Abstract
Binary cardiac transgenic (Tg) overexpression of P2X4 receptors (P2X4R) improved the survival of the cardiomyopathic calsequestrin (CSQ) mice. Here we studied the mechanism of rescue using binary P2X4R/CSQ Tg and CSQ Tg mice as models. Cellular and intact heart properties were determined by simultaneous sarcomere shortening (SS) and Ca2+ transients in vitro and echocardiography in vivo. Similar to a delay in death, binary mice exhibited a slowed heart failure progression with a greater left ventricular (LV) fractional shortening (FS) and thickness and a concomitant lesser degree of LV dilatation in both systole and diastole at 8 or 12 wk. By 16 wk, binary hearts showed similarly depressed FS and thinned out LV and equal enlargement of LV as did 12-wk-old CSQ hearts. Binary cardiac myocytes showed higher peak basal cell shortening (CS) and SS as well as greater basal rates of shortening and relaxation than did the CSQ myocytes at either 8 or 12 wk. Similar data were obtained in comparing the Ca2+ transient. At 16 wk, binary myocytes were like the 12-wk-old CSQ myocytes with equally depressed CS, SS, and Ca2+ transient. CSQ myocytes were longer than myocytes from wild-type and binary mice at 12 wk of age. At 16 wk, the binary myocyte length increased to that of the 12-wk-old CSQ myocyte, parallel to LV dilatation. The data suggest a unique mechanism, which involves a reversal of cardiac myocyte dysfunction and a delay in heart failure progression. It represents an example of targeting the abnormal failing myocyte in treating heart failure.
Keywords: contractility, heart failure, hypertrophy
although significant progress has been made in the pharmacological treatment of heart failure, drug targets have been limited to those that block neurohormonal activation (2, 6, 24). Our recent study showed that cardiac transgenic (Tg) overexpression of P2X4 receptors conferred a survival benefit in the lethal cardiomyopathy of calsequestrin (CSQ) overexpressing mice (25). The activation of the native cardiac P2X receptor by a small molecule agonist could also prolong survival and reduce cardiac hypertrophy in this lethal cardiomyopathy (19). These data provide the initial evidence that the cardiac P2X receptor is a new therapeutic target for treating heart failure, different from those involved in the neurohormonal blockade such as the β-adrenergic receptor pathway or the renin-angiotensin-aldosterone system. It is a target that requires activation rather than inhibition to achieve a beneficial effect in heart failure. The P2X receptor subfamily of the P2 purinergic receptor represents ligand-gated ion channels that are activated by adenine nucleotides such as ATP, ADP (11, 23), or, in our most recent investigation, AMP derivatives (19). The P2X receptor is not G protein coupled, different from the G protein-coupled P2Y receptor (1, 16). Cardiac-restricted overexpression of the P2X4 receptor, which is an important subunit of the native cardiac myocyte P2X receptor (18), can induce an enhanced contractile state of the intact heart (5). Stimulation of the native cardiac myocyte P2X receptor by exogenous agonists such as 2-methylthioATP (2-meSATP) or MRS2339 can induce a P2X current (19) and an increased heart contractility (unpublished data) in not only wild-type (WT) but also CSQ hearts. Although these data demonstrate a cardiac biological phenotype of the P2X receptor, the mechanism by which cardiac P2X receptors rescue heart failure is not known.
Here we studied the mechanism of rescue using binary or double Tg mice with cardiac-specific P2X4 receptor overexpression in the CSQ heart. We tested the hypothesis that a delay in the progression of cardiac myocyte dysfunction, in parallel with a delay in intact heart contractile impairment and in left ventricular (LV) dilatation, is a mechanism by which cardiac P2X4 receptor rescues the lethal heart failure phenotype of CSQ cardiomyopathy. Similar to a delay in death, binary mice exhibited a slowed progression to end-stage heart failure with a greater LV fractional shortening (FS) and thickness and a concomitant lesser degree of LV dilatation in both systole and diastole at 8 or 12 wk of age. However, by 16 wk, binary hearts showed similarly depressed FS and thinned out LV and similar enlargement of LV dimensions as did 12-wk-old CSQ hearts by echocardiography in vivo. Binary cardiac myocytes showed higher peak basal cell shortening (CS) and sarcomere shortening (SS) as well as greater rates of cell shortening and relaxation than did the CSQ cardiac myocytes at either 8 or 12 wk of age. At 16 wk, binary myocytes were more like the 12-wk-old CSQ myocytes with equally depressed CS, SS, and Ca2+ transient.
METHODS
Experimental animals.
The P2X4 receptor Tg, CSQ Tg, and binary Tg mice were generated and bred as previously described (5, 19, 25). The expression of human P2X4 receptor and canine CSQ in Tg mice was verified by polymerase chain reaction of the genomic DNA. Mice of each genotype were used at the various indicated ages. P2X4 receptor overexpressing Tg mice were derived from littermates that overexpressed P2X4 receptors only and likely represented heterozygous animals. More importantly, the P2X4 receptor overexpressing Tg mice maintained a stable 20-fold overexpression of the P2X4 receptor in both the double Tg and the sole P2X4 receptor overexpressing Tg animals (25). Similarly, the level of CSQ overexpression was similar in the double Tg and the sole CSQ overexpressing Tg mice. Animals were handled and maintained according to the approved protocol by the Institutional Animal Care and Use Committee at the University of Connecticut Health Center.
Isolation of adult cardiac ventricular myocytes.
Cardiac ventricular myocytes were obtained from WT, P2X4 Tg, CSQ Tg, and binary Tg mice using an enzymatic dissociation procedure described previously (5). In summary, after obtaining body weight and anesthetization, hearts were rapidly excised. The heart weight was obtained after the aorta was cannulated and lung tissues were removed. Retrograde perfusion via the aorta was carried out with oxygenated (95% O2-5% CO2) Ca2+-free solution containing (in mM) 126 NaCl, 4.4 KCl, 1.0 MgCl2, 18 NaHCO3, 11 glucose, 4 HEPES, and 3 2,3-butanedione monoxime (BDM; pH 7.3 adjusted with NaOH) at 37°C for 5 min at a rate of 2.5 ml/min. Enzymatic dissociation of cardiac myocytes was carried out by perfusing with the same solution except that 25 μM CaCl2 and liberase (70 μg/ml; Roche Molecular Biochemicals) were added. Isolated cardiac myocytes were then exposed to a stepwise increase in extracellular Ca2+ from 0.025, 0.2, and then 1.0 mM, allowed to settle for 30 min at room temperature, and finally suspended with Tyrode's solution containing (in mM) 135 NaCl, 5.4 KCl, 1.0 CaCl2, 1.0 MgCl2, 10 HEPES, and 10 dextrose (pH 7.4 adjusted with NaOH). The experiments were carried out at room temperature (22 to 23°C) and were completed within 3–5 h after myocyte isolation.
Measurements of cell length and width, CS, Ca2+ transient, and SS.
CS was elicited by field stimulation at 0.2, 0.5, 1.0, and 2.0 Hz and was detected by a video edge-detector device (Crescent Electronics, Sandy, UT) (17). The cell image was projected on a high-resolution video monitor through a sequential-scanning video camera attached to the microscope. The cell length and width of cardiac myocytes were measured on the video monitor and were calibrated with 10-μm beads under the same magnification. The distance between the clear edges of the cell ends along the long axis was taken as the cell length, and the distance between the clear edge along the short axis near the middle of the cell was measured as the cell width. The camera was rotated to keep the video detector raster lines parallel with the long axis of the cell. The video dimension analyzer monitored a selected raster line for differences in light intensity between the cell end and the surrounding field. The time constant and apparent spatial resolution of the video analysis system response at 400× magnification were 16.7 ms and 0.15 μm, respectively. The signal from the detector was sent to the computer equipped with AxoScope9.0 (Axon). Five traces were averaged, and the common kinetics and amplitude parameters were analyzed with clampfit 9.0. The amplitude of CS was expressed as the percent resting cell length.
Ca2+ transient and SS were recorded simultaneously with an epifluorescence inverted microscope using Ionoptix software (Ionoptix, Milton, MA) as previously described (20). Myocytes were loaded with 2 μM of fura-2 AM (Molecular Probes, Eugene, OR) for 20 min at room temperature before superfusion with Tyrode's solution and field stimulated at 0.2 Hz. Ca2+ transients were recorded as the fluorescence ratio at 510 nm in response to excitation from 340 and 380 nm as digitized signals for analysis. The background fluorescence, determined by measuring and averaging the autofluorescence of randomly selected 6–9 myocytes not loaded with fura-2, was subtracted. Steady-state recordings were measured under baseline control conditions and after exposure to 2-meSATP (Sigma Chemical, St. Louis, MO). Three to five traces were averaged, and various SS and Ca2+ transient parameters such as amplitude, maximal rates of rise and fall, time-to-90% peak, and time-to-90% decline were measured using Ionoptix curve-fitting algorithm software as previously described (7–10, 12, 15, 17, 20).
Echocardiography.
Transthoracic echocardiography was performed at 12 wk in all four genotypes: WT, P2X4 receptor Tg, CSQ Tg, and binary Tg mice. To study the progression of heart failure, CSQ and binary Tg mice were also investigated at 8 wk of age. Most of the CSQ mice died by 16 wk, whereas the majority of the binary mice were alive at 16 wk of age. At that time, binary heart and cell functions were determined by echocardiography and the simultaneous recording of SS and Ca transient. Echocardiography was determined using a linear 30-MHz transducer according to the manufacturer's instructions (Vevo 660 High Resolution Imaging System; VisualSonics, Toronto, Canada) as previously described (21). In brief, two-dimensional-targeted M mode echocardiographic measurements were carried out at midpapillary muscle level. Mice were anesthetized with 1% isoflurane using a vaporizer. LV end-diastolic diameter (LVEDD) and LV end-systolic diameter (LVESD), LV posterior wall (LVPW) and interventricular septal (IVS) thickness at diastole and systole, and FS (defined as LVEDD − LVESD/LVEDD * 100) were measured. LVPW and IVS were measured digitally on the M mode tracings, and all echocardiogram parameters were averaged from more than three cardiac cycles.
Statistical analysis.
Data were presented as means ± SE. Myocytes in each group were obtained from the indicated number of mice. The significance of differences between means was analyzed with appropriate ANOVA and posttest comparison by Newman-Keuls test. Values with P < 0.05 were considered statistically significant. In other comparisons, a paired or unpaired t-test was used accordingly.
RESULTS
Reversal of cardiac myocyte dysfunction by P2X4 receptor overexpression in the CSQ hearts.
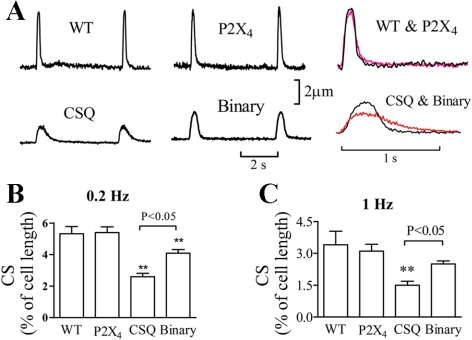
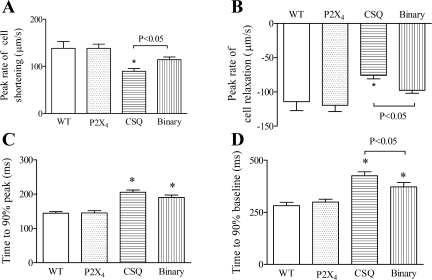
Human P2X4 receptors were overexpressed in the CSQ heart to create a binary or a double Tg mouse. Basal cardiac myocyte function was examined in WT, P2X4 receptor Tg, CSQ Tg, and binary P2X4 receptor/CSQ Tg animals. CS of the cardiac myocyte was used to characterize the baseline cardiac myocyte contractile function. The P2X4 receptor Tg cardiac myocyte, with its overexpression of the receptor, showed normal contractile function as the WT cardiac myocyte (Fig. 1). On the other hand, CSQ myocytes had a markedly abnormal cellular contractile phenotype, showing reduced peak amplitude and a more prolonged duration of CS. The peak rates of shortening and relaxation were less in the CSQ than in the WT or P2X4 receptor Tg myocytes (Fig. 2). In the CSQ cardiac myocyte, time-to-90% peak shortening and time-to-90% recovery to baseline contraction were longer than those in the WT or P2X4 receptor Tg myocytes. Binary Tg overexpression of the P2X receptor in the CSQ background partially improved the abnormal contractile phenotype of the CSQ myocytes. The peak rates of shortening and relaxation were greater in the binary than in the CSQ myocytes, which was associated with a shorter time-to-reach 90% baseline contraction in the binary myocyte (Fig. 2).
Fig. 1.
Improved basal contractile shortening of the binary P2X4 receptor (P2X4R)/calsequestrin (CSQ) cardiac myocyte. A: representative traces of contractile or cell shortening (CS) at 0.2 Hz in myocytes from 12-wk-old wild-type (WT), P2X4 transgenic (Tg), CSQ Tg, and binary Tg hearts were shown. Data were typical of 41 myocytes from 20 WT mice, 59 myocytes of P2X4R Tg, 60 myocytes from 33 CSQ mice, and 54 myocytes from 32 binary mice. CS traces were superimposed for WT and P2X4R Tg as well as for CSQ and binary myocytes in right column. Binary myocytes showed a greater peak CS and a shorter duration of contraction at 0.2 Hz. Average of CS, expressed as percentage of cell length, was shown for myocytes paced at 0.2 Hz (B) or at 1.0 Hz (C). Data and SE were presented. Maximal CS in binary cardiac myocytes was greater than that in CSQ cardiac myocytes (P < 0.05) at both stimulation frequencies. **P < 0.05 when compared with either WT or P2X4 Tg by 1-way ANOVA followed by posttest comparison.
Fig. 2.
Characterization of the kinetics of cell shortening in binary versus CSQ cardiac myocytes. In binary cardiac myocytes (38 myocytes from 25 mice), peak rates of cell shortening (A) and cell relaxation (B) were greater than those in CSQ myocytes (54 myocytes of 28 hearts; P < 0.05). Time-to-90% peak (C) was similar between binary and CSQ myocytes, whereas the time-to-90% baseline (D) in binary Tg myocytes was shorter than that in CSQ myocytes (P < 0.05 by 1-way ANOVA followed by posttest comparison). The kinetics of cell shortening, as measured by rates of shortening and relaxation and by times to 90% peak and to 90% baseline, were similar in WT (34 myocytes from 16 mice) and in P2X4R Tg (45 myocytes from 20 mice) myocytes. *P < 0.05 when compared with either WT or P2X4R Tg by 1-way ANOVA followed by posttest comparison.
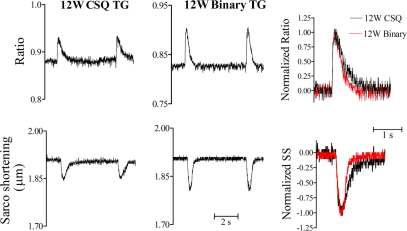
To further characterize the improvement of cardiac myocyte function, simultaneous measurement of SS and Ca2+ transients were compared between the binary and the CSQ myocytes. Binary myocytes showed higher SS amplitude than CSQ myocytes (Fig. 3). When SS and Ca2+ ratios were normalized to their maximal amplitudes with the peak values as 100%, it was evident that the binary myocyte had a shorter duration in both functional parameters. The superimposed contraction tracings highlighted greater peak amplitude of SS and a shorter duration of contraction in the binary myocyte. The restoration of cardiac myocyte function was further supported by data summarized in Table 1. Binary myocyte, compared with the CSQ myocyte, had a greater rate of relaxation and a shorter time to recovery to 90% of the baseline in both SS and Ca2+ transient. The data demonstrated a partial reversal of the abnormal contractile function of the cardiomyopathic myocytes as a result of cardiac P2X4 receptor overexpression.
Fig. 3.
Characterization of an improved cardiac myocyte phenotype in the binary heart by simultaneous sarcomere shortening (SS) and Ca2+ transient determinations. Representative traces of Ca2+ transients and SS in a 12-wk-old (12W) CSQ myocyte (left column) and a 12W binary myocyte (middle column) were shown. Data were typical of 11 CSQ cardiac myocytes (from 10 mice) and 20 binary cardiac myocytes (from 17 mice). SS and Ca2+ transient were normalized to their maximal amplitudes as percentages and were superimposed. The superimposed normalized Ca2+ transient (top row) and SS (bottom row) in the right column showed a faster relaxation in the binary (red line) than the CSQ cardiac myocytes.
Table 1.
Ca2+ transient and sarcomere shortening in binary and CSQ cardiac myocytes
| 12W WT | 12W P2X4 Tg | 12W CSQ Tg | 12W Binary Tg | 16W Binary Tg | |
|---|---|---|---|---|---|
| n | 21 | 22 | 27 | 32 | 12 |
| Sarcomere shortening, μm | 0.11±0.007 | 0.11±0.008 | 0.066±0.003* | 0.094±0.004*† | 0.08±0.01 |
| Shortening velocity, μm/s | 2.26±0.17 | 2.15±0.16 | 1.64±0.14* | 1.86±0.11 | 1.66±0.08 |
| Relaxation velocity, mm/s | 1.81±0.18 | 1.93±0.12 | 1.07±0.07* | 1.37±0.06*† | 1.11±0.06‡ |
| Time-to-90% peak, ms | 89±8.9 | 101±9.6 | 141±12.2* | 123±8.2 | 154±11.8 |
| Time-to-90% baseline, ms | 176±10.9 | 188±11.8 | 296±14.1* | 255±14.8*† | 285±18.8 |
| Ca transient amplitude, unit | 0.063±0.003 | 0.065±0.003 | 0.049±0.005* | 0.053±0.006 | 0.050±0.003 |
| Rising velocity, U/s | 1.40±0.11 | 1.39±0.13 | 0.94±0.11* | 1.07±0.09 | 0.91±0.07 |
| Decaying velocity, U/s | 0.21±0.011 | 0.22±0.012 | 0.14±0.015* | 0.17±0.011*† | 0.13±0.01‡ |
| Time-to-90% peak, ms | 54±6.6 | 53±7.3 | 69±7.4 | 65±6.9 | 68±6.2 |
| Time-to-90% baseline, ms | 443±37.5 | 431±19.7 | 587±53.1* | 481±24.7† | 553±50.2 |
Values are means ± SE; n, number of cardiac myocytes. Sarcomere shortening and Ca2+ transient were measured as described in METHODS. The number of cardiac myocytes is from wild-type (WT; 10 mice), P2X4 receptor transgenic (Tg; 11 mice), calsequestrin (CSQ; 15 mice), and binary (17 mice). 12W, 12 week old; 16W, 16 week old.
P < 0.05 compared with 12W WT and P2X4 Tg, one-way ANOVA and Newman-Keuls posttest comparing 12W WT, P2X4 Tg, CSQ Tg, and binary Tg parameters;
P < 0.05 compared with 12W CSQ Tg by one-way ANOVA and Newman-Keuls;
P < 0.05 compared with 12W binary Tg only by unpaired t-test.
Salutary effect of cardiac P2X4 receptor at the intact heart level.
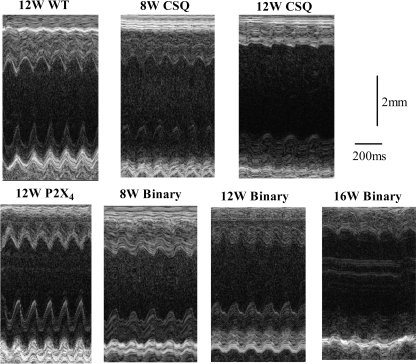
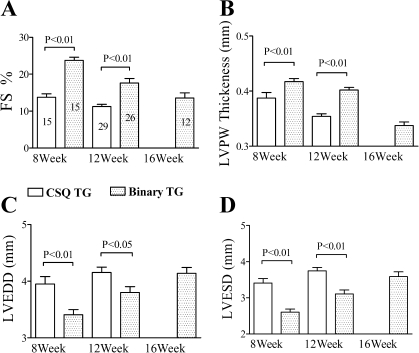
To study whether the improved cardiac myocyte function in vitro is associated with an enhanced intact heart function in vivo, cardiac contractile performance was determined by echocardiography. CSQ mice exhibited significant LV dilatation and thinning with impaired FS compared with those of the WT mice (Fig. 4). P2X4 receptor Tg hearts showed normal contractility without a hyperdynamic or a hypercontractile phenotype. However, binary Tg overexpression of the P2X4 receptor in the CSQ hearts was able to restore the intact heart function toward the level seen in WT hearts (Figs. 4 and 5). Thus, at the same age of either 8 or 12 wk, binary hearts showed less LV dilatation or thinning and better FS than did the CSQ hearts at the corresponding age (Fig. 4). As the binary mice aged, they began to show greater LV dilatation, thinning, and contractile dysfunction. In fact, the aged binary hearts became more like the younger CSQ hearts. Data summarized in Fig. 5A showed a clear progressive decline in FS in both CSQ and binary hearts at increasing age. Although binary hearts showed better FS at 8 and 12 wk than the CSQ hearts of corresponding ages, by 16 wk, binary hearts had similarly impaired FS as the 12-wk-old CSQ hearts. LVPW thickness (Fig. 5B) and septal wall thickness (data not shown) showed an identical pattern of progressive decrease in both binary and CSQ hearts. However, there was a better preserved LV wall thickness in the binary than the CSQ hearts at each age. LV dilatation, measured by LVEDD and LVESD, also showed a progressive increase in both types of hearts but, again, a lesser increase in LVEDD or LVESD in the binary than the CSQ hearts at each age (Fig. 5, C and D). By 16 wk, binary hearts showed similar extent of LV thinning and dilatation as the 12-wk-old CSQ hearts. The data demonstrated a pattern of delayed development of cardiac dysfunction in the binary hearts.
Fig. 4.
A slower progression of heart failure in binary versus CSQ hearts by echocardiography. Two-dimensional-directed M mode echocardiography was carried out as described in methods. Representative M mode echocardiography was shown for hearts from WT, P2X4R Tg, CSQ Tg, and binary Tg mice at the indicated ages. WT and P2X4R Tg hearts showed similar fractional shortening (FS) and left ventricular (LV) dimensions, whereas the CSQ and binary hearts exhibited a progressive decline of FS and LV chamber dilatation. However, the binary hearts showed a slower heart failure progression such that 8-wk-old (8W) binary hearts had better contractile function with less LV dilatation than 8W CSQ hearts. Similar data were obtained in the 12W binary versus 12W CSQ comparison. 12W binary hearts showed similar FS and LV dilatation as the 8W CSQ hearts. By 16 wk (16W), the binary heart function declined and became similar to that of the 12W CSQ heart.
Fig. 5.
A delay in the thinning of LV posterior wall (LVPW) is a novel feature of the rescuing effect in the binary heart. Two-dimensional-directed M mode echocardiography was carried out as described in Fig. 4 legend. The various echocardiography measures were summarized in CSQ and binary mice as means and SE. The number of mice studied in each genotype is indicated within each column. A: FS showed progressive decline in both CSQ and binary animals as they aged. Between successive age groups, the FS was different in both genotypes (8W CSQ vs. 12W CSQ, P < 0.05; 8W binary vs. 12W binary vs. 16W binary was also significant between any pairs of comparison, P < 0.05, 1-way ANOVA followed by posttest comparison). 8W and 12W binary hearts showed better FS than CSQ hearts of corresponding ages (P < 0.05). 16W binary hearts had similar FS as the 12W CSQ hearts (P > 0.1). B: LVPW thickness was characterized. A slowing of the LV wall thinning was also seen in the binary hearts. Results identical to those in A were obtained when LVPW thickness was compared between successive age groups in each genotype and when wall thickness was compared in binary versus CSQ mice. Again, by 16W, the LVPW thickness had declined and became similar to that found in 12W CSQ hearts (P > 0.1). LV dimension at end diastole (LVEDD; C) and at end systole (LVESD; D) also showed progressive increases in each genotype. Results identical to those in A were obtained when either LVEDD or LVESD was compared between successive age groups in each genotype and when these LV dimensions were compared in binary versus CSQ mice. By 16W, the LVEDD or LVESD had declined and became similar to those found in 12W CSQ hearts (P > 0.1).
Comparison of cellular to intact heart properties in binary versus CSQ hearts.
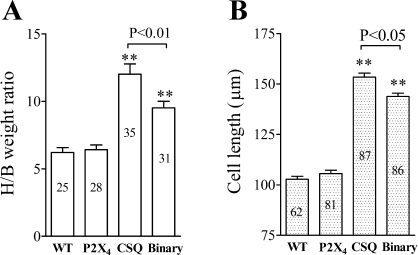
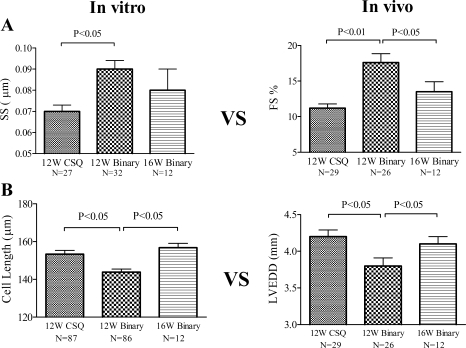
Similar to previous finding (25), CSQ animals had elevated heart weight-to-body weight ratio compared with the WT hearts (Fig. 6A). The greater ratio was associated with a longer cardiac myocyte length in the CSQ than in the WT hearts (Fig. 6B). P2X4 receptor Tg mice showed a normal ratio, consistent with our previous finding (5), and a normal cardiac myocyte length. Overexpression of the P2X4 receptor in the CSQ hearts caused a reduction in the heart weight-to-body weight ratio and in the cardiac myocyte length. There was no difference in the cell width between binary and CSQ cardiac myocytes. Comparison of cardiac myocyte contractile function, as determined by SS, to the intact heart contractile FS showed a parallel pattern of improvement following the overexpression of the P2X4 receptor in the CSQ hearts and a subsequent development of impaired contractile performance at both cardiac myocyte and intact heart levels in the older 16-wk binary animals (Fig. 7A and Table 1). At 16 wk, the binary cardiac myocyte showed impaired sarcomere relaxation velocity and Ca2+ decaying velocity compared with the 12-wk-old binary myocyte (Table 1). In fact, SS and Ca2+ transient properties of the 16-wk binary myocytes had declined to the level of the 12-wk CSQ myocytes (Table 1). Similarly, comparison of cardiac myocyte cell length to the intact heart dimension of LVEDD showed a parallel pattern of improvement following the overexpression of the P2X4 receptor in the CSQ hearts and a subsequent development of myocyte lengthening and cardiac dilatation in the 16-wk binary hearts (Fig. 7B). The data suggested a link between the improvement in cardiac myocyte properties and that of the intact heart function. Since the P2X4 receptor is overexpressed in cardiac myocytes of the Tg animals, the data are consistent with a myocyte origin of the rescuing effect by P2X4 receptor.
Fig. 6.
A: binary hearts showed a decreased cardiac myocyte length and a reduced heart weight-to-body weight ratio (H/B). H/B was obtained from mice of each of the 4 genotypes at 12W. The number of mice is indicated within each column. B: cell lengths in binary and CSQ hearts were measured in cardiac myocytes isolated from each genotype. The number of myocytes studied is indicated within each column, and myocytes were isolated from 12W mice (30 WT mice, 37 P2X4 Tg, 48 CSQ Tg, and 47 binary Tg mice). Data were means and SE. Binary hearts showed lower H/B than did CSQ hearts. Binary cardiac myocytes were shorter than CSQ myocytes. **P < 0.05 when compared with either WT or P2X4R Tg animals by 1-way ANOVA followed by posttest comparison.
Fig. 7.
Comparison of cellular to intact heart properties in binary versus CSQ hearts. Cardiac myocyte contractile function and cell length, measured in vitro (left column), were compared with FS and LVEDD measured in vivo (right column) at the indicated ages for binary and CSQ animals. A: SS (32 myocytes from 17 mice) and FS in 12W binary hearts (n = 26) were both larger than the SS (27 myocytes from 15 mice) and the FS in CSQ (n = 29) hearts at 12W. When the binary animals advanced to 16W, there was a significant decrease in FS compared with that in 12W binary animals (P < 0.05, 1-way ANOVA followed by posttest comparison). B: resting cell length of 12W binary cardiac myocytes (86 myocytes from 47 binary mice) and LVEDD in 12W binary hearts (n = 26) was both less than the corresponding cell length (87 myocytes from 48 mice) and LVEDD (n = 29) of 12W CSQ animals. At 16W, the cell length of binary myocytes (12 myocytes from 8 mice) became longer than that of the 12W binary myocytes (P < 0.05) and was similar to that of the 12W CSQ myocytes (P > 0.1, 1-way ANOVA followed by posttest comparison). Similarly, the LVEDD of 16W binary mice (n = 12) also increased compared with that of 12W binary hearts (n = 26; P < 0.05) and became similar to the LVEDD of the 12W CSQ hearts (P > 0.1, 1-way ANOVA followed by posttest comparison).
DISCUSSION
Current pharmacological therapy for heart failure is aimed at blocking neurohormonal activation or at attenuating its effect. Therefore, drugs such as β-adrenergic receptor blockers, angiotensin converting enzyme inhibitor or angiotensin receptor antagonist, or aldosterone antagonist can inhibit the deleterious cardiovascular effects of catecholamines, angiotensin II, and aldosterone, respectively (2A–4, 13, 14). Despite the success of neurohormonal blockade, new therapeutic targets for the treatment of heart failure are still desirable. Recent studies demonstrated that either cardiac-specific overexpression of a ligand-gated P2X receptor channel or stimulation with an exogenous agonist in intact animal can rescue the lethal cardiomyopathic phenotype of the CSQ Tg mice (19, 25). Although the activation of the cardiac P2X receptor results in an increased cardiac contractility, the mechanism of its rescue effect remains unclear. The present study uses cardiac overexpression of human P2X4 receptors in the CSQ heart as a binary transgenic model to gain insights into this rescue mechanism.
A number of lines of evidence support the concept that reversal of cardiac myocyte dysfunction is a key mechanism by which the cardiac P2X receptor induces its salutary effect in lethal heart failure. First, isolated cardiac myocytes from binary P2X4 receptor/CSQ hearts showed improved function compared with CSQ cardiac myocytes. Binary myocytes had an enhanced basal contractile function with higher peak amplitude of CS and faster rates of cell shortening and relaxation than did CSQ myocytes. Although the time-to-90% recovery of baseline contractile state was longer than that in WT myocytes, the binary myocytes showed shorter relaxation time than CSQ myocytes. Second, using simultaneous recording of SS and Ca2+ transients as additional measures of myocyte function, the binary myocyte exhibited significant improvement in both SS and Ca2+ transients compared with CSQ myocytes of the same age. Similar to the CS data, peak amplitude of SS and rate of relaxation were both better in binary than in CSQ myocytes. The Ca2+ transient decaying velocity was also faster in binary than CSQ myocytes. The amount of time needed to recover to 90% baseline contractile state and diastolic calcium level was both shorter in binary than CSQ myocytes. Finally, cardiac overexpression of the P2X4 receptor in a normal nonfailing cardiac myocyte, that is, the P2X4 receptor Tg cardiac myocyte, had normal function. Whether assessed by CS, SS, or Ca2+ transient, the P2X4 receptor Tg cardiac myocytes were similar to the WT cardiac myocytes. When P2X4 receptors were overexpressed in a pathologically diseased failing myocyte, they were able to reverse the abnormal contractile phenotype of the CSQ cardiac myocyte. Additionally, although cardiac overexpression of P2X4 receptors in a normal myocyte had similar length and width as WT myocytes, P2X4 receptor overexpression in the CSQ cardiac myocyte was associated with a lesser degree of cell lengthening than the CSQ myocyte. Such data link cardiac P2X receptors causally to a more normal, healthier cardiac myocyte.
Additional lines of evidence, obtained in the in vivo heart function study by echocardiography, further supported the concept that restoration toward a healthier cardiac myocyte is an important heart failure rescue mechanism. Again, the P2X4 receptor Tg hearts had normal phenotype in vivo with similar cardiac function by echocardiography as the WT hearts. However, cardiac overexpression of the P2X4 receptor caused better FS and less LV dilatation in both systole and diastole than did the CSQ hearts at both 8 wk (preheart failure stage) and 12 wk (advanced heart failure stage). Of interest is the finding that cardiac overexpression of P2X4 receptors in the CSQ heart resulted in decreased LV thinning. The binary hearts had greater posterior and septal thickness than did the CSQ hearts at either 8 wk or 12 wk of age. The lower heart weight-to-body weight ratio and the shorter cardiac myocyte cell length in the binary heart suggested against cardiac hypertrophy of the binary heart as a reason for its greater LV thickness. Possible explanations for a greater LV thickness are unknown and deserve further investigation.
The ability to characterize cellular and intact heart functions at various ages permitted the opportunity to study the effect on heart failure progression as a result of cardiac P2X receptor overexpression. Although the binary animals showed improved cellular and intact heart functions at 8 and 12 wk, they exhibited significant decline as the heart failure progressed. By 16 wk, the cellular and intact heart functions of the binary animals became as abnormal as those seen in the 12-wk-old CSQ mice. Thus the beneficial effect of cardiac P2X receptors in heart failure appears to be the result of a delay in its progression. Overall, cardiac P2X receptor overexpression in the severely cardiomyopathic CSQ myocytes restored the markedly abnormal myocyte toward normal function in parallel with an improved intact heart function. The study suggests a unique heart failure rescue mechanism, one of a reversal of myocyte dysfunction and a delay in heart failure progression. Targeting the restoration of abnormal cardiac myocytes may be a new approach to treat heart failure.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1-HL48225 (to B. Liang).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International union of pharmacology. Update of the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 59: 281–341, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham WT, Greenberg BH, Yancy CW. Pharmacologic therapies across the continuum of left ventricular dysfunction. Am J Cardiol 102: 21G–28G, 2008. [DOI] [PubMed] [Google Scholar]
- 2A.[Anon]. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 325: 293–302, 1991. [DOI] [PubMed] [Google Scholar]
- 3.β-Blocker Heart Attack Trial Research Group. A randomized trial of propranolol in patients with acute myocardial infarction I. Mortality results. JAMA 247: 1707–1714, 1982. [DOI] [PubMed] [Google Scholar]
- 4.Flather Yusuf S MD, Kober L, Pfeffer M, Hall A, Murray G, Torp-Pedersen C, Ball S, Pogue J, Moye LA, Braunwald E, for the ACE-inhibitor Myocardial Infarction Collaborative Group. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. Lancet 355: 1575–1581, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Hu B, Mei Q, Smith E, Barry WH, Liang BT. A novel cardiac inotropic phenotype with cardiac transgenic expression of human P2X4 receptor transgenic mouse. FASEB J 15: 2739–2741, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Hunt SA ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation, and Management of Heart Failure). J Am Coll Cardiol 46: e1–e82, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Janczewski AM, Kadokami T, Lemster B, Frye CS, McTiernan CF, Feldman AM. Morphological and functional changes in cardiac myocytes isolated from mice overexpressing TNF-α. Am J Physiol Heart Circ Physiol 284: H960–H969, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhefer U, Neumann J, Bers DM, Buchwalow IB, Fabritz L, Hanske G, Justus I, Riemann B, Schmitz W, Jones LR. Impaired relaxation in transgenic mice overexpressing junction. Cardiovasc Res 59: 369–379, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Kondo RP, Dederko DA, Teutsch C, Chrast J, Catalucci D, Chien KR, Giles WR. Comparison of contraction and calcium handling between right and left ventricular myocytes from adult mouse heart: a role for repolarization waveform. J Physiol 571: 131–146, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin SR, Emanuel K, Sears CE, Zhang YH, Casadei B. Are myocardial eNOS and nNOS involved in the β-adrenergic and muscarinic regulation of inotropy? A systematic investigation. Cardiovasc Res 70: 97–106, 2006. [DOI] [PubMed] [Google Scholar]
- 11.North RA Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Olsson MC, Palmer BM, Stauffer BL, Leinwand LA, Moore RL. Morphological and functional alterations in ventricular myocytes from male transgenic mice with hypertrophic cardiomyopathy. Circ Res 94: 201–207, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med 334: 1349–1355, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Pfeffer MA, Braunwald E, Moy LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial. The SAVE Investigator. N Engl J Med 327: 669–677, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Pohlmann L, Kroger I, Vignier N, Schlossarek S, Kramer E, Coirault C, Sultan KR, El-Armouche A, Winegrad S, Eschenhagen T, Carrier L. Cardiac myosin-binding protein C is required for complete relaxation in intact myocytes. Circ Res 101: 928–938, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998. [PubMed] [Google Scholar]
- 17.Shen JB, Jiang B, Pappano AJ. Lack of effect of McN-A-343 on membrane current and contraction in guinea pig ventricular myocytes. J Pharmacol Exp Ther 290: 641–648, 1999. [PubMed] [Google Scholar]
- 18.Shen JB, Pappano A, Liang BT. Extracellular ATP-stimulated current in wild type and P2X4 receptor transgenic mouse ventricular myocytes. Implication for a cardiac physiologic role of P2X4 receptors. FASEB J 20: 277–284, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Shen JB, Cronin C, Sonin D, Joshi BV, Nieto M, Harrison D, Jacobson KA, Liang BT. P2X purinergic receptor-mediated ionic current in cardiac myocytes of calsequestrin model of cardiomyopathy. Implications for the treatment of heart failure. Am J Physiol Heart Circ Physiol 292: H1077–H1084, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen JB, Shutt R, Pappano AJ, Liang BT. Characterization and mechanism of P2X receptor-mediated increase in cardiac myocyte contractility. Am J Physiol Heart Circ Physiol 293: H3056–H3062, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Sonin D, Zhou SY, Cronin C, Sonina T, Wu J, Jacobson KA, Pappano AJ, Liang BT. Role of P2X purinergic receptors in the rescue of ischemic heart failure. Am J Physiol Heart Circ Physiol 295: H1191–H1197, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vassort G Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev 81: 767–806, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Williams RE The effect of neurohormonal antagonists in reducing heart failure hospitalizations. Curr Med Res Opin 22: 139–150, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Yang A, Sonin D, Jones L, Liang BT. A beneficial role of cardiac P2X4 receptors in heart failure: rescuing the calsequestrin-overexpression model of cardiomyopathy. Am J Physiol Heart Circ Physiol 287: H1096–H1103, 2004. [DOI] [PubMed] [Google Scholar]