Abstract
Exposure to a specific pulsed electromagnetic field (PEMF) has been shown to produce analgesic (antinociceptive) effects in many organisms. In a randomized, double-blind, sham-controlled clinical trial, patients with either chronic generalized pain from fibromyalgia (FM) or chronic localized musculoskeletal or inflammatory pain were exposed to a PEMF (400 μT) through a portable device fitted to their head during twice-daily 40 min treatments over seven days. The effect of this PEMF on pain reduction was recorded using a visual analogue scale. A differential effect of PEMF over sham treatment was noticed in patients with FM, which approached statistical significance (P=0.06) despite low numbers (n=17); this effect was not evident in those without FM (P=0.93; n=15). PEMF may be a novel, safe and effective therapeutic tool for use in at least certain subsets of patients with chronic, nonmalignant pain. Clearly, however, a larger randomized, double-blind clinical trial with just FM patients is warranted.
Keywords: Chronic pain, Fibromyalgia, Musculoskeletal pain, Neuromodulation, Pulsed magnetic field therapy, Randomized clinical trial
Abstract
Il est démontré que l’exposition à un champ électromagnétique pulsé (CÉMP) produit des effets analgésiques (antinociceptifs) sur de nombreux organismes. Dans le cadre d’un essai clinique aléatoire à double insu contrôlé contre placebo, des patients atteints de douleurs généralisées chroniques causées par une fibromyalgie (FM) ou d’une douleur inflammatoire ou musculosquelettique chronique localisée ont été exposés à un CÉMP (400 μT) au moyen d’un dispositif portatif fixé sur leur tête pendant deux traitements quotidiens de 40 minutes sur une période de sept jours. Les auteurs ont enregistré l’effet de ce CÉMP sur la réduction de la douleur au moyen d’une échelle analogique visuelle. Les auteurs ont constaté un effet différentiel du CÉMP par rapport au traitement par placebo chez les patients atteints de FM, lequel avoisine une signification statistique (P=0,06) malgré le faible nombre (n=17). Cet effet n’était pas évident chez les personnes sans FM (P=0,93, n=15), Le CÉMP peut être un outil thérapeutique sécuritaire, efficace et novateur qui peut être utilisé chez au moins certains sous-groupes de patients atteints de douleurs chroniques non cancéreuses. Cependant, de toute évidence, un plus grand essai clinique aléatoire à double insu s’impose auprès d’un groupe de patients atteints seulement de FM.
Musculoskeletal (MSK) pain is common worldwide. In economically developed countries such as the United States, between 14% and 26% of the adult population suffers from chronic pain or arthritis (1,2), approximately 11% report chronic, widespread pain (3), and MSK disorders account for 15% of work-loss days (4). The figures are similar elsewhere. In Canada, 15% of adults report chronic MSK pain (5), 7% report chronic, widespread pain (6), and 5% report physical disabilities secondary to MSK illness (7). In Europe, the prevalence of chronic widespread MSK pain varies between 11% and 17% (8–10); and MSK disorders account for between 14% and 17% of work-loss days (11). Chronic pain costs an estimated $61.2 billion a year in the United States (12).
Fibromyalgia (FM) is a common (3,6) and particularly difficult cause of chronic pain. It is associated with chronic, widespread pain, debilitating fatigue and a host of other symptoms (13), and is relatively unresponsive to treatment (14). In recent years, the pain associated with FM is being increasingly attributed to central, rather than peripheral, nerve mechanisms (15,16), thereby distinguishing it from conditions such as osteoarthritis (OA), rheumatoid arthritis (RA) and localized pain disorders. This may be one of the reasons for FM’s relative resistance to many analgesics.
Most chronic pain is treated with pharmacological measures, including acetaminophen and nonsteroidal anti-inflammatory drugs such as ibuprofen, naproxen and the newer cyclooxygenase-2 inhibitors. Acetaminophen is generally effective in managing mild to moderate pain, but its chronic use has long been associated with potential liver toxicity, which can be fatal, even in therapeutic doses (17,18). Nonsteroidal anti-inflammatory drugs are commonly associated with gastrointestinal and renal side effects and, consequently, their use is contraindicated in many individuals (19). Muscle relaxants generally are not prescribed for chronic use because of their sedating effects and, like acetaminophen, generally are more effective for mild to moderate pain (20,21). Antidepressants, such as amitriptyline, have been prescribed chronically for the management of pain, and especially for the generalized pain of FM, but they generally produce a modest, transient benefit and significant side effects (22).
Opioid analgesics comprise a class of drugs that demonstrate a higher level of potency for pain relief than most other drugs, and can be used as alternative treatment for patients with more severe pain or who are intolerant to other pharmacological means. The efficacy of opioid analgesics in chronic, nonmalignant MSK pain has been documented in many publications (23–25). However, besides producing relatively common gastrointestinal side effects, opioid analgesics may produce physical and psychological dependence, so that many doctors and patients are uncomfortable with their use for chronic, nonmalignant pain (26). Consequently, the search continues for novel ways to control chronic pain.
Specific low-frequency pulsed electromagnetic fields (PEMF, 1000 Hz or less), also called complex neuroelectromagnetic pulses (Cnp), comprise a potentially new modality of therapy for chronic pain. They fall within the rapidly developing field of biomagnetics, a field that includes, among other modalities, transcutaneous electrical nerve stimulation (27–29), repetitive transcranial magnetic stimulation (30–35) and deep brain stimulation (36–40). PEMF differ from these other modalities primarily because they are a subthreshold, low-power, low-frequency electromagnetic waveform (equivalently described as an electromagnetic pulse-form) specifically designed to target limbic cells in the brain. The effects of PEMF on pain behaviour already have been reported in rats, mice, snails, pigeons and humans (41–43). In humans, this modality can be administered by means of cranial exposure to PEMF of low strength (±200 μT head surface to ±35 μT deep brain) (44,45). In one tested protocol, the therapy is applied for 40 min, twice daily, by means of a headset containing coils that are positioned bilaterally over the cranium. In a variety of chronic pain states, PEMF exposure is associated with a reduction in pain severity (46–48). The direction of its effect appears to be influenced by the specific magnetic field parameters and by light/dark conditions (49,50). Recently, a significant increase in thermal pain thresholds was reported among human volunteers exposed to PEMF (47). Encouraging work in our laboratory with chronic pain patients, specifically those with RA and FM, has revealed a significant reduction in pain ratings following exposure to PEMF (48). The present study was carried out to further our understanding of the analgesic effects of PEMF exposure on chronic MSK pain in humans. The primary objective was to determine if twice-daily use of a portable PEMF unit produces a reduction in pain severity versus sham treatment in patients with chronic pain. The secondary objective was to assess whether PEMF is more effective in patients with presumed central pain (FM) versus patients with more peripherally localized pain.
METHODS
The following is a randomized, double-blind, sham-controlled trial of PEMF among adult patients with either FM or a variety of other pain conditions associated with localized pain. The primary objective, described more specifically, was to determine if twice-daily use of a portable PEMF unit, for one week, resulted in a clinically meaningful and statistically significant reduction in self-reported pain severity, as measured on a pain severity visual analogue scale (VAS), versus an identical protocol sham treatment. The null hypothesis was that there would be no intergroup difference in the change in pain severity over time. The secondary objective was to assess the differential effect of PEMF in patients with chronic generalized pain secondary to FM versus patients with a variety of localized MSK or inflammatory pain states.
Subjects
Because one of the referring specialists had been heavily involved in FM research for several years, at the time of recruitment, patients with FM comprised roughly 50% of his overall practice. Based upon this large FM patient base and prior referral patterns, it was therefore assumed that approximately one-half of the patients referred for study assessment would have FM. It also was assumed, based upon the clinic’s demographics, that a sizeable majority of the referrals would be female. An a priori decision was made to include both male and female subjects, but to assess for sex effects at the time of data analysis. Consequently, 50 adult patients with chronic MSK pain were recruited either from the outpatient clinic at St Joseph’s Health Care Centre in London, Ontario, or by self-referral. Twenty-seven of the 50 were diagnosed FM patients (see Table 1 for details).
TABLE 1.
Participants versus drop-outs
| Participants | Drop-outs | |
|---|---|---|
| Total, n (%) | 32 (100) | 18 (100) |
| Female, n (%) | 24 (75) | 12 (67) |
| Male, n (%) | 8 (25) | 6 (33) |
| Mean age, years | 54.0 | 50.8 |
| Minimum age, years | 28 | 27 |
| Maximum age, years | 81 | 78 |
| Pain, n (%) | ||
| Fibromyalgia | 17 (53) | 10 (56) |
| Back | 6 (19) | 2 (11) |
| Neck and/or head | 2 (6) | 0 (0) |
| Localized extremity | 3 (9) | 4 (22) |
| Diffuse inflammatory (ie, RA) | 3 (9) | 0 (0) |
| Postoperative | 0 (0) | 1 (6) |
| Other | 1 (3) | 0 (0) |
| Data missing | 0 (0) | 1 (6) |
| Generalized (fibromyalgia) | 17 (53) | 10 (56) |
| Localized (all others) | 15 (47) | 7 (39) |
| Day 0 baseline pain VAS score | ||
| Mean | 8.5 | 7.8 |
| Minimum* | 2.1 | 0 |
| Maximum | 13.1 | 13.7 |
Subject with day 0 score of 2.11 on the 14 cm visual analogue scale (VAS) was included because the day 1 VAS score before treatment was 8.2. RA Rheumatoid arthritis
The patients’ health information, such as past and present diagnoses, sites of pain and medications, were obtained from each patient’s primary specialist. To be eligible, patients had to be at least 18 years of age, had to report chronic pain of at least six month’s duration, and had to have a specialist-established diagnosis to explain their chronic pain. Patients were excluded if they had participated in any PEMF studies previously, because of the concern that prior ‘education’ might bias their responses. Patients were also excluded if they had malignancies or any other potentially rapidly progressive cause of pain, if they had any medical condition that would preclude their participation in the study, if they were deemed to be incompetent to provide informed consent, if they were pregnant or if, for any reason, they were deemed to be unable to understand the fundamental nature of the study or be able to report pain severity without requiring a proxy.
Informed consent was obtained from all subjects after explaining the study verbally and through printed information sheets. The protocol had been preapproved by the University of Western Ontario Review Board for Health Sciences Research Involving Human Subjects.
Intervention and data collection
The observation period was four weeks, although subjects only received PEMF or sham treatment over the first seven days. After a baseline assessment of pain severity (on day 0), and using simple, random sampling, 24 and 26 patients (total n=50) were randomly assigned to the PEMF (treatment) and sham control exposure groups, respectively. Patients assigned to receive PEMF were given a headset containing coils beneath plastic ear coverings that were connected by wire to the portable PEMF-generating unit (Figure 1), which generated extremely weak PEMF (400 μT, 1000 Hz or less, 800 mT/sec) at the contact point of the headset. Headsets given to sham-exposed control patients were identical to those given to PEMF-exposed patients, except that they did not deliver any form of treatment. It is important to note that both the active and sham headsets were entirely silent, and that subjects experienced no sense of sound or vibration when they were ‘on’.
Figure 1).
Headset for delivering pulsed electromagnetic fields or sham treatment
For the first week, patients were asked to wear the headset just above their ears twice daily for seven days only. Subjects were instructed to self-administer the headset, turned on, for an exposure duration of 40 min, and to ensure that there were at least 4 h between successive treatments. The effect of the headsets, with and without PEMF, was measured immediately before and after each session by having the subjects rate their pain severity on a 14 cm VAS. This scale was identical to the one completed at the time of their baseline assessment. On this scale, subjects were asked to draw a line somewhere between the left extreme (no pain) and the right extreme (worst possible pain). During the experimental period, patients were allowed to keep their eyes open or closed, as they chose, and to continue their regular activities, with the exception of activities that could cause wetting of the headset, such as showering, washing their hair or swimming.
Monitoring of the effect of treatment continued for three weeks after treatment was discontinued, assessed daily during the second week of the study (the washout week), and weekly at the beginning of the third and fourth weeks, again asking each subject to rate their pain severity on a VAS. Each patient was also assessed at the end of each week by the investigating team, including an assessment of pain level using the VAS pain severity scale and questions on potential side effects of treatment derived from an a priori derived checklist.
Data analysis
Data analysis was both univariate and multivariate. The primary research objective was addressed by comparing all subjects receiving PEMF versus all subjects receiving sham treatment, with respect to change in VAS pain severity score from pretreatment (day 1) to post-treatment (day 7). Student’s t test for unpaired samples was used to compare the net change in pain severity from pretreatment time D1d1b (representing the first day of treatment, and the first of two doses of treatment that day, just before that initial dose was administered, be it PEMF or sham) to post-treatment time D7d2a (representing the seventh day of treatment and the second of the two daily doses just after that final dose was administered, be it PEMF or sham).
Repeated measures ANOVA was performed to assess the individual effects of each of the 14 treatment doses administered by computing the change in VAS pain severity score from D1d1b to D1d1a, from D1d2b to D1d2a, and so on, up to the change between D7d2b and D7d2a.
To address the second primary objective, the above univariate and repeated measures analyses were repeated, this time separating subjects with FM from those without FM.
To address the issue of potential confounders, univariate analyses were performed to assess the potential effects of age, sex and pretreatment pain score on change in pain severity, using Pearson χ2 analysis to assess the relationship between sex and change in pain severity, and Pearson correlation analyses to assess the relationships for age and pretreatment pain score versus change in pain severity.
All tests were two-tailed, and differences were considered statistically significant when P<0.05. Data were analyzed using SPSS software, version 14.0 (SPSS Inc, USA).
RESULTS
Participants and nonparticipants
Fifty subjects were randomly assigned to receive either PEMF (n=24) or sham treatment (n=26); however, only 32 of the 50 were included in the final analysis: 15 of 24 (63%) randomly assigned to receive PEMF and 17 of 26 (66%) randomly assigned to receive sham treatment. Although the initial intention was to conduct an intent-to-treat analysis, a consensus decision was made not to analyze the 18 drop-outs for the following reasons:
15 of the 18 never returned on day 1 to pick up their headsets; consequently, they received no doses of treatment, either PEMF or sham;
two of the subjects had VAS pain severity scores of 2 or less at baseline;
one subject, assigned to the sham treatment group, had a baseline VAS of 3.6 and stopped all treatment on the third day; and
participants and drop-outs were otherwise demographically and clinically similar (Table 1).
Demographic and baseline clinical characteristics
Tables 2 and 3 present demographic and baseline clinical characteristics for all participating subjects, comparing all sham versus PEMF subjects in Table 2, and all FM versus non-FM subjects in Table 3. Statistical comparisons were not performed, by consensus decision, because such a comparison is unwarranted in a random sample. What is more meaningful than any statistically significant intergroup differences are clinically significant differences. Note that the 17 sham-treated subjects were more than four years older (56.3 versus 51.7 years of age, respectively). Also, the PEMF group had a day 1 pre-treatment score almost 1 cm greater than the sham group (9.3 versus 8.4, respectively) in terms of VAS pain severity. The sex distribution for the two groups was similar (sham group: 13 of 17 (76%) female; PEMF group: 11 of 15 (73%) female.
TABLE 2.
Baseline comparison of subject groups: Sham versus low-frequency pulsed electromagnetic fields (PEMF)
| Sham
|
PEMF
|
|||||
|---|---|---|---|---|---|---|
| FM | Other | Total | FM | Other | Total | |
| Total, n | 9 | 8 | 17 | 8 | 7 | 15 |
| Female, n | 9 | 4 | 13 | 7 | 4 | 11 |
| Male, n | 0 | 4 | 4 | 1 | 3 | 4 |
| Mean age, years | 54.6 | 58.3 | 56.3 | 47.5 | 56.6 | 51.7 |
| Minimum age, years | 41 | 47 | 41 | 28 | 37 | 28 |
| Maximum age, years | 65 | 78 | 78 | 72 | 81 | 81 |
| Day 0 baseline pain VAS score* | 9.0 | 7.7 | 8.4 | 8.9 | 8.4 | 8.6 |
| Day 1 baseline pain VAS score† | 8.5 | 8.4 | 8.4 | 9.6 | 8.9 | 9.3 |
Pain severity on day recruited into study;
Pain severity just before first sham or PEMF treatment. FM Fibromyalgia; VAS Visual analogue scale
TABLE 3.
Baseline comparison of subject groups: Fibromyalgia (FM) versus localized pain
| FM
|
Localized pain
|
|||||
|---|---|---|---|---|---|---|
| Sham | PEMF | Total | Sham | PEMF | Total | |
| Total, n | 9 | 8 | 17 | 8 | 7 | 15 |
| Female, n | 9 | 7 | 16 | 4 | 4 | 8 |
| Male, n | 0 | 1 | 1 | 4 | 3 | 7 |
| Mean age | 54.6 | 47.5 | 51.1 | 58.3 | 56.6 | 57.4 |
| Minimum age | 41 | 28 | 28 | 47 | 37 | 37 |
| Maximum age | 65 | 72 | 72 | 78 | 81 | 81 |
| Day 0 baseline pain VAS score* | 9.0 | 8.9 | 8.9 | 7.7 | 8.4 | 8.0 |
| Day 1 baseline pain VAS score† | 8.5 | 9.6 | 9.0 | 8.4 | 8.9 | 8.6 |
Pain severity on day recruited into study;
Pain severity just before first sham or low-frequency pulsed electromagnetic fields (PEMF) treatment. VAS Visual analogue scale
The sex distribution was disproportionately female in the FM versus localized pain group (94% versus 53%, respectively), consistent with the sex distribution of FM patients in the clinic and the literature (13). FM subjects were more than six years younger (51.1 versus 57.4 years of age, respectively), although the age ranges were similar. FM subjects had a day 0 VAS pain severity score almost 1 cm higher than the non-FM subjects (8.9 versus 8.0, respectively), but the day 1 pretreatment scores were more similar (9.0 versus 8.6, respectively). The day VAS score in FM subjects on PEMF (9.6) was more than one full point (1.1) higher than in FM subjects who then received sham treatment (8.5); among those without FM, this difference was only 0.5 (8.9 versus 8.4, respectively).
PEMF versus sham treatment effects
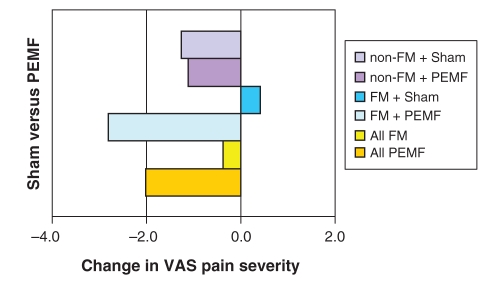
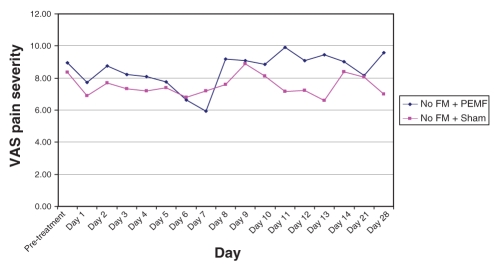
Net change in pain severity from pretreatment (day 1) to post-treatment (day 7) is depicted in Figure 2. Combining FM and non-FM subjects, pretreatment VAS fell from 9.3 cm to 7.3 cm (a net change of −2.0) in the PEMF-treated group, versus a fall from 8.4 to 8.1 (a net change of −0.4 [to two decimal places, from 8.45 to 8.07, of −0.38.]) in those receiving sham treatments; this intergroup difference did not achieve statistical significance. Analyzing FM subjects alone, PEMF-treated subjects experienced a 2.8 cm drop in pain VAS score compared with a 0.4 increase in pain severity among sham-treated subjects; again, this failed to achieve statistical significance (P=0.11) (Figure 3). Non-FM subjects exhibited virtually no difference at all between PEMF and sham-treated individuals, with mean declines in pain severity of −1.1 and −1.3, respectively (P=0.93) (Figure 4).
Figure 2).
Change in pain severity from pretreatment to post-treatment: low-frequency pulsed electromagnetic fields (PEMF) versus sham; fibromyalgia (FM) versus no FM
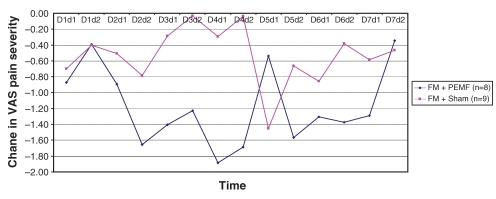
Figure 3).
Change in pain severity from pre- to post-treatment with each dose of pulsed electromagnetic fields (PEMF) in subjects with fibromyalgia (FM). VAS Visual analogue scale
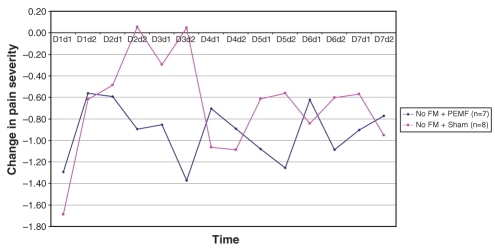
Figure 4).
Change in pain severity from pre- to post-treatment with each dose of pulsed electromagnetic fields (PEMF) in subjects without fibromyalgia (FM)
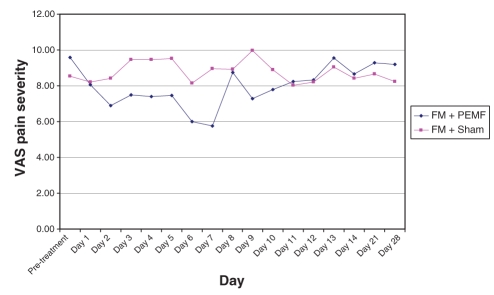
Recalling that each subject received twice-daily treatments (PEMF or sham) over the first seven days, and had pretreatment and post-treatment evaluations each time, each subject, over the first week, received 14 treatments and had 14 corresponding pre- to post-treatment comparisons. The means for each of these 14 data points are presented graphically in Figures 5 and 6, comparing PEMF with sham treatment among FM subjects (Figure 5) and PEMF with sham treatment among non-FM subjects (Figure 6). Repeated measures ANOVA among the FM subjects approached statistical significance for treatment effect (P=0.06), whereas it fell far short of statistical significance in the non-FM group (P=0.46).
Figure 5).
Visual analogue scale (VAS) pain level scores over four weeks in subjects with fibromyalgia (FM) treated with pulsed electromagnetic fields (PEMF) compared with sham
Figure 6).
Visual analogue scale (VAS) pain level scores over four weeks in subjects without fibromyalgia (FM) treated with pulsed electromagnetic fields (PEMF) compared with sham
Assessment of potential confounders
The small sample size prohibited logistic regression to assess the relative contributions of potential confounders, so potential effects were examined using univariate analyses. Examining sex was warranted given the disproportionate number of FM subjects who were female (94% versus 53% among non-FM subjects) and the increased response to treatment among FM versus non-FM subjects. However, the difference between PEMF and sham was greater among males (PEMF −3.37 versus sham −0.77; difference of −2.60) than among females (PEMF −1.53 versus sham −0.25; difference of −1.28); albeit neither difference was statistically significant (P=0.35 and P=0.39, respectively). Two other potential confounders, given group differences observed in group demographics and baseline characteristics, were age (recalling that subjects receiving PEMF and subjects with FM were younger than their counterparts), and pretreatment (day 1) VAS pain severity (recalling that subjects receiving PEMF and subjects with FM had higher pretreatment scores). Both these variables were examined for correlation relative to change in pain from pretreatment to post-treatment. Age was not statistically correlated (R=−0.12, P=0.53); but pre-treatment score was negatively correlated (R=−0.38, P<0.05), indicating that increasing pretreatment VAS score was associated with greater decreases in pain.
Post-treatment washout
During the washout week, there was a reasonably steady rise in pain severity in those with FM who had received PEMF (Figure 5). Conversely, in the sham group, there was no clear trend. Among those without FM, a rapid rise in pain level followed cessation of PEMF, whereas there again was no clear trend in those receiving the sham treatment (Figure 6). Neither trend achieved statistical significance. During the third and fourth weeks, pain level continued to be high in the PEMF groups, whereas it fluctuated among those in the sham group.
Side effects and complications of treatment
No subject in either group reported significant side effects from use of the headset twice daily. Compliance with treatment was fair, with 11 of 15 (73%) in the PEMF group and 16 of 17 (94%) in the sham group using the headset 10 or more times out of 14 available treatments. Only one of four of the less-compliant subjects in the PEMF group had FM, and none of the four had seemed to respond to PEMF (net change from baseline: mean +0.28, range −0.22 to +1.11). Interestingly, the one noncompliant sham subject, who missed six treatments, had a net decline (improvement) in pain severity of 2.22.
DISCUSSION
Chronic pain is one of the most troublesome and disabling conditions that physicians are called upon to treat. However, current options for the treatment of more severe chronic pain are generally flawed by reason of being ineffective in controlling anything more than mild to low-moderate pain, or because they are associated with significant side effects or risks of drug tolerance, dependence and addiction. The rapidly expanding field of biomagnetics potentially offers a variety of therapeutic modalities that may be of clinical value, especially in patients who have pain that is resistant to more traditional therapeutics. Among these modalities, PEMF is unique. To begin with, as opposed to a transcutaneous electrical nerve stimulation unit – an electrical device that is associated with currents, such that electrons actually pass through the tissues to which the device is being applied, thereby providing localized pain relief (27–29) – PEMF is an electromagnetic process that is not associated with currents, but with magnetic fields that can be applied to the brain to generate more global pain relief. Secondly, as opposed to a variety of oscillating fields, such as repetitive transcranial magnetic stimulation, which primarily relies on the physical attributes of the electromagnetic field, PEMF utilizes particular aspects of the pulse-form shape to affect a clinical response. An analogy to clarify this distinction would be to think of oscillating fields as using the percussive effect of a sound wave, and PEMF as using the information carried within a complex form of that wave, such as that created by human speech. The PEMF that we use exclusively are also of lower power and frequency than virtually all other modalities, so that theoretically, they should be associated with fewer adverse effects.
Considerable prior work has demonstrated the beneficial effects of PEMF in various animal models. One of the first notable findings was the apparent attenuation of morphine-induced analgesia in mice by magnetic resonance imaging (52). Subsequently, Prato et al (1995) assessed the potential mechanisms for the previously observed analgesia in the land snail when subjected to a hot plate (53). It was determined that analgesic effects occur only with time-varying magnetic fields and at certain combinations of frequency and amplitude, and that the effects are influenced by the presence or absence of light (54). Specifically, they considered whether the magnetic field effects involve an indirect induced electric current mechanism or a direct effect. Findings suggested that, both in light and dark, the effect of a pulsed extremely low-frequency magnetic field is mediated via a direct magnetic field detection mechanism, rather than an induced current.
In a further study on the land snail, again subjected to extremely low-frequency magnetic fields, it was demonstrated that exposure to a specific electromagnetic field, the Cnp, increased the latency of the snail’s nociceptive response to a hot plate, while a random pulsed low-frequency magnetic field did not (51). Moreover, this Cnp analgesia was significantly decreased by administering the opioid antagonist, naloxone, again suggesting that Cnp’s antinociceptive effects somehow involve the augmentation of endogenous opioids. Subsequently, this same effect was demonstrated in mice (42).
As a result of these early animal studies, in conjunction with more recent animal work, further studies involving humans have documented effects both on standing balance (44,49,55) and pain (41,46–48). The mechanisms by which these effects occur are not fully understood. However, there is evidence that PEMF actually change brain wave activity, suggesting that the symptom-altering effects of electromagnetic waves are the result of a direct effect on central nervous function (45). Moreover, the blocking effects of naloxone strongly suggest that the electromagnetic forces affect the release of endogenous opioids, probably via direct influences upon the brain’s limbic system.
In our most recent research, we have shown that low-frequency PEMF can be delivered to adults with chronic pain by means of a headset, with minimal to no inconvenience or side effects, at least in the short term. Moreover, even though our study was small, the evidence suggests that PEMF, so delivered, may have significant analgesic effects, at least in patients with FM.
That there seemed to be a differential effect between those with FM and those with more localized MSK pain or inflammatory pain was a bit surprising. In an earlier study, patients with chronic knee pain receiving a two-week exposure to a magnetic field experienced a significant improvement in the individual’s levels of self-rated pain and physical functioning (56). On the other hand, given the perceived central neural mechanism operating in FM (15,16), it makes some sense that this population would be most responsive to a therapeutic modality delivered via a headset. The pain associated with OA, for example, is believed to arise from irritation of nociceptors in and around the joint itself. In the joint, tissues containing nociceptors include the joint capsule, ligaments and bone. Nociceptive stimuli are likely to emanate from one or more of these locations in people with OA (57). This peripheral origin of OA pain is the likely reason for its therapeutic response to nonsteroidal anti-inflammatories (58), a response that is not seen in patients with FM (59). In early RA, much of the pain and stiffness likely arises from irritation of the joint capsule (synovium), secondary to inflammation (60–62), whereas later in RA, it also results from bony microfractures and other tissue disruption, similar to what is seen in late OA (62). In both instances, the pain mechanisms seem to originate peripherally, again making relief from a therapeutic modality targeting the brain seem less likely.
Our FM sample was different than those we recruited who did not have FM, in that a much greater percentage were female, the FM patients were somewhat younger, and their baseline pain severity scores were generally higher. It is conceivable, then, that the differential response of FM to PEMF was the result of one of these potential confounders. However, both on univariate and multivariate analyses, none of these variables explained the seemingly selective response of FM to PEMF. Our sample was too small, however, to allow for full multivariate testing. A larger sample would allow for linear or logistic regression to determine the relative impact of each of these variables, including treatment arm, on change in pain.
In the present study, the percentage of pain reduction was not uniform throughout the first week of study in patients exposed to PEMF or to sham treatment, and in either those with FM or without. This fluctuation may have been due to the headsets not fitting each individual correctly, which may have led to discomfort, and therefore, to interference, at least at times during the treatment week. Increased patient training and a longer duration of treatment to allow for enhanced use of the headsets may result in a greater effect of treatment than we observed.
Over the entire four weeks of observation, the group of subjects with FM who received PEMF exhibited changes in VAS pain severity that were most consistent with a treatment response. Specifically, pain severity declined by the end of the first day and continued to decline throughout the seven days of treatment; over the entire week, pain levels were lower than in the sham group, with the intergroup difference increasing steadily as the week progressed. By the end of the first day after cessation of PEMF, pain had increased dramatically, almost to pretreatment levels; pain fell on the second post-treatment day, and then steadily increased through the washout week. Pain VAS remained high at the end of weeks 3 and 4. Subjects without FM receiving PEMF had a somewhat delayed decline in VAS pain severity but to a lesser degree, and it only fell below the pain levels of the sham group by the seventh day. As with those with FM, a rebound increase in pain was noticed on the first day post-treatment, but pain fluctuated thereafter. Graphically, there was no clear trend toward decrease or increase in pain severity in either sham group.
The net reduction in pain on the VAS was equivalent to a low to moderate dose of opioid analgesic in PEMF-exposed patients (63–67). It has often been pointed out that both the endogenous and exogenous opioid systems are influenced by PEMF exposure sessions in animals and humans (68–70). Moreover, when an opiate such as morphine is used in combination with PEMF, the side effects of the opiate may be reduced (42). Consequently, we believe not only that PEMF should be investigated further as a replacement for opioid analgesics in some patients with chronic pain, in particular those with FM, but that PEMF may also warrant investigation as a supplement to opioids, especially in patients with more severe pain.
In our study, the overall net percentage change for PEMF was 20%, corresponding to a percentage change of 24% and 4% for treatment and placebo, respectively. A subset analysis on patients (n=15) who reasonably complied with the protocol (used device 12 or more applications out of 14) and whose intake VAS was seven or higher revealed a net change of 38%. Both values compare favourably with the intent-to-treat responses of 16% to 23% observed with low to low-intermediate dose sustained or immediate-release oxycodone (65–66); and with the 9% to 12% observed with low-dose sustained-release morphine (68) (Table 4).
TABLE 4.
Net analgesic efficacy of opioids in chronic musculoskeletal pain
| Medication | Dosage | Daily morphine equivalent (mg) | Treatment, % change | Placebo, % change | Net % change | Treatment group, n | Placebo group, n | Follow-up period, days | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Avinza* | 30 mg qam | 30 | 27 | 15 | 12% | 46 | 50 | 28 | 3 |
| Avinza* | 30 mg qpm | 30 | 23 | 15 | 8% | 40 | 50 | 28 | 3 |
| MS Contin† | 15 mg bid | 30 | 23 | 15 | 9% | 48 | 50 | 28 | 3 |
| CR Codeine | 159 mg bid | 95 | 44 | 10 | 34 | 31 | 35 | 28 | 4 |
| CR Oxycodone | 10 mg bid | 40 | 28 | 13 | 16 | 20 | 18 | 14 | 5 |
| CR Oxycodone | 20 mg bid | 80 | 35 | 13 | 23 | 25 | 18 | 14 | 5 |
| CR Codeine | 273 mg/day | 82 | 26 | −2 | 28 | 30 | 30 | 7 | 6 |
| CR Oxycodone | 40 mg/day | 80 | 25 | 2 | 23 | 34 | 36 | 28 | 7 |
| IR Oxycodone | 40 mg/day | 80 | 23 | 2 | 21 | 37 | 36 | 28 | 7 |
| Cnp | 40 min bid | N/A | 24 | 4 | 20 | 17 | 17 | 7 | 8 |
| Cnp | 40 min bid | N/A | 33 | −5 | 38 | 7 | 8 | 7 | 8 |
King Pharmaceuticals, USA;
Purdue Pharma, Canada. bid Twice daily; Cnp Complex neuroelectromagnetic pulse; CR Controlled release; IR Immediate release; qam Every morning; qpm Every evening
Having said this, we urge caution to every reader, given that our study has undeniable limitations. To begin with, the study produced only marginally significant results, so that it is possible that the seemingly beneficial effects of PEMF in our study were merely the result of chance. Second, our study only followed patients for a total of four weeks, and we only delivered treatment for one week. It is conceivable, although we think improbable, that use of PEMF may result in significant rebound exacerbation of pain or, alternatively, that PEMF may be subject to tolerance, in the same way that many patients ultimately develop tolerance to the analgesic effects of opioids, consequently requiring higher and higher doses to achieve satisfactory pain relief. Clearly, longer-term follow-up is warranted to address this concern.
One data concern that arises is that there was a day 1 difference in pain VAS score of 1.1 between FM subjects who ultimately received PEMF (VAS=9.6) and FM subjects who received the sham treatment (8.5); this compares to a difference of just 0.5 between subjects without FM who received PEMF (8.9) versus sham treatment (8.4). Because there is a statistical tendency for values to regress to the mean, any such tendency among the FM subjects would likely be greater than among the remaining subjects, which is a potential source of type I error (identifying a difference which does not truly exist). A larger study clearly is warranted to offset this potential bias, because larger, random samples of FM subjects and patients with other sources of pain would tend to reduce any chance differences between the within-disease subgroups (PEMF versus sham) at baseline.
Another source of bias might pertain to the level of physical activity exerted by FM versus non-FM subjects. Given the high rate of debilitating fatigue reported by FM patients (13), it may be that any improvement in pain in this group was associated with less of an increase in activity than among those without FM. If increased activity increases pain, the increased level of activity among non-FM subjects may have offset any analgesic effect, relative to what was experienced by those with FM. This would be another potential source of type I error. Future research should assess outcomes beyond pain severity, including the levels of activity and function, to determine if there is any potentially confounding interactions between pain severity and these other variables.
Finally, 18 of our initially randomly assigned 50 patients dropped out of the study, including 10 with FM. Fifteen never received a single treatment, which is a usual criterion for inclusion in intent-to-treat analyses. The remaining three, two from the PEMF group and one from the sham group, were excluded on the basis of no longer meeting inclusion criteria at the time of their day 1 assessment, due to exceedingly low pain severity scores. Had we performed an intent-to-treat analysis, these three should have been included, because they did receive some treatment. Nonetheless, we felt justified excluding these three subjects because they were almost equally distributed between the two treatment arms and between the FM versus no FM groups (two versus one), because they were so few in number, and because none of the three received more than a few treatments. In addition, ours essentially was a negative study, albeit with enticing results in FM patients which warrant further study.
Consequently, we believe that our study forms another crucial step in the development of a novel therapeutic option for patients with chronic pain and, in particular, for patients with disorders like FM, in which central mechanisms of pain appear to predominate. Traditionally, this has been a group that is poorly served by existing treatments. Our hope is that PEMF may offer a very safe, yet effective alternative for at least some these patients. Clearly, a larger randomized and double-blinded clinical trial, focusing especially on FM patients, is warranted. Based upon the variances determined in our study, we predict that a study with 25 FM subjects per group would demonstrate a 25% reduction in VAS pain severity, even allowing for 35% drop-out rate (so that 16 per group complete the study). However, given the potential confounding effect of pretreatment pain levels, and the preference for intent-to-treat analysis, a study with 25 to 30 subjects per group completing treatment would be preferable.
Acknowledgments
Funding for this study was provided by the Ontario Research and Development Challenge Fund (Ontario, Canada), Canadian Institute of Health Research, Fralex Therapeutics Inc (a spin-off company of the University of Western Ontario and Lawson Health Research Institute), and the University of Western Ontario Interdisciplinary Pain Program. Special thanks to “Real Ghostwriters Ltd” (www.realghostwriters.com) for manuscript preparation and statistical consulting, and to Mr Lynn Keenliside for technical assistance.
Footnotes
DECLARATIONS: Alex W Thomas PhD: Dr Thomas is the Bioelectromagnetics Scientist for Lawson Health Research Institute (LHRI) and St. Joseph’s Hospital (London Ontario Canada), assistant professor with the Departments of Medical Biophysics and Diagnostic Imaging and Nuclear Medicine, Schulich School of Medicine and Dentistry, University of Western Ontario (UWO) and a co-inventor of specific pulsed low frequency magnetic field therapy (neuromodulation, U.S. Patent # 6,234,953 and patents pending.) Dr Thomas holds a noncontrolling interest in Fralex Therapeutics Inc. (a spin-off company of UWO and LHRI), which owns the technology. Dr Thomas also serves as the V-P Research for Fralex Therapeutics Inc. Dr Thomas was Ms Graham’s supervisor for this project. Frank S Prato PhD: Dr Prato is the Chair of Imaging Sciences and Director of Imaging for Lawson Health Research Institute (LHRI) and St Joseph’s Hospital (London Ontario Canada), professor with the Departments of Medical Biophysics and Diagnostic Imaging and Nuclear Medicine, Schulich School of Medicine and Dentistry, University of Western Ontario (UWO) and a co-inventor of specific pulsed low-frequency magnetic field therapy (neuromodulation, U.S. Patent # 6,234,953 and patents pending.) Dr Prato holds a noncontrolling interest in Fralex Therapeutics Inc (a spinoff company of UWO and LHRI), which owns the technology. Dr Prato also serves as the Chair of the Scientific Advisory Committee for Fralex Therapeutics Inc. Karissa Graham (summer student, University of Western Ontario): Ms Graham was the blinded experimenter for this project and after participant enrolment by a physician (Drs Moulin and Morley Forster), performed all of the participant contact and testing. Julia McKay (PhD candidate): Ms McKay is a graduate student of Dr Thomas and contributed significantly to analysis and manuscript preparation. Patricia Morley Forster MD: Dr Morley Forster (anesthetist) is the former Earl Russell Chair in Pain Management (UWO). Dr Morley Forster and Dr Moulin are not currently affiliated with the technology or Fralex Therapeutics Inc, although Dr Morley Foster is a former consultant to Fralex Therapeutics. Sesh Chari MD: Dr Chari (general practitioner) is the Chief Operating Officer with Fralex Therapeutics Inc. and provided the comparisons to pharmaceuticals. Drs Thomas, Prato, and Chari did not have any participant contact. None of the authors had access to the code-key during the active protocol. Participants were randomly assigned and all data were blinded by a third party outside of the study group and the code broken after the completion of all subjects. Statistical analysis was done by a contracted third party expert in rheumatology and biostatistics outside of the study group.
REFERENCES
- 1.Lawrence RC, Hochberg MC, Kelsey JL, et al. Estimates of prevalence of selected arthritic and musculoskeletal diseases in the United States. J Rheumatol. 1989;16:427–41. [PubMed] [Google Scholar]
- 2.Magni G, Marchetti M, Moreschi C, Merskey H, Luchini SR. Chronic musculoskeletal pain and depressive symptoms in the National Health and Nutrition Examination. I. Epidemiologic follow-up study. Pain. 1993;53:163–8. doi: 10.1016/0304-3959(93)90076-2. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 4.Felts W, Yelin E. The economic impact of the rheumatic diseases in the United States. J Rheumatol. 1989;16:867–84. [PubMed] [Google Scholar]
- 5.Lee P, Helewa A, Smythe HA, Bombardier C, Goldsmith CH. Epidemiology of musculoskeletal disorders (complaints) and related disability in Canada. J Rheumatol. 1985;12:1169–73. [PubMed] [Google Scholar]
- 6.White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: The prevalence of fibromyalgia syndrome in London, Ontario. J Rheumatol. 1999;26:1570–6. [PubMed] [Google Scholar]
- 7.Reynolds DL, Chambers LW, Badley EM, et al. Physical disability among Canadians reporting musculoskeletal diseases. J Rheumatol. 1992;19:1020–30. [PubMed] [Google Scholar]
- 8.Andersson HI, Ejlertsson G, Leden I, Rosenberg C. Chronic pain in a geographically defined general population: Studies of differences in age, gender, social class, and pain localization. Clin J Pain. 1993;9:174–82. doi: 10.1097/00002508-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Hagen JB, Kvien TK, Bjorndal A. Musculoskeletal pain and quality of life in patients with non-inflammatory joint pain compared to rheumatoid arthritis: a population study. J Rheumatol. 1997;24:1703–9. [PubMed] [Google Scholar]
- 10.Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. J Rheumatol. 1993;20:710–3. [PubMed] [Google Scholar]
- 11.Wood PHN, Badley EM. Rheumatic disorders. In: Miller DL, Farmer RDT, editors. Epidemiology of Diseases. Oxford: Blackwell Scientific Publications; 1982. pp. 333–46. [Google Scholar]
- 12.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–54. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe F. The clinical syndrome of fibrositis. Am J Med. 1986;81:7–14. doi: 10.1016/0002-9343(86)90866-1. [DOI] [PubMed] [Google Scholar]
- 14.Ledingham J, Doherty S, Doherty M. Primary fibromyalgia syndrome – an outcome study. Br J Rheumatol. 1993;32:139–42. doi: 10.1093/rheumatology/32.2.139. [DOI] [PubMed] [Google Scholar]
- 15.Staud R, Domingo M. Evidence for abnormal pain processing in fibromyalgia syndrome. Pain Med. 2001;2:208–15. doi: 10.1046/j.1526-4637.2001.01030.x. [DOI] [PubMed] [Google Scholar]
- 16.Staud R. Evidence of involvement of central neural mechanisms in generating fibromyalgia pain. Curr Rheumatol Rep. 2002;4:299–305. doi: 10.1007/s11926-002-0038-5. [DOI] [PubMed] [Google Scholar]
- 17.Larson AM, Polson J, Fontana RJ, et al. Acute Liver Failure Study Group Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 18.Bolesta S, Haber SL. Hepatotoxicity associated with chronic acetaminophen administration in patients without risk factors. Ann Pharmacother. 2002;36:331–3. doi: 10.1345/aph.1A035. [DOI] [PubMed] [Google Scholar]
- 19.Henry D. Assessing the benefits and risks of drugs. The example of NSAIDs. Aust Fam Physician. 1990;19:385–7. [PubMed] [Google Scholar]
- 20.Tofferi JK, Jackson JL, O’Malley PG. Treatment of fibromyalgia with cyclobenzaprine: A meta-analysis. Arthritis Rheum. 2004;51:9–13. doi: 10.1002/art.20076. [DOI] [PubMed] [Google Scholar]
- 21.Childers MK, Borenstein D, Brown RL, et al. Low-dose cyclobenzaprine versus combination therapy with ibuprofen for acute neck or back pain with muscle spasm: A randomized trial. Curr Med Res Opin. 2005;21:1485–93. doi: 10.1185/030079905X61938. [DOI] [PubMed] [Google Scholar]
- 22.Carette S, Bell MJ, Reynolds WJ, et al. Comparison of amitriptyline, cyclobenzaprine, and placebo in the treatment of fibromyalgia: a randomized, double-blind clinical trial. Arthritis Rheum. 1994;37:32–40. doi: 10.1002/art.1780370106. [DOI] [PubMed] [Google Scholar]
- 23.France RD, Urban BJ, Keefe FK. Long term use of narcotic analgesics in chronic pain. Soc Sci Med. 1984;19:1379–82. doi: 10.1016/0277-9536(84)90027-3. [DOI] [PubMed] [Google Scholar]
- 24.Zenz M, Strumpf M, Tryba M. Long-term oral opioid therapy in patients with chronic nonmalignant pain. J Pain Symptom Manage. 1992;7:69–77. doi: 10.1016/0885-3924(92)90116-y. [DOI] [PubMed] [Google Scholar]
- 25.Schofferman J. Long-term use of opioid analgesics for the treatment of chronic pain of non-malignant origin. J Pain Symptom Manage. 1993;7:279–88. doi: 10.1016/0885-3924(93)90156-p. [DOI] [PubMed] [Google Scholar]
- 26.Moulin DE. Opioid analgesics for chronic nonmalignant pain. Can J CME. 1996;2:137–43. [Google Scholar]
- 27.Long DM. Electrical stimulation of the nervous system for pain control. Electroencephalogr Clin Neurophysiol Suppl. 1978;34:343–8. [PubMed] [Google Scholar]
- 28.Long DM. Fifteen years of transcutaneous electrical stimulation for pain control. Stereotact Funct Neurosurg. 1991;56:2–19. doi: 10.1159/000099388. [DOI] [PubMed] [Google Scholar]
- 29.Rushton DN. Electrical stimulation in the treatment of pain. Disabil Rehabil. 2002;24:407–15. doi: 10.1080/09638280110108832. [DOI] [PubMed] [Google Scholar]
- 30.Leo RJ, Latif F. Repetitive transcranial magnetic stimulation (rTMS) in experimentally induced and chronic neuropathic pain: A review. J Pain. 2007;8:453–9. doi: 10.1016/j.jpain.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with non-invasive brain stimulation techniques. Lancet Neurol. 2007;6:188–91. doi: 10.1016/S1474-4422(07)70032-7. [DOI] [PubMed] [Google Scholar]
- 32.Lefaucheur JP. The use of repetitive transcranial magnetic stimulation (rTMS) in chronic neuropathic pain. Neurophysiol Clin. 2006;36:117–24. doi: 10.1016/j.neucli.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Lefaucheur JP. Transcranial magnetic stimulation in the management of pain. Suppl Clin Neurophysiol. 2004;57:748. doi: 10.1016/s1567-424x(09)70415-5. [DOI] [PubMed] [Google Scholar]
- 34.Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76:833–8. doi: 10.1136/jnnp.2004.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Nguyen JP. Neuropathic pain controlled for more than a year by monthly sessions of repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol Clin. 2004;34:91–5. doi: 10.1016/j.neucli.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Bussone G, Franzini A, Proietti Cecchini A, et al. Deep brain stimulation in craniofacial pain: seven years' experience. Neurol Sci. 2007;28:S146–9. doi: 10.1007/s10072-007-0768-2. [DOI] [PubMed] [Google Scholar]
- 37.Rasche D, Rinaldi PC, Young RF, Tronnier VM. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus. 2006;21:E8. doi: 10.3171/foc.2006.21.6.10. [DOI] [PubMed] [Google Scholar]
- 38.Osenbach RK. Motor cortex stimulation for intractable pain. Neurosurg Focus. 2006;21:E7. doi: 10.3171/foc.2006.21.6.12. [DOI] [PubMed] [Google Scholar]
- 39.Hamani C, Schwalb JM, Rezai AR, Dostrovsky O, Davis KD, Lozano AM. Deep brain stimulation for chronic neuropathic pain: Long-term outcome and the incidence of insertional effect. Pain. 2006;125:188–96. doi: 10.1016/j.pain.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Bittar RG, Kar-Purkayastha I, Owen SL, et al. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12:515–9. doi: 10.1016/j.jocn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Shupak NM, Prato FS, Thomas AW. Therapeutic uses of pulsed magnetic field exposure: A review. URSI: The Radio Science Bulletin. 2003;307:9–32. [Google Scholar]
- 42.Shupak NM, Hensel JM, Cross-Mellor SK, Kavaliers M, Prato FS, Thomas AW. Analgesic and behavioural effects of a 100 T specific pulsed extremely low frequency magnetic field on control and morphine treated CF-1 mice. Neurosci Lett. 2004;354:30–3. doi: 10.1016/j.neulet.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 43.Prato FS, Kavaliers M, Thomas AW. Extremely low frequency magnetic fields can either increase or decrease analgesia in the land snail depending on field and light conditions. Bioelectromagnetics. 2000;4:287–301. doi: 10.1002/(sici)1521-186x(200005)21:4<287::aid-bem5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 44.Thomas AW, Drost DJ, Prato FS. Human subjects exposed to a specific pulsed (200 microT) magnetic field: Effects on normal standing balance. Neurosci Lett. 2001;297:121–4. doi: 10.1016/s0304-3940(00)01688-8. [DOI] [PubMed] [Google Scholar]
- 45.Cook CM, Thomas AW, Prato FS. Resting EEG is affected by exposure to a pulsed ELF magnetic field. Bioelectromagnetics. 2004;25:196–203. doi: 10.1002/bem.10188. [DOI] [PubMed] [Google Scholar]
- 46.Sartucci F, Bonfiglio L, Del Seppia C, et al. Changes in pain perception and pain-related somatosensory evoked potentials in humans produced by exposure to oscillating magnetic field. Brain Res. 1997;769:362–6. doi: 10.1016/s0006-8993(97)00755-5. [DOI] [PubMed] [Google Scholar]
- 47.Shupak NM, Prato FS, Thomas AW. Human exposure to a specific pulsed magnetic field: Effects on thermal sensory and pain thresholds. Neurosci Lett. 2004;363:157–62. doi: 10.1016/j.neulet.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 48.Shupak NM, McKay JC, Nielson WR, Rollman GB, Prato FS, Thomas AW. Exposure to a specific low frequency magnetic field: A double-blind placebo-controlled study of effects on acute pain ratings in rheumatoid arthritis and fibromyalgia pain populations. Pain Res Manag. 2006;11:85–92. doi: 10.1155/2006/842162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prato FS, Thomas AW, Cook CM. Human standing balance is affected by exposure to pulsed ELF magnetic fields: Light intensity-dependent effects. Neuroreport. 2001;12:1501–5. doi: 10.1097/00001756-200105250-00040. [DOI] [PubMed] [Google Scholar]
- 50.Koziak AM, Desjardins D, Keenliside LD, Thomas AW, Prato FS. Light alters nociceptive effects of magnetic field shielding. Bioelectromagnetics. 2006;27:10–5. doi: 10.1002/bem.20170. [DOI] [PubMed] [Google Scholar]
- 51.Thomas AW, Kavaliers M, Prato FS, Ossenkopp KP. Antinociceptive effects of a pulsed magnetic field in the land snail, Cepaea nemoralis. Neurosci Lett. 1997;222:107–10. doi: 10.1016/s0304-3940(97)13359-6. [DOI] [PubMed] [Google Scholar]
- 52.Prato FS, Ossenkopp KP, Kavaliers M, Sestini E, Teskey GC. Attenuation of morphine-induced analgesia in mice by exposure to magnetic resonance imaging: Separate effects of the static, radiofrequency and time-varying magnetic fields. Magn Reson Imaging. 1987;5:9–14. doi: 10.1016/0730-725x(87)90478-4. [DOI] [PubMed] [Google Scholar]
- 53.Prato FS, Carson JJ, Ossenkopp KP, Kavaliers M. Possible mechanisms by which extremely low frequency magnetic fields affect opioid function. FASEB. 1995;9:807–14. doi: 10.1096/fasebj.9.9.7601344. [DOI] [PubMed] [Google Scholar]
- 54.Prato FS, Kavaliers M, Cullen AP, Thomas AW. Light-dependent and -independent behavioral effects of extremely low frequency magnetic fields in a land snail are consistent with a parametric resonance mechanism. Bioelectromagnetics. 1997;18:284–91. doi: 10.1002/(sici)1521-186x(1997)18:3<284::aid-bem13>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 55.Thomas AW, White KP, Drost DJ, Cook CM, Prato FS. A comparison of rheumatoid arthritis and fibromyalgia patients and healthy controls exposed to a pulsed (200 microT) magnetic field: Effects on normal standing balance. Neurosci Lett. 2001;309:17–20. doi: 10.1016/s0304-3940(01)02009-2. [DOI] [PubMed] [Google Scholar]
- 56.Hinman H, Ford J, Heyl H. Effects of static magnets on chronic knee pain and physical function: a double blind study. Altern Ther Health Med. 2002;8:50–5. [PubMed] [Google Scholar]
- 57.Felson T. The sources of pain in knee osteoarthritis. Curr Opin Rheumatol. 2005;17:624–8. doi: 10.1097/01.bor.0000172800.49120.97. [DOI] [PubMed] [Google Scholar]
- 58.Hinz B, Brune K. Pain and osteoarthritis: New drugs and mechanisms. Curr Opin Rheumatol. 2004;16:628–33. doi: 10.1097/01.hco.0000136130.95746.14. [DOI] [PubMed] [Google Scholar]
- 59.Yunus MB, Masi AT, Aldag JC. Short term effects of ibuprofen in primary fibromyalgia syndrome: A double blind, placebo controlled trial. J Rheumatol. 1989;16:527–32. [PubMed] [Google Scholar]
- 60.Tak PP, Smeets TJ, Daha MR, et al. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–25. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- 61.Wong HY. Neural mechanisms of joint pain. Ann Acad Med Singapore. 1993;22:646–50. [PubMed] [Google Scholar]
- 62.O’Dell JR. Rheumatoid arthritis: The clinical picture. In: Koopman WJ, editor. Arthritis and Allied Conditions. Philadelphia: Lippincott, Williams & Wilkins; 2001. pp. 1153–86. [Google Scholar]
- 63.Peloso PM, Bellamy N, Bensen W, et al. Double blind randomized placebo control trial of controlled release codeine in the treatment of osteoarthritis of the hip or knee. J Rheumatol. 2000;27:764–71. [PubMed] [Google Scholar]
- 64.Arkinstall W, Sandler A, Goughnour B, Babul N, Harsanyi Z, Darke AC. Efficacy of controlled-release codeine in chronic non-malignant pain: A randomized, placebo-controlled clinical trial. Pain. 1995;62:169–78. doi: 10.1016/0304-3959(94)00262-D. [DOI] [PubMed] [Google Scholar]
- 65.Roth SH, Fleischmann RM, Burch FX, et al. Around-the-clock, controlled release oxycodone therapy for osteoarthritis-related pain. Arch Intern Med. 2000;160:853–60. doi: 10.1001/archinte.160.6.853. [DOI] [PubMed] [Google Scholar]
- 66.Caldwell JR, Hale ME, Boyd RE. Treatment of osteoarthritis pain with controlled release oxycodone or fixed combination oxycodone plus acetaminophen added to nonsteroidal anti-inflammatory drugs: A double blind, randomized multicenter placebo controlled trial. J Rheumatol. 1999;26:862–9. [PubMed] [Google Scholar]
- 67.Caldwell JR, Rapoport RJ, Davis JC, et al. Efficacy and safety of a once-daily morphine formulation in chronic, moderate-to-severe osteoarthritis pain: Results from a randomized placebo-controlled double-blind trial and open-label extension trial. J Pain Symptom Manage. 2002;23:278–91. doi: 10.1016/s0885-3924(02)00383-4. [DOI] [PubMed] [Google Scholar]
- 68.Kavaliers M, Ossenkopp KP. Opioid systems and magnetic field effects in the land snail, Cepaea nemoralis. Biol Bull. 1991;180:301–9. doi: 10.2307/1542401. [DOI] [PubMed] [Google Scholar]
- 69.Papi F, Ghione S, Rosa C, Del Seppia C, Luschi P. Exposure to oscillating magnetic fields influences sensitivity to electrical stimuli. II. Experiments on humans. Bioelectromagnetics. 1995;16:295–300. doi: 10.1002/bem.2250160505. [DOI] [PubMed] [Google Scholar]
- 70.Jeong JH, Choi KB, Yi BC, et al. Effects of extremely low frequency magnetic fields on pain thresholds in mice: Roles of melatonin and opioids. J Autonomic Pharmacology. 2000;20:259–64. doi: 10.1046/j.1365-2680.2000.00189.x. [DOI] [PubMed] [Google Scholar]