Abstract
Background
Schizophrenia and related psychoses are associated with brain structural abnormalities. Recent findings in ‘at risk’ populations have identified progressive changes in various brain regions preceding illness onset, while changes especially in prefrontal and superior temporal regions have been demonstrated in first-episode schizophrenia patients. However, the timing of the cortical changes and their regional extent, relative to the emergence of psychosis, has not been clarified. We followed individuals at high-risk for psychosis to determine whether structural changes in the cerebral cortex occur with the onset of psychosis. We hypothesized that progressive volume loss occurs in prefrontal regions during the transition to psychosis.
Methods
35 individuals at ultra-high risk (UHR) for developing psychosis, of whom 12 experienced psychotic onset by 1-year follow-up (‘converters’), participated in a longitudinal structural MRI study. Baseline and follow-up T1-weighted MR images were acquired and longitudinal brain surface contractions were assessed using Cortical Pattern Matching.
Results
Significantly greater brain contraction was found in the right prefrontal region in the ‘converters’ compared with UHR cases who did not develop psychosis (‘non-converters’).
Conclusions
These findings show cortical volume loss is associated with the onset of psychosis, indicating ongoing pathological processes during the transition stage to illness. The prefrontal volume loss is in line with structural and functional abnormalities in schizophrenia, suggesting a critical role for this change in the development of psychosis.
Keywords: schizophrenia, MRI, brain mapping, longitudinal, prodrome, ultra-high risk
1. Introduction
Schizophrenia and related psychoses are associated with brain structural and functional abnormalities. In structural magnetic resonance imaging (MRI) studies, brain volume reductions have been found in both chronic and first-episode schizophrenia patients (Shenton et al. 2001) and also in affective disorder (Strakowski et al. 2005), but whether there is ongoing brain tissue loss prior to, or following, onset of these psychoses, is less clear (Mathalon et al. 2003; Weinberger and McClure 2002). Although accumulating data have shown that various brain regions experience progressive tissue loss after the first psychotic onset of schizophrenia (Ho et al. 2003; Lieberman et al. 2005; Nakamura et al. 2007; Salisbury et al. 2007; van Haren et al. 2007), the timing and regional pattern of these changes remains unclear, and it is unknown whether these changes are caused by the effect of long-term illness or medications (Pantelis et al. 2005).
Two studies to date have attempted to address these questions by identifying individuals at heightened risk for psychosis due to mental state change and/or family history, and by tracking brain changes from pre-onset stages through transition to illness. Pantelis and colleagues (Pantelis et al. 2003a) examined gray matter changes in individuals at ultra-high risk (UHR) for psychosis followed for one year to assess those converting to psychosis (‘converters’) compared to ‘non-converters’. The converters showed gray matter loss in the left inferior frontal region, left medial and inferior temporal regions and bilaterally in the cingulate, but the group by time interaction did not reach significance. Job and colleagues (Job et al. 2005) reported gray matter loss in the left inferior temporal gyrus, left uncus and the right cerebellum in their genetic high-risk subjects who developed schizophrenia during or beyond a 2-year follow-up period. However, no significant group differences in gray matter loss were found in a direct comparison to subjects who did not develop schizophrenia or healthy controls. Both studies suggested that tissue loss occurs over time among individuals at risk for psychosis, but neither study was adequately powered to compare rates of loss among converters relative to non-converters. Since the brain undergoes developmental changes during adolescence and early adulthood, particularly in prefrontal cortex, the observed gray matter reduction may not be pathologic but, rather, developmental in nature (Pantelis et al. 2007). Therefore, whether the changes are related to the onset of psychosis remains unanswered, and can only be determined by a between-group comparison in a longitudinal design.
In the present study, we assessed subtle cortical changes during illness transition, using higher resolution MRI images in a larger sample of the Melbourne UHR subjects. We used Cortical Pattern Matching to directly compare longitudinal brain surface contractions in converters with non-converters followed for one year. Cortical Pattern Matching is an advanced brain warping technique that can achieve accurate anatomical correspondence between brain surfaces, after which point-wise statistical comparisons were performed. It has proven successful in detecting longitudinal changes of cortical gray matter density and thickness in normal and pathological development and degeneration (Thompson et al. 2004). Brain surface contraction is defined as the subvoxel-resolution distance between anatomically corresponding brain surface points of baseline and follow-up brain scans, the measure of which is an integrated part of Cortical Pattern Matching (Sowell et al. 2001). We hypothesized that greater brain surface contraction occurs in UHR converters compared with non-converters and that these changes are most pronounced in frontal brain regions previously shown to be affected in schizophrenia (Cannon et al. 2002; Ho et al. 2003; Lieberman et al. 2005; van Haren et al. 2007).
2. Method
2.1. Participants
Participants were recruited from the Personal Assessment and Crisis Evaluation (PACE) Clinic, a clinical research program for prodromal patients in Melbourne, Australia (Yung et al. 2004). Criteria for identification of the UHR subjects are listed in Table 1. Validated criteria for onset of acute psychosis have also been developed (Table 1) (Yung et al. 2003). Trained psychologists or psychiatrists conducted the clinical interviews and determined whether participants met UHR intake criteria and criteria for acute psychosis (Yung et al. 2004).
Table 1.
Ultra-High-Risk Intake and Exit Criteria
Intake criteriaGroup 1: Attenuated psychotic symptoms
|
BPRS=brief psychiatric rating scale; CASH=comprehensive assessment of symptoms and history; GAF=global assessment of function scale. These are the criteria for identifying people as high risk for psychosis. People are included if they meet criteria for one or more of the three groups.
The UHR subjects were aged between 14 and 30 years at intake, had not experienced a previous psychotic episode or received neuroleptic medication, did not have a neurological disorder, and were not intellectually disabled (IQ>70).
The study was approved by the local research and ethics committee in Melbourne, Australia, and by Internal Review Board at the University of California, Los Angeles, USA. After complete description of the study to the subjects, written informed consent was obtained.
Clinical assessments and MRI scans were conducted at two time points. The planned follow-up time was 1 year, unless onset of psychosis occurred earlier. In the latter cases, the second assessment was conducted as soon as possible after transition. In practice, however, the interval time varied for reasons including difficulty in maintaining contact with the participants, and treatment needs taking priority.
Thirty-five UHR participants who had both baseline and follow-up scans were included. Twelve participants developed a full psychotic episode over the follow-up period. The diagnostic breakdown of psychotic disorders in converters was: schizophrenia (n=4); schizoaffective disorder (1); bipolar disorder (2); major depression with mood incongruent psychotic symptoms (3); brief psychotic episode (1); and psychosis not otherwise specified (1). At follow-up, non-converters had either no psychiatric diagnosis (n=14), or were diagnosed with non-psychotic disorders (major depression 4; dysthymia 2; obsessive-compulsive disorder 1; general anxiety disorder 1; eating disorder 1). There was no significant difference in gender, handedness, premorbid IQ, age, and inter-scan interval between the converters and non-converters (Table 2). Complete information on medications was not available. At follow-up scans, one converter was on risperidone (3mg/day), one on chlorpromazine (150mg/day) and valporate (1,500mg/day), and the other was on citalopram (20mg/day) and valporate (1,500mg/day); one non-converter was on venlafaxine (225mg/day) at follow-up. Three converters and 6 non-converters had records for drug use (including tetrahydrocannabinol, cocaine, and LSD) during the follow-up period. No head injuries were recorded.
Table 2.
Demographic Data of Ultra-High-Risk individuals
| Converters (n=12) | Non-converters (n=23) | P Value | |
|---|---|---|---|
| Sex (male/female) | 7/5 | 12/11 | 0.728 |
| Handedness (right/mixed/left) | 10/0/2 | 18/1/4 | 0.760 |
| Premorbid IQ | 94.3±13.6 | 93.3±13.7 | 0.947 |
| Age at 1st scan (years) | 19.5±5.1 (13.9–29.1) | 20.2±4.0 (14.3–27.5) | 0.662 |
| Age at 2nd scan (years) | 20.7±5.2 (15.4–30.9) | 21.6±4.0 (15.4–28.5) | 0.581 |
| Days between scans | 443±186 (237–826) | 511±289 (329–1361) | 0.469 |
| Age of onset (years) | 20.1±5.0 (15.3–29.5) | ||
| Days between 1st scan and onset | 211±141 (84–538) | ||
| Days between onset and 2nd scan | 232±144 (15–534) |
Data are mean±SD (range) unless otherwise stated.
All but one subject (n=20) who participated in a previous longitudinal study using low-resolution T2-weighted and proton density images (Pantelis et al. 2003a) were included in the present analysis; the one case was excluded because T1-weighted images were not available. The present study incorporates 15 new participants (3 converted, 12 non-converted) and utilizes previously unpublished high-resolution T1-weighted anatomical images obtained at baseline and follow-up.
2.2. Image Processing and Analysis
All participants were scanned twice on a 1.5T General Electric Signa MRI scanner. A three-dimensional volumetric spoiled gradient recalled echo (SPGR) sequence generated 124 contiguous, 1.5 mm coronal slices. Imaging parameters were: TE=3.3 msec; TR=14.3 msec; flip angle, 30°; matrix size, 256 × 256; field of view, 24 × 24 cm matrix; voxel dimensions, 0.938 × 0.938 × 1.5 mm. A numerical code was used to ensure blind analysis of data.
For each scan, a radio-frequency bias field correction was performed. The cerebral image was extracted from the remainder of each head image, and was manually refined for further accuracy. The baseline and follow-up brain images were coregistered to a standard 3D coordinate space (Mazziotta et al. 1995) with 9 degree-of-freedom linear transformations, while image scales were corrected using intracranial volumes. Cortical Pattern Matching was performed in the standard space.
For each scan, a cortical surface model was extracted using an automated method (MacDonald et al. 2000). In this process, a spherical mesh surface was continuously deformed to fit the cortical surface that best differentiated brain tissue and cortical CSF, and a high-resolution surface model representing 65 536 brain surface points was created. On each hemisphere of the surface model, 17 major anatomical landmark curves were manually traced following major sulci. In addition, a set of 8 control curves along the longitudinal fissure that delineate the lateral surface and the medial surface were traced. The tracing protocol is available on the Internet (http://www.loni.ucla.edu/~esowell/edevel/new_sulcvar.html). All sulci and control curves were traced by an image analyst blind to subject demographics and diagnosis. The reliability of tracing was tested on 6 standard brain surfaces, and the average distance between the sulcal curves tracing by the current analyst and the standard sulcal curves was <2mm in most regions.
The brain surfaces and curves were then flattened to a 2D plane, and average curves were created by averaging the positions of the same curves across all subjects. The brain surfaces were elastically warped to each other based on matching individual curves to their corresponding average curves in the 2D plane, while the coordinate positions of each surface point in their 3D space were preserved.
The distance between a center position of the brain (the origin of the standard space, approximately at the midline decussation of the anterior commissure) and each brain surface point was calculated (Sowell et al. 2001). The difference of the above radial distances between the follow-up and baseline brain surfaces at each anatomically corresponding point was defined as brain surface contraction, which reflected local tissue loss or growth over time. The resulting values at all brain surface voxels were then assigned to the corresponding voxel location in the group average surface to create composite maps of brain surface contraction.
2.3. Statistical analysis
Individual brain surface contraction was annualized by dividing the contraction value in millimeters by the between-scan interval in years, and this surface contraction rate was taken as the primary variable for the following statistical analyses. The contraction rate was used as a parsimonious control for different between-scan intervals among subjects, and should not be extrapolated beyond the range of the follow-up period. T-tests were performed to compare surface contraction rates between the two UHR groups at each brain surface points, and P-maps were generated. Standard permutation tests were conducted to confirm that the significant changes were not purely by chance. We conducted 100 000 randomized permutations (randomly assigning subjects to groups) in the frontal, parietal, occipital, and temporal lobes. Between-group differences were considered significant if less than 5% (p<0.05) of the results from all random permutations exceeded the difference detected in the groupings. In addition, we compared the converters with the non-converters with no psychiatric diagnoses at follow-up, using the same statistical method as above.
3. Results
3.1. Between-group difference
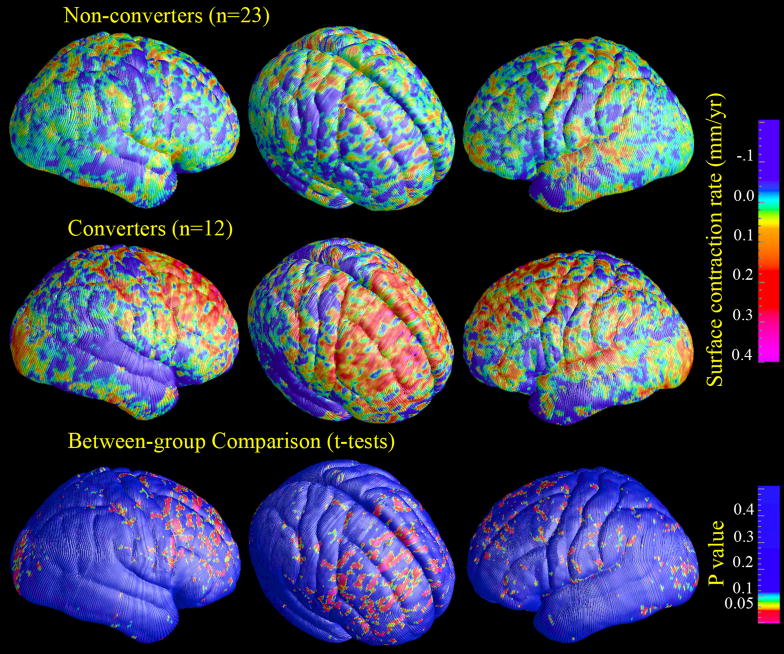
Figure 1 shows the average brain surface contraction rate (mm/year) both for individuals who converted to psychosis and individuals who did not convert and the maps of statistical significance (P values, uncorrected for multiple-voxel comparisons) in between-group comparison. In contrast to the average rate of surface contraction in non-converters, the magnitude of which was less than 0.2 mm/year (upper row), the converters showed greater brain surface contraction in bilateral dorsolateral prefrontal regions, with a maximum magnitude of 0.4 mm/year (middle row). In the between-group comparison, prefrontal regions showed the most prominent difference. There was no major region where converters showed less contraction or greater expansion than non-converters.
Figure 1.
(Colored). Maps of average brain surface contraction rates and uncorrected p-maps of converters v.s. non-converters comparison.
Using permutation tests with p-value at 0.05 as the predefined threshold, the between-group difference in right frontal lobe remained significant (p=0.030), while the differences in left frontal lobe showed trends towards significance (p=0.077). No significant results appeared in other regions of interest.
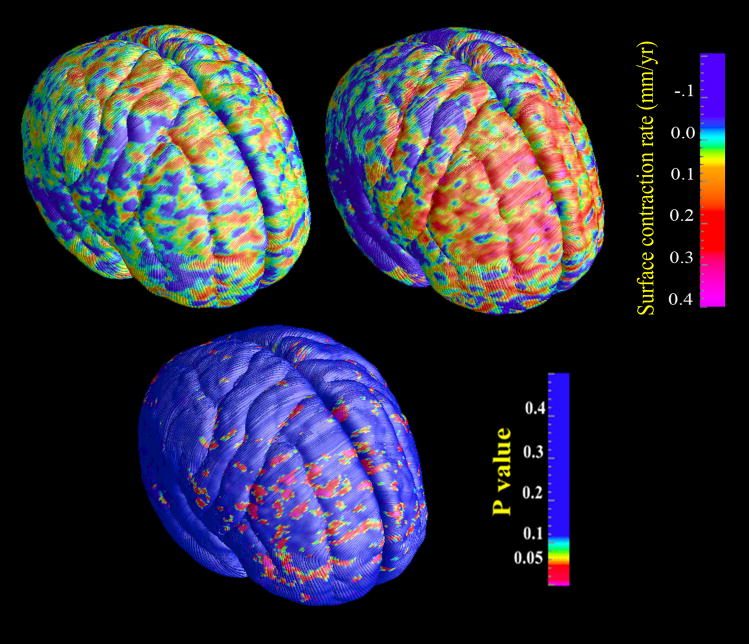
Compared to the 14 non-converters with no psychiatric diagnoses, the converters also showed greater contraction rate in the prefrontal region, but with the smaller sample size, the difference was not significant in the permutation tests (Fig. 2).
Figure 2.
(Colored). Maps of average brain surface contraction rates and uncorrected p-values in the comparison between converters v.s. non-converters with no follow-up psychiatric diagnoses.
3.2. Method Validation
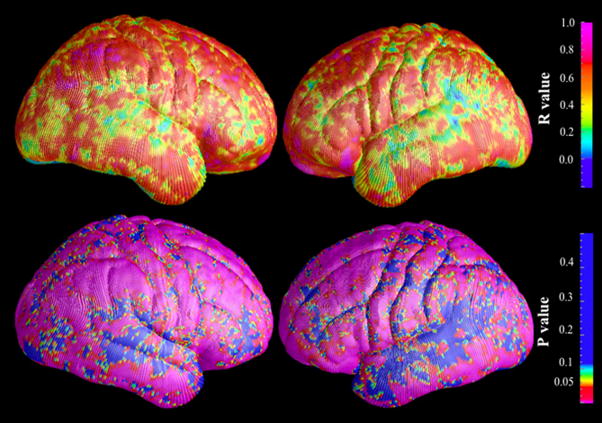
Because the rate of change in brain surface contraction detected in this study was below the level of intrinsic image resolution, it would not have been detected using an approach dependent on voxel-level quantitation such as gray matter density. To determine whether the measure of brain surface contraction is related to changes in brain volume, we quantified local cortical brain tissue density (i.e., gray and white matter in contiguous 5mm spheres) using the Cortical Pattern Matching method, and correlated this metric with that for brain surface contraction across all individuals included in this study. Highly significant correlations were observed covering most brain surface areas, as shown in the maps of Pearson’s R values and P values (Figure 3). This result supports the hypothesis that the brain surface contraction was caused by local brain tissue loss. It is not possible in this method to determine whether the local brain tissue loss is driven principally by reductions in gray matter, white matter, or both. It should be noted that the difference in brain tissue density between the two groups did not achieve statistical significance.
Figure 3.
(Colored). Maps of correlation between brain surface contraction and volume reduction of local brain tissue (n=35).
4. Discussion
This is the first study to show statistically significant MRI brain structural change around the time of onset of psychosis using a direct between-group analysis comparing converted to non-converted UHR subjects. We found greater brain surface contraction rate in UHR subjects who converted to psychosis compared to those who did not during a one-year follow-up. Converters showed increased brain contraction in the right prefrontal region, while left prefrontal and left and right occipital regions showed trends towards significance.
The limitations of the study included the relatively small sample size of the UHR converter group (n = 12) and the lack of a healthy control group. Although the included UHR non-converter group was the most appropriate to control for potential influence of the prodromal status on the brain, a healthy control group would further address the deviation from normal brain changes. In addition, the follow-up scans were not acquired immediately after the psychosis onset, so the possibility that factors secondary to psychosis or its treatment contributed to the greater brain change in the converters can not be excluded. These limitations need to be examined in future studies.
In a prior report using lower-resolution T2 and proton density dual-echo image data on a subset of the subjects from this study, no significant differences were found in gray matter changes between converters and non-converters (Pantelis et al. 2003a). In the present study, additional converters and non-converters were involved in the comparison, in which higher resolution T1 scans were used, thereby providing additional sensitivity and statistical power. Further, a new parameter of longitudinal brain change, brain surface contraction, with resolution at a sub-voxel level, was adopted, in contrast to the gray matter density analysis used in the previous voxel-based morphometry analysis. In combination with the cortical pattern matching method, which provides a precise brain surface registration across subjects, the surface contraction measurement showed an advantage in detecting subtle changes. The maximum group-mean local brain surface contraction rate was less than 0.3 mm/year, which is within one third of the image voxel size. Such a subtle change could not be detected by any metric dependent on a voxel-level or coarser resolution, e.g., gray matter density. The measure of gray matter density also relies on the accuracy of image tissue classification, which can be confounded by partial volume effects. Although in the prior study significant gray matter reduction was found for converters (but not in the comparison between converters and non-converters) in several brain regions including the cingulate, parahippocampal gyrus, and the cerebellum, these regions were not examined in this surface-based approach.
Although the mixed diagnoses of the converters at follow-up make it difficult to associate the observed changes with a particular psychotic disorder, it is intriguing to note that the progressive surface contraction in the prefrontal region is in line with findings on schizophrenia from several perspectives. The prefrontal cortex is involved in working memory, executive function and other higher-order cognitive functions, all of which are impaired in schizophrenia, with evidence for such deficits in high-risk cohorts (Brewer et al. 2006). In postmortem neuropathological studies, the prefrontal region shows increased neuronal packing (Selemon et al. 1995) and reduced dendritic spines (Glantz and Lewis 2000) without significant decrease in neuronal number (Pakkenberg 1993). Defects in oligodendrocytes and myelin in the prefrontal region have also been reported in schizophrenia and in bipolar disorder (Tkachev et al. 2003). Cortical mapping in twins discordant for schizophrenia shows both genetic and disease-specific influences on gray matter deficits in the prefrontal region (Cannon et al. 2002), and several susceptibility genes for schizophrenia have been found to be associated with prefrontal structural or functional abnormalities (Cannon et al. 2005; Egan et al. 2001). In longitudinal MRI studies of schizophrenia, frontal volume reduction has also been found (Ho et al. 2003; Lieberman et al. 2005; Nakamura et al. 2007; Sun et al. 2008; van Haren et al. 2007). Non-schizophrenic psychotic disorders, including affective forms of psychosis, may share some susceptibility genes and underlying neuropathological features in common with schizophrenia (Craddock et al. 2006). Therefore, it is plausible that the prefrontal change is shared by schizophrenia and other psychotic disorders.
The observation of progressive brain tissue loss during the period of onset of psychosis is predicted by theories of schizophrenia that emphasize involvement of abnormal neurodevelopmental processes (Cannon et al. 2003; Feinberg 1982–83; Pantelis et al. 2003b; Woods 1998). For example, it has been suggested that the onset of schizophrenia may be associated with an abnormal acceleration of the neuroregressive processes that occur as part of normal brain development in adolescence, such as synaptic pruning. It is of interest in this regard that gray matter reduction in the region detected as showing differential surface contraction among the high-risk cases who converted to psychosis (superior frontal gyrus) has been linked to schizophrenia-related sequence variations in the Disrupted-In-Schizophrenia-1 (DISC1) gene (Cannon et al. 2005), which is known to regulate neurite outgrowth, synaptogenesis, and other aspects of brain growth and plasticity (Porteous et al. 2006).
By including non-converters who could not be differentiated from subjects who were pre-psychotic at intake as a comparison group, some identifiable trait or state factors shared by these two cohorts were controlled for. These factors include prodromal symptoms, recreational drug use, and developmental brain changes that are normally occurring during adolescence and early adulthood. However, the follow-up scans were not acquired during or immediately after the psychosis onset, so it is possible that factors secondary to psychosis or its treatment contributed to the greater brain change in the converters.
Elevated levels of stress hormones have been considered a source of hippocampal volume reduction in depression and other psychiatric conditions, including schizophrenia (Phillips et al. 2006). In a recent study of a larger sample of the UHR subjects, larger pituitary volumes were found in those who were pre-psychotic, and this was associated with proximity to transition to illness (Garner et al. 2005), suggesting stress-related changes occur even prior to psychosis onset. Stress and other disease processes may interact to cause the brain structural changes and the emergence/exacerbation of psychosis (Phillips et al. 2006). This is consistent with a multiple-hit model of psychosis (Pantelis et al. 2005), and has important implications for the prevention and intervention of psychotic disorders.
While we cannot rule out an effect of antipsychotic medications, drug treatment is not likely to be a major source of the observed changes. The results from primate studies examining antipsychotic effects on brain volume are conflicting. While one study showed proliferation of glial cells but not neuronal density change following chronic antipsychotic drug exposure (Selemon et al. 1999), another study reported brain weight loss and volume reduction in animals after chronic exposure (Dorph-Petersen et al. 2005). Antipsychotic medications have also been associated with brain volume changes in human subjects. Typical and atypical drugs have shown different effects in volumetric studies (Lieberman et al. 2005), with the latter being associated with less brain volume reduction or even volume increase. In the present study the majority of converted subjects received low-dose risperidone (an atypical antipsychotic drug) after onset of psychosis.
In summary, by using advanced methods optimized for detecting subtle local surface contraction, we found significant prefrontal volume loss around the time of psychosis onset in a unique ultra-high-risk cohort. These findings encourage further study to isolate the cellular and molecular mechanisms underlying the onset of a psychotic illness and to determine whether such changes are differential in the developmental course of different clinically defined syndromes involving psychotic symptoms.
Acknowledgments
This research and the clinical research structure of PACE were supported by project grants from the National Health & Medical Research Council (NHMRC; grant IDs: 145627, 145737, 970598, 981112, 970391), NHMRC Program Grant (ID: 350241), Victorian Health Promotion Foundation, the Stanley Foundation, and Ian Potter Foundation. Drs Velakoulis and Wood were supported as Research Officers with funding from the NHMRC. Dr McGorry was supported by a NARSAD Distinguished Investigator Award. Dr Wood is currently supported by a Clinical Career Development Award from the NHMRC (ID: 359223) and a NARSAD Young Investigator Award. Dr Thompson is supported by NIH grants AG016570, LM05639, EB01651, and RR019771. Image analysis and statistical analysis were supported by NIH grants MH65078 to Dr Cannon and GM072978 (Center for Computational Biology) to Dr Toga.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brewer WJ, Wood SJ, Phillips LJ, Francey SM, Pantelis C, Yung AR, Cornblatt B, McGorry PD. Generalised and specific cognitive performance in clinical high-risk cohorts: A review highlighting potential vulnerability markers for psychosis. Schizophr Bull. 2006;32(3):538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Hennah W, van Erp TG, Thompson PM, Lonnqvist J, Huttunen M, Gasperoni T, Tuulio-Henriksson A, Pirkola T, Toga AW, Kaprio J, Mazziotta J, Peltonen L. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry. 2005;62(11):1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, Lonnqvist J, Standerskjold-Nordenstam CG, Narr KL, Khaledy M, Zoumalan CI, Dail R, Kaprio J. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci U S A. 2002;99(5):3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, Huttunen MO, Keshavan MS, Seidman LJ, Tsuang MT. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull. 2003;29(4):653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- Craddock N, O’Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32(1):9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Phychiatr Res. 1982–83;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B, Brewer WJ, Smith DJ, Dazzan P, Berger GE, Yung AR, van den Buuse M, Murray R, McGorry PD, Pantelis C. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry. 2005;58(5):417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60(6):585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25(4):1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62(4):361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- MacDonald JD, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12(3):340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Rapoport JL, Davis KL, Krystal JH. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry. Arch Gen Psychiatry. 2003;60:846–848. doi: 10.1001/archpsyc.60.8.846. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2(2):89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Salisbury DF, Hirayasu Y, Bouix S, Pohl KM, Yoshida T, Koo MS, Shenton ME, McCarley RW. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol Psychiatry. 2007;62(7):773–783. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B. Total nerve cell number in neocortex in chronic schizophrenics and controls estimated using optical disectors. Biol Psychiatry. 1993;34(11):768–772. doi: 10.1016/0006-3223(93)90065-l. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003a;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, Wood SJ, Yucel M, Yung AR, Phillips LJ, Sun DQ, McGorry PD. Neuroimaging and emerging psychotic disorders: the Melbourne ultra-high risk studies. Int Rev Psychiatry. 2007;19(4):371–381. doi: 10.1080/09540260701512079. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, McGorry PD, Velakoulis D. Early and late neurodevelopmental disturbances in schizophrenia and their functional consequences. Aust N Z J Psychiatry. 2003b;37(4):399–406. doi: 10.1046/j.1440-1614.2003.01193.x. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, Stuart GW, Yung A, Phillips L, McGorry PD. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31(3):672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, McGorry PD, Garner B, Thompson KN, Pantelis C, Wood SJ, Berger G. Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry. 2006;40(9):725–741. doi: 10.1080/j.1440-1614.2006.01877.x. [DOI] [PubMed] [Google Scholar]
- Porteous DJ, Thomson P, Brandon NJ, Millar JK. The genetics and biology of DISC1--an emerging role in psychosis and cognition. Biol Psychiatry. 2006;60:123–131. doi: 10.1016/j.biopsych.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64(5):521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Lidow MS, Goldman-Rakic PS. Increased volume and glial density in primate prefrontal cortex associated with chronic antipsychotic drug exposure. Biol Psychiatry. 1999;46(2):161–172. doi: 10.1016/s0006-3223(99)00113-4. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52(10):805–818. doi: 10.1001/archpsyc.1995.03950220015005. discussion 819–820. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10(1):105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Sun D, Stuart GW, Jenkinson M, Wood SJ, McGorry PD, Velakoulis D, van Erp TG, Thompson PM, Toga AW, Smith DJ, Cannon TD, Pantelis C. Brain surface contraction mapped in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Mol Psychiatry advance online publication 8. 2008 July; doi: 10.1038/mp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, Doddrell DM, Wang Y, van Erp TG, Cannon TD, Toga AW. Mapping cortical change in Alzheimer’s disease, brain development, and schizophrenia. Neuroimage. 2004;23(Suppl 1):S2–18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362(9386):798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Mandl RC, Collins DL, Evans AC, Kahn RS. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32(10):2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, McClure RK. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry: what is happening in the schizophrenic brain? Arch Gen Psychiatry. 2002;59(6):553–558. doi: 10.1001/archpsyc.59.6.553. [DOI] [PubMed] [Google Scholar]
- Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry. 1998;155(12):1661–1670. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- Yung A, Phillips L, McGorry PD. Treating Schizophrenia in the Prodromal Phase. London: Taylor & Francis; 2004. [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, Francey SM, McFarlane CA, Hallgren M, McGorry PD. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60(1):21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]