Abstract
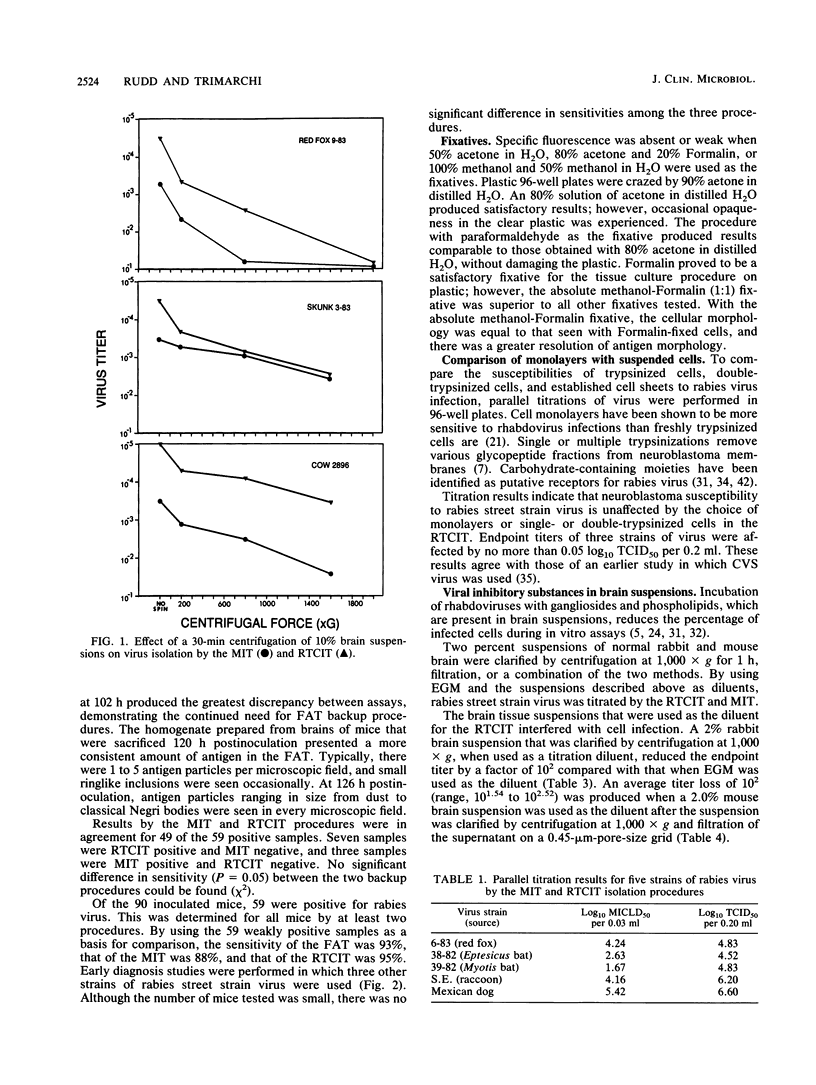
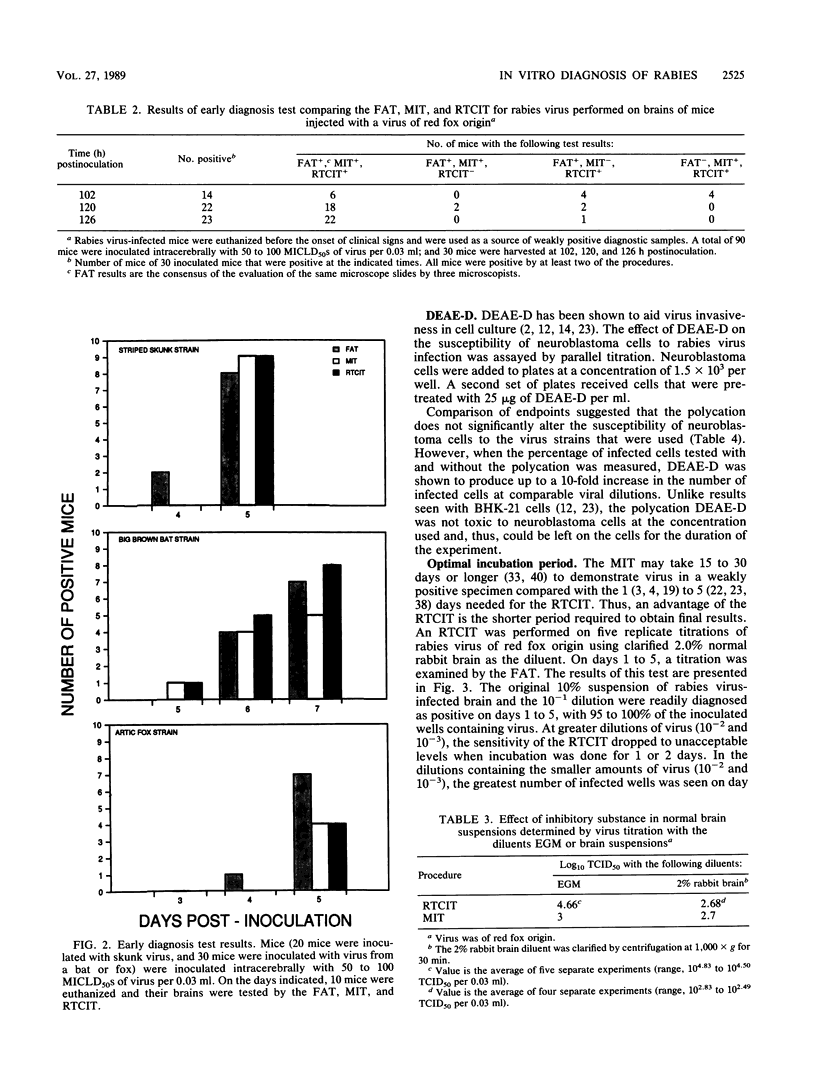
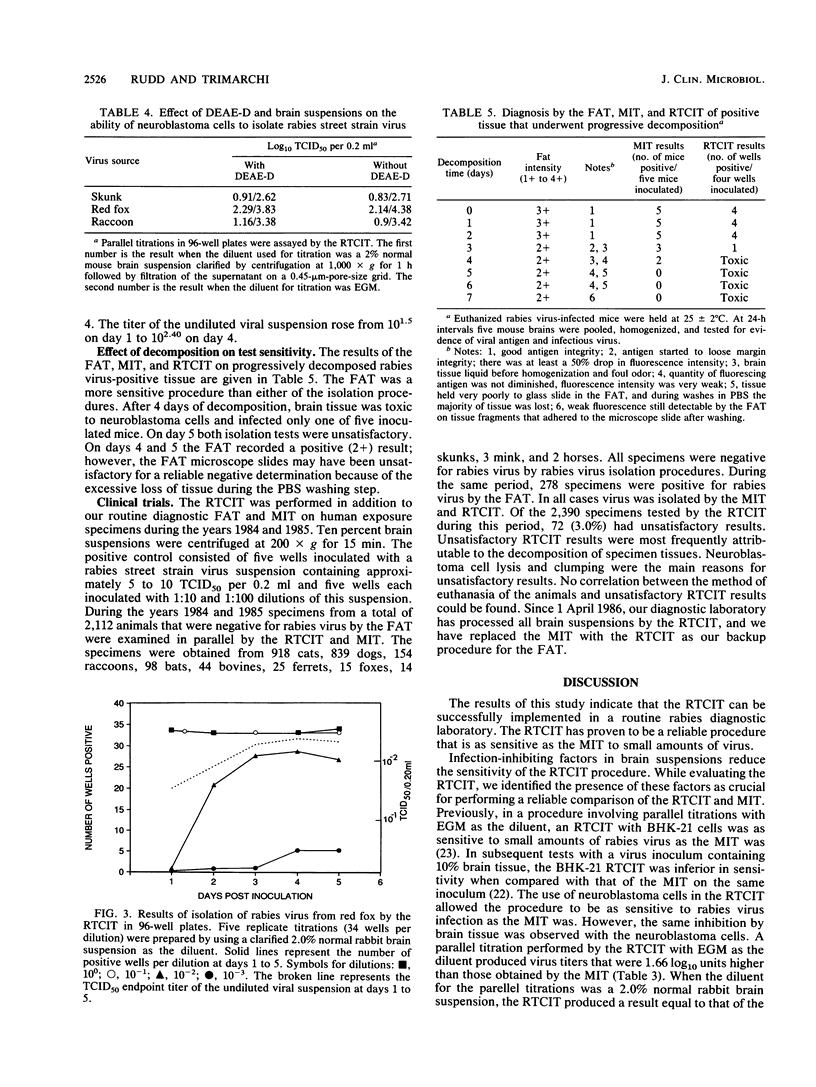
A tissue culture virus isolation procedure for rabies street strain virus on mouse neuroblastoma cells is described. Parameters for the optimum sensitivity of the procedure were determined to include a minimum 4-day incubation of virus in tissue culture and the use of diethylaminoethyl-dextran for increased cell susceptibility. The in vitro procedure performed well in a comparison with the fluorescent-antibody test and the mouse inoculation test (MIT) on weakly positive brain tissue. Decomposed specimens and virus inhibitors present in brain suspensions were found to interfere with the in vitro procedure. A Formalin-methanol fixative was found to be superior on plastic 96-well plates to previously used fixatives. A 2-year clinical trial of the procedure in parallel with the MIT demonstrated the practicality of the procedure. Accordingly, the New York State rabies diagnostic laboratory has replaced the MIT with the in vitro procedure as a backup for the fluorescent-antibody test in the routine diagnosis of rabies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. A., Miller D. K., Lenard J. Effects of DEAE-dextran on infection and hemolysis by VSV. Evidence that nonspecific electrostatic interactions mediate effective binding of VSV to cells. Virology. 1984 Feb;133(1):111–118. doi: 10.1016/0042-6822(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Bourhy H., Rollin P. E., Vincent J., Sureau P. Comparative field evaluation of the fluorescent-antibody test, virus isolation from tissue culture, and enzyme immunodiagnosis for rapid laboratory diagnosis of rabies. J Clin Microbiol. 1989 Mar;27(3):519–523. doi: 10.1128/jcm.27.3.519-523.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitra L., Pandit V., Kalyanaraman V. R. Use of murine neuroblastoma culture in rapid diagnosis of rabies. Indian J Med Res. 1988 Feb;87:113–116. [PubMed] [Google Scholar]
- Conti C., Hauttecoeur B., Morelec M. J., Bizzini B., Orsi N., Tsiang H. Inhibition of rabies virus infection by a soluble membrane fraction from the rat central nervous system. Arch Virol. 1988;98(1-2):73–86. doi: 10.1007/BF01321007. [DOI] [PubMed] [Google Scholar]
- Glick M. C., Kimhi Y., Littauer U. Z. Glycopeptides from surface membranes of neuroblastoma cells. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1682–1687. doi: 10.1073/pnas.70.6.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough P. M., Dierks R. E., Russell R. M., Archer B. G. Characterization of brain-associated rabies neutralizing substance. J Infect Dis. 1974 Apr;129(4):456–460. doi: 10.1093/infdis/129.4.456. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y., Clark H. F. Rabies virus infection in mouse neuroblastoma cells. Lab Invest. 1977 Jun;36(6):578–574. [PubMed] [Google Scholar]
- Kaplan M. M., Giard D. J., Blattner W. A., Lubiniecki A. S., Fraumeni J. F., Jr An improved method of fixation for immunofluorescent detection of SV40 T-antigen in infected human fibroblasts. Proc Soc Exp Biol Med. 1975 Mar;148(3):660–664. doi: 10.3181/00379727-148-38605. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Maes R. F., Campbell J. B., Koprowski H. Effect of polyions on the infectivity of rabies virus in tissue culture: construction of a single-cycle growth curve. J Virol. 1967 Feb;1(1):145–151. doi: 10.1128/jvi.1.1.145-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H. Laboratory techniques in rabies: the mouse inoculation test. Monogr Ser World Health Organ. 1973;(23):85–93. [PubMed] [Google Scholar]
- Laurila P., Virtanen I., Wartiovaara J., Stenman S. Fluorescent antibodies and lectins stain intracellular structures in fixed cells treated with nonionic detergent. J Histochem Cytochem. 1978 Apr;26(4):251–257. doi: 10.1177/26.4.207770. [DOI] [PubMed] [Google Scholar]
- Lewis V. J., Thacker W. L. Limitations of deteriorated tissue for rabies diagnosis. Health Lab Sci. 1974 Jan;11(1):8–12. [PubMed] [Google Scholar]
- McMorris F. A., Ruddle F. H. Expression of neuronal phenotypes in neuroblastoma cell hybrids. Dev Biol. 1974 Aug;39(2):226–246. doi: 10.1016/0012-1606(74)90237-1. [DOI] [PubMed] [Google Scholar]
- Parker R. L., Sikes R. K. Development of rabies inhibiting substance in skunks infected with rabies virus. Public Health Rep. 1966 Oct;81(10):941–944. [PMC free article] [PubMed] [Google Scholar]
- Rudd R. J., Trimarchi C. V., Abelseth M. K. Tissue culture technique for routine isolation of street strain rabies virus. J Clin Microbiol. 1980 Oct;12(4):590–593. doi: 10.1128/jcm.12.4.590-593.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd R. J., Trimarchi C. V. Comparison of sensitivity of BHK-21 and murine neuroblastoma cells in the isolation of a street strain rabies virus. J Clin Microbiol. 1987 Aug;25(8):1456–1458. doi: 10.1128/jcm.25.8.1456-1458.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Tralka T. S., Willingham M. C., Pastan I. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell. 1983 Feb;32(2):639–646. doi: 10.1016/0092-8674(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger H. R., Wells H. J., Hummeler K. Comparison of the lipids of intracellular and extracellular rabies viruses. J Virol. 1973 Nov;12(5):1028–1030. doi: 10.1128/jvi.12.5.1028-1030.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shope T. C., Klein-Robbenhaar J., Miller G. Fatal encephalitis due to Herpesvirus hominis: use of intact brain cells for isolation of virus. J Infect Dis. 1972 May;125(5):542–544. doi: 10.1093/infdis/125.5.542. [DOI] [PubMed] [Google Scholar]
- Sitthi-Amorn C., Jiratanavattana V., Keoyoo J., Sonpunya N. The diagnostic properties of laboratory tests for rabies. Int J Epidemiol. 1987 Dec;16(4):602–605. doi: 10.1093/ije/16.4.602. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Tignor G. H., Emmons R. W., Woodie J. D. Isolation of field rabies virus strains in CER and murine neuroblastoma cell cultures. Intervirology. 1978;9(6):359–361. doi: 10.1159/000148958. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Tignor G. H., Mifune K., Motohashi T. Isolation and assay of rabies serogroup viruses in CER cells. Intervirology. 1977;8(2):92–99. doi: 10.1159/000148883. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Reid-Sanden F. L., Roumillat L. F., Trimarchi C., Clark K., Baer G. M., Winkler W. G. Demonstration of antigenic variation among rabies virus isolates by using monoclonal antibodies to nucleocapsid proteins. J Clin Microbiol. 1986 Oct;24(4):573–580. doi: 10.1128/jcm.24.4.573-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti F., Hauttecoeur B., Morelec M. J., Goldoni P., Bizzini B., Tsiang H. Involvement of gangliosides in rabies virus infection. J Gen Virol. 1986 Jan;67(Pt 1):47–56. doi: 10.1099/0022-1317-67-1-47. [DOI] [PubMed] [Google Scholar]
- Superti F., Seganti L., Tsiang H., Orsi N. Role of phospholipids in rhabdovirus attachment to CER cells. Brief report. Arch Virol. 1984;81(3-4):321–328. doi: 10.1007/BF01310002. [DOI] [PubMed] [Google Scholar]
- Tsiang H., Koulakoff A., Bizzini B., Berwald-Netter Y. Neurotropism of rabies virus. An in vitro study. J Neuropathol Exp Neurol. 1983 Jul;42(4):439–452. doi: 10.1097/00005072-198307000-00006. [DOI] [PubMed] [Google Scholar]
- Umoh J. U., Blenden D. C. Comparison of primary skunk brain and kidney and raccoon kidney cells with established cell lines for isolation and propagation of street rabies virus. Infect Immun. 1983 Sep;41(3):1370–1372. doi: 10.1128/iai.41.3.1370-1372.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster W. A. A tissue culture infection test in routine rabies diagnosis. Can J Vet Res. 1987 Jul;51(3):367–369. [PMC free article] [PubMed] [Google Scholar]
- Webster W. A., Casey G. A., Charlton K. M. The mouse inoculation test in rabies diagnosis: early diagnosis in mice during the incubation period. Can J Comp Med. 1976 Jul;40(3):322–325. [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Koprowski H. Antigenic variants of rabies virus. J Exp Med. 1980 Jul 1;152(1):99–112. doi: 10.1084/jem.152.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunner W. H., Reagan K. J., Koprowski H. Characterization of saturable binding sites for rabies virus. J Virol. 1984 Jun;50(3):691–697. doi: 10.1128/jvi.50.3.691-697.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


