Abstract
We used a heterotopic transplanted working heart model to probe the collaborative role of bone marrow-derived progenitor cells (BPCs) and stromal cell-derived factor (SDF)-1α in attenuating tissue remodeling in recipient and transplanted hearts. BPCs from male transgenic rats expressing green fluorescent protein (GFP+ BPCs, 2 × 106 cells) were injected intravenously into myeloablated female rats. One month later, heterotopic heart transplantation was performed. The left anterior descending coronary artery (LAD) of the recipient heart was occluded permanently. Mesenchymal stem cells (MSCs; 2 × 106 cells) with a null gene (null group) or overexpressing SDF-1α (SDF-1α group) were injected intramyocardially in the LAD perfusion region of both recipient and transplanted hearts. Recipient and transplanted hearts (n = 10 hearts/group) were harvested 21 days later for analysis. The survival of transplanted hearts was assessed daily by palpation in additional animals (n = 7). Five days after LAD occlusion, subpopulations of GFP+ BPCs in the circulation were significantly higher in the SDF-1α group. Y chromosome, 5-bromo-2′-deoxyuridine, Ki67-positive nuclei, newly formed vessels, and GFP+ cells significantly increased in transplanted hearts of the SDF-1α group at 21 days after the injection of MSCs overexpressing SDF-1α, whereas fewer TUNEL-positive nuclei were found. The survival of transplanted hearts was also markedly increased in the SDF-1α group (P < 0.05). Supplementation of endogenous cytokines released from the ischemic myocardium with exogenous MSCs overexpressing SDF-1α significantly increased BPC homing to acutely ischemic recipient and progressively ischemic transplanted hearts. BPC recruitment resulted in the regeneration of new cardiomyocytes and blood vessels and extended survival of the transplanted hearts.
Keywords: stromal cell-derived factor-1α, heterotopic heart transplantation, stem cells, cell migration
ischemic heart disease is the leading cause of heart failure and death (1). Conventional therapeutic options for the treatment of myocardial infarction (MI) are limited to preventing the progression of ventricular remodeling and congestive heart failure (22). Currently, the only successful treatment for end-stage heart failure is heart transplantation (9, 18). Even if a suitable donor is found, cardiac allograft vasculopathy characterized by a diffuse process of concentric narrowing of the whole arterial allograft microvasculature may result in high morbidity and mortality among long-term heart transplant recipients (36). Recently, approaches using cell therapies have gained credibility as an alternative treatment for repair of the infarcted myocardium. Stem cell transplantation for the regeneration of the myocardium has been studied in various animal models (19). Unfortunately, the native process of stem cells homing to the infarcted myocardium is inefficient; only limited generation of angiogenic cells and cardiac myocytes has been documented (27). This approach is, therefore, not yet effective for correcting ischemic tissue damage leading to full recovery of heart function.
The heterotopic transplanted working heart model [chronic progressive diffused vasculopathy and global ventricular underperfusion in the transplanted heart combined with acute regional myocardial ischemia created by left anterior descending coronary artery (LAD) ligation in the recipient heart] is valuable in examining interventions with putative salutary myogenic and angiogenic actions. In addition, peripheral progenitor cells recruited to the ischemic myocardium by locally released cytokines can be identified using both sex- and fluorescent-tagged bone marrow (BM)-derived progenitor cells (BPCs).
Several reports have indicated that the chemokine stromal cell-derived factor (SDF)-1α and its G protein-coupled receptor (CXCR4) play important roles in stem cell release and recruitment from the BM and homing to remote sites of tissue injury (16, 21). Increasing evidence has implicated the role(s) of SDF-1α/CXCR4 in cardiac regeneration (9) and in blood vessel growth and development (6). Moreover, Carr et al. (5) recently demonstrated that circulating endothelial precursor cells (EPCs) express CXCR4 and that local, intramuscular administration of SDF-1α coupled with systemic injections of isolated human EPCs improved collateral blood flow in mice with femoral artery resection. Other laboratories (24, 37) have recently reported that SDF-1α contributes to neovascularization of the ischemic mouse heart. Taking into consideration these unique properties of SDF-1α, we hypothesized that 1) direct action of SDF-1α on differentiated endothelial cells and recruitment of BPCs contribute to SDF-1α-mediated angiogenesis in ischemic areas of both recipient and transplanted hearts and 2) homing of stem cells due to constant release of SDF-1α by lentiviral SDF-1α-transduced mesenchymal stem cells (MSCs) in addition to endogenous stimuli is augmented, resulting in greater influx of green fluorescent protein (GFP)-positive (GFP+) cells in donor and recipient hearts.
MATERIALS AND METHODS
Experiments using animals or materials were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996) and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Preparation of MSCs.
Male Spraque-Dawley rats (8 wk old) were euthanized, and BM cells were flushed and collected from tibias and femurs. After being washed, centrifuged cells were resuspended in normal culture medium to a final concentration of 5 × 105 viable cells/ml in a T75 flask. Normal culture medium consisted of DMEM (GIBCO-BRL) containing 10% (vol/vol) FBS and penicillin (100 U/ml)-streptomycin (100 μg/ml). The T75 flask was equilibrated in a humidified 5% CO2 incubator at 37°C for 72 h before being seeded with cells. Culture medium was changed every 3–4 days, and nonadherent cells were removed during the medium changes. Passage 2–4 MSCs were used in experiments.
Generation of the recombinant lentivirus vector and SDF-1α labeling of MSCs.
We generated the pLenti6/V5-SDF-1-internal ribosome entry site (IRES)-enhanced red fluorescent protein (ERFP) construct and lentiviral stock. In brief, the SDF-1-IRES-ERFP blunt-end PCR product was generated by PCR and cloned into the vector of pLenti6/V5-d-TOPO. Primers for the SDF-1-IRES-ERFP blunt end were synthesized with forward primer 5′-CACCATGGACGCCAAGGTCG-3′ and reverse primer 5′-TCAGGGCAAGGCGGAGCCGG-3′. The reaction was mixed gently and incubated for 5 min at room temperature. The TOPO cloning reaction was transformed into One Shot Stbl3 competent Esherichia coli. Lentiviral plasmid DNA was isolated using the S.N.A.P MidiPrep Kit, and the correct orientation of the sequence was confirmed. The lentiviral stock was produced by cotransfection of the plasmid mixture. This plasmid mixture and pLenti6/V5-SDF-1-IRES-ERFP constructs were cotransfected into 293FT cells. 293FT cells (5 × 106 cells) were plated in 10-cm tissue culture plates containing 10 ml of growth medium. Diluted DNA and Lipofectamine 2000 were mixed and incubated for 20 min at room temperature to form DNA-Lipofectamine 2000 complexes. Complexes were added to each plate of the cells. Cells were incubated for overnight at 37°C, and the medium containing the DNA-Lipofectamine 2000 complexes was removed and replaced by culture medium. Supernatants were centrifuged at 3,000 rpm for 15 min at 4°C to pellet debris. Viral supernatants were put into cryovials. The lentiviral stock was stored at −80°C until it was used. Lentiviral vector without SDF-1α (null) or with the SDF-1α gene was used. MSC cultures were initiated in six-well plates. MSCs were transduced overnight using dilutions of concentrated virus equivalent to 1 × 107 infectious units in non-FBS medium. MSCs transduced with the lentiviral vector null gene served as controls. The transfection efficiency of MSCs with the viral system was examined by flow cytometry.
Animal model and experimental design.
Two-month-old female Sprague-Dawley rats were myeloablated by cesium irradiation (600 cGy × 2 times, total: 1,200 cGy) followed by an intravenous injection of BPCs from donor male transgenic rats expressing GFP (GFP+ BPCs; 2 × 106 cells) as previously described (33). One month later, animals with BM chimeras were subjected to heterotopic cardiac transplantation with female rat hearts. Rats were assigned to one of two groups to receive an intramyocardial injection of either MSCs (2 × 106 cells) engineered to express the null gene (null group) or engineered to overexpress SDF-1α (SDF-1α group) into the peri-infarcted area of recipient hearts and into the LAD perfusion area of transplanted hearts (Fig. 1A). Immediately after abdominal heart transplantation, the recipient heart was exposed by limited left lateral thoracotomy. The LAD was permanently occluded using a 6-0 polyester suture in the recipient heart of both groups to trigger endogenous SDF-1α release into the surrounding tissue with spillover into the systemic circulation, as previously described (34). Injection of 5- bromo-2′-deoxyuridine (BrdU; 50 mg·kg−1·day−1 ip) was started at 2 wk after heart transplantation and continued for 7 days until animals were killed. After heart function measurements, recipient and transplanted hearts (n = 10 hearts/group) were harvested at 21 days for immunohistological and biochemical analyses. Additional animals (n = 7 animals/group) were used to study transplanted heart viability as assessed by palpable contractions. Additional animals (n = 3 animals/group) were used to study the time course of myocardial expression of SDF-1α in recipient and transplanted hearts with or without infarction of recipient hearts.
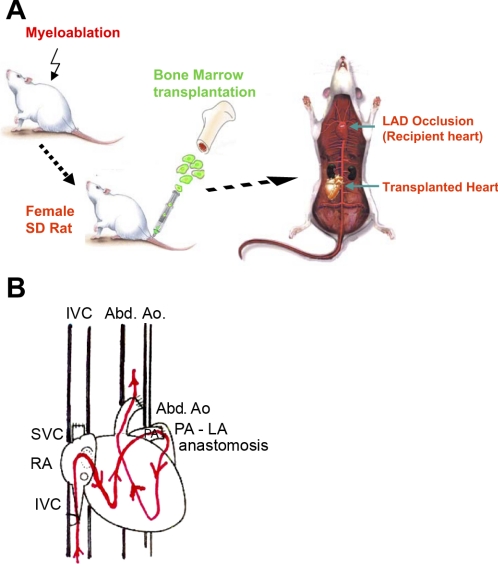
Fig. 1.
A and B: experimental design. A: female Sprague-Dawley (SD) rats were myoabalated by irradiation followed by the injection of bone marrow-derived progenitor cells (BPCs) from donor male transgenic rats expressing green fluorescent protein (GFP) via the tail vein. One month later, animals underwent a heterotrophic cardiac transplantation (working heart model) from female SD rats. Recipient hearts were then subject to permanent occlusion of the left anterior descending coronary artery (LAD) to produce regional myocardial infarction (MI). Both recipient and transplanted female hearts received either mesenchymal stem cells (MSCs) engineered to overexpressing stromal cell-derived factor (SDF)-1α or null gene. B: working heart model. In this volume-loaded heterotopic heart transplantation model, the right ventricle is filled (preload) from the recipient's inferior vena cava (IVC) via the superior vena cava (SVC) of the graft to the right atrium (RA). The left atrium (LA) is filled by the right ventricle with the same volume via the direct anastomosis of the pulmonary artery (PA) to the LA. The left ventricle (LV) ejects volume through the aorta of the graft into the abdominal aorta (Abd Ao) of the recipient, facing the afterload of the systemic circulation.
Heterotopic heart transplantation.
Procedures for the volume-loaded modified heterotopic heart transplantation were similar to those described previously for nonvolume-loaded heart transplantation (3, 34) with a small modification in the heart-harvesting procedure (Fig. 1B). The superior vena cava (SVC) was tied 5 mm cranial to the junction of the right atrium to permit anastomosis to the inferior vena cava. The suture line of the SVC ligature as well as that around the left lung hilus was 5 mm long to allow anastomosis of the pulmonary artery to the left atrium. This pulmonary artery-left atrium connection was performed in cardioplegic solution at 4°C. The heart was secured by fixation of the SVC and left lung hilus ligatures. Donor preparation was identical to that of the traditional transplantation model. The aorta was incised close to the distal clamp, an end-to-side anastomosis of the donor aorta to the recipient's abdominal aorta was begun, and the heart was inverted to complete the anastomosis. The donor's SVC was anastomosed to the recipient's inferior vena cava. The abdomen was then closed.
Real-time PCR.
The mRNA level of SDF-1α in the myocardium was examined by RT-PCR and real-time PCR. Total RNA was isolated using TRIzol reagent (Invitrogen) followed by DNAse treatment and purification using an RNeasy mini column kit (Qiagen). cDNA was synthesized using SuperScript III RNase H-Reverse Transcriptase (Invitrogen) in a 20-μl reaction mixture. An aliquot of cDNA was amplified using Taq DNA polymerase (2.5 units, Invitrogen) in the presence of sense and antisense primers (1 μM). The forward primer for SDF-1α was 5′-TTGCCAGCACAAAGACACTCC-3′ and the reverse primer was 3′-CTCCAAAGCAAACCGAATACAG-5′. Quantitative real-time PCR was carried out on the iQ5 real-time system (Bio-Rad) with iQ SYBR Supermix (Bio-Rad). The expression of each target mRNA relative to GAPDH under experimental and control conditions was calculated based on the threshold cycle (CT) as follows: r = 2{−Δ(ΔCT)}, where ΔCT = CT target − CT GAPDH and Δ(ΔCT) = ΔCT Experimental − ΔCT control.
Flow cytometric analyses.
On day 5 after the intramyocardial injection of MSCs with or without lentivirus-based SDF-1α gene expression, a peripheral blood sample was obtained from the tail vein for cytometric analysis. Briefly, peripheral blood cells were washed twice and incubated with specific antibodies added at saturating concentrations, including the following: granulocyte (Gr)-1 (Abcam), CD31 (Becton Dickinson), CXCR4 (Abcam), c-Kit (R&D Systems), and SDF-1 α (Abcam). Labeled cells were resuspended in staining medium containing propidium iodide in preparation for flow cytometry. Cells were analyzed and sorted by multiparameter flow cytometry on either a modified two-laser FACS Vantage (Becton Dickinson) or a modified three-laser cytometer (Cytomation and Becton Dickinson). Flow cytometry data were analyzed using FloJo software (Trestar).
Echocardiography.
We performed transthoracic echocardiography at 3 wk after MI using an iE33 Ultrasound System (Phillips) with a 15-MHz probe. Left ventricular (LV) parameters were obtained from two-dimensional images and M-mode interrogation in the long-axis view. The LV ejection fraction (EF) was calculated as follows: LV EF (%) = [(LVIDd)3 − (LVIDs)3]/(LVIDd)3 × 100, where LVIDd is the LV end-diastolic dimension and LVISd is the LV end-systolic dimension. In addition, fractional shortening (FS) was obtained by the following formula: FS (%) = (LVIDd − LVIDs)/LVIDd.
Histological examination of transplanted hearts.
Transplanted hearts were then fixed in 10% formalin at 3 wk after heart transplantation, processed for paraffin embedding, sectioned at 5 μm, stained with picrosirius red, and examined by polarized light microscopy. Heart minor axis cross sections were also stained with Masson's trichrome. Image of the LV area of each slide was prepared by an Olympus BX41 microscope with a charge-coupled device (MagnaFire, Olympus) camera. Fibrosis and the total LV area of each image were measured using Image-Pro Plus (Media Cybernetics, Carlsbad, CA), and the percentage of the fibrotic area was calculated as follows: (fibrotic area/total LV area) × 100.
Immunohistochemical analysis of myocardial tissue.
Immunohistochemical experiments were performed at 3 wk after heart transplantation on sections from transplanted hearts incubated with primary antibodies against CXCR4 (AnaSpec), c-Kit (Dako), Ki-67 (Novocastra Laboratory), BrdU (Roche Diagnostics), GFP (Santa Cruz Biotechnology), α-smooth muscle actin (SMA; Dako), and α-sarcomeric actin (Sigma). 4′,6-Diamino-2-phenylindole (DAPI; Sigma) was used to stain nuclei (32). Fluorescent in situ hybridization (FISH) analysis of heart tissue samples was carried out using the STAR× FISH Rat 12/Y Paints Protocol (CA-1631) as previously described (34). Fluorescent imaging was performed with an Olympus BX41 microscope (Olympus America) equipped with epiflouresence microscopy, and images were recorded using a digital camera with MagnaFire 2.1 software. Confocal images were obtained with a Leitz DMRBE fluorescence microscope equipped with TCS 4D confocal scanning attachments (Leica).
Picrosirius red and TUNEL staining of cardiac tissue.
To visualize collagen deposits, hearts were harvested on day 21 after transplantation, embedded in paraffin, sectioned, and then treated with picrosirius red staining.
To study the degree of apoptotic cell death, transplanted hearts (an additional n = 4 hearts/group), were harvested on day 4 after heart transplantation. The TUNEL assay was performed on deparaffinized tissue sections (5 μm thick) with the MEBSTAIN Apoptosis Kit II (Medical and Biological Laboratories) as previously described (31, 32). Sections were then stained with DAPI to visualize nuclei and photographed with an Olympus BX41 microscope (Olympus America) equipped with a digital camera.
Statistical analysis.
Data are expressed as means ± SE. Statistical analysis of differences was compared by ANOVA with Bonferroni's correction for multiple comparisons. P values of <0.05 were considered statistically significant.
RESULTS
SDF-1α increased early recruitment of BPCs into the peripheral blood circulation.
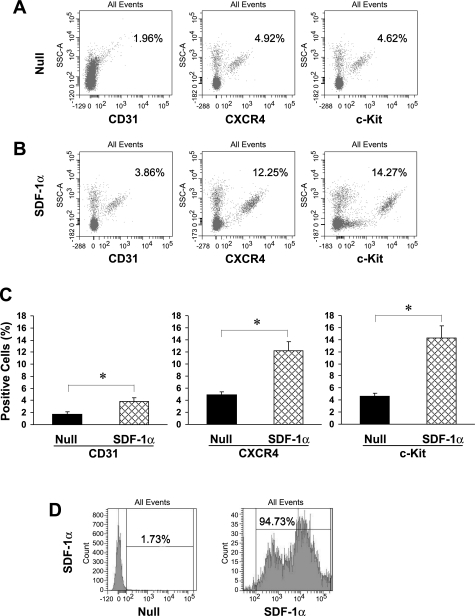
A substantial increase in recruitment of BPCs into the peripheral blood of the SDF-1α group was confirmed on day 5 by Gr-1 antibody-labeled specific myeloid differentiation antigen Ly-6G using flow cytometry. The fraction of granulocytes increased from 25.6% in the null group to 41.2% in the SDF-1α group (data not shown). CD31-, CXCR4-, and c-Kit-positive cells, detected in peripheral blood from the null group (Fig. 2A), were also significantly increased (P < 0.05) in peripheral blood of the SDF-1α group (Fig. 2B) on day 5 (Fig. 2C). The transfection efficiency of SDF-1α was confirmed as the fraction of MSCs expressing SDF-1α was 90-fold greater (94.73%) in the lentiviral SDF-1α group (Fig. 2D, right) than in the lentiviral null group for SDF-1α (1.73%; Fig. 2D, left).
Fig. 2.
Recruitment of SDF-1α-mobilized BMCs assayed by flow cytometry. Flow cytometry of peripheral blood samples showed that subpopulations of BPCs expressed higher levels of CD31 (3.86%), CXCR4 (12.25%), and c-Kit (14.27%) in the SDF-1α group (B) than the null group (A) for CD31 (1.96%), CXCR4 (4.92%), and c-Kit (4.62%), respectively. C: quantitative analysis of mobilized cells in peripheral blood. Data are means ± SE. *P < 0.05 vs. the null group. D: transfection efficiency of MSCs by flow cytometry in the null and SDF-1α group.
SDF-1α expression in both recipient and transplanted hearts.
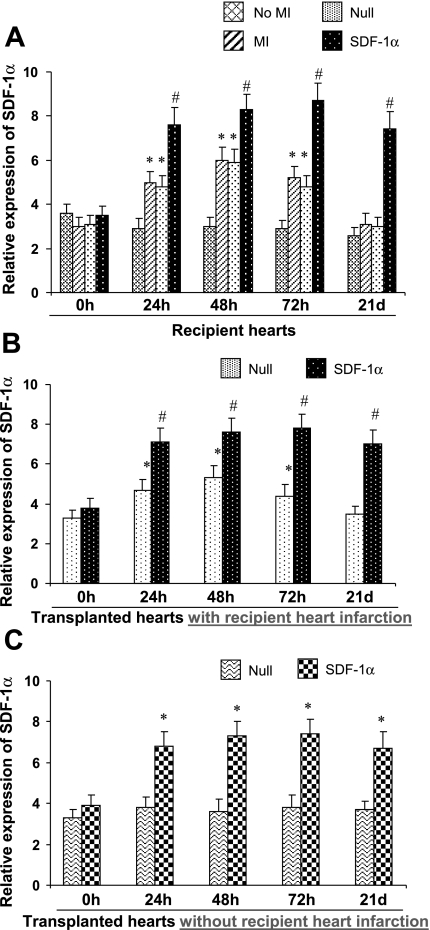
Real time-PCR was performed on LV tissues harvested up to 21 days from recipient and transplanted hearts to ascertain the role of upregulated expression of SDF-1α in BPC recruitment to the myocardium. SDF-1α mRNA levels were unchanged over 21 days in recipient ventricular myocardium samples from hearts with an intact coronary circulation and sex-matched sham control rats (n = 3 hearts/time point without irradiation or heterotopic heart transplantation; Fig. 3A). However, myocardial tissue expression of SDF-1α was significantly upregulated as early as 24 h after LAD occlusion, peaked at 48 h, subsided by 72 h, and returned to baseline at 3 wk. On day 21, there were no significant differences in SDF-1α between LAD-occluded recipient hearts and sham controls without LAD occlusion. In LAD-occluded recipient hearts receiving null MSCs, the temporal SDF-1α expression pattern was similar to that observed in recipient hearts with LAD occlusion only. In contrast, elevated SDF-1α mRNA levels were sustained in LV myocardial tissue samples for at least 3 wk in recipient hearts with LAD occlusion that received MSCs overexpressing SDF-1α (Fig. 3A).
Fig. 3.
Time course of myocardial expression of SDF-1α in the various treatment groups. MI in recipient hearts was produced by LAD ligation. A: recipient hearts; *P < 0.05 vs. no MI; #P < 0.01 vs. MI only. B: transplanted hearts with recipient heart infarction; *P < 0.05 vs. 0 h, #P < 0.01 vs. null group. C: transplanted hearts without recipient heart infarction; *P < 0.05 vs. null group. 0 h, hearts before various treatments. Data are means ± SE.
In a similar manner, the SDF-1α mRNA level in LVs of transplanted hearts that received MSCs overexpressing SDF-1α was significantly elevated starting on day 1 and continuing through day 21 (Fig. 3B). The SDF-1α mRNA temporal response in transplanted hearts that received null MSCs was identical to that observed in recipient hearts after LAD occlusion with or without injection of null MSCs (Fig. 3B).
In contrast to the group with LAD ligation of recipient hearts, the SDF-1α mRNA level in transplanted hearts that received null MSCs and no LAD ligation in recipient hearts was unchanged over 21 days, suggesting that infarction of the recipient heart influences the SDF-1α level of transplanted hearts. However, SDF-1α expression was increased over a period of 21 days in transplanted hearts that received MSCs overexpressing SDF-1α whether recipient hearts underwent infarction or not (Fig. 3, B and C).
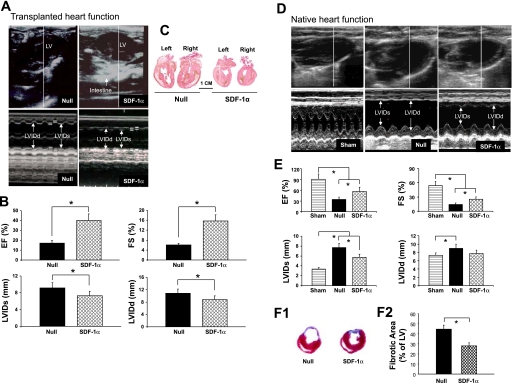
Effect of SDF-1α treatment on LV functional changes after MI and LV remodeling in transplanted and recipient hearts.
There was no significant difference in LV function and heart sizes between recipient and transplanted hearts after 1 wk (data not shown). After 3 wk, the LV was grossly dilated in the null group of transplanted hearts, as shown by the parasternal long-axis view (Fig. 4A, top left). SDF-1α treatment prevented the dilation of the heart (Fig. 4A, top right). Heart function was preserved in the SDF-1α group of transplanted hearts as assessed by EF and FS (40.1 ± 5.2% and 16.1 ± 3.8%, respectively) compared with the null group (18.6 ± 3.7% and 5.3 ± 0.8%, respectively; Fig. 4B). M-mode measurements of LVIDs and LVIDd in the null group (9.2 ± 0.7 mm and 10.8 ± 0.8 mm, respectively) and the SDF-1α group (7.6 ± 0.9 mm and 8.9 ± 0.6 mm, respectively) indicated gross dilatation in the null group of transplanted hearts. The structural preservation of the transplanted heart by SDF-1α treatment was further confirmed by sagittal sections of transplanted hearts (Fig. 4C).
Fig. 4.
Echocardiographic measurements and morphological changes in hearts after the various treatments. A: echocardiographic findings for the null group (top left and bottom left) and SDF-1α group (top right and bottom right) of transplanted hearts. LVIDd, LV end-diastolic dimension; LVIDs, LV end-systolic dimension. B: Quantitative data for ejection fraction (EF), fractional shortening (FS), LVIDs, and LVIDd in transplanted hearts. Data are means ± SE; n = 6 hearts/group. *P < 0.05 vs. the null group. C: gross morphological changes of transplanted hearts from the null group (left) and SDF-1α-treated group (right). D: representative two-dimensional and M-mode images for sham, null, and SDF-1α-treated recipient hearts. Sham animals underwent the surgical operation with loose suture around the LAD. E: quantitative data for EF, FS, LVIDs, and LVIDd in recipient hearts. Data are means ± SE; n = 6 hearts/group. *P < 0.05 vs. the null group. F: Masson's trichrome staining (1) and quantitave analysis (2) in recipient hearts. Hearts treated with the null gene served as controls. Data are means ± SE; n = 6 hearts/group. *P < 0.05 vs. the null group.
LV function of recipient hearts revealed significant improvement in the SDF-1α group compared with the null group (Fig. 4, D and E). There was significant improvement in EF and FS in the SDF-1α group (59.1 ± 6.7% and 28.6 ± 4.5%, respectively) compared with the null group (32.3 ± 4.7% and 14.6 ± 1.6%, respectively). M-mode measurements for LVIDs and LVIDd in the null group (7.6 ± 0.5 and 8.9 ± 0.5 mm, respectively) and the SDF-1α group (5.6 ± 0.6 and 7.8 ± 0.8 mm, respectively) revealed gross dilation in the null group of transplanted hearts at week 3, indicating that SDF-1α treatment prevented the deterioration of cardiac function in postinfarcted hearts.
A marked reduction in LV fibrosis was observed in the SDF-1α group at 21 days after LAD occlusion compared with the null group (Fig. 4F, 1). The percentage of the fibrotic area in SDF-1α-treated hearts was markedly reduced to 28.4 ± 2.7% compared with the null group (46.2 ± 5.6%, P < 0.05; Fig. 4F, 2).
Myocardial regeneration and immunohistochemical analysis of transplanted hearts.
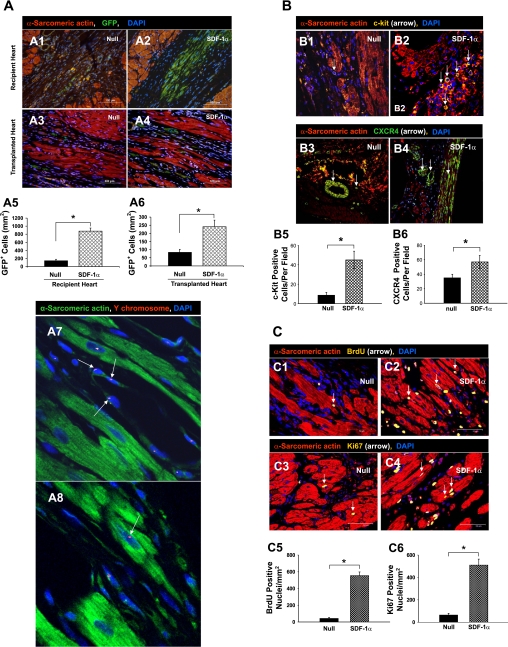
New myocytes were identified by enhanced GFP (green), which was unequivocal evidence for the origin of MSCs forming new myocytes. Compared with the null group of recipient hearts (Fig. 5A, 1) or transplanted hearts (Fig. 5A, 3), homing of GFP+ MSCs to the damaged cardiac area was increased in the SDF-1α group in both recipient (Fig. 5A, 2) and transplanted hearts (Fig. 5A, 4). The migration of MSCs was the highest in recipient hearts that received SDF-1α-treated MSCs (Fig. 5A, 2). Quantitative data demonstrated that the number of GFP+ cells was significantly higher in both SDF-1α-treated recipient and transplanted hearts compared with the null groups. However, cell migration was increased in the damaged area of recipient hearts (Fig. 5A, 5) more than in transplanted hearts (Fig. 5A, 6). Tissue sections clearly showed male cells with Y chromosome-positive nuclei (red fluorescence, arrows) interspersed in nuclei (blue fluorescence, stained with DAPI) of blood vessels (Fig. 5A, 7) or cardiac myocytes (Fig. 5A, 8) in SDF-1α-treated transplanted hearts.
Fig. 5.
A: representative immunofluorescent microscopic findings in hearts after the various treatments. 1–4, Migration of GFP-positive (GFP+) cells in infarcted recipient hearts (1 and 2) and transplanted hearts (3 and 4). Red, α-sarcometric actin; green, GFP; blue, 4′,6-diamino-2-phenylindole (DAPI). All nuclei were identified by DAPI staining. Scale bar = 100 μm. Magnification: ×200. 5 and 6, The average number of transplanted GFP+ cells per unit area (in mm2) was quantified in the peri-infarcted area of recipient hearts (5) and in transplanted hearts (6). Data are means ± SE; n = 6 hearts/group. *P < 0.05 vs. the null group. 7 and 8, Y chromosome-positive nuclei (red; arrow) in differentiated blood vessels (7) and myocytes (8) are shown for the SDF-1α group of transplanted hearts (data in other groups not shown). Green, α-sarcometric actin; blue, DAPI. Original magnification: ×630 (confocal imaging). B, 1–4: mobilization and distribution of c-Kit-positive (1 and 2) and CXCR4-positive (3 and 4) cells for the null group (1 and 3) and SDF-1α-treated group (2 and 4). Red, α-sarcomeric actin (for cardiomyocytes); yellow, c-Kit (arrows); blue, DAPI (for all nuclei). 5, Average number of c-Kit-positive cells per field. 6, Average number of CXCR4-positive cells per field after the various treatments. Data are means ± SE; n = 6 hearts/group. Magnification in B, 1–4: ×400. *P < 0.05 vs. the null group. C: myocardial regeneration and distribution of 5-bromo-2′-deoxyuridine (BrdU)- and Ki67-positive Cells. 1–4, BrdU-positive (1 and 2) and Ki67-positive (3 and 4) nuclei in the null group (1 and 3) and SDF-1α group (2 and 4) were found in transplanted hearts, suggesting regeneration of cardiac myocytes 21 days after recipient hearts underwent LAD ligation. Red, α-sarcometric actin; yellow, BrdU in 1 and 2 and Ki67 in 3 and 4; blue, DAPI. 5 and 6, Graphs showing numbers of BrdU-positive (5) and Ki67-positive (6) nuclei per unit area (in mm2) in donor heart tissue. Data are means ± SE; n = 6 hearts/group. *P < 0.05 vs. the null group. Scale bar = 50 μm. Magnification: ×400.
LV tissue sections were immunostained with primary and fluorochrome-conjugated secondary antibodies for the detection of c-Kit and CXCR4. Very few c-Kit positive cells were observed in the null group (Fig. 5B, 1). In contrast, a large number of c-Kit positive cells was mobilized into transplanted hearts in the SDF-1α group (45.9 ± 8.6 cells/field, P < 0.05; Fig. 5B, 2) compared with the null group (8.6 ± 2.1 cells/field; Fig. 5B, 5).
The number of cytokine receptor CXCR4-positive cells was increased in the SDF-1α group (Fig. 5B, 4) compared with the null group (Fig. 5B, 3). CXCR4 expression was predominantly observed in vascular endothelial cells in damaged hearts of the SDF-1α group (57.3 ± 9.4 cells/field) compared with the null group (34.7 ± 4.6 cells/field, P < 0.05; Fig. 5B, 6).
Cell proliferation, i.e., actively dividing cells, was determined with BrdU, which was detected immunohistochemically. The presence of BrdU- and Ki67-positive nuclei suggested regeneration of cardiac myocytes when assessed 21 days after heart transplantation. The proliferation of stem cells was determined with BrdU, which was incorporated into the DNA of dividing cells and was detected in their progeny (34). Additionally, Ki67, a nuclear protein expressed by cells in G1, S, G2, and M phases, provided a means to estimate the percentage of cells in cycle at the time of death (26). Ki67- and BrdU-positive nuclei were primarily observed in the damaged area of transplanted hearts (Fig. 5C, 1–4). Significant numbers of BrdU- and Ki67-positive nuclei were observed in the SDF-1α group (Fig. 5C, 2 for BrdU and Fig. 5C, 4 for Ki67) compared with the null group (Fig. 5C, 1 for BrdU and Fig. 5C, 3 for Ki67). Quantitative analysis confirmed significant increases in the numbers of BrdU- and Ki67-positive nuclei in the SDF-1α group (553.2 ± 24.6 and 509.7 ± 19.3 cells/mm2, respectively) compared with the null group (BrdU: 42.5 ± 6.1 cells/mm2, P < 0.05, Fig. 5C, 5; and Ki67: 63.2 ± 5.8 cells/mm2, P < 0.05, Fig. 5C, 6).
Blood vessel density in transplanted and recipient hearts.
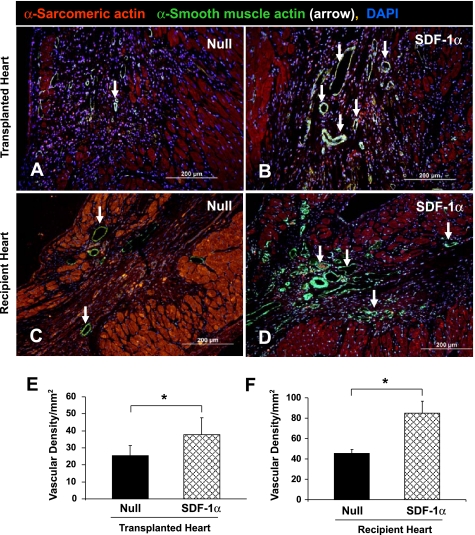
Vascular density, as indicated by labeling of SMA, a marker for vascular smooth muscle cells, was significantly higher in SDF-1α-treated transplanted hearts (Fig. 6B) compared with hearts in the null group (Fig. 6A of recipient heart). In contrast to transplanted hearts, blood vessel density was significantly increased in the SDF-1α group (Fig. 6D) compared with the null group (Fig. 6C). Vascular density, detected at low-power magnification with SMA immunostaining, was 38.2 ± 3.3 cells/mm2 in transplanted hearts and 88.6 ± 7.2 cells/mm2 in recipient hearts of the SDF-1α treated group, respectively. This was significantly higher (P < 0.05) than the null transplanted group (24.8 ± 1.9 cells/mm2; Fig. 6E) and recipient hearts (48.3 ± 3.6 cells/mm2; Fig. 6F).
Fig. 6.
Assessment of blood vessel density in transplanted (A and B) and recipient (C and D) hearts. Blood vessels were stained for α-smooth muscle actin (SMA) in transplanted hearts of the null group (A) and SDF-1α group (B) and in recipient hearts of the null group (C) and SDF-1α group (D). Red, α-sarcomeric actin; green, SMA (arrows); blue, DAPI. E and F: quantitative analysis of vascular density after the different treatments in transplanted (E) and recipient (F) hearts. Data are means ± SE; n = 6 hearts/group. *P < 0.05 vs. the null group. Original magnification: ×200.
Collagen deposition and apoptosis in the various treatment groups.
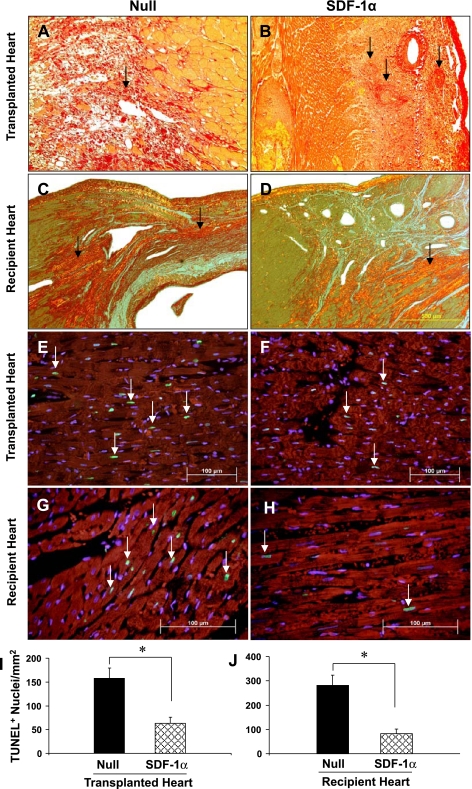
Photomicrographs of picrosirius red-stained tissue in the null group of transplanted hearts showed heavy collagen deposition. Large numbers of infiltrated mononuclear cells were observed in the null group in transplanted hearts (Fig. 7A). Despite observations of perivascular collagen deposits, concentric intimal thickening, narrowing of whole arterial allograft microvasculature (Fig. 7B, arrow), and LV interstitial fibrilla collagen in the SDF-1α treated group (Fig. 7B), the degree of vascular damage was much less in the SDF-1α treated group than in the null group. In contrast to transplanted hearts, the distribution of collagen deposition was mainly limited to infarcted or peri-infarcted areas. The degree of collagen deposition was obviously higher in the null group (Fig. 7C) compared with the SDF-1α group (Fig. 7D) in recipient hearts.
Fig. 7.
Fibrosis and TUNEL analysis in transplanted and recipient hearts. A–D: collagen deposits (dark red brick color) indicated by picrosirius red staining under polarized light microscopy at 3 wk in transplanted (A and B) and recipient (C and D) hearts. Yellow, viable myocardium. Magnification: ×200. E–H: TUNEL-positive nuclei in transplanted (E and F) and recipient (G and H) hearts. Large numbers of TUNEL-positive nuclei (green; white arrows) in transplanted (E) and recipient (G) hearts of the null control group were observed compared with SDF-1α-treated transplanted (F) and recipient (H) hearts. Red, α-sarcomeric actin (for cardiomyocytes); blue, DAPI (for all nuclei). Scale bar = 100 μm. Magnification: ×200. I and J: quantitative estimate of TUNEL-positive nuclei in transplanted (I) and recipient (J) hearts. Data are means ± SE; n = 6 hearts/group. *P < 0.05 vs. the null group.
Large numbers of myocytes underwent apoptosis in the null group (157.4 ± 15.2 positive nuclei/mm2; Fig. 7, E and I) compared with transplanted SDF-1α-treated hearts (61.6 ± 9.5 positive nuclei/mm2, P < 0.05; Fig. 7, F and I). After infarction of recipient SDF-1α-treated hearts (Fig. 7, G and H), positive nuclei were significantly reduced in the SDF-1α group (98.7 ± 8.1 positive nuclei/mm2; Fig. 7, H and J) of recipient hearts compared with the null group (287.4 ± 13.1 positive nuclei/mm2; Fig. 7G).
Survival of transplanted hearts.
The mean heart transplant survival time of 31.6 ± 9.2 days for the SDF-1α group (n = 7) was significantly longer (P < 0.027) than the 21.3 ± 5.4 days observed in the null group (n = 7; Table 1).
Table 1.
Effect of SDF-1α treatment on transplanted heart survival
| Group |
Graft Survival |
|
|---|---|---|
| Days | Mean ± SE, days | |
| Null | 11, 18, 21 22, 24, 26, 27 | 21.3±5.4 |
| SDF-1α | 14, 26, 31, 34, 36, 38, 42 | 31.6±9.2* |
n = 7 hearts/group in the null and stromal cell-derived factor (SDF)-1α groups.
P = 0.027.
DISCUSSION
The heterotopic abdominal heart transplantation rat model, first described by Ono and Lindsey (20), is widely used in cell therapy experiments (34). Perhaps the major drawback of the technique results from a lack of hemodynamic loading (11, 13) in the original nonworking heart transplantation model. This nonphysiological condition can result in rapid remodeling and atrophy at the organ and molecular level (11, 12). To overcome this limitation, we used a working transplantation heart model (17, 35) where volume loading and ejection occurred (Fig. 1).
SDF-1α is involved in stress-induced recruitment of BPCs to the damaged myocardium. SDF-1α is upregulated in the heart immediately after coronary artery occlusion (34). We (34) have previously demonstrated that myocardial ischemic injury triggered mobilization of cells into transplanted hearts and participated in the repair process. However, the expression and release of SDF-1α from a region of myocardial injury lasted for only a brief period of time. Our present results illustrate that peripheral cells are recruited and differentiate into cardiac and new blood vessels when SDF-1α expression (initiated either from endogenous tissue sites evoked by acute MI or sustained by supplemental exogenous lentivirus-mediated SDF-1α release) is maintained. Consequently, cell homing induced by sustained SDF-1α expression leads to substantially prolonged allograft survival. Thus, injection of SDF-1α-overexpressing MSCs in the ischemic myocardium provides a novel strategy whereby the recruitment of CXCR4-positive progenitor cells may be enhanced so as to facilitate repair of the injured myocardium. However, long-term overexpression of SDF-1α has been reported to cause chronic allograft deterioration associated with the development of transplant arteriosclerosis (23). Accordingly, further study on the duality of SDF-1α action is clearly needed.
The results from our present study demonstrate that local myocardial injection of SDF-1α-overexpressing MSCs increases the number of c-Kit-positive cells in injured areas of transplanted hearts (Fig. 5B). This is of potential importance because c-Kit-positive cells play a significant contributing role in cardiac repair and restoration of heart function after myocardial injury (4). Furthermore, it has been reported that c-Kit-positive but not c-Kit-negative BPCs produce high levels of VEGF, which promotes their differentiation into endothelial cells (10). The observation that c-Kit-deficient cardiac progenitor cells in mast cell-deficient mutant mice prevented cell differentiation during aging or after injury and that c-Kit-positive cells were also associated with enhanced angiogenesis highlights the importance of c-Kit-positive cells (30). In addition, significant expression of CXCR4 in damaged areas of SDF-1α-treated transplanted hearts provides important information on the potential role of SDF-1α/CXCR4 receptor interactions in vivo. Here, CXCR4 is strongly expressed in the heart endothelium, which is suggestive of a possible role for CXCR4 receptors in endothelial cell migration and repair. CXCR4 activation in some cell types results in Akt activation and stimulation of cell proliferation and survival (14). It is therefore conceivable that the SDF-1α/CXCR4 axis supported by our data plays a vital role in the reparative process of cardiac allograft.
SDF-1α possesses antiapoptotic activity and prevents γ-irradiation damage (7). In a recent study (25), Akt upregulated by SDF-1α resulted in the prevention of apoptosis. SDF-1α has also been demonstrated to attract endothelial progenitor cells to ischemic regions in a hindlimb injury model (2). Cells overexpressing SDF-1α display an increased capacity for cellular growth and protection against IL-4-induced apoptosis (14). We observed enhanced neovascularization in transplanted hearts in the SDF-1α treated group compared with the null group.
Several mechanisms have been proposed to contribute to the improved myocardial neovascularization. Development of cytokine-rich milieu in the injured myocardium after myocardial ischemia has been shown to stimulate the intrinsic release of cytokines in the injured heart with a special emphasis on the role of the CXCR4 and SDF-1α axis (15). Saxena et al. (25) reported that SDF-1α induces Akt phosphorylation and upregulates VEGF protein after coronary occlusion in vivo. Recently, Sasaki et al. (24) reported that SDF-1α promotes infarcted heart function via angiogenesis in mice model. The advantage of local production and release of chemotactic factors to drive stem cell recruitment to the injured myocardium is supported by a recent report (28) in which chemokines were overexpressed by gene transfer. Endothelial progenitor cells pretreated with SDF-1α before transplantation increased their proangiogenic potential, and SDF-1α augments the efficiency of cell therapy for ischemic vascular diseases (37). In addition, this model of working heart transplantation with SDF-1α treatment may also support recipient hearts in a similar manner to that of an intra-aortic balloon pump (29). An increase in the functionality of recipient hearts treated with SDF-1α may correlate with improved functionality of the transplanted hearts. Whether through regeneration or paracrine mechanisms (8), it appears that SDF-1α is important for myocardial protection.
In conclusion, supplementation of transient endogenous cytokine release during acute and chronic myocardial injury with sustained SDF-1α release from lentivirus-engineered MSCs injected intramyocardially enhances angiogenesis and cardiac myogenesis, reduces apoptosis, and actively participates in the reparative process of transplanted hearts. The sum of these effects leads to enhanced survival of transplanted hearts. This attenuation of remodeling occurs in both transplanted and recipient hearts as a consequence of the sustained in situ release of SDF-1α from MSCs overexpressing SDF-1α. Thus, massive homing of progenitor cells appears to play a key role in cardiac regeneration.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants HL-089824 and HL-081859 (to Y. Wang) and HL-080686 and HL-087246 (to M. Ashraf).
Acknowledgments
The authors thank Dr. Muhammad Rizwan Afzal for echocardiography and Christian Paul and Mahyar Pourriahi for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Almsherqi ZA, McLachlan CS, Slocinska MB, Sluse FE, Navet R, Kocherginsky N, Kostetski I, Shi DY, Liu SL, Mossop P, Deng Y. Reduced cardiac output is associated with decreased mitochondrial efficiency in the non-ischemic ventricular wall of the acute myocardial-infarcted dog. Cell Res 16: 297–305, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Asfour B, Hare JM, Kohl T, Baba HA, Kass DA, Chen K, Tjan TD, Hammel D, Weyand M, Hruban RH, Scheld HH, Byrne BJ. A simple new model of physiologically working heterotopic rat heart transplantation provides hemodynamic performance equivalent to that of an orthotopic heart. J Heart Lung Transplant 18: 927–936, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, Higuchi K, Siatskas C, Lee P, Lim H, Zhang J, Cukerman E, Stanford WL, Medin JA, Liu PP. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci USA 103:2304–2309, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr AN, Howard BW, Yang HT, Eby-Wilkens E, Loos P, Varbanov A, Qu A, DeMuth JP, Davis MG, Proia A, Terjung RL, Peters KG. Efficacy of systemic administration of SDF-1 in a model of vascular insufficiency: support for an endothelium-dependent mechanism. Cardiovasc Res 69: 925–935, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25: 2739–2749, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Herodin F, Bourin P, Mayol JF, Lataillade JJ, Drouet M. Short-term injection of antiapoptotic cytokine combinations soon after lethal gamma-irradiation promotes survival. Blood 101: 2609–2616, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell-derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation 116: 654–663, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James N, Smith M. Treatment of heart failure in children. Curr Pediatr 15: 539–548, 2005. [Google Scholar]
- 10.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 97: 3422–3427, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein I, Hong C, Schreiber SS. Cardiac atrophy in the heterotopically transplanted rat heart: in vitro protein synthesis. J Mol Cell Cardiol 22: 461–468, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Klein I, Ojamaa K, Samarel AM, Welikson R, Hong C. Hemodynamic regulation of myosin heavy chain gene expression. Studies in the transplanted rat heart. J Clin Invest 89: 68–73, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korecky B, Zak R, Schwartz K, Aschenbrenner V. Role of thyroid hormone in regulation of isomyosin composition, contractility, and size of heterotopically isotransplanted rat heart. Circ Res 60: 824–830, 1987. [DOI] [PubMed] [Google Scholar]
- 14.Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood 105: 3793–3801, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ildstad ST, Bolli R, Ratajczak MZ. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res 95: 1191–1199, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia 16: 1992–2003, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Maruyama T, Swartz MT, McBride LR, Pennington DG. Working heart model of heterotopic heart-lung transplantation in rats. J Thorac Cardiovasc Surg 107: 210–215, 1994. [PubMed] [Google Scholar]
- 18.Massad MG Current trends in heart transplantation. Cardiology 101: 79–92, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 12: 459–465, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg 57: 225–229, 1969. [PubMed] [Google Scholar]
- 21.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res 77: 134–142, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Rubio PA, Guinn GA. Myocardial infarction following carotid endarterectomy. Cardiovasc Dis 2: 402–404, 1975. [PMC free article] [PubMed] [Google Scholar]
- 23.Sakihama H, Masunaga T, Yamashita K, Hashimoto T, Inobe M, Todo S, Uede T. Stromal cell-derived factor-1 and CXCR4 interaction is critical for development of transplant arteriosclerosis. Circulation 110: 2924–2930, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki T, Fukazawa R, Ogawa S, Kanno S, Nitta T, Ochi M, Shimizu K. Stromal cell-derived factor-1alpha improves infarcted heart function through angiogenesis in mice. Pediatr Int 49: 966–971, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, DiMaio JM, Srivastava D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation 117: 2224–2231, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholzen T, Gerdes J. The ki-67 protein: from the known and the unknown. J Cell Physiol 182: 311–322, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz Y, Kornowski R. Autologous stem cells for functional myocardial repair. Heart Fail Rev 8: 237–245, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation 116: 1683–1692, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R, Dirschinger J, Kastrati A, Schömig A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 52: 1584–1588, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Wang CH, Anderson N, Li SH, Szmitko PE, Cherng WJ, Fedak PW, Fazel S, Li RK, Yau TM, Weisel RD, Stanford WL, Verma S. Stem cell factor deficiency is vasculoprotective: unraveling a new therapeutic potential of imatinib mesylate. Circ Res 99: 617–625, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Ahmad N, Wang B, Ashraf M. Chronic preconditioning: a novel approach for cardiac protection. Am J Physiol Heart Circ Physiol 292: H2300–H2305, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Ahmad N, Wani MA, Ashraf M. Hepatocyte growth factor prevents ventricular remodeling and dysfunction in mice via Akt pathway and angiogenesis. J Mol Cell Cardiol 37: 1041–1052, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Haider HK, Ahmad N, Xu M, Ge R, Ashraf M. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol 40: 736–745, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Haider HK, Ahmad N, Zhang D, Ashraf M. Evidence for ischemia induced host-derived bone marrow cell mobilization into cardiac allografts. J Mol Cell Cardiol 41: 478–487, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Yokoyama H, Ohmi M, Murata S, Nakame T, Tabayashi K, Mohre H. Proposal of a working left heart model with a heterotopic transplantation technique in rats. J Heart Lung Transplant 14: 706–712, 1995. [PubMed] [Google Scholar]
- 36.Zeltsman D, Acker MA. Surgical management of heart failure: an overview. Annu Rev Med 53: 383–391, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Zemani F, Silvestre JS, Fauvel-Lafeve F, Bruel A, Vilar J, Bieche I, Laurendeau I, Galy-Fauroux I, Fischer AM, Boisson-Vidal C. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol 28: 644–650, 2008. [DOI] [PubMed] [Google Scholar]