Abstract
Both static and dynamic exercise are known to increase cardiac pump function as well as arterial blood pressure. Feedforward control by central command and feedback control by the exercise pressor reflex are thought to be the neural mechanisms causing these effects during exercise. It remains unknown as to how each mechanism activates cardiac sympathetic nerve activity (CSNA) during exercise, especially at its onset. Thus we examined the response of CSNA to stimulation of the mesencephalic locomotor region (MLR, i.e., central command) and to static muscle contraction of the triceps surae muscles or stretch of the calcaneal tendon in decerebrate cats. We found that MLR stimulation immediately increased CSNA, which was followed by a gradual increase in heart rate, mean arterial pressure, and ventral root activity in a stimulus intensity-dependent manner. The latency of the increase in CSNA from the onset of MLR stimulation ranged from 67 to 387 ms. Both static contraction and tendon stretch also rapidly increased CSNA. Their latency from the development of tension in response to ventral root stimulation ranged from 78 to 670 ms. These findings suggest that both central command and the muscle mechanoreflex play a role in controlling cardiac sympathetic outflow at the onset of exercise.
Keywords: mechanoreflex, renal sympathetic nerve activity, group III and IV afferents
both static and dynamic exercise are known to increase cardiac pump function as well as arterial blood pressure. Feedforward control by central command originating from the posterior hypothalamus and midbrain and feedback control by the exercise pressor reflex (23) are thought to be important neural mechanisms causing these exercise-induced effects. Central command is thought to be capable of rapidly stimulating cardiovascular function at the onset of exercise (22). For example, sympathetic outflow to the heart (33) and kidneys (3, 7, 17, 26) increased rapidly and was followed by an increase in blood pressure and heart rate (HR) at the onset of spontaneous locomotion or forced treadmill exercise in conscious or decerebrated animals.
Feedback control by the exercise pressor reflex, especially by the component arising from mechanoreceptors, is also capable of contributing to the rapid cardiovascular adaptation at the onset of exercise (13). In fact, the static contraction of the triceps surae muscles evoked a rapid sympathetic discharge to the heart, kidneys, and adrenal glands in anesthetized or decerebrated animals (15, 19, 20, 35, 38). The average latencies of the increase in cardiac (CSNA) or renal (RSNA) sympathetic nerve activities from the onset of static contraction were 1.8 and 0.8 s, respectively, in chloralose-anesthetized cats (19, 35). Also, static contraction increased RSNA with the latency of 0.4 s in midcollicular decerebrated unanesthetized rats (15). Although the latencies of these sympathetic responses at the onset of contraction varied among different studies, the short latencies suggest that a mechanoreflex arising from muscles plays a role in the initial cardiovascular response to exercise. However, the mechanisms that activate CSNA during exercise, especially at its onset, are not clear.
The purpose of this study was to clarify the roles played by central command and the exercise pressor reflex in activating sympathetic nerve activity during exercise. To eliminate the confounding factors such as anesthetic drugs, intact or decerebrated brain condition, and the differences of animal species, we examined the effect of central command and the muscle reflex on the sympathetic efferent activity in the same decerebrated unanesthetized cats. Because it has been reported that the different level of decerebration affects the exercise pressor reflex (12), we also examined the effect of the static contraction on sympathetic efferent activity in both precollicular and midcollicular preparations.
METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University, Hershey Medical Center.
Surgical Preparation
Adult cats (n = 22, 3.0 ± 0.1 kg) were anesthetized with a mixture of 5% isoflurane and oxygen. The right jugular vein and common carotid artery were cannulated for the delivery of drugs and fluids and the measurement of arterial blood pressure, respectively. The carotid arterial catheter was connected to a pressure transducer (model P23 XL, Statham). HR was calculated beat to beat from the arterial pressure pulse (Gould Biotach). The trachea was cannulated, and the lungs were ventilated mechanically (Harvard Apparatus). Arterial blood gases and pH were measured by an automated blood-gas analyzer (model ABL-700, Radiometer). Pco2 and arterial pH were maintained within a normal range by adjusting either the ventilation or intravenous administration of sodium bicarbonate (8.5%). A rectal temperature probe was inserted, and the core body temperature of the animal was maintained at 37–38°C by a water-perfused heating pad and a lamp. The left triceps surae muscles, left popliteal artery, and tibial nerve were isolated. We sectioned all visible nerves supplying the left hindlimb, except the tibial nerve, which supplies the triceps surae muscles.
The cat was placed in a Kopf stereotaxic frame. In 6 of the 22 cats, a precollicular decerebration was performed; in the remaining 16 cats, a midcollicular decerebration was performed. All neural tissue rostral to the section was removed, bleeding was controlled, and the cranial vault was filled with agar. Dexamethasone (4 mg) was injected intravenously just before the decerebration procedure to minimize brain edema. The left common carotid artery was tied off to reduce bleeding. The left renal nerve was exposed using a retroperitoneal approach. A renal nerve bundle was carefully isolated from the renal plexus and surrounding connective tissue near the renal artery and vein. The nerve was cut and its central end was draped over a pair of silver wire electrodes (bare diameter, 76 μm) insulated with Teflon (A-M Systems) for recording RSNA. The nerve-electrode complex was then covered with a mixture of silicone gel (Kwik Sil, World Precision Instruments). The electrode wire for recording RSNA was placed outside of the incision site, and the abdominal wound was closed.
The third or fourth intercostal space was opened while the cat was placed in a lateral posture. A branch of the inferior cardiac nerve that innervated the left side of the heart was carefully isolated from the surrounding connective tissue near the aortic arch. The left inferior cardiac nerve contained no vagal nerve component (25). The nerve was cut and the nerve-electrode complex was covered with a mixture of silicone gel. The electrode wire for recording CSNA was placed outside the incision site, and the chest wound was then closed under positive end-expiratory pressure to avoid pneumothorax.
The recording electrodes were attached in series with a high-impedance probe (model HIP 511, Grass) and then amplified (model P511, Grass). Both amplified CSNA and RSNA were displayed on a storage oscilloscope (Hewlett-Packard) and made audible. The amplifiers were filtered between 30 Hz and 3 kHz. The cardiac and renal postganglionic sympathetic activities were confirmed by an intravenous injection of hexamethonium bromide (20 mg/kg) at the end of data collection.
The lower lumbar and sacral portions of the spinal cord were exposed, after which the cat was placed in the Kopf spinal unit. The dura of the cord was cut and reflected, allowing a visual identification of the spinal roots. Both the left and the right L7 and S1 ventral roots were identified and cut. Using the skin on the back, we formed a pool that was filled with warm (37°C) mineral oil. Once the surgeries were completed, the anesthesia was withdrawn, and the lungs were ventilated with room air. The calcaneal bone was severed, and its tendon was attached to a force transducer (model FT 10, Grass), which in turn was attached to a rack and pinion. The knee joint was secured to a post. The tendon was stretched so that baseline tension was set between 0.5 and 1.0 kg.
Experimental Protocols
To examine the effect of static contraction and central command on sympathetic outflows and cardiovascular responses, three stimuli were assessed: 1) the static contraction of the left triceps surae muscles; 2) the stretch of the left calcaneal (Achilles) tendon, which, in turn, stretched the triceps surae muscles; and 3) the electrical stimulation of the mesencephalic locomotor region (MLR). Static contraction is a combined mechanical and metabolic stimulus, tendon stretch is solely a mechanical stimulus, and stimulation of MLR is used as a central command stimulus.
Protocol 1: comparison between the effects of static contraction and central command on CSNA.
In six precollicular decerebrated cats, we examined in the same cats the effects of both static contraction and central command on CSNA. Static contraction was evoked by electrically stimulating (40 Hz, 0.1 ms, <2 times motor threshold) the cut peripheral ends of the left L7 and S1 ventral roots. The stimulation of the peripheral cut ends of the left ventral roots was performed with three current intensities. After the cats were paralyzed with vecuronium bromide (0.1 mg/kg iv), a muscle mechanoreceptor reflex (9) was evoked by stretching the calcaneal tendon by manually turning the rack and pinion, which was placed in series with the force transducer. Baseline tension was set between 0.5 and 1.0 kg. Both muscle contraction and tendon stretch lasted for 60 s.
The central command was evoked by the electrical stimulation of the MLR. The tip of a monopolar stainless steel electrode (model SNEX-300; Rhodes) was positioned 4 mm lateral to the midline and 1 mm caudal to the anterior margin of the inferior colliculi. The electrode was lowered to a depth of 2 mm below the surface of one inferior colliculus, and current was then passed through the electrode while advancing it in 0.5-mm increments from 2 to 10 mm below the collicular surface. The electrode was considered to be optimally placed when low-intensity (10–70 μA) stimulation increased arterial blood pressure, HR, and ventral root activity while the cat was paralyzed. The criterion for identifying the MLR in the decerebrate paralyzed cat closely parallels that described by Grillner and Shik (6). After the cardiovascular variables had become stable, monophasic electrical pulses (40 Hz, 0.5 ms, 10–126 μA) were delivered for 60 s to the MLR. Stimulation of the MLR was performed at three current intensities, which averaged 44 ± 9, 62 ± 13, and 83 ± 17 μA. Ventral root activity was recorded as an index of central command output. The cut central ends of either the right L7 or S1 ventral roots were separated into small bundles, and one of their small bundles was draped over a pair of silver wire electrodes.
Protocol 2: control of cardiac and renal sympathetic outflows by the exercise pressor reflex.
In 16 midcollicular decerebrated cats, we determined whether the responses of CSNA to static contraction were different than those in precollicular decerebrated cats. In this protocol, we simultaneously recorded both CSNA and RSNA to examine the differences of the effect of static contraction on the cardiac and renal sympathetic outflows.
Data Analysis.
CSNA, RSNA, blood pressure, HR, muscle tension, and ventral root activity were recorded with a Spike 2 data acquisition system (CED, Cambridge) and stored on a computer hard drive (Dell). Mean arterial pressure (MAP) is expressed in millimeters of mercury, HR in beats per minute, and sympathetic nerve activities as percentage of baseline. Recordings of CSNA and RSNA were rectified and integrated (Spike 2), and the initial 60-s values were used to compare the differences between baseline and the response to each maneuver. The integrated voltage found after hexamethonium injection was subtracted from the integrated CSNA and RSNA values. In addition, the time course of the CSNA, RSNA, and MAP responses to static muscle contraction, tendon stretch, and MLR stimulation were analyzed at 2, 5, 10, 20, 40, and 60 s following the onset of each maneuver. The tension-time index (TTI; in kg·s) was calculated by integrating the area between the tension trace and the baseline level (Spike 2). The onset latency of the sympathetic nerve response to either MLR stimulation or static contraction was determined by replaying an expanded version of the Spike 2 record.
All values are expressed as means ± SE. Two-way repeated-measure ANOVA followed by Tukey post hoc tests were used to determine statistical significance, except in the case of TTIs, in which case one-way repeated-measure ANOVA were used to determine significance. We also used the Bonferroni post hoc tests to determine the significant differences between time-course means. The criterion for statistical significance was set at P < 0.05.
RESULTS
Comparison between the Effects of Static Contraction and Central Command on CSNA: Protocol 1
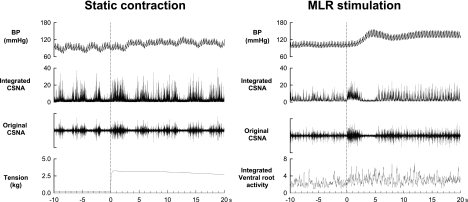
In each of the six cats that were decerebrated at the precollicular level, the CSNA, MAP, and HR increased during both static contraction and MLR stimulation (Figs. 1 and 2). Static contraction immediately increased CSNA, followed by increases in blood pressure and HR. These increases in CSNA, blood pressure, and HR were abolished by cutting the L4-S1 dorsal roots (data not shown). Electrical stimulation of MLR also immediately increased CSNA and was followed rapidly by increases in contralateral ventral root activity, arterial blood pressure, and HR (Figs. 1 and 2).
Fig. 1.
Examples of the responses of arterial blood pressure (BP), integrated and original cardiac sympathetic nerve activity (CSNA), and muscle tension or integrated ventral root activity during static contraction or mesencephalic locomotor region (MLR) stimulation in 1 cat decerebrated at the precollicular level. Both contraction and MLR stimulation rapidly activated CSNA. Both maneuvers started at time 0; their onsets are signaled by the dotted vertical lines.
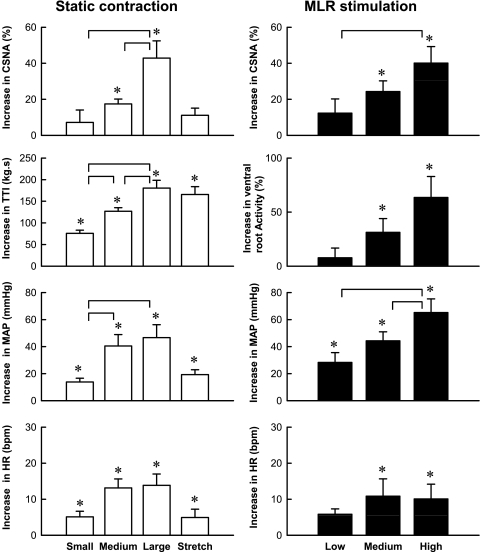
Fig. 2.
Mean responses of CSNA, tension time index (TTI), ventral root activity, mean arterial pressure (MAP), and heart rate [HR, in beats/min (bpm)] over 60 s of static contraction (left) or MLR stimulation (right) in 6 cats decerebrated at the precollicular level. *P < 0.05, increase is significantly greater than its corresponding baseline. Horizontal brackets signify that the increases are significantly different from each other (P < 0.05).
Static contraction induced by ventral root stimulation increased CSNA, TTI, MAP, and HR in a manner that was directly dependent on the stimulus intensity. In contrast, passive stretch of the triceps surae muscles did not significantly increase CSNA, even though the TTI was similar to that of the TTI of the large static contraction. As expected, stretch did significantly increase MAP and HR (Fig. 2). When the cats were paralyzed, the ventral root stimulation did not increase any of the variables measured (n = 6; data not shown). During each of the three levels of static contraction, CSNA rapidly increased and peaked at 2 s after the onset of tension development. The peak CSNA response to contraction increased as the TTI increased (Fig. 3). For each of the three levels of contraction tested, CSNA decreased from its peak levels after about 5 s of tension development.
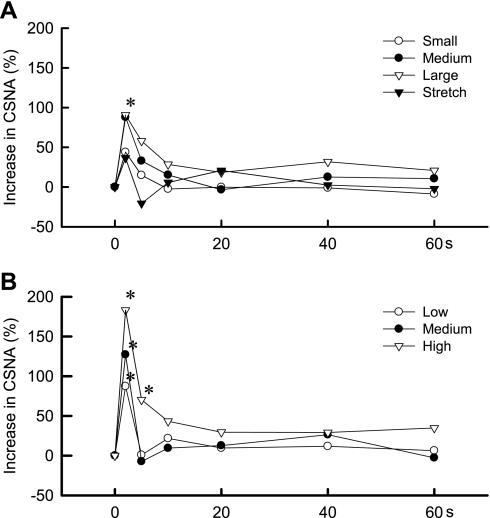
Fig. 3.
Time courses of the average changes in CSNA during static contraction (A) and MLR stimulation (B) in 6 cats decerebrated at the precollicular level. Both static contraction and MLR stimulation increased CSNA within 2 s from the onset of each maneuver. *P < 0.05, increase is significantly greater than baseline. Both static contraction and MLR stimulation lasted for 60 s and started at time 0.
MLR stimulation increased CSNA, ventral root activity, MAP, and HR in a current-dependent manner. CSNA immediately increased and peaked at 2 s after the onset of MLR stimulation. The peak responses in CSNA were also dependent on the stimulation intensities. The peak values were not sustained during the 60 s of MLR stimulation. Hexamethonium, injected intravenously (20 mg/kg), abolished baseline CSNA, and subsequent MLR stimulation had no effect on any of the variables measured.
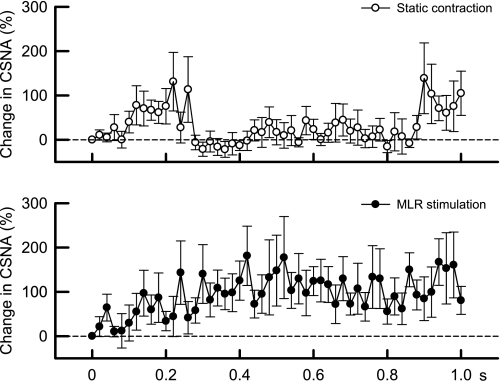
We plotted the initial increases in CSNA over 1 s for both static contraction and MLR stimulation for the six cats that were decerebrated at the precollicular level (Fig. 4). In general, the onset latencies of the CSNA responses to either static contraction or MLR stimulation were well within 0.5 s. Specifically, the latency to the onset of the CSNA response to static contraction was shortest for the highest TTI and longest for the lowest TTI (Fig. 5), whereas the onset of the CSNA response to MLR stimulation was not dependent on the current intensity applied to this midbrain area. The increase in CSNA induced by static contraction was transient and was followed by a return to baseline levels until about 0.9 s when it increased again and remained elevated during static contraction. MLR stimulation increased CSNA within 0.2 s (Fig. 4), but unlike static contraction, CSNA remained elevated for the entire first second of the maneuver.
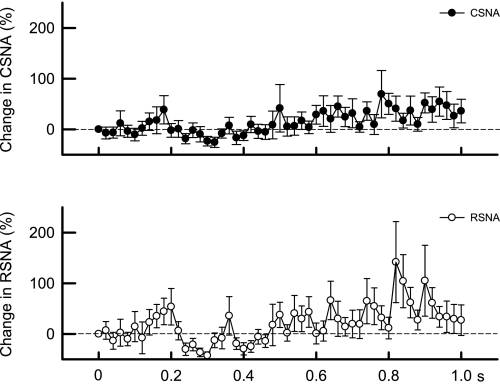
Fig. 4.
Time courses of the average changes in CSNA during the first second of static contraction (○) and MLR stimulation (•) in 6 cats decerebrated at the precollicular level. CSNA began to increase around 0.1 s in both maneuvers. Thereafter, CSNA remained elevated during MLR stimulation, whereas CSNA returned to baseline levels until ∼0.9 s after the onset of contraction when it started to increase again. The sample size at each point was 6.
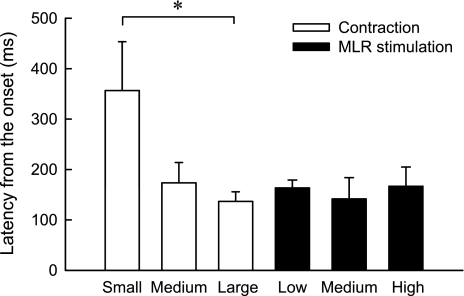
Fig. 5.
Mean CSNA onset latencies evoked by static contraction and MLR stimulation in 6 cats decerebrated at the precollicular level. *P < 0.05, significant difference between the onset latency of the small contraction and the onset latency of the large contraction. No other pairwise comparisons were significant.
Differential Responses of CSNA and RSNA during the Muscle Reflex: Protocol 2
In 16 cats decerebrated at the midcollicular level, static contraction increased CSNA, RSNA, MAP, and HR (Fig. 6). During the large contraction, both RSNA and CSNA increased significantly above their respective baseline levels, but these increases did not differ significantly from each other (P = 0.15). Likewise, during tendon stretch, the increases in CSNA and RSNA did not differ from each other (P > 0.84). When the cats were paralyzed, ventral root stimulation no longer increased arterial pressure, HR (data not shown), and RSNA and CSNA (Fig. 7).
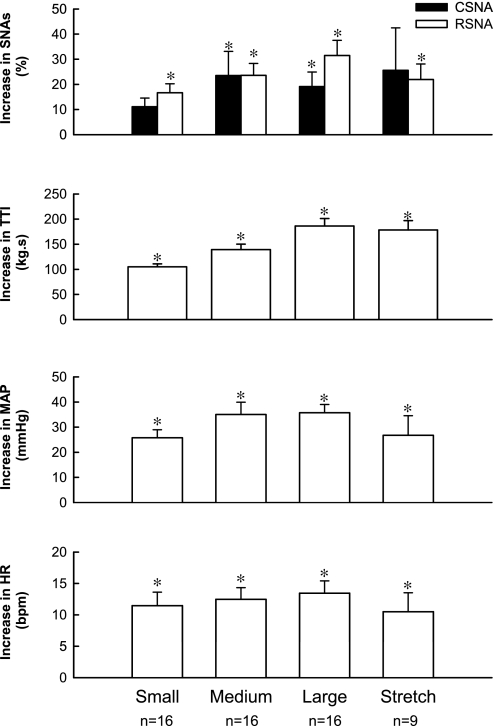
Fig. 6.
Mean peak increases in CSNA, renal sympathetic nerve activity (RSNA), MAP, and HR evoked by 60 s of static contraction and tendon stretch in cats decerebrated at the midcollicular level. *P < 0.05, increase is significantly greater than its corresponding baseline; n, number of data points in each group. SNAs, sympathetic nerve activities.
Fig. 7.
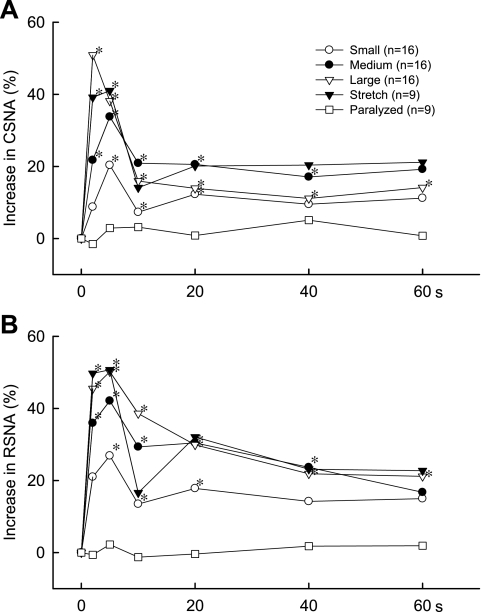
Time courses of the mean changes in CSNA (A) and RSNA (B) evoked by static contraction for 60 s in cats decerebrated at the midcollicular level. *P < 0.05, increase over baseline level; n, number of cats/group.
During either static contraction or tendon stretch, both CSNA and RSNA increased rapidly, reaching their peak values within 5 s of the onset of each maneuver (Fig. 7). This was the case for each of the three different TTIs developed during contraction. After reaching the peak well within 5 s (Figs. 8 and 9), both CSNA and RSNA decreased and then leveled off above baseline levels for the remainder of the contraction or stretch (Fig. 7). In the midcollicular preparation, the onset latencies of both the cardiac and renal sympathetic nerve responses to static contraction were difficult to discern and appeared to consist of a gradual increase in whole nerve discharge. This gradual increase contrasted markedly with the sudden, and easy to discern, onset of cardiac sympathetic nerve response to static contraction in the precollicular cats (Fig. 1). Overall, the onset latency of the CSNA response to the large static contraction in the precollicular preparation averaged 137 ± 19 ms (n = 6), whereas it averaged 733 ± 65 ms (n = 16; P < 0.0001) for the CSNA response to the large static contraction in the midcollicular preparation.
Fig. 8.
Time courses of the mean changes in CSNA (•) and RSNA (○) during the first second of the large static contraction in cats decerebrated at the midcollicular level. Note that the rapid increase in SNA observed in precollicular decerebrated cats at the precollicular level was eliminated in cats decerebrated at the midcollicular level.
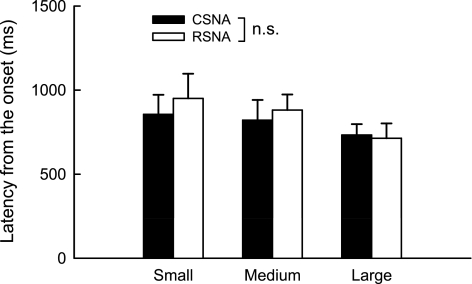
Fig. 9.
Mean onset latencies of CSNA and RSNA evoked by static contraction in 16 cats decerebrated at the midcollicular level. Horizontal bracket signifies that the difference in onset latency between CSNA and RSNA in the small contraction was greater than the difference in onset latency between CSNA and RSNA in the large contraction. There was no significant (NS) difference between CSNA and RSNA at any level of contraction.
DISCUSSION
The major new finding of our experiments is that central command, evoked by the electrical stimulation of the MLR, increased CSNA in paralyzed cats that were decerebrated at the precollicular level. We also found that static contraction of the triceps surae muscles, evoked by an electrical stimulation of the cut peripheral ends of the ventral roots, increased CSNA in these cats, a finding which agrees with that reported previously by Matsukawa et al. (19). Moreover, the magnitude of the increases in CSNA evoked by either MLR stimulation or by static contraction was dependent on the intensity of the stimulation.
The onset latency of the CSNA response to static contraction, regardless of whether the preparation was decerebrated at the midcollicular or precollicular level, was always <1 s and, as a consequence, is attributable to the stimulation of mechanoreceptors in contracting skeletal muscle. The mechanoreceptors in skeletal muscle responsible for causing the reflex autonomic responses to contraction are innervated usually by thinly myelinated afferent fibers belonging to the group III category (8, 13, 21). Some of these afferents have been found in cats to respond within 100 ms after the onset of static contraction, and a few have been found to respond within 30 ms (13). Thus it seems quite reasonable to speculate that these mechanoreceptors are responsible for the rapid activation of CSNA within 150 ms during static contraction in the precollicular cat.
The CSNA response to static contraction appeared to depend partly on the level of decerebration performed in our experiments. Specifically, the magnitude of the responses was larger in the precollicular cats than in the midcollicular cats. Moreover, the onset latencies were shorter in the precollicular cats than in the midcollicular ones. Many neural structures and pathways may be responsible for these differences in the CSNA responses to contraction. Nevertheless, the one that comes to mind is the pedunculopontine nucleus (27) of the midbrain, which is very close to the plane of section when the cat is decerebrated at the midcollicular level. We speculate that the pedunculopontine nucleus, which has been suggested to be the anatomical substrate for the MLR (4, 5, 27), was functional in the precollicular cats but was either removed or damaged in the midcollicular cats.
An important limitation of our findings concerns the function of the sympathetic nerves from which we recorded impulse activity. Specifically, we do not know whether the increases in cardiac sympathetic nerve discharge evoked by either MLR stimulation or static contraction were causing either an increase in cardiac rate and contractility or coronary vasoconstriction (1, 24). Likewise, we do not know whether the increases in renal sympathetic nerve discharge evoked by either MLR stimulation or by static contraction were causing renin release, sodium reabsorption, or renal vasoconstriction (2).
Using stretch to evoke the muscle mechanoreflex in anesthetized cats (29, 32), Matsukawa et al. (19) concluded that the reflex increased the cardiac sympathetic outflow more than it increased the renal sympathetic outflow. Their conclusion was based on recordings of cardiac and renal sympathetic activity and was performed in different cats (18, 20). In contrast, we found that when cardiac and renal sympathetic activity were recorded in the same unanesthetized cat, which was decerebrated at the midcollicular level, that stretch of the triceps surae muscles and calcaneal tendon increased both sympathetic outflows almost equally. Perhaps one explanation for this difference is that we used smaller stretches than did Matsukawa et al. (19). Alternatively, our use of a decerebrated unanesthetized preparation may have resulted in different central neural processing of muscle and tendon stretch information than that which occurred in the chloralose urethane-anesthetized preparation used by Matsukawa et al. (19).
Regardless of the cause of this difference, stretch stimulates a different population of group III mechanoreceptors in decerebrate cats than does static contraction (9). For example, of 14 group-III afferents stimulated by tendon stretch, only seven were stimulated by static contraction (9). Consequently, we believe that it is not appropriate to use stretch to assess the mechanoreceptor component of the exercise pressor reflex. We believe, instead, that this assessment is better served by quantifying the sympathetic response to the first 2 s of static contraction. During this period of time, group-III afferents often discharge a burst of impulses, whereas a few group-IV afferents discharge at best a single impulse or two (13, 14), the latter suggesting that a few of these unmyelinated afferents have some minimal sensitivity to mechanical stimuli.
During exercise the sympathetic outflow to the vascular beds supplying skeletal muscle and skin appears to be regulated differently from the sympathetic outflow supplying vascular beds in the viscera. For example, the sympathetic outflow to skeletal muscle appears to be mostly under the control of the exercise pressor reflex (10, 34, 36); central command appears to play a role in activating this outflow only when the muscles are contracting at 75% of maximum voluntary force (37). In contrast, the sympathetic outflow to the skin appears to be mostly under the control of central command (11, 16, 30, 39), with the exercise pressor reflex playing a small role in activating this outflow (31). In contrast, the sympathetic outflow to visceral organs, such as the kidneys and the heart, appears to be controlled by both central command and the exercise pressor reflex (19, 20, 35).
The increase in HR at the onset of exercise is commonly believed to be caused by a withdrawal of vagal tone to the heart (28, 29). This concept was challenged by Tsuchimochi et al. (33), who recorded CSNA and HR in conscious cats exercising on a treadmill. They found that CSNA increased about 3 s after the onset of exercise and preceded the increase in HR by about 4 s. These findings suggest that the sympathetic supply to the heart can cause at least some of the initial cardiac response to exercise. Our findings are consistent with this suggestion because they demonstrate that either central command, induced by MLR stimulation, or the exercise pressor reflex, induced by static contraction, are capable of increasing cardiac sympathetic activity well within a half a second of their onset. In conclusion, our results suggest that both central command and muscle mechanoreflex play a pivotal role in controlling cardiac pumping functions via the activation of cardiac sympathetic outflow at the onset of exercise.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-051503.
Acknowledgments
We thank Jennifer Probst for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aung-Din R, Mitchell JH, Longhurst JC. Reflex α-adrenergic coronary vasoconstriction during hindlimb static exercise in dogs. Circ Res 48: 502–509, 1981. [DOI] [PubMed] [Google Scholar]
- 2.DiBona GF Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am J Physiol Regul Integr Comp Physiol 279: R1517–R1524, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol 59: 313–337, 1985. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Rill E, Houser CR, Skinner RD, Smith W, Woodward DJ. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res Bull 18: 731–738, 1987. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Rill E, Kinjo N, Atsuta Y, Ishikawa Y, Webber M, Skinner RD. Posterior midbrain-induced locomotion. Brain Res Bull 24: 499–508, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Grillner S, Shik ML. On the descending control of the lumbosacral spinal cord from the “mesencephalic locomotor region”. Acta Physiol Scand 87: 320–333, 1973. [DOI] [PubMed] [Google Scholar]
- 7.Hajduczok G, Hade JS, Mark AL, Williams JL, Felder RB. Central command increases sympathetic nerve activity during spontaneous locomotion in cats. Circ Res 69: 66–75, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Circ Physiol 280: H2153–H2161, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol 99: 1891–1896, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Hill JM, Adreani CM, Kaufman MP. Muscle reflex stimulates sympathetic postganglionic efferents innervating triceps surae muscles of cats. Am J Physiol Heart Circ Physiol 271: H38–H43, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Hill JM, Kaufman MP. Central command, but not muscle reflex, stimulated cutaneous sympathetic efferents of cats. Am J Physiol Heart Circ Physiol 274: H1552–H1559, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Iwamoto GA, Waldrop TG, Kaufman MP, Botterman BR, Rybicki KJ, Mitchell JH. Pressor reflex evoked by muscular contraction: contributions by neuraxis levels. J Appl Physiol 59: 459–467, 1985. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- 14.Kindig AE, Hayes SG, Hanna RL, Kaufman MP. P2 antagonist PPADS attenuates responses of thin fiber afferents to static contraction and tendon stretch. Am J Physiol Heart Circ Physiol 290: H1214–H1219, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Koba S, Xing J, Sinoway LI, Li J. Differential sympathetic outflow elicited by active muscle in rats. Am J Physiol Heart Circ Physiol 293: H2335–H2343, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Leuenberger UA, Mostoufi-Moab S, Herr M, Gray K, Kunselman A, Sinoway LI. Control of skin sympathetic nerve activity during intermittent static handgrip exercise. Circulation 108: 2329–2335, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Matsukawa K, Murata J, Wada T. Augmented renal sympathetic nerve activity by central command during overground locomotion in decerebrate cats. Am J Physiol Heart Circ Physiol 275: H1115–H1121, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Matsukawa K, Wall PT, Wilson LB, Mitchell JH. Neurally mediated renal vasoconstriction during isometric muscle contraction in cats. Am J Physiol Heart Circ Physiol 262: H833–H838, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Matsukawa K, Wall PT, Wilson LB, Mitchell JH. Reflex stimulation of cardiac sympathetic nerve activity during static muscle contraction in cats. Am J Physiol Heart Circ Physiol 267: H821–H827, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Matsukawa K, Wall PT, Wilson LB, Mitchell JH. Reflex responses of renal nerve activity during isometric muscle contraction in cats. Am J Physiol Heart Circ Physiol 259: H1380–H1388, 1990. [DOI] [PubMed] [Google Scholar]
- 21.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell JH J. B. Wolffe memorial lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc 22: 141–154, 1990. [PubMed] [Google Scholar]
- 23.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell JH, Reardon WC, McCloskey DI. Reflex effects on circulation and respiration from contracting skeletal muscle. Am J Physiol Heart Circ Physiol 233: H374–H378, 1977. [DOI] [PubMed] [Google Scholar]
- 25.Ninomiya I, Matsukawa K, Honda T, Nishiura N, Shirai M. Cardiac sympathetic nerve activity and heart rate during coronary occlusion in awake cats. Am J Physiol Heart Circ Physiol 251: H528–H537, 1986. [DOI] [PubMed] [Google Scholar]
- 26.O'Hagan KP, Bell LB, Mittelstadt SW, Clifford PS. Effect of dynamic exercise on renal sympathetic nerve activity in conscious rabbits. J Appl Physiol 74: 2099–2104, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Plowey ED, Kramer JM, Beatty JA, Waldrop TG. In vivo electrophysiological responses of pedunculopontine neurons to static muscle contraction. Am J Physiol Regul Integr Comp Physiol 283: R1008–R1019, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Robinson BF, Epstein SE, Beiser GD, Braunwald E. Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ Res 19: 400–411, 1966. [DOI] [PubMed] [Google Scholar]
- 29.Rowell L, O'Leary D. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69: 407–418, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Saito M, Naito M, Mano T. Different responses in skin and muscle sympathetic nerve activity to static muscle contraction. J Appl Physiol 69: 2085–2090, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Silber DH, Sinoway LI, Leuenberger UA, Amassian VE. Magnetic stimulation of the human motor cortex evokes skin sympathetic nerve activity. J Appl Physiol 88: 126–134, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol 65: 1539–1547, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Tsuchimochi H, Matsukawa K, Komine H, Murata J. Direct measurement of cardiac sympathetic efferent nerve activity during dynamic exercise. Am J Physiol Heart Circ Physiol 283: H1896–H1906, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res 65: 468–476, 1989. [DOI] [PubMed] [Google Scholar]
- 35.Victor RG, Rotto DM, Pryor SL, Kaufman MP. Stimulation of renal sympathetic activity by static contraction: evidence for mechanoreceptor-induced reflexes from skeletal muscle. Circ Res 64: 592–599, 1989. [DOI] [PubMed] [Google Scholar]
- 36.Victor RG, Seals DR, Mark AL. Differential control of heart rate and sympathetic nerve activity during dynamic exercise. J Clin Invest 79: 508–516, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Victor RG, Secher NH, Lyson T, Mitchell JH. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circ Res 76: 127–131, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Vissing J, Wilson LB, Mitchell JH, Victor RG. Static muscle contraction reflexly increases adrenal sympathetic nerve activity in rats. Am J Physiol Regul Integr Comp Physiol 261: R1307–R1312, 1991. [DOI] [PubMed] [Google Scholar]
- 39.Vissing SF, Scherrer U, Victor RG. Stimulation of skin sympathetic nerve discharge by central command. Circ Res 69: 228–238, 1991. [DOI] [PubMed] [Google Scholar]