Abstract
During a whole body heat stress, stroke volume is either maintained or slightly elevated despite reduced ventricular filling pressures and central blood volume, suggestive of improved cardiac diastolic and/or systolic function. Heat stress improves cardiac systolic and diastolic function in patients with congestive heart failure, although it remains unknown whether similar responses occur in healthy individuals, which is the hypothesis to be tested. Nine male volunteers underwent a whole body heat stress. Echocardiographic indexes of diastolic and systolic function were performed following a supine resting period, and again following an increase in internal temperature of ∼1.0°C via passive heat stress. Despite previous reports of heat stress-induced decreases in ventricular filling pressures and central blood volume, no changes in indexes of diastolic function were identified during heating [i.e., unchanged early diastolic mitral annular tissue velocity (E′), mitral inflow during the early diastolic phase (E), the E/E′ ratio, and isovolumetric relaxation time]. Heat stress increased late diastolic septal (P = 0.03) and lateral (P = 0.01) mitral annular tissue velocities (A′), mitral inflow velocity during atrial contraction (P < 0.001), and the relative contribution of atrial contraction to left ventricular filling during diastole (P = 0.01), all indicative of improved atrial systolic function. Furthermore, indexes of ventricular systolic function were increased by heat stress [i.e., increased septal (P = 0.001) and lateral (P = 0.01) mitral annular systolic velocities and isovolumic acceleration at the septal (P = 0.03) and lateral (P < 0.001) mitral annulus]. These data are suggestive of improved atrial and ventricular systolic function by the heat stress. Together these data support previous findings, which used the less precise measure of ejection fraction, that heat stress improves indexes of systolic function, while diastolic function is maintained.
Keywords: cardiac systolic function, stroke volume
a pronounced increase in internal body, or core, temperature imposes a significant stress to the human cardiovascular system (28). To optimize heat dissipation, there is a profound increase in cutaneous blood flow that has been estimated to reach values as high as 7,500 ml/min compared with ∼300 ml/min during normothermia (27, 28). Maintenance of arterial blood pressure during heat stress conditions, in the face of profound increases in cutaneous vascular conductance, requires an increase in cardiac output along with reductions in vascular conductance from noncutaneous beds (26, 27, 29). During pronounced heat stress, cardiac output can more than double, approaching values as high as 13 l/min, with 50% or more of that value being directed toward skin (27, 28). Interestingly, this large increase in cardiac output is predominately mediated through heat stress-induced increases in heart rate (HR), since stroke volume is typically unchanged or only slightly elevated (11, 20, 28, 36). Furthermore, passive heat stress in humans reduces central blood volume, which is accompanied by a 3- to 5-mmHg reduction in central venous pressure and pulmonary capillary wedge pressure (6, 7, 20, 28, 36). Reduced central blood volume and ventricular filling pressures, accompanied with preserved or slightly elevated stroke volumes, have led to the suggestion that heat stress increases the inotropic state of the heart (17, 27). Consistent with this hypothesis, heat stress increases sympathetic activity to noncardiac regions such as the skeletal muscle (5, 9), splanchnic, and renal vascular beds (27, 29) and increases an index of cardiac sympathetic activity (8).
Heat stress increased cardiac index, stroke index, and ejection fraction in a group of chronic heart failure patients (33). Additionally, Frey and Kenney (13) reported a decrease in various “indirect” indexes of cardiac systolic function, including left ventricular ejection time and preejection period (both are indicative of increased cardiac systolic function) following an increase in oral temperature of 0.8°C in healthy individuals. More recently, work from our laboratory has shown that whole body heat stress increases ejection fraction, further suggestive of improved cardiac systolic function (7). Although the findings of the aforementioned investigations suggest that heat stress improves cardiac systolic function, they must be interpreted cautiously because of a combination of the indirect nature of measurements (13) and the load dependency of ejection fraction (7), resulting in an imprecise measure of systolic function, particularly given changes in ventricular loading status during a heat stress.
In addition to systolic function, the effectiveness of cardiac diastolic function to provide adequate ventricular filling contributes to the overall stroke volume. Although the effects of heat stress on diastolic function in healthy individuals are unknown, Kisanuki et al. (18) found that heat stress, induced by sauna therapy, improved diastolic function in patients with congestive heart failure. Taken together, heightened cardiac systolic and diastolic function would contribute to the maintenance of stroke volume during heat stress despite reduced left ventricular filling pressures.
Although findings in healthy individuals suggest that heat stress increases cardiac systolic function (7, 13), whether this occurs remains speculative. Furthermore, while the reports of improved cardiac diastolic function in patients with congestive heart failure are compelling, the effects of heat stress on this parameter in a healthy population are unknown. Therefore, the aim of this study was to test the hypothesis that heat stress improves cardiac systolic and diastolic function in a healthy population assessed using echocardiographic techniques.
METHODS
Nine healthy normotensive male subjects participated in this study. Average subject characteristics were (mean ± SD): age, 38 ± 10 yr; height, 182 ± 9 cm; and weight, 86 ± 12 kg. Subjects were not taking medications and were free of any known cardiovascular, metabolic, or neurological diseases. Subjects were informed of the purpose and risks of the study before providing their informed written consent. The study protocol and consent were approved by the University of Texas Southwestern Medical Center and Presbyterian Hospital of Dallas. Subjects refrained from alcohol, caffeine, and exercise for 24 h before the study. The protocol was performed in accordance with the Declaration of Helsinki.
Instrumentation and Measurements
Following arrival to the laboratory, each subject swallowed a telemetry pill for the measurement of intestinal temperature (HQ, Palmetto, FL). Mean skin temperature was measured from the weighted average of six thermocouples attached to the skin (32). Each subject was fitted with a water-perfused tube-lined suit (Med-Eng, Ottawa, Canada) that covered the entire body except for the head, face, hands, one forearm, and feet. This suit permitted the control of skin and internal temperatures by adjusting the temperature of the water perfusing the suit. Heart rate was continuously obtained from an electrocardiogram (HP Patient Monitor; Agilent, Santa Clara, CA) interfaced with a cardiotachometer (CWE, Ardmore, PA). Intermittent blood pressure measurements were obtained by auscultation of the brachial artery via electrosphygmomanometry (SunTech, Raleigh, NC). Skin blood flow was measured at the exposed forearm via laser-Doppler flowmetry using an integrating flow probe (MoorLab Laser Doppler Perfusion Monitor; Moor Instruments, Wilmington, DE). Cutaneous vascular conductance was calculated from the ratio of laser Doppler flux to mean arterial pressure.
Echocardiography
Echocardiographic images were obtained using commercially available ultrasound equipment (iE33, Philips Ultrasound, Bothell, WA). All examinations were performed during supine resting conditions with the subject in the left lateral decubitus position, and measurements were obtained during quiet guided expiration. All images were obtained by an experienced sonographer and stored on the iE33 hard drive and were later exported for blinded offline analysis by an experienced sonographer using commercially available software (Xcelera; Philips Ultrasound).
Tissue Doppler Imaging
Measurements of septal and lateral mitral annular early diastolic (E′), late diastolic (A′), and systolic velocities were obtained to provide indexes of left ventricular diastolic function (24), left atrial systolic function (21, 22), and left ventricular systolic function (14, 30), respectively, via standard tissue Doppler imaging techniques, as previously described (24). Additionally, isovolumic acceleration, which is an another index of left ventricular systolic function (10), was determined as the slope of the presystolic velocity curve at the septal and lateral mitral annulus (10). Tissue Doppler measurements were obtained from the apical four-chamber view with a 4.0-mm sample volume positioned at the junction of the septal mitral annulus and the left ventricular wall, as well as the junction of the lateral mitral annulus and the left ventricular wall (24).
Mitral Inflow Velocities
Mitral inflow velocities were assessed from the apical four-chamber view using pulsed wave Doppler with a sample volume of 2.0 mm positioned over the mitral valve leaflet tips. From these data measurements were obtained of the peak inflow velocity during the early phase of left ventricular relaxation (E), which provides an index of left ventricular diastolic function (4, 15, 19), and during left atrial contraction (A), which provides an index of left atrial systolic function (4). These values were subsequently used to further assess diastolic function via the E/A ratio (15, 19, 24) as well as left atrial systolic function via the relative contribution of A to left ventricular filling during diastole using the following formula: [A/(E + A)] × 100.
Isovolumetric Relaxation Time
Isovolumetric relaxation time (IVRT) represents the time interval between aortic outflow during systole and the opening of the mitral valve during diastole and thus is commonly used as an index of left ventricular relaxation (24). IVRT was determined using a five-chamber apical view with the sample volume set at 4.0 mm. Because of complications obtaining IVRT in one subject, the data illustrated for this variable are representative of eight subjects.
Experimental Protocol
Following instrumentation, subjects rested quietly in the supine position for ∼30 min while normothermic water (34°C) circulated through the suit. After this 30-min period, 6 min of baseline thermal and hemodynamic data were collected, during spontaneous respiration, which was followed by echocardiographic assessment of the aforementioned variables. Subjects were then exposed to a whole body heat stress by perfusing 49°C water through the suit until core temperature was elevated by ∼1°C. Once this target core temperature was achieved, the temperature of the water circulating through the suit was lowered to ∼47°C to limit further increases in core temperature. Next, the aforementioned 6 min of baseline data collection were obtained followed by the indicated echocardiographic assessments.
Data Analysis
Thermal and hemodynamic data were sampled at 50 Hz via a data-acquisition system (Biopac System, Santa Barbara, CA). Data from the last 60 s of the 6-min baseline period were averaged and compared between thermal conditions. Echocardiographic analyses were performed using commercially available software (Xcelera; Philips Ultrasound). The average of four measurements obtained from four consecutive cardiac cycles of each echocardiographic parameter was compared between thermal conditions. All statistical comparisons were performed via paired t-tests between thermal conditions using a commercially available statistical software package (SigmaStat 3.11, Chicago, IL). All values are reported as means ± SD. P values <0.05 were considered statistically significant.
RESULTS
Thermal and Hemodynamic Data
Thermal and hemodynamic responses to the whole body heat stress are shown in Table 1. As expected, heat stress significantly elevated core temperature, mean skin temperature, heart rate, and forearm cutaneous vascular conductance relative to normothermia (P ≤ 0.001 for all variables), whereas systolic (P = 0.63) and mean (P = 0.55) arterial pressures were unchanged.
Table 1.
Cardiovascular and thermal responses during normothermia and whole body heat stress
| Normothermia | Heat Stress | P Value | |
|---|---|---|---|
| Heart rate, beats/min | 57±9 | 84±17 | <0.001 |
| Systolic blood pressure, mmHg | 110±13 | 108±10 | 0.63 |
| Mean blood pressure, mmHg | 81±11 | 79±9 | 0.55 |
| Core temperature, °C | 36.9±0.3 | 37.8±0.3 | <0.001 |
| Mean skin temperature, °C | 34.2±0.4 | 38.0±0.7 | <0.001 |
| Forearm CVC, AU/mmHg | 0.20±0.1 | 0.85±0.5 | 0.001 |
Data are expressed as means ± SD. CVC, cutaneous vascular conductance; AU, arbitrary units.
Echocardiographic Parameters
Mitral annular early and late diastolic velocities.
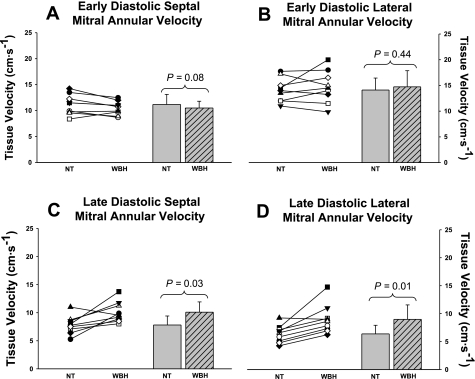
The early (E′) and late (A′) diastolic velocities of the septal and lateral mitral annulus during both thermal conditions are shown in Fig. 1. Septal (P = 0.08; Fig. 1A) and lateral (P = 0.44; Fig. 1B) mitral annular early diastolic velocities were unchanged during heat stress relative to normothermia. Both the septal (P = 0.03; Fig. 1C) and the lateral (P = 0.01; Fig. 1D) mitral annular late diastolic velocities were increased during heat stress relative to normothermia.
Fig. 1.
Early and late diastolic septal and lateral mitral annular velocities. Individual (left) and group averaged (right) echocardiographic measurements of early diastolic septal mitral annular velocity (A), early diastolic lateral mitral annular velocity (B), late diastolic septal mitral annular velocity (C), and late diastolic lateral mitral annular velocity (D) during normothermic (NT) and whole body heat stress (WBH) conditions. Early diastolic septal annular and lateral annular velocities were unchanged, whereas late diastolic septal annular and lateral annular velocities were increased during heat stress relative to normothermia. The significant increase in late diastolic tissue velocities is indicative of increased left atrial systolic function. Group data are means ± SD.
Mitral inflow velocities.
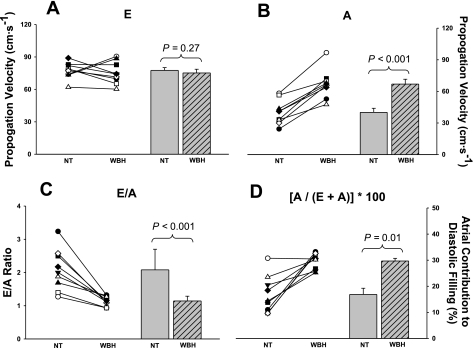
The effect of heat stress on mitral inflow blood velocities during different stages of left ventricular diastolic filling are shown in Fig. 2. Heat stress had no effect on mitral inflow blood velocity during early diastolic filling (P = 0.27; Fig. 2A), whereas inflow blood velocity during atrial contraction (Fig. 2B) was elevated significantly relative to normothermia (P < 0.001). As a result, heat stress decreased the E/A ratio (P < 0.001; Fig. 2C), which was manifested by an increase in the relative contribution of atrial contraction to left ventricular diastolic filling during heat stress (P = 0.01; Fig. 2D).
Fig. 2.
Mitral inflow velocities during different phases of diastolic filling. Individual (left) and group averaged (right) echocardiographic measurements of mitral inflow velocity during early diastolic filling (E wave; A), mitral inflow velocity during atrial contraction (A wave; B), the ratio of E/A (C), and the contribution of atrial contraction to diastolic filling (D) during NT and WBH conditions. Heat stress increased A velocity but not E velocity, which resulted in an increased relative contribution of atrial filling during diastole and a decrease in the E/A ratio. The significant increase in A velocity suggests that heat stress augments atrial systolic function. Moreover, heat stress increases the dependence on atrial booster to maintain left ventricular filling in the face of reduced ventricular filling pressures. Group data are means ± SD.
Relationship between early diastolic inflow velocity and early diastolic tissue velocity (E/E′).
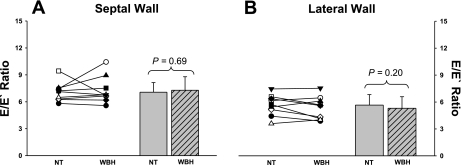
Whole body heat stress had no effect on the E/E′ ratio at either the septal wall (P = 0.69; Fig. 3A) or the lateral wall (P = 0.20; Fig. 3B).
Fig. 3.
Relationship between early diastolic inflow velocity and early diastolic tissue velocity (E/E′) at the septal and lateral wall. Individual (left) and group averaged (right) calculations of the E/E′ ratio at the septal (A) and the lateral (B) wall during NT and WBH conditions. The E/E′ ratio at both the septal and lateral walls were unchanged during heat stress relative to normothermia.
IVRT.
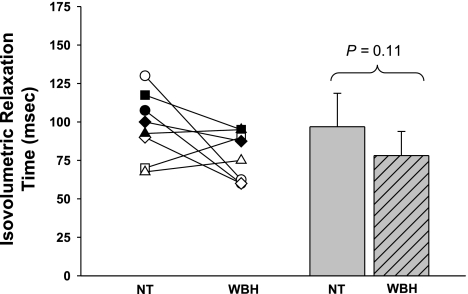
Figure 4 shows the IVRT during both thermal conditions. IVRT during normothermia was unaffected by whole body heating (P = 0.11).
Fig. 4.
Isovolumetric relaxation time. Individual (left) and group averaged (right) isovolumetric relaxation times during NT and WBH conditions. The isovolumetric relaxation time was unchanged during heat stress relative to normothermia. Group data are means ± SD.
Mitral annular systolic velocities.
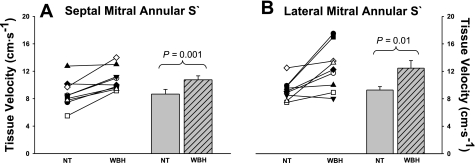
The peak septal and lateral mitral annular tissue systolic velocities during both thermal conditions are shown in Fig. 5. Both the septal (P = 0.001; Fig. 5A) and the lateral (P = 0.01; Fig. 5B) mitral annular systolic velocities were elevated during the heat stress relative to normothermia.
Fig. 5.
Peak septal and lateral mitral annular systolic velocities (S′). Individual (left) and group averaged (right) echocardiographic measurements of peak septal mitral annular systolic velocity (A) and peak lateral mitral annular systolic velocity (B) during NT and WBH conditions. Septal annular and lateral annular systolic velocities were increased during heat stress relative to normothermia, thereby indicating an increase in cardiac systolic function. Group data are means ± SD.
Isovolumic acceleration.
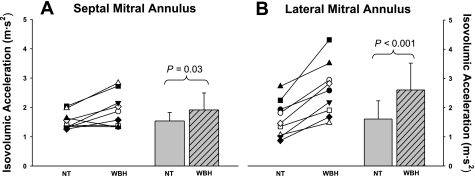
Isovolumic acceleration was increased following whole body heat stress at both the septal mitral annulus (P = 0.03; Fig. 6A) and at the lateral mitral annulus (P < 0.001; Fig. 6B).
Fig. 6.
Isovolumic acceleration of the septal and lateral mitral annulus. Individual (left) and group averaged (right) calculations of isovolumic acceleration at the septal mitral annulus (A) and the lateral mitral annulus (B) during NT and WBH conditions. Isovolumic acceleration was significantly increased at both locations during heat stress relative to normothermia, which is indicative of increased cardiac systolic function. Group data are means ± SD.
DISCUSSION
There are a number of primary findings resulting from this study. 1) Heat stress has no effect on indexes of diastolic function as indicated by an unchanged velocity of blood during the early phase of left ventricular filling, early diastolic mitral annular velocity, and the ratio of blood velocity/mitral annular velocity during the early phase of diastole. However, the preservation of these indexes of diastolic function, despite heat stressed-induced decreases in ventricular filling pressures (6, 20, 28, 36) and central blood volume, leaves room for speculation that diastolic function is perhaps improved during heat stress. 2) Heat stress significantly increases left ventricular systolic function as evidenced by an increase in peak septal and lateral mitral annular systolic velocities and isovolumic acceleration. 3) Heat stress significantly increases left atrial systolic function as evidenced by an increased velocity of blood during the atrial contraction phase of left ventricular diastolic filling and an increased velocity of the septal and lateral mitral annulus during the late phase of diastolic relaxation relative to normothermia.
It is well established that stroke volume is maintained or slightly elevated during passive whole body heating (11, 20, 28, 36), despite reductions in central blood volume (7) and cardiac filling pressures (6, 20, 28, 36). These findings suggest that cardiac systolic function is augmented during heat stress conditions. Recently, using the technique of multiple-gated acquisition, Crandall et al. (7) reported that ejection fraction was increased by ∼13% following an increase in internal temperature of ∼1.3°C, which is consistent with the current findings, while left ventricular end diastolic volume was unchanged. Furthermore, this increase in ejection fraction and maintenance of left ventricular end diastolic volume occurred in spite of significant reductions in central venous pressure and central blood volume (7). These observations further suggest that cardiac systolic function is augmented while diastolic function is maintained during whole body heating. However, there are a number of limitations to relying solely on those indexes of cardiac systolic and diastolic function.
Diastolic Function
Doppler echocardiographic evaluation of mitral inflow velocities was used in the assessment of left ventricular diastolic function (4, 15, 19). The velocity of the blood flowing from the left atrium in the left ventricle during the early phase of diastolic filling (E) is primarily dependent on the magnitude of the suction created by left ventricular relaxation, whereas the velocity of blood flow during the late phase of diastolic filling (A) is dependent on left atrial contraction and left ventricular compliance (4). In healthy individuals, the E/A ratio is >1.0 (4, 15, 19). However, various cardiovascular disease states as well as normal healthy aging are associated with a reduction in the E/A ratio to values <1.0, which is manifested by impaired ventricular relaxation (i.e., reduced E), thus increasing the reliance on the atrial systolic contribution to ventricular filling (i.e., increased A) (15, 19, 24). Ultimately, the increase in atrial systolic function is not able to offset the detrimental effect of reduced ventricular relaxation, which contributes in part to the decrease in cardiac output that is common in the aforementioned conditions. In the present study, we observed an ∼50% reduction in the E/A ratio (normothermia: 2.1 ± 0.6; hyperthermia: 1.1 ± 0.1, P < 0.001) during heat stress relative to normothermia. However, unlike what occurs in cardiovascular disease states and the normal aging process, the observed reduction in the E/A ratio during heating was solely due to an approximate doubling of the velocity of blood during A, since E was unchanged (Fig. 2, A and B). A decrease in the E/A ratio associated with a preserved E velocity indicates that left ventricular relaxation is unaffected by heat stress.
Other parameters of left ventricular relaxation, including mitral annular tissue velocity during early diastole (E′; Fig. 1, A and B), the ratio between E/E′ (Fig. 3, A and B), and IVRT (Fig. 4), were unaffected by heat stress, providing additional support that left ventricular relaxation was unchanged and that these indexes were unaffected by the observed increases in HR. However, echocardiographic measures of diastolic function are dependent on a variety of factors, including volume status and left atrial pressure. Recently, in healthy normothermic subjects, Prasad et al. (24) reported that reductions in pulmonary capillary wedge pressure and central blood volume induced by lower body negative pressure impaired ventricular relaxation. Additionally, the early (E) mitral inflow velocity has been shown to be highly preload dependent, with significant reductions in E accompanying reductions in preload in human (22, 25) and animal (16, 21) models. In the present study, there was no difference in any of the indexes of left ventricular diastolic function between normothermia and heat stress conditions, despite heat stress reducing central blood volume and ventricular filling pressures (6, 7, 20, 29, 36). Therefore, unchanged indexes of diastolic function despite reduced ventricular filling pressures and central blood volume raise the speculation that perhaps diastolic function (i.e., left ventricular relaxation and/or suction) is improved with heat stress. This hypothesis is in agreement with a recent finding of increased untwisting rate, i.e., increased suction, of the left ventricle during diastole in heat stress conditions (31); findings of little to no reduction in left ventricular end diastolic volume during heat stress conditions (7) despite pronounced reductions in left ventricular filling pressures (6, 20, 29, 36); as well as findings of improved diastolic function in congestive heart failure patients with elevated internal temperatures (18).
Systolic Function
The other interesting aspect of the pattern of the reduction in the E/A ratio is the approximate doubling of blood velocity during A (Fig. 2B), which indicates that left atrial systolic function is augmented by heat stress. Additionally the rate of relaxation of the septal and lateral mitral annulus during the late phase of diastolic relaxation (A′), which is the result of passive ventricular distension primarily mediated by ventricular filling during atrial contraction (21, 22), was also increased, thus providing additional evidence of a heat stress-induced increase in atrial systolic function and/or left ventricular compliance. This is contrary to the findings of no effect of sauna heating on A velocity in patients with congestive heart failure (18). It is most likely that this discrepancy is related to the use of healthy subjects in the present study relative to congestive heart failure patients in the study by Kisanuki et al. (18). Ultimately, the maintenance of early diastolic filling and significantly increased blood velocity during atrial contraction is likely important in ensuring the left ventricle receives an adequate blood supply to maintain stroke volume despite reduced filling pressures in heat stress conditions.
Tissue Doppler evaluation of the mitral annulus provides information regarding movement of the myocardial wall in the longitudinal axis, i.e., shortening of the myocardial fibers during ventricular systole (21), and is an acceptable measurement of cardiac systolic function (14, 30). For example, mitral annular systolic velocity increases during inotropic stimulation (14, 30). Previously, we reported that heat stress increases ejection fraction, suggestive of improved cardiac contractile function (7). However, ejection fraction is an imprecise measure of cardiac contractility, and in the aforementioned study (30) a significant increase in ejection fraction was not observed until a higher dose of dobutamine (3 mg·kg−1·min−1) was administered, relative to the dose required to change mitral annular velocities (1 mg·kg−1·min−1). These observations, and others, suggest that tissue Doppler assessment of mitral annular velocities is a reliable and sensitive indicator of left ventricular systolic function (1, 2, 35). In the present study, the peak septal and lateral mitral annular systolic velocities were both significantly augmented during heat stress relative to normothermia (Fig. 5, A and B), thus providing further support of a heat stress-induced increase in cardiac systolic function.
In normothermic subjects, systolic mitral annular tissue velocity is reduced following reductions in preload induced by a variety of maneuvers, including lower body negative pressure (22), parabolic flight at 1.8 gravitational acceleration (3), and hemodialysis (12). Like the aforementioned conditions, heat stress also reduces central blood volume and ventricular filling pressures (6, 7, 20, 23, 28, 36). Despite reductions in indexes of preload during heat stress, we observed increases in late mitral inflow blood velocity (Fig. 2B) as well as peak systolic septal and lateral mitral annular velocities (Fig. 5, A and B), indicating a significant increase in both left atrial and left ventricular systolic function during heat stress. Furthermore, isovolumic acceleration has recently been demonstrated to be a preload-independent index of cardiac systolic function in normal healthy individuals (10). In the present study, isovolumic acceleration increased during heat stress relative to normothermia (Fig. 6, A and B), thereby providing further support that heat stress increases cardiac systolic function and contractility.
Study Limitations
In the present study, systolic and mean arterial blood pressures were not affected by heat stress (Table 1). However, heat stress does result in reduced total peripheral resistance (20); thus, the possibility that the observed increases in indexes of systolic function were because of reductions in systemic afterload cannot be ruled out. On the contrary, several investigations have demonstrated that various indexes of systolic function, including systolic mitral annular velocity (1) and isovolumic acceleration (34), are afterload independent. Therefore, it is unlikely than the observed increases in left ventricular systolic/contractile function in the present study are solely due to decreases in systemic afterload.
In conclusion, the present data demonstrate that whole body heating increases left ventricular and left atrial systolic function, whereas indexes of left ventricular diastolic function are preserved with heat stress, despite decreases in central blood volume and cardiac filling pressures. These findings provide possible mechanisms of preserved, and in some cases elevated, stroke volume in healthy individuals exposed to elevated internal temperatures.
GRANTS
This project was supported by National Heart, Lung, and Blood Institute Grant HL-61388.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ama R, Segers P, Roosens C, Claessens T, Verdonck P, Poelaert J. The effects of load on systolic mitral annular velocity by tissue Doppler imaging. Anesth Analg 99: 332–338, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bach DS Quantitative Doppler tissue imaging as a correlate of left ventricular contractility. Int J Card Imaging 12: 191–195, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Caiani EG, Weinert L, Takeuchi M, Veronesi F, Sugeng L, Corsi C, Capderou A, Cerutti S, Vaida P, Lang RM. Evaluation of alterations on mitral annulus velocities, strain, and strain rates due to abrupt changes in preload elicited by parabolic flight. J Appl Physiol 103: 80–87, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Cohen GI, Pietrolungo JF, Thomas JD, Klein AL. A practical guide to assessment of ventricular diastolic function using Doppler echocardiography. J Am Coll Cardiol 27: 1753–1760, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Crandall CG, Farr D, Etzel RA. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 277: H2348–H2352, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Crandall CG, Levine BD, Etzel RA. Effect of increasing central venous pressure during passive heating on skin blood flow. J Appl Physiol 86: 605–610, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Crandall CG, Wilson TE, Marving J, Vongelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol 586: 293–301, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crandall CG, Zhang R, Levine BD. Effects of whole body heating on dynamic baroreflex regulation of heart rate in humans. Am J Physiol Heart Circ Physiol 279: H2486–H2492, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Cui J, Wilson TE, Crandall CG. Baroreflex modulation of sympathetic nerve activity to muscle in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol 282: R252–R258, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Dalsgaard M, Snyder EM, Kjaergaard J, Johnson BD, Hassager C, Oh JK. Isovolumic acceleration measured by tissue Doppler echocardiography is preload independent in healthy subjects. Echocardiography 24: 572–579, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Damato AM, Lau SH, Stein E, Haft JI, Kosowsky B, Cohen SI. Cardiovascular response to acute thermal stress (hot dry environment) in unacclimatized normal subjects. Am Heart J 76: 769–774, 1968. [DOI] [PubMed] [Google Scholar]
- 12.Drighil A, Madias JE, Mathewson JW, El Mosalami H, El Badaoui N, Ramdani B, Bennis A. Haemodialysis: effects of acute decrease in preload on tissue Doppler imaging indices of systolic and diastolic function of the left and right ventricles. Eur J Echocardiogr 9: 530–535, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Frey MA, Kenney RA. Cardiac response to whole-body heating. Aviat Space Environ Med 50: 387–389, 1979. [PubMed] [Google Scholar]
- 14.Gorcsan J, 3rd Deswal A, Mankad S, Mandarino WA, Mahler CM, Yamazaki N, Katz WE. Quantification of the myocardial response to low-dose dobutamine using tissue Doppler echocardiographic measures of velocity and velocity gradient. Am J Cardiol 81: 615–623, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Hatle L Doppler echocardiographic evaluation of diastolic function in hypertensive cardiomyopathies. Eur Heart J 14, Suppl J: 88–94, 1993. [PubMed] [Google Scholar]
- 16.Jacques DC, Pinsky MR, Severyn D, Gorcsan J 3rd. Influence of alterations in loading on mitral annular velocity by tissue Doppler echocardiography and its associated ability to predict filling pressures. Chest 126: 1910–1918, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of Physiology: Adaptations to the Environment, edited by Blatteis C and Fregly M. Bethesda, MD: Am Physiol Soc, 1996, p. 215–243.
- 18.Kisanuki A, Daitoku S, Kihara T, Otsuji Y, Tei C. Thermal therapy improves left ventricular diastolic function in patients with congestive heart failure: a tissue doppler echocardiographic study. J Cardiol 49: 187–191, 2007. [PubMed] [Google Scholar]
- 19.Labovitz AJ, Pearson AC. Evaluation of left ventricular diastolic function: clinical relevance and recent Doppler echocardiographic insights. Am Heart J 114: 836–851, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters cardiovascular response to direct passive heating. J Appl Physiol 84: 1323–1332, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Nagueh SF, Sun H, Kopelen HA, Middleton KJ, Khoury DS. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J Am Coll Cardiol 37: 278–285, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Pela G, Regolisti G, Coghi P, Cabassi A, Basile A, Cavatorta A, Manca C, Borghetti A. Effects of the reduction of preload on left and right ventricular myocardial velocities analyzed by Doppler tissue echocardiography in healthy subjects. Eur J Echocardiogr 5: 262–271, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Peters JK, Nishiyasu T, Mack GW. Reflex control of the cutaneous circulation during passive body core heating in humans. J Appl Physiol 88: 1756–1764, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol 99: 1629–1636, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regolisti G, Coghi P, Orlandini G, Zoni A, Guariglia A, Vinci S, Borghetti A. Effects of reduced preload on diastolic filling in essential hypertensive patients with increased left ventricular mass. Am J Hypertens 10: 447–453, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Rowell LB Hyperthermia: a hyperadrenergic state. Hypertension 15: 505–507, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Rowell LB Thermal stress. In: Human Circulation Regulation During Physical Stress. New York, NY: Oxford Univ Press, 1986, p. 174–212.
- 28.Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 27: 673–680, 1969. [DOI] [PubMed] [Google Scholar]
- 29.Rowell LB, Detry JR, Profant GR, Wyss C. Spanchnic vasoconstriction in hyperthermic man: role of falling blood pressure. J Appl Physiol 31: 864–869, 1971. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu Y, Uematsu M, Shimizu H, Nakamura K, Yamagishi M, Miyatake K. Peak negative myocardial velocity gradient in early diastole as a noninvasive indicator of left ventricular diastolic function: comparison with transmitral flow velocity indices. J Am Coll Cardiol 32: 1418–1425, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Stohr E, Gonzalez-Alonso J, Pearson J, Ali L, Barker H, Shave R. Effects of heat stress on left ventricular rotation and rotation rate in resting humans (Abstract). Proc Physiol Soc 11: C64, 2008. [Google Scholar]
- 32.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y, Tanaka N. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 91: 2582–2590, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Vogel M, Cheung MM, Li J, Kristiansen SB, Schmidt MR, White PA, Sorensen K, Redington AN. Noninvasive assessment of left ventricular force-frequency relationships using tissue Doppler-derived isovolumic acceleration: validation in an animal model. Circulation 107: 1647–1652, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Waggoner AD, Bierig SM. Tissue Doppler imaging: a useful echocardiographic method for the cardiac sonographer to assess systolic and diastolic ventricular function. J Am Soc Echocardiogr 14: 1143–1152, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Wilson TE, Tollund C, Yoshiga CC, Dawson EA, Nissen P, Secher NH, Crandall CG. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. J Physiol 585: 279–285, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]