Abstract
The sphingosine kinase (SphK)/sphingosine 1-phosphate (S1P) pathway, known to determine the fate and growth of various cell types, can enhance cardiac myocyte survival in vitro and provide cardioprotection in acute ex vivo heart preparations. However, the relevance of these findings to chronic cardiac pathology has never been demonstrated. We hypothesized that S1P signaling is impaired during chronic remodeling of the uninfarcted ventricle during the evolution of post-myocardial infarction (MI) cardiomyopathy and that a therapeutic enhancement of S1P signaling would ameliorate ventricular dysfunction. SphK expression and activity were measured in the remote, uninfarcted myocardium (RM) of C57Bl/6 mice subjected to coronary artery ligation. The mRNA expression of S1P receptor isoforms was also measured, as was the activation of the downstream S1P receptor mediators. A cardioprotective role for S1P1 receptor agonism was tested via the administration of the S1P1-selective agonist SEW2871 during and after MI. As a result, the expression data suggested that a dramatic reduction in SphK activity in the RM early after MI may reflect a combination of posttranscriptional and posttranslational modulation. SphK activity continued to decline gradually during chronic post-MI remodeling, when S1P1 receptor mRNA also fell below baseline. The S1P1-specific agonism with oral SEW2871 during the first 2-wk after MI reduced apoptosis in the RM and resulted in improved myocardial function, as reflected in the echocardiographic measurement of fractional shortening. In conclusion, these results provide the first documentation of alterations in S1P-mediated signaling during the in situ development of cardiomyopathy and suggest a possible therapeutic role for the pharmacological S1P receptor agonism in the post-MI heart.
Keywords: sphingosine 1-phosphate, apoptosis
the pathological remodeling of the remaining myocardium remote to the area of infarction contributes importantly to the development of dilated, post-myocardial infarction (MI) cardiomyopathy, the leading cause of heart failure. This remodeling involves changes in the structure, composition, and function of the surviving myocardium, which compensates for tissue lost to infarction by a progressive increase in chamber volume. Ventricular remodeling is now increasingly understood to involve changes in critical signaling pathways within cardiac myocytes (CMs) that help to determine both the cellular fate and function (2, 6, 7, 22, 23). These discoveries have led to the hope that the activation of the prosurvival signals could ameliorate or even reverse the inexorable progression to post-MI heart failure.
Formed as a result of sphingosine kinase (SphK) activation in response to diverse stimuli, the bioactive sphingolipid metabolite sphingosine 1-phosphate (S1P) has been implicated in many biological processes, including cell growth, suppression of apoptosis, stress responses, calcium homeostasis, cell migration, angiogenesis, and vascular maturation (5, 8, 27, 29, 36). The S1P signaling cascade has recently been found to play an important role in regulating CM survival and growth (11, 12, 15, 33, 39, 45). Both intracellular and extracellular pathways have been identified for S1P signaling, and at least three S1P receptor isoforms have been reported in the heart. These receptors are Gi protein coupled and have been shown to activate antiapoptotic pathways, such as phosphatidylinositol 3-kinase (PI3K)/Akt, and to reduce levels of the proapoptotic molecules Bax and Bad (3, 25, 30, 32, 36, 42, 44). A paradigm of intracellular S1P generation, particularly by the enzyme SphK, coupled with the autocrine and paracrine stimulation of cell surface S1P receptors, has emerged as a potential mechanism for enhanced cell survival and other potential therapeutic effects, including a β-blocker-like reduction in CM chronotropy and inotropy (1, 9, 13, 16, 18, 24, 26, 31, 35, 37).
Studies in our laboratory and others have demonstrated that S1P is involved in CM survival signaling during proapoptotic stress in vitro and in cardioprotection achieved via ischemic preconditioning in ex vivo perfusion (Langendorff) experiments (11, 12, 15, 25, 39, 45). Enhanced S1P signaling, achieved either through an exposure to exogenous S1P or through an induction of increased intracellular SphK activity, has also been found to inhibit CM apoptosis in vitro and to limit ischemic injury in the ex vivo heart (11, 12, 15). We therefore hypothesized that the alterations in endogenous myocardial S1P receptors, signaling, and SphK activity would correlate with the progressive loss of CMs in remote myocardium (RM) during the evolution of ventricular dilatation and fibrosis associated with post-MI cardiomyopathy in vivo. We further tested the hypothesis that the therapeutic enhancement of S1P survival signaling through a pharmacological stimulation of the most abundant cardiac S1P receptor, S1P1, would reduce myocardial apoptosis and improve left ventricular (LV) function after MI.
MATERIALS AND METHODS
Mouse MI.
Male C57Bl/6 mice (25 g) were anesthetized with 1.5% inhaled isoflurane using a rodent ventilator (Harvard) at 115 breaths/min. A left lateral thoracotomy incision was placed at the level of the fourth intercostal space to expose the left ventricle and left atrial appendage, and a 7-0 polypropylene suture was used to ligate the left anterior descending coronary artery (LAD) approximately one-third the distance from the base to the apex of the heart to generate a myocardial infarction (MI) encompassing 30–40% of the left ventricle. In sham-operated controls, the thoracotomy was closed without coronary ligation. Although a postoperative mortality of ∼10% is observed in this model, the rodent heart consistently exhibits a substantial capacity for functional compensation in the face of a loss of ventricular wall and aneurysm formation. Despite very severe infarctions of 30–40% of the LV wall, which would often lead to acute heart failure or death in humans, fractional shortening in the myocardium remote to the infarct and global parameters of function such as cardiac output are well compensated in mice. As such, permanent coronary ligation models of MI in this species represent models of post-MI remodeling, but not necessarily frank heart failure.
The S1P1 receptor agonist SEW2871 (Biomol, Plymouth Meeting, PA) or vehicle (Tween 20/DMSO) was administered via oral gavage (40 mg·kg−1·day−1) 1 h before LAD ligation and daily afterward for 2 wk or until death in some mice, according to the methods of Lien et al. (19) who demonstrated an ameliorative effect of SEW2871 administration on ischemic injury in the kidney.
At death, myocardial tissue from the remote free ventricular wall and septum was separated from the area of gross infarction. To avoid a contamination with infarcted tissue, a border zone of ∼1 mm was left with the infarcted region. Although the border zone pathology itself and the infarct extension/expansion in particular represent an intriguing target for the study of S1P signaling, any attempt to accurately isolate the border zone from the infarcted mouse hearts was felt to introduce a prohibitive sampling error. To gauge the effect of MI on S1P signaling, the measurements taken during post-MI remodeling were compared with those in tissue from sham-operated controls. To assess the ability of therapeutic S1P agonism to reverse post-MI abnormalities, the measurements from remote myocardium (RM) in SEW2871-treated hearts were compared with both those in the RM from the infarcted hearts treated with vehicle and those in normal, uninfarcted myocardium. All procedures conformed with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996) and were approved by the Institutional Animal Care and Use Committee of the San Francisco Veterans Affairs Medical Center.
SphK activity.
Tissues were homogenized in assay buffer containing 0.13 M KCl, 20 mM HEPES (pH 7.4), 1 mM EGTA, 1 μg/l leupeptin, and 0.25 μg/l each of chymostatin and pepstain A. The homogenate was centrifuged at 45,000 g for 30 min, and the supernatant was collected for SphK assay. SphK activity was assayed as previously described in our laboratory (40).
Protein expression.
Tissues were homogenized in a lysis buffer containing 0.13 M KCl, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 5 mM NaF, 20 mM HEPES, and a protease inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany). BCA protein assay reagent kit (Pierce, Rockford, IL) was used to measure protein concentration. Samples containing equal amounts of protein were separated by NuPAGE Novex Bis-Tris Gels (Invitrogen, Carlsbad, CA) and transferred to PVDF membranes (Invitrogen). Blots were probed with antibodies specific for SphK1 (generous gift from Dr. Yoshiko Banno), SphK2 (Santa Cruz Biotech, Santa Cruz, CA), phospho- and total Akt, and total p70S6 kinase, (Cell Signaling, Beverly, MA) with appropriate horseradish peroxidase-conjugated antibodies as secondary antibodies (Cell Signaling). SuperSignal West Femto Maximum Sensitivity substrate (Pierce) was used for the chemiluminescent visualization of proteins. Exposed films were then subjected to density analysis using Scion Image (Scion, Frederick, MD).
RNA isolation and quantitative real-time RT-PCR.
RNA samples were extracted from tissue with Total RNA Purification System (Invitrogen). TaqMan real-time RT-PCR analyses of mouse S1P1, S1P2, S1P3, SphK1, and SphK2 in the RNA samples was conducted in an ABI 7700 sequence detection system (Applied Biosystems, Foster City, CA) with mouse-specific primers and 5′-FAM/3′-TAMRA-labeled probes purchased from Applied Biosystems [Assay ID for S1P1, Mm00514644_m1; S1P2, Mm02620208_s1; S1P3, Mm00515669_m1; mSphK1, Mm00448841_g1; SphK2, Mm00445021_m1; and hypoxanthine-guanine phosphoribosyltransferase (HPRT), Mm01545399_m1]. Relative levels of mRNA expression of each gene were normalized separately using the respective HPRT gene for endogenous loading controls.
Echocardiography.
A transthoracic echocardiography was performed in conscious mice using an Acuson Sequoia 512 machine and a 13-MHz probe. A two-dimensional short-axis view of the left ventricle was obtained at the level of the papillary muscles, i.e., in myocardium remote to the infarction, and two-dimensional M-mode tracings were also recorded. LV fractional shortening was calculated as (LVDd − LVDs)/LVDd × 100, where LVDd is LV diastolic dimension and LVDs is LV systolic dimension (14, 34).
Histology and apoptosis.
Five-micrometer sections of pressure-fixed, paraffin-embedded hearts were stained with Gomori trichrome, and computerized image analysis (Scion, Frederick, MD) was used to assess the infarct size and wall thickness. Fibrosis scores of 0–4, based on a combination of staining intensity and distribution according to the method of Jacoby et al. (10), were assigned in a blinded fashion to four Gomori trichrome-stained sections of RM from each heart, and an average score was calculated. Myocyte size was evaluated via Texas red-conjugated wheat germ agglutinint (Invitrogen). Thin sections were also subjected to terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) staining (ApopTag peroxidase in situ apoptosis detection kit, Chemicon, Temecula, CA), and apoptotic cells and total cells identified via hematoxylin nuclear counterstaining were counted in three sections per heart. Ligase staining (ApopTag peroxidase ISOL kit, Chemicon) of selected adjacent sections was used to confirm the specificity of TUNEL staining. Hematoxylin-eosin staining was used to distinguish the morphology of cells containing apoptotic nuclei on adjacent sections. The level of apoptosis is expressed as apoptotic cells as a percentage of total nuclei.
Statistics.
Data are reported as means ± SE. Comparisons among groups were made using ANOVA, followed by Neuman-Keuls post hoc testing. A P value of <0.05 was considered to denote statistical significance.
RESULTS
mRNA expression of S1P receptors and of SphK1 and -2 in mouse heart.
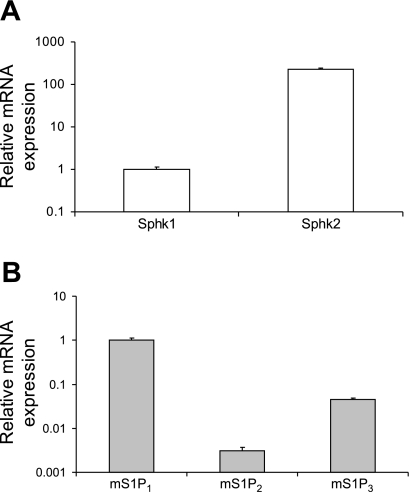
Quantitative analyses of mRNA via real-time RT-PCR indicated that the SphK2 mRNA level is over 200-fold greater than that of SphK1 in the normal unoperated mouse heart (Fig. 1A). Given our previous observation that SphK2 accounts for roughly 40% of total SphK activity in the C57Bl/6 heart (41), these data and those of others (4) suggest that SphK1 is a more efficient kinase and that a substantial degree of posttranscriptional and posttranslational control of SphK2 activity is exerted in CMs. Consistent with previous reports, mRNA for S1P receptor isoforms S1P1–3 was identified in mouse heart lysates, whereas the expression of S1P4 or S1P5 was undetectable (data not shown). Among these receptors, S1P1 mRNA was most abundant (>95% of total receptor RNA expression), with S1P3 mRNA representing most of the remainder (4.6% of the total, Fig. 1B). Only very low levels of S1P2 mRNA were measured in normal hearts.
Fig. 1.
Relative abundance of mRNA for sphingosine kinases (SphK; A) and sphingosine 1-phosphate (S1P) receptors (B) in normal mouse (m) heart (n = 4 animals).
SphK expression and activity in the post-MI myocardium.
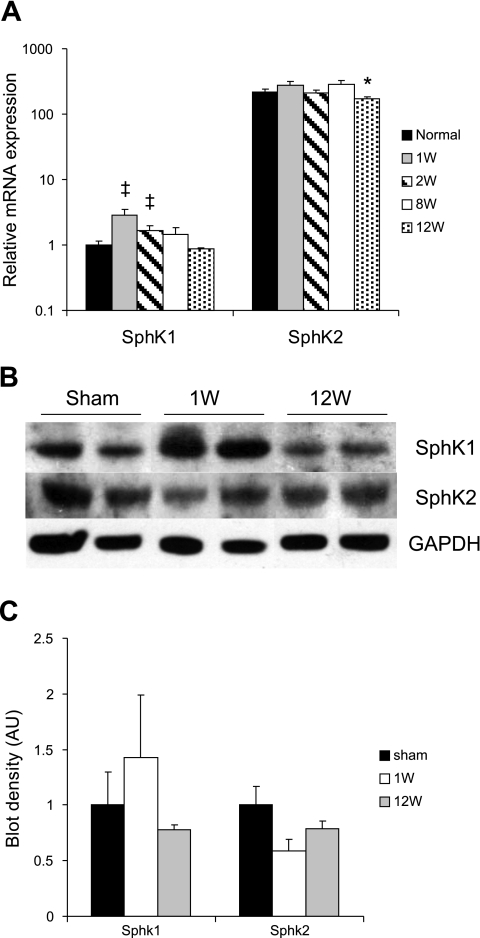
Our model of mid-LAD ligation (43) results in the infarction of 30–40% of the LV wall (Fig. 2A) and in a progressive pattern of both LV dilatation and reduction of LV function, as reflected in the fractional shortening in the RM over a 12-wk period (Table 1). Consistent with previous reports, increased levels of myocardial apoptosis were also observed in the RM during chronic post-MI remodeling (Fig. 2B). Total SphK activity was significantly reduced in the uninfarcted RM at 1 wk after LAD ligation during the early phase of this post-MI remodeling process, and this activity level continued to decline during the subsequent period of progressive LV dilatation and functional deterioration (Fig. 2C).
Fig. 2.
A: Gomori trichrome stain of infracted mouse myocardium at 1 and 12 wk (W) post-myocardial infarction (MI). B and C: changes in myocardial apoptosis (B) and total SphK activity (C) in the remote myocardium during the evolution of post-MI remodeling in mice. *P < 0.05; n = 10–14 animals for A and n = 4–6 animals for B and C.
Table 1.
Assessment of cardiac parameters by echocardiography
| Sham | 1–3 wk | 4–6 wk | 7–9 wk | 10–17 wk | |
|---|---|---|---|---|---|
| n | 15 | 63 | 24 | 22 | 43 |
| FS, % | 57±1 | 46±1 | 43±2 | 42±2 | 41±2* |
| LVEDV, μl | 35±2 | 45±2 | 49±2 | 50±2 | 53±2* |
| LVESV, μl | 6±1 | 14±2 | 17±3 | 19±3 | 18±4 |
Values are means ± SE; n, number of animals. FS, fractional shortening; LVEDV and LVESV, left ventricular diastolic and systolic volume, respectively.
P < 0.05 vs. group 1–3 wk.
The drop in total SphK activity early after MI may have been explained by a corresponding decline observed at that time in SphK2 protein, but not mRNA, levels (Fig. 3). The discrepancy between the stability in SphK2 mRNA expression and the lower level of SphK2 protein suggests a high degree of posttranscriptional regulation. Alternatively, the decline in total SphK activity may have also been mediated by a posttranslational regulation of SphK1, as has been reported in cell culture systems (38). In fact, SphK1 expression, both at the mRNA and protein levels, actually increased during the first week after MI compared with sham-operated uninfarcted hearts and then gradually decreased to slightly below that of sham-operated controls at 12 wk after infarction (Fig. 3, A and C). It remains interesting to note that SphK2 has been associated in some studies with a proapoptotic effect (20, 21, 28).
Fig. 3.
A: mRNA expression of SphK1 and SphK2 in the remote, uninfarcted myocardium (RM) after MI in mouse hearts over time (*P < 0.05, ‡P = 0.08, n = 4–6 animals). B: Western blot of SphK1 and Sphk2 in lysates from the RM, early and late after MI, compared with sham-operated controls. C: SphK1 and -2 protein expression in lysate from the RM, early and late after MI, compared with sham-operated controls.
S1P receptor expression and downstream signaling after MI.
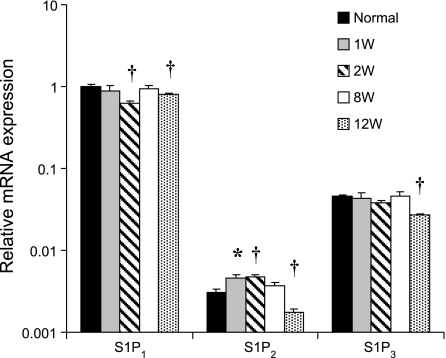
As has been reported previously (17), quantitative RT-PCR indicated that S1P1 is the most abundantly expressed S1P receptor isoform in the myocardium. At 2 wk post-MI, S1P1 mRNA expression was significantly reduced in the RM compared with the levels in uninfarcted sham-operated control hearts (Fig. 4). An increase in mRNA expression of the much less abundant S1P2 isoform was observed early after MI (Fig. 4). By 12 wk post-MI, both S1P1 and S1P2 mRNA expression had declined to levels that were below baseline. A significant change in the low levels of expression of S1P3 mRNA was observed only very late after MI (Fig. 4). Phosphorylation (i.e., activation) of Akt, a known downstream prosurvival kinase activated by S1P1 receptor signaling (45), was also depressed at 1 wk after MI (Fig. 5A). In contrast, in what may be a compensatory reaction to proapoptotic post-MI signaling, ERK phosphorylation was increased in the RM compared with levels seen in normal, uninfarcted hearts.
Fig. 4.
mRNA expression of S1P1, S1P2, and S1P3 receptors in the RM of post-MI mouse hearts over time. *P < 0.05 and †P < 0.01; n = 4–6 animals.
Fig. 5.
A: Akt, p70S6 kinase, and ERK1/2 phosphorylation at 2 wk after MI in the RM of mouse hearts treated either with SEW2871 (SEW) or vehicle (Veh) (*P < 0.05 and †P < 0.01; n = 5 animals). B: apoptosis in SEW2871- and vehicle-treated mice at 2 wk after MI compared with uninfarcted control (†P < 0.01). C: fibrosis scores from Gomori trichrome-stained sections of remote myocardium (*P = 0.03). D: wall thickness of the remote myocardium in SEW2871- and vehicle-treated mice at 2 wk after MI (P = 0.08). E: myocyte cross-sectional surface area in the remote myocardium in Veh- and SEW-treated hearts, 1 and 2 wk after MI. N, normal; LV, left ventricular.
S1P receptor agonism after MI.
We hypothesized that the decline observed in myocardial SphK activity in the surviving RM after MI, together with the reduction in the mRNA expression of S1P1, the most abundant S1P receptor isoform, would contribute to a reduction in antiapoptotic signaling in the RM and play an important role both in CM loss and in the observed reduction of post-MI LV function. We therefore postulated that the therapeutic restoration of S1P1 receptor stimulation in the heart could inhibit apoptosis in the RM and ameliorate the decline in LV dysfunction.
The S1P1-specific agonist SEW2871 has been shown to be pharmacologically active in both stressed and unstressed CM in vitro (39, 45). SEW2871 was administered via oral gavage according to a dosing regimen previously reported to yield an amelioration of ischemic renal injury (18). Interestingly, slightly worsened fractional shortening was observed in contemporaneous control animals receiving oral gavage of vehicle (Table 2) compared with historical animals undergoing LAD ligation without gavage (Table 1). This observation may have been due to experimental or biological variation or in part due to added stress on the myocardium related to the stress of daily forced gavage.
Table 2.
Assessment of cardiac parameters by echocardiography
| Sham |
1 wk |
2 wk
|
|||
|---|---|---|---|---|---|
| Vehicle | SEW2871 | Vehicle | SEW2871 | ||
| n | 15 | 11 | 15 | 25 | 12 |
| EF, % | 81±1 | 72±2 | 74±2 | 71±2 | 76±1* |
| FS, % | 57±1 | 43±2 | 45±2 | 43±2 | 47±1* |
| LVEDV, μl | 35±2 | 38±5 | 33±2† | 38±4 | 34±5† |
| CO, ml/min | 17±1 | 16±1 | 14±1 | 15±1 | 15±2 |
Values are means ± SE; n, number of animals. CO, cardiac output.
P < 0.05 vs. vehicle.
Not significant.
An oral administration of SEW2871 in this study was associated with a significant reduction of the decline in Akt phosphorylation in the RM during post-MI remodeling at 2 wk after MI (Fig. 5A), indicating a pharmacological effect. Although phosphorylation (i.e., activation) of p70S6 kinase did not decline after MI in vehicle-treated animals below the already low levels observed in uninfarcted controls, SEW2871 treatment was associated with an increase in activation of this downstream mediator of Akt signaling, further suggesting an upregulation of the SphK/S1P cascade. There was no statistically significant change in ERK phosphorylation with SEW2871 treatment, although a nonsignificant increase was also observed.
A pharmacological S1P1 receptor stimulation was associated with an inhibition of programmed cell death in the RM (Fig. 5B). It is not clear whether apoptosis contributes significantly to infarct size in the type of nonreperfused MI model used in this study, and, in fact, infarct size was not affected by oral SEW2871 administration (37.9 ± 6.2% vs. 40.4 ± 5.0% in SEW2871- and vehicle-treated hearts, respectively, P = 0.75). Nevertheless, the increase in S1P signaling and the reduction in myocardial apoptosis noted in the RM was associated with an 11% improvement in LV function at 2 wk post-MI, as evidenced by echocardiographic measurements of ejection fraction and of fractional shortening in the remote myocardium (Table 2). Taken together, these data support the significance of remote myocardial remodeling in the progression of post-MI cardiomyopathy and suggest a therapeutic amelioration of that remodeling via S1P receptor stimulation. Furthermore, there was a reduction in myocardial fibrosis (P = 0.03) and a nonsignificant trend toward the preservation of RM wall thickness was also observed in SEW2871-treated mice at 2 wk after MI (Fig. 5, C and D). Myocyte size, however, was not found to be increased in SEW2871-treated hearts at either 1 or 2 wk after MI (Fig. 5E), suggesting that an increase in wall thickness may have been related to CM preservation rather than hypertrophy.
Interestingly, in contrast to persistent therapy with SEW2871, a short course of this agent, beginning just before LAD ligation and continuing for 72 h, failed to yield an improvement in LV function at either 1 or 2 wk after MI (data not shown). Changes in myocardial signaling events related to the substantial inflammatory response at that early post-MI time point did not allow for a confirmation of signaling changes due to SEW2871 administration with this short course of therapy. Nevertheless, these observations may reflect a need for ongoing S1P signaling to prevent longer-term post-MI remodeling in the RM and may indicate that the beneficial impact of S1P receptor agonism is not due simply to an inhibition of early post-MI apoptosis/necrosis.
DISCUSSION
The major findings of this study regarding the remote myocardium after MI include the following: 1) SphK activity is decreased; 2) S1P1 mRNA expression is reduced; 3) apoptosis is increased; 4) survival signaling is dampened; and 5) contractile function, as reflected in fractional shortening, is impaired. An additional key observation is that at least some of the functional abnormalities are ameliorated by a systemic administration of a selective S1P1 receptor agonist. These results are the first to implicate significant changes in key elements of the SphK/S1P signaling cascade in pathological changes during chronic LV remodeling and provide early proof of the concept for the therapeutic modulation of this pathway.
Relatively little is known regarding the possible role of SphK2 in regulating CM fate. Data from embryonic fibroblasts suggest that the intracellular localization of this SphK isoform may actually contribute to a paradoxical proapoptotic effect (27). Some discrepancies were observed in this study between changes in levels of mRNA and protein expression of SphK2 after MI; an even more substantial discrepancy is noted between the ratios of SphK2 and SphK1 mRNA levels measured here in the mouse and the previously described ratio of activity levels of these two isoforms in the rat heart (4, 40). These observations may reflect a very complex regulation of this enzymatic pathway, including posttranscriptional and posttranslational modification. Sun et al. (38) have demonstrated posttranslational regulation of SphK1 in cultured CM mediated both by the LIM-only factor FHL2 and endothelin-1; the disconnection observed between SphK expression and activity may result from posttranslational inhibition in the peri-MI period.
S1P-mediated signaling also involves a complex combination of intracellular pathways as well as a paracrine and autocrine activation of cell surface receptors and corresponding downstream mediators. A recent report in cultured wild-type mouse CM has indicated a cardioprotective, antiapoptotic role of S1P1 stimulation, mediated through downstream PI3K/Akt activation (44). Other studies in a double S1P2/S1P3 knockout model, however, have suggested that these receptor isoforms, and not S1P1, are primarily responsible for cardioprotective Akt activation (24). In addition, S1P1 and S1P3 have both been implicated in a negative inotropic and chronotropic effect on CM, possibly mediated by a muscarinic receptor-activated inward rectifier K+ current (17, 23, 25). This functional effect on CMs may mimic the therapeutic effect of β-blockade after an ischemic insult. While further studies with a more extensive array of specific receptor agonists and antagonists may be necessary to sort out this complex myocardial signaling pathway, the simple systemic administration of an S1P1-specific agonist reported here does suggest a cardioprotective role for the stimulation of this receptor in the working wild-type mouse heart that was at least in part mediated through an increase in Akt signaling.
The changes in SphK activity and in S1P receptor RNA expression documented in this study suggest a role for both intracellular and receptor-mediated S1P pathways in pathological cardiac remodeling. Because of the potential confounding effects of the systemic administration on a wide variety of cell types, even of relatively specific receptor and enzyme agonists and antagonists, a clearer elucidation of either the critical elements in myocardial S1P signaling or the potential targets for therapeutic intervention may require the study of regulatable, cardiac-specific genetic models. Our use of a murine model for these in vivo investigations, therefore, provides a particularly appropriate basis for such future studies.
Our results also provide very early support for novel S1P-based therapies to enhance myocardial preservation during the evolution of chronic heart failure. Interestingly, S1P receptor agonism affected both CM survival and myocardial function remote from the infarct, as evidenced by an improvement in fractional shortening in this region. The absence of a significant effect of a 3-day administration of SEW2871 suggests that S1P receptor agonism does not simply inhibit acute or subacute cell loss from necrosis or apoptosis but can enhance CM survival and preserve LV function even during later post-MI remodeling.
Treatment with SEW2871 in this study represented an early proof of the concept for the ability of S1P receptor agonism to influence molecular and cellular events that might contribute to physiological changes during post-MI remodeling. The magnitude of the effects observed on parameters of LV function was modest with this relatively simple treatment regimen. Other regimens that facilitate more prolonged therapy or that allow a modulation of the timing of S1P agonism relative to an acute coronary event and to the time course of chronic post-MI remodeling may have an even more pronounced effect. In addition, the stimulation of S1P2 or S1P3 receptors may also play a cardioprotective role after MI, and a less specific S1P receptor agonism with agents such a FTY720 may therefore prove even more potent than SEW2871 therapy. Other means of altering myocardial S1P signaling, including local delivery systems and even myocardial SphK1 gene transfer, may also provide new avenues for a much-needed improvement of both the prevention and treatment of chronic post-MI heart failure.
GRANTS
This work was supported by National Institutes of Health Grants 1R01-HL-083118 (to M. J. Mann), 1K08-HL-079239 (to M. J. Mann), and 5R01-HL-31809 (to E. J. Goetzl) and American Heart Association Grant 0465090Y (to M. J. Mann).
Acknowledgments
We thank Dr. Yoshiko Banno (Gifu University, Japan) for providing the Sphk1 antibody and Michael Kelly and Richard Tu for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab 18: 300–307, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong PW Defining myocardial infarction: a work in progress: ischemic heart disease. Heart 94: 1076–1079, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Betito S, Cuvillier C. Regulation by sphingosine 1-phosphate of Bax and Bad activities during apoptosis in a MEK-dependent manner. Biochem Biophys Res Commun 340: 1273–1277, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem 278: 47408–47415, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381: 800–803, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev 12: 181–188, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med 358: 1370–1380, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci USA 100: 10664–10669, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ipatova OM, Torkhovskaya TI, Zakharova TS, Khalilov EM. Sphingolipids and cell signaling: involvement in apoptosis and atherogenesis. Biochemistry 71: 713–722, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, Ji L, Iwamoto Y, Li E, Schneider M, Russell KS, Fu XY. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci USA 100: 12929–12934, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation 110: 1980–1989, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Jin ZQ, Karliner JS. Low dose N,N-dimethylsphingosine is cardioprotective and activates cytosolic sphingosine kinase by a PKCepsilon dependent mechanism. Cardiovasc Res 71: 725–734, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Johnson KR, Johnson KY, Becker KP, Bielawski J, Mao C, Obeid LM. Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. J Biol Chem 28: 34541–34547, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Kanno S, Lerner DL, Schuessler RB, Betsuyaku T, Yamada KA, Saffitz JE, Kovacs A. Echocardiographic evaluation of ventricular remodeling in a mouse model of myocardial infarction. J Am Soc Echocardiogr 15: 601–609, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol 33: 1713–1717, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Kluk MJ, Hla T. Role of the sphingosine 1-phosphate receptor EDG-1 in vascular smooth muscle cell proliferation and migration. Circ Res 89: 496–502, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Landeen LK, Aroonsakool N, Giles WR. Expression of sphingosine-1-phosphate receptors in neonatal and adult mouse heart (Abstract). FASEB J 20: A321, 2006. [Google Scholar]
- 18.Landeen LK, Dederko DA, Kondo CS, Hu BS, Aroonsakool N, Haga JH, Giles WR. Mechanisms of the negative inotropic effects of sphingosine-1-phosphate on adult mouse ventricular myocytes. Am J Physiol Heart Circ Physiol 294: H736–H749, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Lien YH, Yong KC, Cho C, Igarashi S, Lai LW. S1P1-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int 69: 1601–1608, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem 278: 40330–40336, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH Jr, Milstien S, Spiegel S. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem 280: 37118–37129, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Mann DL Basic mechanisms of left ventricular remodeling: the contribution of wall stress. J Card Fail 10: 202–206, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Mann DL Left ventricular size and shape: determinants of mechanical signal transduction pathways. Heart Fail Rev 10: 95–100, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Means CK, Miyamoto S, Chun J, Brown JH. S1P1 receptor localization confers selectivity for Gi-mediated cAMP and contractile responses. J Biol Chem 283: 11954–11963, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 292: H2944–H2951, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Ochi R, Momose Y, Oyama K, Giles WR. Sphingosine-1-phosphate effects on guinea pig atrial myocytes: alterations in action potentials and K+ currents. Cardiovasc Res 70: 88–96, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 4: 604–616, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, Gao S, Miwa N, Jahangeer S, Nakamura S. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem 280: 36318–36325, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Olivera A, Spiegel S. Sphingosine-1-phosphate as a second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 365: 557–560, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Pham TC, Fells JI Sr, Osborne DA, North EJ, Naor MM, Parrill AL. Molecular recognition in the sphingosine 1-phosphate receptor family. J Mol Graph 26: 1189–1201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pyne S, Pyne N. Sphingosine 1-phosphate signalling in mammalian cells. Pharmacol Ther 88: 115–131, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Radeff-Huang J, Seasholtz TM, Matteo RG, Brown JH. G protein mediated signaling pathways in lysophospholipid induced cell proliferation and survival. J Cell Biochem 92: 949–966, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Robert P, Tsui P, Laville MP, Livi GP, Sarau HM, Bril A, Berrebi-Bertrand I. EDG1 receptor stimulation leads to cardiac hypertrophy in rat neonatal myocytes. J Mol Cell Cardiol 33: 1589–1606, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58: 1072–1083, 1978. [DOI] [PubMed] [Google Scholar]
- 35.Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans 31: 1216–1219, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4: 397–407, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Stunff HL, Galve-Roperh I, Peterson C, Milstien S, Spiegel S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J Cell Biol 158: 1039–1049, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun JX, Yan GJ, Ren AX, You B, Liao JK. FHL2/SLIM3 decreases cardiomyocyte survival by inhibitory interaction with sphingosine kinase-1. Circ Res 99: 468–476, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tao R, Zhang JQ, Vessey DV, Honbo N, Karliner JS. Deletion of the sphingosine kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc Res 74: 56–63, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Vessey DA, Kelley M, Karliner JS. A rapid radioassay for sphingosine kinase analytical biochemistry. Anal Biochem 337: 136–142, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Vessey DA, Li L, Kelley M, Zhang J, Karliner JS. Sphingosine can pre- and post-condition heart and utilizes a different mechanism from sphingosine 1-phosphate. J Biochem Mol Toxicol 22: 113–118, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Weigert A, Johann AM, Knethen A, Schmidt H, Geisslinger G, Brüne B. Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood 108: 1635–1642, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Yeh CC, Malhotra D, Li H, Nicholas S, Tu R, Mann MJ. Surgical ventricular reconstruction in mice: elucidating potential targets for combined molecular/surgical intervention. J Thor Cardiovasc Surg. In press. [DOI] [PMC free article] [PubMed]
- 44.Young N, Van Brocklyn JR. Signal transduction of sphingosine-1-phosphate G protein-coupled receptors. Scientific World Journal 11: 946–966, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang JQ, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol 293: H3150–H3158, 2007. [DOI] [PubMed] [Google Scholar]