. Vanhoutte's group demonstrated that endothelium-dependent contractions are COX1 dependent (35), and in vascular diseases such as hypertension, COX1 and COX2 activity can affect endothelium-dependent contractions (36). However, the mechanism that contributes to the diminished receptor-induced endothelium-dependent relaxation in Gla−/0 mice is unknown, and our initial observation pertaining to endothelium-dependent contractions in Gla−/0 mice led us to hypothesize that the vasculopathy in Gla−/0 mice may be due to a vasoactive COX-derived product.
To test this hypothesis, vascular reactivity experiments were performed in isolated thoracic aortic rings from wild-type (Gla+/0) and Gla−/0 mice. We observed that specific and nonspecific COX inhibition in endothelium-intact aortic ring preparations caused changes in vasopressor responses to phenylephrine (PE), whereas COX inhibition had no effects on the endothelium-denuded preparations from Gla−/0 mice. The nonspecific COX inhibitor indomethacin restored ACh-mediated relaxation in endothelium-intact aortic rings from Gla−/0 mice. Specific inhibition of COX1 improved endothelium-dependent relaxation in aortas from Gla+/0 and Gla−/0 mice, whereas COX2 inhibition exacerbated the endothelial dysfunction in Gla−/0 mice. These data indicate that the blunted vascular responses associated with Gla−/0 mice are due to a vasoactive COX derivative and suggest that vascular glycosphingolipids may have a role in the regulation of arachidonic acid metabolism via the COX enzymes.
MATERIALS AND METHODS
Mice.
Male C57Bl/6 mice [wild-type (Gla+/0); 12–19 wk old] were obtained from Jackson Laboratories (Bar Harbor, ME). Male Gla−/0 mice were bred from mice provided by Drs. Ashok Kulkarni and Roscoe Brady (National Institutes of Health, Bethesda, MD) (26). These mice were backcrossed at least five generations to the C57Bl/6 strain. All mice were maintained on normal chow in specific-pathogen-free facilities.
The procedures with mice were approved by and performed in accordance with guidelines of the University of Michigan Committee on the Use and Care of Animals. The University of Michigan Unit for Laboratory Animal Medicine provided veterinary care. The University of Michigan is accredited by the American Association of Laboratory Animal Care. The animal care and use program conformed to the standards in the National Institutes of Health Guide for the Care and Use of laboratory Animals [Publication No. (NIH) 86-23].
Vascular reactivity experiments.
Thoracic aortas were cut into rings (2 mm long), and endothelium was left intact or removed by perfusion of the ring lumen with 100 μl of 0.1% Triton X-100 in PBS. Aortic rings were mounted in a myograph system (Danish Myo Technology, Aarhus, Denmark) and bathed with warmed (37°C), aerated (95% O2-5% CO2) physiological salt solution (PSS, mmol/l: 130 NaCl, 4.7 KCl, 1.18 KHPO4, 1.17 MgSO4, 1.6 CaCl2, 14.9 NaHCO3, 5.5 dextrose, 0.03 CaNa2 EDTA). The aortic rings were set at 700 mg passive tension and equilibrated for 1 h with washes every 20 min. Before concentration-response curves were obtained, rings were subjected to a wake-up protocol consisting of two consecutive contractions with K+-PSS (mmol/l: 14.7 NaCl, 100 KCl, 1.18 KHPO4, 1.17 MgSO4, 1.6 CaCl2, 14.9 NaHCO3, 5.5 dextrose, 0.03 CaNa2 EDTA) and contraction with PE (10−6 mol/l in endothelium-intact or 10−7 mol/l in endothelium-denuded rings). Endothelial integrity was tested with ACh (10−5 mol/l) after PE-induced contraction. After the wake-up protocol, a PE concentration response (10−9–10−5 mol/l) was performed in the endothelium-intact and -denuded rings, and the PE EC80 was determined for each vascular preparation with an intact endothelium. After the PE concentration response, rings with intact, functional endothelium were washed, contracted with the calculated PE EC80 for each ring, and allowed to reach a stable plateau. ACh (10−10–10−5 mol/l) or sodium nitroprusside (SNP; 10−11–10−6 mol/l) was added cumulatively to the bath to examine endothelium-dependent (ACh) or -independent (SNP) relaxation. We, as well as others, demonstrated previously that ACh-mediated relaxation in the mouse thoracic aorta is endothelium dependent (19, 27). ACh and SNP relaxation were expressed as a percentage of the PE EC80 contraction. Reactivity to all agonists also was examined in the presence of the nonspecific COX inhibitor indomethacin (10−5 mol/l), the irreversible COX1-specific inhibitor valeryl salicylate (VS, 3 × 10−3 mol/l), or the COX2-specific inhibitor NS-398 (10−6 mol/l). When ACh and SNP concentration responses were determined in the presence of a COX inhibitor, the PE contraction-response curves and calculated PE EC80 were determined in the presence of the COX inhibitor.
Mouse aortic endothelial cell cultures.
Primary mouse aortic endothelial cells (MAECs) were isolated, cultured, and maintained as described previously (31). Cultured MAECs were studied at passages 3–4 or frozen at a density of 1.0–1.5 × 106 cells/ml in frozen medium consisting of 50% FBS, 10% DMSO, and 40% RPMI 1640 medium. After they were thawed at 37°C for 2–3 min, frozen MAECs were recovered in RPMI 1640 medium supplemented with 15% FBS only for 3–4 h to allow cells to adhere to the culture dishes or preincubated in RPMI 1640 medium containing 15% FBS, 2 mM l-glutamine, and 1× nonessential amino acid overnight, if necessary. Recovery medium and unattached cells were then removed by replacement with RPMI 1640 medium containing 15% FBS, 2 mM l-glutamine, 1× nonessential amino acid, 0.05 mg/ml endothelial cell growth supplement, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.05 mg/ml heparin. Under those conditions, the recovery rate of frozen MAECs was usually >90%.
Preparation of mouse tissue and MAEC lysates.
Aortas of 4- to 12-mo-old Gla+/0 and Gla−/0 mice were isolated and dissected as described previously (31). The minced aortas or kidneys were lysed in 0.3 ml of ice-cold lysis buffer consisting of 25 mM Tris·HCl (pH 7.4), 137 mM NaCl, 2 mM EDTA, 2 mM Na3VO4, 20 mM NaF, 1% Triton X-100, 10% glycerol, 5% P2714 (a protease inhibitor cocktail with a broad specificity for the inhibition of serine, cysteine, and metalloproteases; Sigma), and 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (a water-soluble and more stable form of PMSF; Sigma) for 10 min at 4°C. A probe sonicator was used to sonicate the tissue five times for 1 s at 4°C. Tissue debris was removed by centrifugation at 12,000 g for 10 min at 4°C. Each lysate sample was pooled from three aortas. MAEC lysates were prepared from cultured Gla+/0 and Gla−/0 MAECs at passage 1 without heparin. The MAECs cultured in 100-mm culture dishes were lysed in 0.5 ml of 1% Triton X-100 lysate buffer as described above for 10 min at 4°C and collected by scraping. After a brief sonication (3 times for 1 s each), the cell debris was precipitated by centrifugation at 12,000 g for 10 min. The clarified aortic and MAEC lysates were subjected to immunoblot analysis after the determination of protein concentration by bicinchoninic acid assay, with BSA used as a standard.
Immunoblot analysis.
Immunoblots were performed as previously described (32). Briefly, equal amounts of protein from aorta or cell lysates were resolved by SDS-PAGE using a 7–13% gradient and transferred to a nylon membrane. The blots were blocked in 5% skimmed milk in TBS buffer [20 mM Tris·HCl (pH 7.6) and 150 mM NaCl] for 1 h at room temperature and probed with anti-COX1 and -COX2 antibodies diluted in Tris-buffered saline containing 1% skimmed milk. The immunoreactive bands were detected with the ECL-plus system (PerkinElmer Life Sciences).
Chemicals.
PE, ACh, SNP, indomethacin, and all salts for PSS were purchased from Sigma (St. Louis, MO). VS and NS-398 were purchased from Cayman Chemical (Ann Arbor, MI).
Data and statistical analysis.
Agonist EC50 values were calculated with a nonlinear regression analysis with the algorithm [effect = maximum response/1 + (EC50/agonist concentration)] in the computer program GraphPad Prism (San Diego, CA). Hill slope values were also derived from GraphPad Prism. The PE EC80 values were calculated from the following equation: logEC50 = logECF − (1/Hill slope) * log[F/(100 − F)], where F = 80. Values are means ± SE, and concentration-response data were analyzed using two-way ANOVA followed by Bonferroni's post hoc test. Differences in EC50 values were evaluated using one-way ANOVA with Newman-Keuls multiple comparison post hoc test. Differences in Emax values between two groups were analyzed by Student's t-test. P < 0.05 was considered significant.
RESULTS
PE in the presence of endothelium.
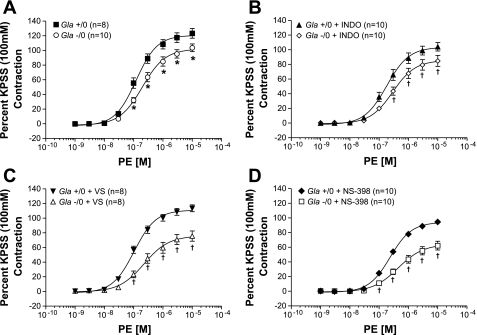
PE induced concentration-dependent contractions in endothelium-intact rings (Fig. 1), and the PE-mediated contractions in rings not exposed to COX inhibitors from Gla−/0 mice were significantly blunted compared with those in rings from Gla+/0 mice. This is illustrated by the decreased PE Emax values (Fig. 1A; 103.6 ± 5.3 and 123.3 ± 6.6%, respectively, P < 0.05), as well as the significantly decreased EC50, representing a decrease in sensitivity to PE in the rings from Gla−/0 mice (Table 1). These data are consistent with previously reported PE reactivity in these animals (27).
Fig. 1.
Phenylephrine (PE)-mediated vascular contraction in endothelium-intact mouse aortic rings from α-galactosidase A (Gla) wild-type (Gla+/0) and Gla-knockout (Gla−/0) mice in the absence of cyclooxygenase (COX) inhibitor (A) or in the presence of 10−5 mol/l indomethacin (INDO; B), 3 mmol/l valeryl salicylate (VS; C), or 10−6 mol/ NS-398 (D). Data are expressed as percentage of contraction elicited by 100 mmol/l KCl-containing physiological salt solution (KPSS). *P < 0.05 vs. Gla−/0 (by 2-way ANOVA followed by Bonferroni's post hoc test). †P < 0.05 vs. Gla+/0 + respective treatment (by 2-way ANOVA followed by Bonferroni's post hoc test).
Table 1.
Potency of PE in thoracic aortas from Gla+/0 and Gla−/0 mice in the presence of COX inhibitors with and without endothelium
|
−log EC50, M |
||||
|---|---|---|---|---|
|
+Endo |
−Endo
|
|||
| Gla+/0 | Gla−/0 | Gla+/0 | Gla−/0 | |
| Control | 6.92±0.06(8) | 6.70±0.06† (10) | 7.75±0.05 (5) | 7.80±0.09 (7) |
| Indomethacin | 6.74±0.07*(10) | 6.54±0.09 (10) | 7.90±0.04 (5) | 7.84±0.07 (7) |
| Valeryl salicylate | 6.98±0.05 (8) | 6.64±0.11† (8) | 7.68±0.06 (5) | 7.54±0.09 (7) |
| NS-398 | 6.62±0.04*(10) | 6.35±0.11†‡ (10) | 7.87±0.03 (5) | 7.82±0.06 (7) |
Values are means ± SE for number of animals in parentheses. +Endo, endothelium intact; −Endo, endothelium denuded; Gla+/0 and Gla−/0, α-galactosidase A (Gla) wild-type and Gla-knockout mice, respectively.
P < 0.05 vs. Gla+/0 control (by 1-way ANOVA with Newman-Keuls multiple comparison post hoc test).
P < 0.05 vs. Gla+/0 in respective treatment group (by unpaired t-test).
P < 0.05 vs. Gla−/0 control (by 1-way ANOVA with Newman-Keuls multiple comparison post hoc test).
PE Emax was decreased in Gla+/0 and Gla−/0 mice in the presence of indomethacin, however, compared with their respective untreated controls (Fig. 1B). PE Emax in Gla−/0 mice was still similarly depressed compared with that in Gla+/0 mice, although the difference was not statistically significant (104.1 ± 5.8 and 84.7 ± 7.5%, respectively, P = 0.55). Similarly, PE EC50 was shifted to the right, but not significantly (Table 1).
The specific COX1 inhibitor VS did not alter PE Emax (123.3 ± 6.6% and 113.5 ± 4.7% in Gla+/0 and Gla+/0 + VS, respectively) or EC50 (Table 1) in rings from Gla+/0 mice. However, VS caused a further decrease in PE Emax (Fig. 1C) and a greater rightward shift in PE EC50 in rings from Gla−/0 mice (Table 1).
Similar PE reactivity was observed in rings exposed to the COX2-specific inhibitor NS-398 (Fig. 1D), but NS-398 caused significant changes in both groups, as illustrated by significant decreases in PE Emax and a rightward shift in PE EC50 compared with their respective untreated controls, as well as compared with each other.
PE in the absence of endothelium.
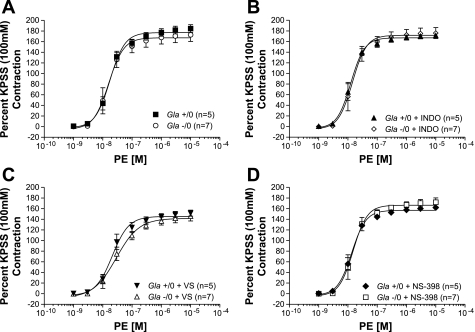
PE mediated concentration-dependent contractions with greater sensitivity in endothelium-denuded rings (Fig. 2). Similar to previous results (27), PE produced contractions in endothelium-denuded rings from Gla−/0 mice identical to those in rings from Gla+/0 mice not exposed to COX inhibitors (Fig. 2A, Table 1). COX inhibition with indomethacin (Fig. 2B, Table 1), VS (Fig. 2C, Table 1), or NS-398 (Fig. 3C, Table 1) did not alter reactivity in the endothelium-denuded rings from Gla−/0 mice compared with similarly treated endothelium-denuded rings from Gla+/0 mice, suggesting that any differences in PE-mediated contractility between Gla+/0 and Gla−/0 mice involve COX and that the majority of the COX effect on contractility is associated with the endothelium.
Fig. 2.
PE-mediated vascular contraction in endothelium-denuded mouse aortic rings from Gla+/0 and Gla−/0 mice in the absence of COX inhibitor (A) or in the presence of 10−5 mol/l indomethacin (B), 3 mmol/l VS (C), or 10−6 mol/l NS-398 (D). Data are expressed as percentage of contraction elicited by 100 mmol/l KPSS.
Fig. 3.
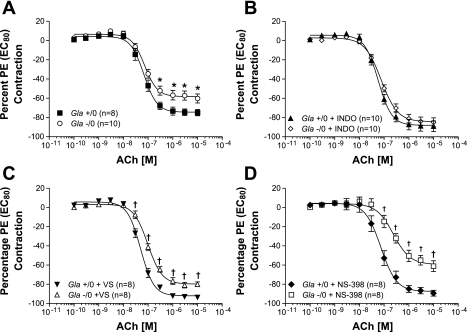
ACh-mediated endothelium-dependent relaxation in endothelium-intact mouse aortic rings from Gla+/0 and Gla−/0 mice precontracted with an EC80 concentration of PE in the absence of COX inhibitor (A) or in the presence of 10−5 mol/l indomethacin (B), 3 mmol/l VS (C), or 10−6 mol/l NS-398 (D). Data are expressed as percentage of contraction elicited by PE EC80. *P < 0.05 vs. Gla−/0 (by 2-way ANOVA followed by Bonferroni's post hoc test). †P < 0.05 vs. Gla+/0 + respective treatment (by 2-way ANOVA followed by Bonferroni's post hoc test).
ACh-mediated relaxations.
ACh caused a concentration-dependent relaxation in endothelium-intact thoracic aortic rings from Gla+/0 and Gla−/0 mice (Fig. 3). Maximal endothelium-dependent relaxation to ACh without pharmacological intervention in the rings from Gla−/0 mice (Emax = 60.3 ± 4.7%) was significantly blunted compared with that in rings from Gla+/0 mice (Fig. 3A; Emax = 75.0 ± 3.2%, P < 0.05), while no change in sensitivity (EC50) was observed (Table 2). These data are consistent with previously reported ACh-mediated, endothelium-dependent relaxation in this mouse model of Fabry disease (27).
Table 2.
Potency of ACh in thoracic aortas from Gla+/0 and Gla−/0 mice in the presence of COX inhibitors with endothelium
|
−log EC50, M |
||
|---|---|---|
| Gla+/0 | Gla−/0 | |
| Control | 7.17±0.05 (8) | 7.14±0.06 (10) |
| Indomethacin | 7.24±0.05 (10) | 7.12±0.05 (10) |
| Valeryl salicylate | 7.24±0.05 (8) | 7.12±0.05 (8) |
| NS-398 | 7.13±0.07 (8) | 6.63±0.10†‡ (8) |
Values are means ± SE for number of animals in parentheses.
P < 0.05 vs. Gla+/0 in respective treatment group (by unpaired t-test).
P < 0.05 vs. Gla−/0 control (by 1-way ANOVA with Newman-Keuls multiple comparison).
In the presence of indomethacin (10 μmol/l), maximal endothelium-dependent relaxation to ACh was normalized to responses similar to those observed in rings from Gla+/0 mice, both in terms of Emax (Fig. 3B) and sensitivity or EC50 (Table 2).
VS improved maximal ACh endothelium-dependent relaxation in thoracic aortic rings from Gla+/0 and Gla−/0 mice, as illustrated in Fig. 3C. The maximal endothelium-dependent relaxation (Vmax) to ACh for rings from Gla+/0 mice after VS improved to 93.7 ± 0.7%. ACh-mediated endothelium-dependent relaxation in aortic rings from Gla−/0 mice also improved after VS (Vmax = 80.9 ± 02.9%) to a level similar to that in aortic rings from Gla+/0 without COX1 inhibition (Vmax = 75.0 ± 3.2%). COX1 inhibition with VS did not change the sensitivity of ACh in aortic rings from Gla+/0 or Gla−/0 mice compared with their respective untreated controls (Table 2). VS, however, did cause a rightward shift in the ACh EC50 in a comparison of Gla+/0 + VS with Gla−/0 + VS (Table 2).
The specific COX2 inhibitor NS-398 did not alter maximal endothelium-dependent relaxation or change sensitivity to ACh in thoracic aortic rings from Gla+/0 mice (Fig. 3D). COX2 inhibition, however, caused a significant rightward shift in the endothelium-dependent relaxation to ACh in aortic rings from Gla−/0 mice compared with thoracic aortic rings from Gla−/0 mice not exposed to NS-398 (Table 2), whereas maximal relaxation responses were maintained in the two Gla−/0 groups (Fig. 3D). This rightward shift in the EC50 for ACh in the presence of COX2 inhibition suggests that COX2 inhibition further exacerbates the endothelial dysfunction in the Gla−/0 mice.
Endothelium-independent relaxation to SNP.
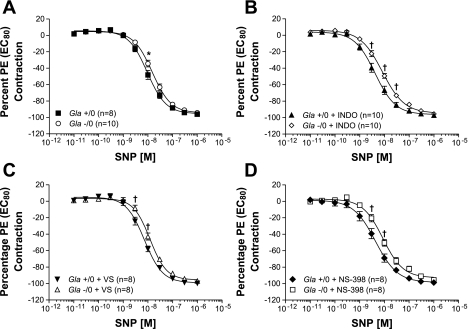
SNP caused a concentration-dependent relaxation in thoracic aortic rings from Gla+/0 and Gla−/0 mice (Fig. 4). Maximum endothelium-independent relaxation to SNP without pharmacological intervention in the rings from Gla−/0 mice (Vmax = 94.3 ± 1.4%) was similar to that observed in rings from Gla+/0 mice (Vmax = 95.6 ± 1.1%, P > 0.05; Fig. 4A). However, sensitivity to SNP (EC50) was less in the aortic rings from Gla−/0 than Gla+/0 mice (Table 3). In the presence of indomethacin (10 μmol/l), maximum endothelium-dependent relaxation to SNP was unchanged in aortic rings from Gla+/0 and Gla−/0 mice (Fig. 4B) compared with rings from the two respective groups of mice not exposed to indomethacin (Fig. 4A). Indomethacin caused an increase in SNP sensitivity in Gla+/0 and Gla−/0 mice compared with untreated respective groups, but indomethacin-treated Gla+/0 mice remained more sensitive to SNP than indomethacin-treated Gla−/0 mice (Table 3).
Fig. 4.
Endothelium-independent relaxation mediated by sodium nitroprusside (SNP) in endothelium-intact mouse aortic rings from Gla+/0 and Gla−/0 mice precontracted with an EC80 concentration of PE in the absence of COX inhibitor (A) or in the presence of 10−5 mol/l indomethacin (B), 3 mmol/l VS (C), or 10−6 mol/l NS-398 (D). Data are expressed as percentage of contraction elicited by 100 mmol/l KPSS. *P < 0.05 vs. Gla−/0 (by 2-way ANOVA followed by Bonferroni's post hoc test). †P < 0.05 vs. Gla+/0 + respective treatment (by 2-way ANOVA followed by Bonferonni's post hoc test).
Table 3.
Potency of SNP in thoracic aortas from Gla+/0 and Gla−/0 mice in the presence of COX inhibitors with endothelium
|
−log EC50, M |
||
|---|---|---|
| Gla+/0 | Gla−/0 | |
| Control | 8.03±0.04 (8) | 7.82±0.03† (10) |
| Indomethacin | 8.38±0.05*(10) | 8.09±0.03†‡ (10) |
| Valeryl salicylate | 8.14±0.03 (8) | 7.91±0.03† (8) |
| NS-398 | 8.36±0.06*(8) | 8.06±0.05†‡ (8) |
Values are means ± SE for the number of animals in parentheses. SNP, sodium nitroprusside.
P < 0.05 vs. Gla+/0 control (by 1-way ANOVA with Newman-Keuls multiple comparison post hoc test).
P < 0.05 vs. Gla+/0 in respective treatment group (by unpaired t-test).
P < 0.05 vs. Gla−/0 control (by 1-way ANOVA with Newman-Keuls multiple comparison).
COX1 inhibition with VS did not change maximal SNP-mediated endothelium-independent relaxation in thoracic aortic rings from Gla+/0 and Gla−/0 mice, as illustrated in Fig. 4C. Inhibition of COX1 with VS did not further change the sensitivity to SNP in aortic rings from Gla+/0 or Gla−/0 mice compared with their respective groups not treated with COX inhibitor, whereas the difference between Gla+/0 mice in the presence of VS vs. Gla−/0 mice in the presence of VS was maintained (Table 3).
The specific COX2 inhibitor NS-398 did not alter maximal endothelium-independent relaxation to SNP in thoracic aortic rings from Gla+/0 or Gla−/0 mice (Fig. 4D). NS-398 caused an increase in SNP sensitivity in Gla+/0 and Gla−/0 mice compared with untreated respective groups, but Gla+/0 mice treated with NS-398 remained more sensitive to SNP than Gla−/0 mice in the presence of the COX2 inhibitor (Table 3).
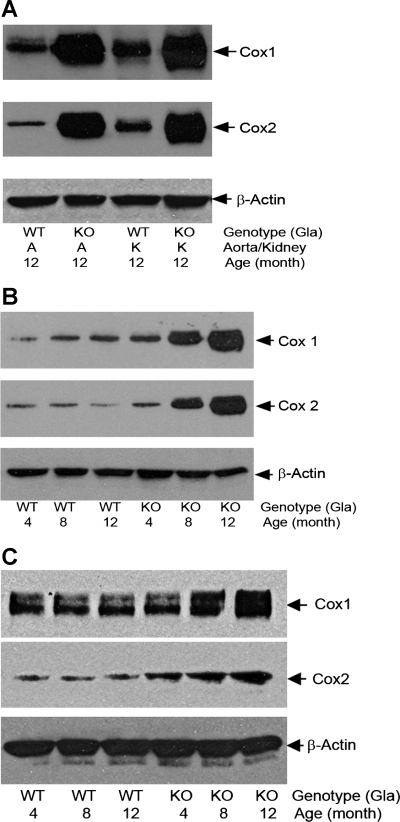
To ascertain the role of the differential expression of COX1 and COX2 in the differences in vascular reactivity, Western blots were performed on the lysates from kidneys and aortas of Gla+/0 and Gla−/0 mice (Fig. 5A). These tissues have previously been demonstrated to be major sites of Gb3 accumulation. A marked increase in the expression of COX1 and COX2 was observed in the Gla−/0 mice compared with the Gla+/0 mice. The age-dependent differences in COX1 and COX2 expression were studied in aortas from 4-, 8-, and 12-mo-old mice (Fig. 5B). Although COX2 levels were not detectably different in 4-mo-old aortas, COX1 levels were significantly elevated. The levels of COX1 and COX2 increased markedly in the Gla−/0 mice as a function of age, with no detectable differences in the Gla+/0 mice. Finally, to determine whether these changes differed between whole aorta and the aortic endothelial cells, Western blots were performed on cultured MAECs obtained from 4-, 8-, and 12-mo-old Gla+/0 and Gla−/0 mice (Fig. 5C). Under these conditions, COX2 levels were significantly elevated in 4-mo-old mice, with an age-dependent increase in expression. COX1 levels were not detectably different at 4 mo but increased in the older Gla−/0 mice.
Fig. 5.
A: COX1 and COX2 in kidney and aortic lysates from 12-mo-old Gla+/0 (WT) and Gla−/0 (KO) mice. Immunoreactivities of COX1 and COX2 in mouse tissues were detected with an anti-COX1 monoclonal antibody (5F6/F4, Abcam, Cambridge, MA) or a monoclonal antibody against COX2 (BD Transduction Laboratories, Lexington, KY). Proteins were reduced in sample loading buffer containing 0.5% β-mercaptoethanol, heated at 80°C for 5 min, separated in SDS-PAGE with a 7–13% gradient, and probed with anti-COX1 and -COX2 antibodies. β-Actin served as internal loading controls. Blots are representative of 3 independent experiments that yielded equivalent results. B: age-dependent accumulation of COX1 and COX2 proteins in aortas of Gla+/0 and Gla−/0 mice. Blots are representative of 3 independent experiments that yielded equivalent results. C: age-dependent accumulation of COX1 and COX2 proteins in mouse aortic endothelial cells isolated from Gla−/0 mice. Proteins in mouse aortic endothelial cell lysates were nonreduced, but they were heated at 80°C for 5 min before they were loaded into SDS-PAGE. β-Actin served as internal loading controls. Results are representative of 3 independent experiments.
DISCUSSION
Premature mortality associated with Fabry disease is commonly manifest as cardiovascular complications such as stroke or myocardial infarction (10), clinical syndromes where endothelial dysfunction is emerging as a primary risk factor (13). Previous studies utilizing the Gla−/0 mouse as a model for Fabry disease have revealed an underlying vascular endothelial dysfunction (15, 27) that correlates with a markedly increased susceptibility to oxidant-induced thrombosis (11) as well as accelerated plaque formation in a model of atherosclerosis (4). The excessive accumulation of Gb3 in the vascular endothelium has been implicated as the basis for the development of endothelial dysfunction associated with Fabry disease, but whether Gb3 is really involved or other underlying mechanisms contribute to the endothelial dysfunction associated with Fabry disease is relatively unknown. We previously characterized vasopressor and endothelium-dependent relaxation defects, including depressed vasopressor responses, endothelial dysfunction, and depressed endothelium-dependent contractions mediated by ACh (27), a phenomenon previously characterized by Tang et al. to be COX dependent (35), in the Gla−/0 mice. Because of this novel observation related to endothelium-dependent contractions, we hypothesized that the vasculopathy in the Gla−/0 mice may be due to a vasoactive COX-derived product.
In this study, we report that specific COX1 or COX2 inhibition in endothelium-intact aortic rings from Gla−/0 mice causes further depressed vasopressor contractility to PE. COX inhibition, however, had no effect on PE reactivity in endothelium-denuded aortic rings from Gla−/0 mice, suggesting that the effects of COX-derived products on vascular smooth muscle contractility are endothelium derived and contractile in nature, since COX1 or COX2 inhibition resulted in further depressed contractility. In addition, nonspecific COX or specific COX1 inhibition improved endothelium-dependent relaxation responses, whereas specific COX2 inhibition worsened endothelial function in already compromised vascular preparations from Gla−/0 mice, suggesting that COX1 may be producing a vasopressor-type product, whereas COX2 may be producing a vasorelaxant agent as a compensatory mechanism for compromised endothelial function. These observations are important, because they provide further insight into the mechanisms by which vasculopathy in Fabry disease occurs, suggesting that the defect and the COX-derived products originate from the endothelium.
Although COX1 and COX2 have been demonstrated to play a role in vascular dysfunction (3, 14, 17, 22, 36), the observations reported in the present study are the first demonstration that COX1 and COX2 have a role in modulating vascular function in a model of Fabry disease. Studies in models of hypertension have demonstrated that the endothelium is capable of synthesizing COX-derived vasopressors and may be a source of COX-derived products (2, 5, 36). The vascular smooth muscle as an important source of COX-derived products cannot be ruled out, since COX1 and COX2 can be expressed in vascular smooth muscle (2, 7, 8, 34). However, the reactivity experiments performed in endothelium-denuded aortas from the Gla−/0 mice demonstrated no effects of COX inhibitors on PE-mediated contractions, suggesting that the effects of COX-derived vasoactive metabolites are derived from the endothelium. These considerations are further complicated by the observation that COX1 and COX2 expression increases as a function of age in the Gla−/0, but not Gla+/0, mice. In addition, the increase in COX2 is observed earliest in the Gla−/0 aortic endothelial cells, but not whole aorta, whereas COX1 elevations occur earliest in the whole aorta.
In addition, these data suggest that COX1 and COX2 activities are increased in Fabry disease, but their involvement in regulating vascular contraction in Fabry disease appears to be different from that in other vascular diseases. For example, COX appears to contribute to augmented spontaneous tone during hypertension (23). Similarly, COX appears to increase the sensitivity of other vasopressors in hypertension (9, 37) as well as diabetes (14). We, however, have observed that COX1- and/or COX2-derived products appear to have more of a compensatory role by preserving vascular contractility or endothelium-dependent relaxation in Fabry disease. In hypertension and diabetes, COX enzymes participate in augmented endothelium-dependent contraction, where the COX-derived product originates from the endothelium and acts at the thromboxane A2/prostaglandin H2 (TP) receptor on the vascular smooth muscle (29, 36). In Fabry disease, direct stimulation of the TP receptor with the TP receptor agonist U46619 in endothelium-intact aortic ring preparations results in normal vascular smooth muscle contractility (27), whereas the observations in the present study demonstrate that modulation of the COX enzymes in Fabry disease reveals a compensatory role of COX-derived products in an attempt to maintain normal vascular smooth muscle contractility. These combined observations suggest that the differences in TP receptor-mediated contractility in Fabry disease are due to changes in endothelium-derived COX activity, presumably as a result of increased glycosphingolipid accumulation, especially Gb3, in the endothelium. Further studies, however, are needed to further elucidate the role of glycosphingolipids in regulating endothelial COX activity and arachidonic acid metabolism through this pathway.
Glycosphingolipids have also been demonstrated to be significant modulators of signal transduction through G protein-coupled receptors and receptor tyrosine kinases (30, 33). Because Gb3 accumulates in lysosomal and extralysosomal compartments in endothelial cells, specifically in caveolar-associated plasma membranes, an additional role for Gb3 in the signaling of COX products through their cognate receptors must be considered (32).
In addition to its effects on contractility, specific COX inhibition revealed differential roles of COX1 and COX2 in endothelium-dependent relaxation. Nonspecific COX inhibition with indomethacin revealed that COX was playing a role in the endothelial dysfunction associated with Fabry disease, since indomethacin was able to fully restore ACh-mediated relaxation. This observation is consistent with other studies in aging and hypertension, where COX inhibition reversed endothelial dysfunction (17, 22). Specific COX1 inhibition also improved ACh-mediated relaxation in the vascular preparations from Gla−/0 mice, as well as in the Gla+/0 controls, but the differences between Gla+/0 and Gla−/0 mice were maintained, suggesting that COX1 is producing an arachidonic acid metabolite that antagonizes endothelium-dependent relaxation and is most likely a vasopressor, since these data are consistent with the observation that COX1 inhibition also depressed PE-induced contractions in the vascular preparations from the Gla−/0 mice.
Specific COX2 inhibition, however, had an opposite effect on endothelium-dependent relaxation compared with specific COX1 antagonism. Inhibition of COX2 worsened the existing endothelial dysfunction. This particular observation has clinical relevance, since COX2 inhibitors have been demonstrated to increase the risk of cardiovascular complications (6, 25), probably by inhibiting the production of endothelium-derived prostacyclin in vessels such as the coronary arteries (16, 18). Inhibition of COX2 could also increase the risk of cardiovascular events by not only affecting endothelium-dependent relaxation, but by also increasing the likelihood of a thrombotic episode (18) such as stroke. However, further studies are needed to identify the specific COX1 and COX2 products that may be responsible for influencing endothelial function.
In conclusion, Gla deficiency is associated with overall vascular dysfunction, which manifests as depressed vascular smooth muscle contraction as well as endothelial dysfunction. The aberrant vascular reactivity in this mouse model of Fabry disease, in part, can be attributed to changes in endothelial COX1 and COX2 activity, but COX1 and COX2 appear to contribute to the vasculopathy in different ways. These data suggest that glycosphingolipids may play an important role in regulating vascular function by influencing vascular COX activity.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 5RO1 DK-055823-06 (J. A. Shayman) and partially by American Heart Association Scientist Development Grant 0430045N (J. L. Park).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Altarescu G, Moore DF, Pursley R, Campia U, Goldstein S, Bryant M, Panza JA, Schiffmann R. Enhanced endothelium-dependent vasodilation in Fabry disease. Stroke 32: 1559–1562, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez Y, Briones AM, Balfagon G, Alonso MJ, Salaices M. Hypertension increases the participation of vasoconstrictor prostanoids from cyclooxygenase-2 in phenylephrine responses. J Hypertens 23: 767–777, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, Kaley G. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol 25: 1610–1616, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bodary PF, Shen Y, Vargas FB, Bi X, Ostenso KA, Gu S, Shayman JA, Eitzman DT. α-Galactosidase A deficiency accelerates atherosclerosis in mice with apolipoprotein E deficiency. Circulation 111: 629–632, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bratz IN, Kanagy NL. Nitric oxide synthase-inhibition hypertension is associated with altered endothelial cyclooxygenase function. Am J Physiol Heart Circ Physiol 287: H2394–H2401, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 352: 1092–1102, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Chen D, Balyakina EV, Lawrence M, Christman BW, Meyrick B. Cyclooxygenase is regulated by ET-1 and MAPKs in peripheral lung microvascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 284: L614–L621, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Connolly E, Bouchier-Hayes DJ, Kaye E, Leahy A, Fitzgerald D, Belton O. Cyclooxygenase isozyme expression and intimal hyperplasia in a rat model of balloon angioplasty. J Pharmacol Exp Ther 300: 393–398, 2002. [DOI] [PubMed] [Google Scholar]
- 9.da Cunha V, Rossoni LV, Oliveira PA, Poton S, Pretti SC, Vassallo DV, Stefanon I. Cyclooxygenase inhibition reduces blood pressure elevation and vascular reactivity dysfunction caused by inhibition of nitric oxide synthase in rats. Clin Exp Hypertens 22: 203–215, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Desnick R, Ioannou Y, Eng C. α-Galactosidase A deficiency: Fabry disease. In: The Metabolic and Molecular Bases of Inherited Disease (8th ed.), edited by Scriver C, Beadet A, Sly W, and Valle D. New York: McGraw-Hill, 2001, p. 3733–3774.
- 11.Eitzman DT, Bodary PF, Shen Y, Khairallah CG, Wild SR, Abe A, Shaffer-Hartman J, Shayman JA. Fabry disease in mice is associated with age-dependent susceptibility to vascular thrombosis. J Am Soc Nephrol 14: 298–302, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Elliott PM, Kindler H, Shah JS, Sachdev B, Rimoldi OE, Thaman R, Tome MT, McKenna WJ, Lee P, Camici PG. Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with α-galactosidase A. Heart 92: 357–360, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goligorsky MS Endothelial cell dysfunction: can't live with it, how to live without it. Am J Physiol Renal Physiol 288: F871–F880, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, Gong MC. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res 67: 723–735, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Heare T, Alp NJ, Priestman DA, Kulkarni AB, Qasba P, Butters TD, Dwek RA, Clarke K, Channon KM, Platt FM. Severe endothelial dysfunction in the aorta of a mouse model of Fabry disease: partial prevention by N-butyldeoxynojirimycin treatment. J Inherit Metab Dis 30: 79–87, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Hennan JK, Huang J, Barrett TD, Driscoll EM, Willens DE, Park AM, Crofford LJ, Lucchesi BR. Effects of selective cyclooxygenase-2 inhibition on vascular responses and thrombosis in canine coronary arteries. Circulation 104: 820–825, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Heymes C, Habib A, Yang D, Mathieu E, Marotte F, Samuel J, Boulanger CM. Cyclo-oxygenase-1 and -2 contribution to endothelial dysfunction in ageing. Br J Pharmacol 131: 804–810, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong TT, Huang J, Barrett TD, Lucchesi BR. Effects of cyclooxygenase inhibition on canine coronary artery blood flow and thrombosis. Am J Physiol Heart Circ Physiol 294: H145–H155, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Jiang F, Gibson AP, Dusting GJ. Endothelial dysfunction induced by oxidized low-density lipoproteins in isolated mouse aorta: a comparison with apolipoprotein-E deficient mice. Eur J Pharmacol 424: 141–149, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Kalliokoski RJ, Kalliokoski KK, Sundell J, Engblom E, Penttinen M, Kantola I, Raitakari OT, Knuuti J, Nuutila P. Impaired myocardial perfusion reserve but preserved peripheral endothelial function in patients with Fabry disease. J Inherit Metab Dis 28: 563–573, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kalliokoski RJ, Kantola I, Kalliokoski KK, Engblom E, Sundell J, Hannukainen JC, Janatuinen T, Raitakari OT, Knuuti J, Penttinen M, Viikari J, Nuutila P. The effect of 12-mo enzyme replacement therapy on myocardial perfusion in patients with Fabry disease. J Inherit Metab Dis 29: 112–118, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Luscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension 8: 344–348, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Michel FS, Man RY, Vanhoutte PM. Increased spontaneous tone in renal arteries of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 293: H1673–H1681, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Moore DF, Scott LT, Gladwin MT, Altarescu G, Kaneski C, Suzuki K, Pease-Fye M, Ferri R, Brady RO, Herscovitch P, Schiffmann R. Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy. Circulation 104: 1506–1512, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, Boyce SW, Verburg KM. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 352: 1081–1091, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Ohshima T, Murray GJ, Swaim WD, Longenecker G, Quirk JM, Cardarelli CO, Sugimoto Y, Pastan I, Gottesman MM, Brady RO, Kulkarni AB. α-Galactosidase A-deficient mice: a model of Fabry disease. Proc Natl Acad Sci USA 94: 2540–2544, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JL, Whitesall SE, D'Alecy LG, Shu L, Shayman JA. Vascular dysfunction in the α-galactosidase A-knockout mouse is an endothelial cell-, plasma membrane-based defect. Clin Exp Pharmacol Physiol 35: 1156–1163, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shayman JA, Killen PD. Fabry disease. In: Molecular and Genetic Basis of Renal Disease, edited by Mount DB and Pollak MR. Philadelphia: Saunders Elsevier, 2008, p. 195–199.
- 29.Shepherd JT, Katusic ZS. Endothelium-derived vasoactive factors. I. Endothelium-dependent relaxation. Hypertension 18 Suppl III: 76–85, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Shu L, Lee L, Shayman JA. Regulation of phospholipase C-γ activity by glycosphingolipids. J Biol Chem 277: 18447–18453, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Shu L, Murphy HS, Cooling L, Shayman JA. An in vitro model of Fabry disease. J Am Soc Nephrol 16: 2636–2645, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Shu L, Shayman JA. Caveolin-associated accumulation of globotriaosylceramide in the vascular endothelium of α-galactosidase A null mice. J Biol Chem 282: 20960–20967, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Shu L, Shayman JA. Src kinase mediates the regulation of phospholipase C-γ activity by glycosphingolipids. J Biol Chem 278: 31419–31425, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Sun D, Liu H, Yan C, Jacobson A, Ojaimi C, Huang A, Kaley G. COX-2 contributes to the maintenance of flow-induced dilation in arterioles of eNOS-knockout mice. Am J Physiol Heart Circ Physiol 291: H1429–H1435, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang EH, Ku DD, Tipoe GL, Feletou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and COX2−/− knockout but not COX1−/− knockout mice. J Cardiovasc Pharmacol 46: 761–765, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol 144: 449–458, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang D, Chabrashvili T, Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane prostanoid receptors, and endothelium. Circ Res 94: 1436–1442, 2004. [DOI] [PubMed] [Google Scholar]