Abstract
BACKGROUND:
Multiple sclerosis (MS) is a neurological disease affecting approximately 50,000 Canadians. Although studies have described overall MS costs, none have focused specifically on MS-related pain.
OBJECTIVES:
To estimate the prevalence of MS-related pain in Canada, the proportion of patients treated and responding to treatment for MS-related pain, and the associated economic burden.
METHODS:
Results were captured through physician and patient surveys. Patients were recruited through MS clinics and the MS Society. Patient-reported outcomes and resource utilization over the previous six months were collected by telephone interview. Costs were measured in 2004 Canadian dollars. The economic burden was extrapolated to the population using national demographics and prevalence. Spearman’s ρ assessed the relationship between cost and pain severity.
RESULTS:
Physicians estimated that 46% of their MS patients experienced MS-related pain, and that 35% received treatment for pain. Pain was reported to be relieved somewhat in 29%±10% of their patients, adequately in 26%±19% and poorly in 27%±13%, while 17%±9% received no relief. Two hundred ninety-seven participants completed the patient survey. Seventy-one per cent (211 of 297 patients) experienced MS-related pain. Eighty per cent of patients reported taking some type of medication to manage their pain, and of these, 82% reported some reduction in pain. The mean ± SD direct cost per patient of MS-related pain was $2,528±5,695. The mean ± SD indirect cost per patient was $669±875. Total costs were positively correlated with levels of self-reported pain (ρ=0.291, P<0.0001). The estimated six-month burden of pain of MS patients in Canada was $79,444,888.
CONCLUSIONS:
The prevalence of pain is high in MS patients. This condition may be underdiagnosed and undertreated, and results in a significant economic burden on society.
Keywords: Burden of illness, Multiple sclerosis, Pain, Patient-reported outcomes, Prevalence
Abstract
HISTORIQUE :
La sclérose en plaques (SP) est une maladie neurologique dont souffrent environ 50 000 Canadiens. Bien que des études aient déjà décrit les coûts globaux de la SP, aucune n’a porté directement sur les douleurs reliées à la SP.
OBJECTIFS :
Évaluer la prévalence des douleurs reliées à la SP au Canada, la proportion de patients traités et réagissant au traitement contre les douleurs reliées à la SP et le fardeau économique s’y rapportant.
MÉTHODOLOGIE :
On a obtenu les résultats au moyen de sondages auprès de médecins et de patients. On a recruté les patients par l’entrem-ise de cliniques de la SP et de la Société de la SP. À l’aide d’entrevues téléphoniques, on a colligé les issues et l’utilisation des ressources d’après les déclarations des patients, au cours des six mois précédents. On a mesuré les coûts en dollars canadiens de 2004. On a extrapolé le fardeau économique à la population au moyen de la démographie et de la prévalence nationales. Le ρ de Spearman a permis d’évaluer le lien entre le coût et la gravité de la douleur.
RÉSULTATS :
Les médecins estiment que 46 % de leurs patients atteints de SP avaient des douleurs reliées à la SP et que 35 % étaient traitées contre la douleur. Ils ont déclaré que 29 %±10 % de leurs patients soulageaient quelque peu leur douleur, 26 %±19 % d’entre eux la soulageaient correctement et 27 %±13 % la soulageaient mal, tandis que 17 %±9 % n’étaient pas soulagés. Deux cent quatre-vingt-dix-sept participants ont rempli le sondage auprès des patients. Soixante et onze pour cent (211 des 297 patients souffraient de douleurs reliées à la SP. Quatrevingts pour cent des patients ont déclaré prendre un certain type de médicament pour prendre leur douleur en charge et, de ce nombre, 82 % ont déclaré une certaine réduction de la douleur. Le coût direct moyen±ÉT de la douleur reliée à la SP par patient était de 2 528 $± 5 695. Le coût indirect moyen±ÉT par patient s’élevait à 669 $±875. Les coûts totaux étaient corrélés positivement avec des taux de douleur autodéclarés (ρ=0,291, P<0,0001). Le fardeau estimatif de la douleur dont souffrent les patients atteints de SP au Canada sur une période de six mois est de 79 444 888 $.
CONCLUSIONS :
La prévalence de la douleur est élevée chez les patients atteints de SP. Ce problème peut être sous-diagnostiqué et sous-traité, ce qui représente un fardeau économique important pour la société.
Multiple sclerosis (MS) is a chronic neurological disease of the central nervous system that involves the brain and spinal cord (1,2). There is strong evidence supporting an autoimmune etiopathogenesis (3). Symptoms associated with MS include pain, spasticity, weakness, muscle spasms, numbness, tremors or lack of coordination, bladder problems, fatigue and sexual dysfunction (2,4). Pain associated with MS can be described as either musculoskeletal and/or nerve related (5).
There are four described clinical forms of MS: relapsing-remitting, primary progressive, secondary progressive and progressive relapsing (4). Progression of the disease can be assessed through the Expanded Disability Status Scale (EDSS) (6) a generic method of quantifying disability in MS. Prognosis as to the severity of the disease, progression and the specific symptoms of MS cannot be predicted at the time of diagnosis (2).
An estimated 50,000 Canadians live with MS (2). In Canada, the prevalence of this disease ranges from 100 per 100,000 population to as high as 240 per 100,000 population (2,7). The reported prevalence of pain varies from 10% to 80% in patients living with MS (5,8–13).
Beard et al (14) performed a systematic literature review of treatments currently available for spasticity and pain in MS. Their review noted that published evidence regarding the effectiveness of treatments for pain was limited to review articles, small case series and individual case reports. Furthermore, no consistency was observed in how effectiveness was gauged. That review was able to identify only one uncontrolled study of gabapentin that incorporated a validated instrument to assess pain (14).
Another review by Pollman et al (15) reported that anticonvulsants such as carbamazepine, lamotrigine, gabapentin and oxcarbazepine were prescribed for the treatment of trigeminal neuralgia and painful tonic spasms. Tricyclic antidepressants or carbamazepine were prescribed for painful ‘burning’ dysaesthesiae, whereas gabapentin or lamotrigine were used as alternative treatments. Opioids were prescribed in cases where escalation therapy was deemed appropriate. Treatment options that indirectly managed MS-related pain included anti-spasticity agents such as baclofen or tizanidine and, alternatively, gabapentin (15).
Archibald et al (5) studied 85 patients in Canada with MS. They found that 53% of those patients experienced MS-related pain. However, no attempt to correlate the prevalence of pain with disease characteristics was made. Sixty-five per cent of patients with pain reported taking medications for pain. Nevertheless, patients with pain reported poorer mental health and a higher level of discomfort (5).
Although there have been several studies published that describe the overall cost of MS and its treatment (16–23), none has focused specifically on the pain component. Therefore, a need exists to further examine the economic implications of this disorder by estimating the frequency and severity of pain in MS and quantifying the resources consumed in its treatment.
The objectives of the present study were threefold. The first was to estimate the overall prevalence of pain in the MS population in Canada. The second objective was to determine the percentage of MS patients receiving pain treatments and the proportion of those patients who responded to their treatment. A third objective was to estimate the overall economic burden of pain associated with MS by quantifying the average cost due to pain and listing the resource utilization associated with that pain.
METHODS
Standardized questionnaires were designed a priori and used to prospectively collect data from physicians specializing in MS or neurology, as well as from patients diagnosed with MS. Physician surveys were mailed in, while patient data were collected by telephone interviews.
Physician survey
The physician survey was created to collect data pertaining to the diagnosis of pain and the clinical management of pain associated with MS, in addition to the resource utilization attributed to the treatment. A questionnaire was mailed or faxed to a selected list of neurologists or pain specialists from various Canadian provinces, which was provided by Bayer HealthCare, Canada. Physicians inferred their consent to participate by completing the questionnaire. Responses from participating physicians were analyzed using descriptive statistics such as mean, median, SD and/or range with respect to the following topics: pain prevalence, level of pain and its management.
Patient survey
The patient survey targeted three areas of interest. The first set of questions gathered data on subject demographics, the second part retrieved information related to the subjects’ MS and the third area of interest focused on MS-related pain. Age, sex, income and employment status were examples of the components of the demographics part of the survey. The second set of questions retrieved data on type of MS and severity, as well as MS-related symptoms. Questions related to types and severity of pain, as well as treatment and resource utilization attributed to the management of MS-related pain, were included in the final section of the survey.
Responders who had experienced pain in the six months before the survey were asked approximately 65 questions, while those who did not experience pain were asked 13 questions.
Pain measurement:
Pain was measured using two validated scales: the Box Score-11 (BS-11) scale (24,25) and an adapted version of the pain attribute of the Health Utilities Index Mark 3 (HUI-3) (24).
Subject recruitment:
Subjects were recruited through either a referral from MS clinics or the Canadian MS Society. Subjects who were referred from clinics and who expressed an interest in the study were asked to sign an informed consent form at the clinic and were given a toll-free number to call. The Canadian MS Society informed its members of the study through announcements in the bi-monthly MS Society newsletter, and by posting information about the ongoing study on their Web page. Subjects recruited through the society were asked for verbal consent before beginning the survey. This process was approved by a research ethics board.
Sample size:
Based on previously reported prevalence rates of approximately 50% (5,8–13), a sample size of 384 study participants was targeted to achieve 95% confidence, allowing a 5% alpha error (25,26). Patients were recruited either through four MS clinics or through the MS Society of Canada. They were included in the present study if they had a diagnosis of MS (regardless of severity or clinical form), had experienced MS-related pain in the previous six months, and were Canadian residents older than 18 years. Those who were unable to understand either French or English were excluded. Those eligible and willing to participate were asked to sign an informed consent form. Unconditional approval was granted by an independent ethics review board, as well as from the individual hospital-based ethics review boards of the clinics participating in the study.
Responses were analyzed using descriptive statistics such as mean, median, SD and/or range with respect to the following variables: age; duration of disease; presence, duration and severity of pain episodes; pain treatment; and the use of cannabis. Differences in participant characteristics measured at ordinal, or interval or ratio level (eg, age, education, duration of disease) between those reporting pain and those reporting the absence of pain were assessed with a Wilcoxon rank sum test, and χ2 tests were used on those measured at the nominal level (eg, sex, use of cannabis). Correlations between total cost and forms or severity of MS, as well as severity of pain, were assessed by calculating Spearman’s rank correlation (ρ). All tests were considered significant at a level of P≤0.05.
Based on the questions on the disease-specific resource utilization, the mean total cost attributed to this Canadian population of MS patients experiencing pain was derived by multiplying the quantity of resources used by the unit price of the respective resources. The average cost of illness per patient was calculated first and then projected to the entire population in Canada. The overall burden of illness for Canada was determined based on the following formula:
The pain prevalence in patients with MS was determined by calculating the proportion of participants who answered the questionnaire and reported experiencing pain in the previous six-month period. Resource utilization and mean burden of illness were calculated for the corresponding six-month period.
Unit costs were calculated based on 2004 Canadian dollars and were applied to each resource, based on standard reference lists of unit costs. Costs were determined from the societal perspective, including both direct and indirect costs. Costs associated with any time loss due to pain in MS were calculated using the average Canadian hourly wage reported by Statistics Canada (27). Costs for transportation were self-reported (ie, patients reported the dollar value of what they spent) or based on public transportation costs (ie, when they reported frequencies of travel). Only expenditures related to MS pain were included – not those that were assumed to be related to the underlying MS. The latter types of costs, such as those for home renovation or for MS medications, were excluded from the present analysis.
RESULTS
Physician survey
Among the 13 specialists who responded to the questionnaire, 12 were neurologists (nine MS specialists, two pain specialists and one general neurologist) and one was a pain specialist. On average, physicians reported that 71±29% of their MS patient visits included a discussion of pain, while 46±22% of their patients were suffering from MS-related pain. Of their MS patients, 35±22% were being treated for pain (62±31% of them with pharmacotherapy). Of these patients, 29±10% had their pain somewhat relieved, 27±13% had their pain poorly relieved and 26±19% had their pain adequately relieved, while 17±9% did not get relief from their pain.
Physicians prescribed anticonvulsants in 43% of MS-related pain cases, antidepressants in 23%, analgesics in 14%, anti-spastics in 11%, antiemetics in 4%, and other drugs in 5%. The most prescribed drugs were amitriptyline (21%), followed by gabapentin (20%), carbamazepine (14%) and baclofen (9%), respectively. On average, physicians responded that 19.5±11.2% of patients used cannabis to manage their pain related to MS.
The mean proportion of patients by severity level, stratified by the HUI-2 pain attribute as reported by physicians, is presented in Table 1. This is contrasted with the mean proportion of patient self-reported pain, stratified by the HUI-3 pain attribute.
TABLE 1.
Pain severity reported by physicians for their patients and by multiple sclerosis (MS) patients*
| Pain severity from the physician perspective
|
Pain severity from the patients’ survey
|
||
|---|---|---|---|
| Pain severity level (from HUI-2 pain attribute) | Proportion of patients (%) | Pain severity level (from HUI-3 pain attribute) | Reported by MS patients (%) |
| 1 – Free of pain and discomfort | 17 | 1 – I was free from pain and pain-associated discomfort | 0 |
| 2 – Occasional pain; discomfort relieved by non-prescription drugs or self control activity without disruption of normal activities | 24 | 2 – The pain discomfort associated with MS did not disrupt any social or work-related functions | 13 |
| 3 – Frequent pain; discomfort relieved by oral medications with occasional disruptions of normal activities | 30 | 3 – The pain discomfort associated with MS occasionally disrupted social or work-related functions | 26 |
| 4 – Frequent pain; frequent disruptions of normal activities; discomfort required prescription narcotics for relief | 17 | 4 – The pain discomfort associated with MS frequently disrupted social or work-related functions | 39 |
| 5 – Severe pain; pain not relieved by drugs and constantly disrupted normal activities | 10 | 5 – The pain discomfort associated with MS disrupted all social or work-related functions | 22 |
Comparison between physician and patient perspective was for illustrative purposes only, because two different scales were used (HUI Health Utility Index [HUI]-2 versus HUI-3), as well as two different time frames (recall period for physicians was one month and for patients was six months)
Patient survey
Prevalence:
Data were collected from a total of 297 patients with MS. Their mean age was 49±11 years, ranging from 26 to 84 years. The ratio of men to women was 23:77. Most of the participants were unemployed (77%). The largest proportion of the respondents was located in Ontario (55%), followed by British Columbia (16%) and Quebec (10%). There were 211 persons who indicated that they had experienced some level of MS-related pain during the previous six months, for an overall prevalence of 71%.
There were no significant differences (P>0.05) in age, sex or employment status between participants who had experienced pain in the previous six months and those who had not. A significant difference (P=0.002) was observed in the level of education between MS patients with pain and MS patients without pain. Those having pain tended to have somewhat lower levels of education. A comprehensive summary of the demographic characteristics of the study population is presented in Table 2.
TABLE 2.
Demographic characteristics of the study participants
| Demographic | Patients with pain | Patients without pain | Overall | P* |
|---|---|---|---|---|
| Participants, n (%) | 211 (71) | 86 (29) | 297 (100) | – |
| Age (mean ± SD)† | 48.7±10.0 | 49.2±12.5 | 48.8±10.8 | 0.772 |
| Sex, n (%)‡ | ||||
| Male | 47 (22) | 21 (24) | 68 (23) | 0.691 |
| Female | 164 (78) | 65 (74) | 229 (77) | |
| Highest education level, n (%)‡ | ||||
| High school | 69 (33) | 18 (21) | 87 (29) | 0.002 |
| College | 61 (29) | 21 (24) | 82 (28) | |
| Undergraduate | 46 (22) | 19 (22) | 65 (22) | |
| Graduate | 32 (15) | 28 (33) | 60 (20) | |
| Other | 3 (1) | 0 (0) | 3 (1) | |
| Employment status, n (%)‡ | ||||
| Full time | 28 (13) | 20 (23) | 48 (16) | 0.103 |
| Part time | 15 (7) | 6 (7) | 21 (7) | |
| Unemployed | 168 (80) | 60 (70) | 228 (77) | |
| Yearly individual income, n (%)‡ | ||||
| $0–$19,999 | 97 (46) | 25 (29) | 122 (41) | 0.082 |
| $20,000–$39,999 | 63 (30) | 26 (30) | 89 (30) | |
| $40,000–$59,999 | 27 (13) | 16 (19) | 43 (15) | |
| $60,000–79,999 | 12 (6) | 5 (6) | 17 (6) | |
| $80,00–$99,999 | 4 (2) | 4 (5) | 8 (3) | |
| Over $100,000 | 2 (1) | 3 (4) | 5 (2) | |
| Refused to answer | 6 (3) | 7 (8) | 13 (4) | |
Comparison of patients with and without pain;
Student’s t test;
Wilcoxon rank sum test
Fifty per cent of respondents reported having a relapsing-remitting form of MS. Pain prevalence ranged from 64% to 90% for individual clinical forms. The proportions of patients experiencing pain versus no pain did not differ across clinical forms (Z=0.139, P=0.890). The numbers and percentages of participants reporting their clinical forms of MS, as well as prevalence of pain, are presented in Table 3.
TABLE 3.
Clinical forms of multiple sclerosis (MS) in study population and associated pain
| Clinical forms | Overall, n (%) | Patients without pain, n (%) | Patients with pain, n (%) |
|---|---|---|---|
| Relapsing-remitting MS | 149 (50) | 41 (28) | 108 (72) |
| Secondary progressive MS | 80 (27) | 29 (36) | 51 (64) |
| Primary progressive MS | 42 (14) | 11 (26) | 31 (74) |
| Primary relapsing MS | 10 (3) | 1 (10) | 9 (90) |
| Unknown/No answer | 16 (5) | 5 (31) | 11 (69) |
The prevalence of pain by EDSS level ranged from 56% to 82%. No difference was observed between EDSS levels and the number of patients reporting pain versus no pain (Z=1.21, P=0.227). The overall number and percentage of respondents in each EDSS level, along with associated levels of pain, are presented in Table 4.
TABLE 4.
Expanded Disability Status Scale (EDSS) score of study population and associated pain
| EDSS score | Overall, n (%) | Patients without pain, n (%) | Patients with pain, n (%) |
|---|---|---|---|
| 0 | 18 (6) | 8 (44) | 10 (56) |
| 1 | 45 (15) | 19 (42) | 26 (58) |
| 2 | 38 (13) | 8 (21) | 30 (79) |
| 3 | 25 (8) | 8 (32) | 17 (68) |
| 4 | 22 (7) | 4 (18) | 18 (82) |
| 5 | 54 (18) | 12 (22) | 42 (78) |
| 6 | 42 (14) | 10 (24) | 32 (76) |
| 7 | 37 (12) | 14 (38) | 23 (62) |
| 8 | 7 (2) | 2 (29) | 5 (71) |
| 9 | 9 (3) | 2 (22) | 7 (78) |
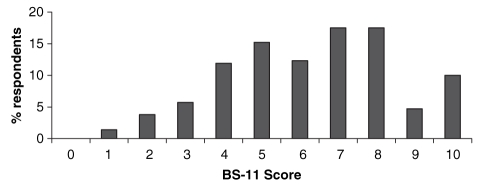
The distribution of the severity of pain reported by participants is presented in Figure 1. The mean ± SD severity level was 6.3±2.2, as measured by the BS-11 scale, with a median score of 6 (interquartile [IQR] range 5 to 8). Pain in joints, back pain, pins and needles sensation, and muscle spasms or cramps were the most commonly reported types of pain, affecting more than 60% of participants. Bladder spasms were the most commonly reported recurring type of pain, with a mean ± SD of 23.9±63.1 episodes in the past six months. There was a weak correlation between the severity of the pain and its duration (ρ=0.157, P=0.022).
Figure 1).
Distribution of pain severity scores in participants reporting pain (n=211). BS-11 Box Score-11
Treatment:
Eighty per cent of participants reported the use of some type of medication to manage their pain. There were more than 100 different types of either prescribed or over-the-counter medications reportedly used by participants. Acetaminophen was the most commonly used medication to manage pain, with 44% of the respondents reporting its use, followed by marijuana and ibuprofen (both 25%), baclofen (19%), gabapentin (18%), any opioid (15%), amitriptyline (9%) and tizanidine (7%). Oxybutynin chloride, acetylsalicylic acid and naproxen were used by 5% of the participants. All other reported medications were used by less than 5% of the respondents.
Forty-one per cent of the respondents used at least one type of nonpharmacological treatment to manage their pain. Of the 16 different treatments reported, massage was the most common (25% of the respondents), followed by acupuncture (6%), chiropractic treatments (4%) and yoga (3%). All other types of reported treatment were used by 1% or less of the participants.
Eighty-two per cent of the respondents reported a reduction in pain of at least two points on the BS-11 scale with treatment. Ninety-three per cent of participants reported clinically important pain (ie, a BS-11 level 4 or greater) before any treatment and 53% reported clinically important pain after any treatment. Respondents were also asked to rate any pain treatment (pharmacological or nonpharmacological) used. Cannabis was reported to be the most effective treatment for relieving pain, with a mean ± SD score of 2.7±0.7 on a scale from 0 (not relieved) to 3 (adequately relieved).
Resource utilization and burden:
A large proportion (85%) of patients who reported experiencing pain had consulted a health care provider about their pain. For the six-month study period, participants reported a mean ± SD of 2.3±10.7 visits per patient to a physical therapist for pain, whereas the average number of visits to a pain clinic was only 0.1±0.4 per patient (Table 5). Hospital admissions (hospitalizations and emergency room visits) and home care visits due to pain attributed to MS were reported by 6% and 13% of the respondents with pain, respectively. On average, each patient had 0.1±0.6 admissions and 0.3±1.0 emergency room visits. Of the 211 patients with pain, 26 (12.3%) required home care visits for their pain, with an average of 61.9±64.9 visits each. Laboratory tests were performed on 25% of the respondents with pain, with a mean of 5.4±8.6 tests per patient. Diagnostic tests, such as magnetic resonance imaging and computed tomography scans were performed on 8% of the patients, with an average of 1.6±1.3 and 1.5±0.9 tests per patient, respectively. Table 5 summarizes the self-reported data pertaining to resource utilization.
TABLE 5.
Self-reported resources utilized and time lost by multiple sclerosis patients for the management of their pain in a six-month period (n=211)
| Parameter | Per patient, mean ± SD |
|---|---|
| Health care professionals | |
| Family physician visits, n | 1.8±3.0 |
| Multple sclerosis specialist visits, n | 0.4±0.9 |
| Pain specialist visits, n | 0.1±0.4 |
| Therapist visits, n | 2.3±10.7 |
| Hospitalizations | |
| Admission visits, n | 0.1±0.6 |
| Length of stay, days | 12.3±18.3 |
| Emergency room visits, n | 0.3±1 |
| Professional care giving | |
| Homecare visits, n | 61.9±64.9 |
| Duration of homecare, h | 1.4±0.9 |
| Laboratory and diagnostic tests, n | |
| Laboratory tests | 5.4±8.6 |
| Magnetic resonance imaging | 1.6±1.3 |
| Computed tomography scan | 1.5±0.9 |
| Travel | |
| Travel times, n | 6.2±10.1 |
| Travel duration, h | 47.5±71.7 |
| Distance, km | 46.3±102.2 |
| Time lost, h | |
| Time lost from work | 4.7±17.7 |
| Time lost from leisure | 33.4±47.8 |
| Time lost from volunteers | 18±35.9 |
The estimated mean ± SD direct cost for pain in MS patients over a six-month period was $2,528±5,695 (median $753, IQR $209 to $2,061) and the mean ± SD indirect cost was $669±875 (median $264, IQR= $1 to $933). The mean ± SD total cost per patient was $3,197±5,965 (median $1,496, IQR=$606 to $3,354). Hospitalization and drug management of pain were the two most important contributors to the total cost. A summary of the direct and indirect costs appears in Table 6.
TABLE 6.
Summary of total direct and indirect costs of pain in multiple sclerosis (MS) over a six-month period* (n=211)
| Parameter | Total | Cost per patient
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | 25% quartile | 75% quartile | Minimum† | Maximum | Daily | ||
| Direct costs | ||||||||
| Health care visits | ||||||||
| Family physician | $15,084 | $72±205 | $0 | $0 | $46 | $0 | $1,666 | $0.39 |
| Specialist | $5,082 | $24±54 | $0 | $0 | $0 | $0 | $372 | $0.13 |
| Pain clinic | $908 | $4±22 | $0 | $0 | $0 | $0 | $151 | $0.02 |
| Physical therapist | $5,941 | $28±131 | $0 | $0 | $0 | $0 | $1,220 | $0.16 |
| Total | $27,016 | $129±320 | $0 | $0 | $139 | $0 | $2,362 | $0.70 |
| Hospitalizations and ER visits | ||||||||
| Hospitalizations | $149,350 | $711±4,170 | $0 | $0 | $0 | $0 | $43,500 | $3.90 |
| ER visits | $7,200 | $34±122 | $0 | $0 | $0 | $0 | $1,200 | $0.19 |
| Total | $156,550 | $745±4,196 | $0 | $0 | $0 | $0 | $43,620 | $4.08 |
| Drug management of pain | ||||||||
| Prescribed | $144,300 | $684±2,121 | $130 | $0 | $528 | $0 | $20,318 | $3.75 |
| Over the counter | $5,318 | $25±53 | $2 | $0 | $25 | $0 | $390 | $0.14 |
| Total | $149,618 | $709±2,118 | $165 | $32 | $560 | $0 | $20,318 | $3.89 |
| Nondrug management of pain | $92,850 | $440±1,059 | $0 | $0 | $385 | $0 | $8,927 | $2.41 |
| Laboratory/diagnostic services | $8,017 | $38±92 | $0 | $0 | $20 | $0 | $685 | $0.21 |
| Home care services | $67,853 | $323±1,606 | $0 | $0 | $0 | $0 | $15,030 | $1.77 |
| Travel costs | $30,044 | $143±500 | $130 | $0 | $528 | $0 | $6,400 | $0.78 |
| Total direct costs | $531,948 | $2,528±5,695 | $761 | $210 | $2,076 | $0 | $49,804 | $13.85 |
| Indirect costs | ||||||||
| Lost time | ||||||||
| Lost time from work | $16,945 | $82±312 | $0 | $0 | $0 | $0 | $2,112 | $0.45 |
| Lost time from leisure | $121,622 | $588±841 | $264 | $0 | $660 | $0 | $3,168 | $3.22 |
| Total | $138,567 | $669±875 | $264 | $1 | $933 | $0 | $3,168 | $3.67 |
| Total indirect costs | $138,567 | $669±875 | $264 | $1 | $933 | $0 | $3,168 | $3.67 |
| Direct and indirect costs | $670,515 | $3,197±5,965 | $1,496 | $606 | $3,354 | $0 | $52,339 | $17.52 |
Unless stated otherwise, all costs are for a six-month period;
Four participants reported pain but incurred zero cost for all items. ER Emergency room
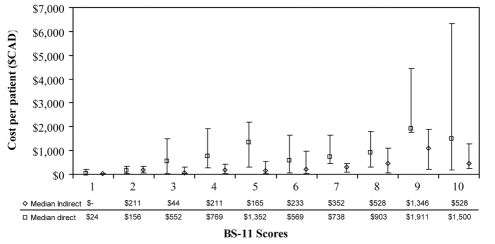
Figure 2 displays the variation of direct and indirect median costs per BS-11 level. A positive trend was observed between the total cost and increasing levels of pain severity (ρ=0.291, P<0.0001).
Figure 2).
Direct and indirect costs (median and interquartile range) per Box Score-11 (BS-11) level. Spearman rank correlation: ρ=0.291, P<0.0001
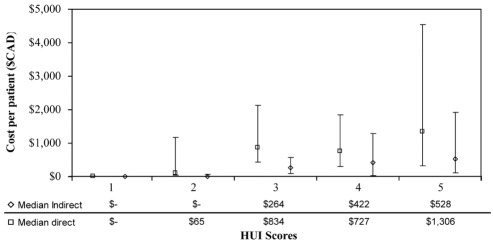
Figure 3 depicts the variation of direct and indirect median costs according to HUI-3 pain attribute severity score. A positive trend was again observed with increasing pain severity (ρ=0.294, P<0.0001). The only deviation was seen when the median indirect costs increased between HUI-3 level 4 and HUI-3 level 5, whereas the median direct costs slightly decreased between the two categories, possibly indicating a plateau effect.
Figure 3).
Direct and indirect costs (median and interquartile range) per Health Utility Index (HUI)-3 pain attribute severity scores. Spearman rank correlation: ρ=0.294, P<0.0001
The estimated six-month burden of pain of MS patients in Canada was $79,444,888. This amount was calculated from the total cost of the cohort ($670,515) divided by the number of respondents (n=211) and multiplied by the estimated number of patients with MS suffering from pain in the country (50% of 50,000 MS patients = 25,000 patients). However, if the prevalence of pain found in the present study was representative of the true prevalence of pain in the population, the six-month burden of illness would amount to $112,881,741.
DISCUSSION
The prevalence of pain due to MS in the present study sample was 71%. This rate is at the higher end of the prevalences of pain reported in the literature (10% to 80%) (5–28). As for the mean estimated total cost per patient experiencing pain, it was $3,197 over a six-month period. The projected total six-month burden of illness for all Canadian patients with pain due to MS was therefore $79,444,888.
In 1998, the Canadian Burden of Illness Study Group published a paper estimating the annual cost of MS from the Canadian societal perspective (16). In that study, data were collected from a total of 198 patients, in whom symptoms were described as mild in 62 patients, moderate in 68 and severe in the remaining 68. Costs were positively correlated with EDSS scores from all analytic perspectives. That study concluded that, in Canada, MS was associated with substantial direct and indirect costs, with patients carrying most of the economic burden (74% to 88% of total costs). The reported annualized societal costs per patient were $14,523 for the mild, $21,698 for the moderate and $37,024 for the severe EDSS groups. Indirect costs, such as lost daily activity or leisure time and lost productivity were the major societal cost drivers (16). That yielded an average daily cost of approximately $66 to treat MS. In comparison, our study estimated daily costs of approximately $17 for pain due to MS only, which amounts to approximately one-quarter of the total costs of MS as determined by the previous study.
In our study, 80% of the patients experiencing pain reported using some sort of drug product (either prescription or over-the-counter) to manage MS-related pain. That rate is somewhat higher than the 64% reported by Archibald et al (5). Pain medications that were mentioned most frequently in our study (ie, acetaminophen, ibuprofen, baclofen, gabapentin, amitriptyline, tizanidine, oxybutynin chloride, acetylsalicylic acid and naproxen) overlapped with the findings of the review by Pollman et al (15), who reported the use of carbamazepine, lamotrigine, gabapentin, oxcarbazepine and tricyclic antide-pressants, as well as opioids. Twenty-five per cent of patients in our study reported the use of marijuana, which is also comparable to the rate of 16% published by Clark et al (29).
Eighty-two percent of respondents who took medication in the present study reported a reduction in the severity of their pain of at least two points on the BS-11 scale as a result of their respective treatments. Ninety-three per cent of the patients reported to be at BS-11 level 4 or higher before taking any medication. Despite the high number of respondents who reported at least a two-point reduction on the BS-11 scale with their medication, 53% reported that their pain severity was still equal to or above BS-11 level 4. The pain reduction was attained mainly by patients with very severe pain (BS-11 levels 9 and 10), and the overall pain was not always well controlled by the available medications, as reported by the MS patients and physicians who participated in the surveys.
No associations were observed in our study between severity of pain and the different clinical forms of pain, nor did it show any correlation between severity of pain and disease severity. These findings confirm the results presented by Archibald et al (5). Cohorts in our study were subdivided by each level of the BS-11 scale as well as by all five rankings of the HUI-3 pain component scale. Predictably, the study samples were larger for each HUI-3 pain ranking than for each level of the BS-11 scale. Correlation tests demonstrated overall trends between higher levels of pain severity and higher costs, regardless of the manner in which the cohort was subdivided.
The ratio of male to female participants in our study was 23:77. This finding corresponds with those published by the MS Society of Canada (2), indicating that two to three times more women are affected by MS than are men. The mean ± SD age at which participants in this study were diagnosed with MS was 37±10 years. This result also falls within the range of disease onset reported by the Canadian MS Society (2).
The rate of unemployment in our study sample was 77%, which was within the range of unemployment rates of 50% to 80% as described by Orlewska et al (30). The Canadian MS Society reported that relapsing-remitting MS was the most frequently presented form of MS, affecting 75% of patients, compared with 50% in our study.
A statistically significant difference (P=0.004) was found between the 71% rate of MS-related pain reported by patients and the 46% rate estimated by physicians. The exact reason for this discrepancy is unknown, but may be due to sampling or to a clustering effect. It could also be due to an underestimation of the pain by treating neurologists.
In the analysis of the costs, we used nonparametric statistics due to the non-normality and skewness of the data. As can be seen in several of the tables in the results section, the data were greatly skewed, with the SD greater than the mean. In such cases, nonparametric statistics are used, because they do not require normality in the distributions being contrasted and remain robust when applied to skewed data.
Limitations
A limitation of the present study is that the patients’ reporting of their EDSS scores was subject to memory recall by the participants. Therefore, the frequencies of each EDSS level may be not as accurate as they would be if the data were provided directly by physicians or obtained from chart reviews. The method by which EDSS data were collected may have led to recall bias.
Also, the geographic distribution in our study sample was not even among provinces. The largest proportion of our participants was from Ontario, with more callers from British Columbia than from Quebec. We are not certain whether our study sample represents the average Canadian MS patient who suffers from pain and, therefore, care must be taken when generalizing the findings from the present study.
Although questions were specifically designed to capture pain-related costs, there may have been an overestimation of the reported costs, because patients may have reported costs not related specifically to pain in MS but to their overall condition. Furthermore, participants may not have been able to distinguish between MS-related pain and pain due to a comorbid condition or any other cause.
Home renovation costs associated with MS-related pain were collected; however, we suspect that respondents may have included costs that were related to their overall MS condition rather than only to pain. Therefore, this cost component was not included in the analysis to avoid overestimating costs attributed specifically to pain. Furthermore, costs of medications used in clinical practice for the treatment of MS and reported as taken for pain by the respondents were not included in costs attributed to pain. Laboratory and diagnostic tests, as well as home care, were excluded for the same reason.
Because the number of patients recruited was somewhat less than the targeted sample size of 384, we performed a post hoc power analysis. The original analysis was based on an expected 50% prevalence, and we observed a 71% prevalence of patients experiencing pain due to MS; the resulting power was 94%. The achieved precision level of 5.2% was considered acceptable for the purposes of the study.
CONCLUSIONS
The prevalence of pain among MS patients is substantial at 71%. Comparison of results from the physician and patient survey demonstrate that MS-attributed pain may be under-diagnosed and undertreated. Eighty per cent of participants reported the use of some sort of medication to manage their pain, with 82% of those taking a medication reporting a reduction of at least two points on the BS-11 scale with their current pain treatment. The estimated average cost of managing pain in MS patients was $17.52 per patient per day based on a six-month analysis, resulting in an estimated six-month economic burden of pain in MS patients of $79,444,888 for the Canadian society.
Acknowledgments
This study was sponsored by Bayer HealthCare, Canada.
REFERENCES
- 1.Bruck G, Stadelmann C. Inflammation and degeneration in multiple sclerosis. Neurol Sci. 2003;24:S265–7. doi: 10.1007/s10072-003-0170-7. [DOI] [PubMed] [Google Scholar]
- 2.Multiple Sclerosis Society of Canada MS Information – Frequently Asked Questions<www.mssociety.ca/en/information/faq.htm?> (Version current at April 13, 2007).
- 3.Brassington J, March N. Neuropsychological aspects of multiple sclerosis. Neuropsychol Rev. 1998;8:43–77. doi: 10.1023/a:1025621700003. [DOI] [PubMed] [Google Scholar]
- 4.Brichetto G, Messmer Uccelli M, Mancardi G, Solaro C. Symptomatic medication use in multiple sclerosis. Mult Scler. 2003;9:458–60. doi: 10.1191/1352458503ms957oa. [DOI] [PubMed] [Google Scholar]
- 5.Archibald C, McGrath P, Ritvo P. Pain prevalence, severity and impact in a clinical sample of multiple sclerosis patients. Pain. 1994;58:89–93. doi: 10.1016/0304-3959(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 6.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 7.Beck C, Metz L, Svenson L, Patten S. Regional variation of multiple sclerosis prevalence in Canada. Mult Scler. 2005;11:516–9. doi: 10.1191/1352458505ms1192oa. [DOI] [PubMed] [Google Scholar]
- 8.Solaro C, Lunardi G, Mancardi G. Pain and MS. Int MS J. 2003;10:14–9. [PubMed] [Google Scholar]
- 9.Solaro C, Brichetto G, Amato M, et al. The prevalence of pain in multiple sclerosis. Neurology. 2004;63:919–21. doi: 10.1212/01.wnl.0000137047.85868.d6. [DOI] [PubMed] [Google Scholar]
- 10.Burks J. A review of current medical aspects of multiple sclerosis. J Neurol Rehabil. 1992;6:131–9. [Google Scholar]
- 11.Indaco A, Iachetta C, Nappi C, Socci L, Carrieri P. Chronic and acute pain syndromes in patients with multiple sclerosis. Acta Neurol Scand. 1994;16:97–102. [PubMed] [Google Scholar]
- 12.Moulin D. Pain in central and peripheral demyelinating disorders. Neurol Clin. 1998;16:889–97. doi: 10.1016/s0733-8619(05)70103-1. [DOI] [PubMed] [Google Scholar]
- 13.Maloni H. Multiple sclerosis and pain. Consortium for multiple sclerosis. < www.mscare.org/cmsc/CMSC-Multiple-Sclerosis-and-Pain.html> (Version current at April 23, 2004).
- 14.Beard S, Hunn A, Wight J. Treatments for spasticity and pain in multiple sclerosis: A systematic review. Health Technol Assess. 2003;7:1–111. doi: 10.3310/hta7400. [DOI] [PubMed] [Google Scholar]
- 15.Pollman W, Feneberg W, Steinbrecher A, Haupts M, Henze T. Therapy of pain syndromes in multiple sclerosis: An overview with evidence-based recommendations. Fortschr Neurol Psychiatr. 2005;73:268–85. doi: 10.1055/s-2004-830193. [DOI] [PubMed] [Google Scholar]
- 16.The Canadian Burden of Illness Study Group Burden of Illness of Multiple Sclerosis: Part I: Cost of Illness. Can J Neurol Sci. 2006;25:23–30. doi: 10.1017/s0317167100033448. [DOI] [PubMed] [Google Scholar]
- 17.Grima D, Torrance G, Francis G, Rice G, Rosner A, Lafortune L. Cost and health related quality of life consequences of multiple sclerosis. Mult Scler. 2000;6:91–8. doi: 10.1177/135245850000600207. [DOI] [PubMed] [Google Scholar]
- 18.Amato M, Battaglia M, Caputo D, et al. The costs of multiple sclerosis: A cross-sectional, multicenter cost-of-illness study in Italy. J Neurol. 2002;249:152–63. doi: 10.1007/pl00007858. [DOI] [PubMed] [Google Scholar]
- 19.Whetten-Goldstein K, Sloan F, Goldstein L, Kulas E. A comprehensive assessment of the cost of multiple sclerosis in the United States. Mult Scler. 1998;4:419–25. doi: 10.1177/135245859800400504. [DOI] [PubMed] [Google Scholar]
- 20.Henriksson F, Fredrikson S, Masterman T, Jönsson B. Costs, quality of life and disease severity in multiple sclerosis: A cross-sectional study in Sweden. Eur J Neurol. 2001;8:27–35. doi: 10.1046/j.1468-1331.2001.00169.x. [DOI] [PubMed] [Google Scholar]
- 21.Miltenburger C, Kobelt G. Quality of life and cost of multiple sclerosis. Clin Neurol Neurosurg. 2002;104:272–5. doi: 10.1016/s0303-8467(02)00051-3. [DOI] [PubMed] [Google Scholar]
- 22.Grudzinski A, Hakim Z, Cox E, Bootman J. The economics of multiple sclerosis: Distribution of costs and relationship to disease severity. Pharmacoeconomics. 1999;15:229–40. doi: 10.2165/00019053-199915030-00003. [DOI] [PubMed] [Google Scholar]
- 23.Clanet M, Brassat D. The management of multiple sclerosis patients. Curr Opin Neurol. 2000;13:263–70. doi: 10.1097/00019052-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Feeny D, Furlong W, Boyle M, Torrance GW. Multi-attribute health status classification systems: Health utilities index. Pharmacoeconomics. 1995;7:490–502. doi: 10.2165/00019053-199507060-00004. [DOI] [PubMed] [Google Scholar]
- 25.Aday L. A Comprehensive Guide. San Francisco: Jossey-Bass Publishers; 1996. Designing and Constructing Health Surveys. [Google Scholar]
- 26.Cochran W. Wiley Series in Probability and Mathematical Statistics – Applied. New York: John Wiley & Sons; 1977. Sampling Techniques. [Google Scholar]
- 27.StatsCan Statistics Canada<www40.statcan.ca/l01/cst01/labr69a.htm> (Version current April 13, 2007). [Google Scholar]
- 28.Ehde D, Gibbons L, Chwastiak L, Bombardier C, Sullivan M, Kraft G. Chronic pain in a large community sample of persons with multiple sclerosis. Mult Scler. 2003;9:605–11. doi: 10.1191/1352458503ms939oa. [DOI] [PubMed] [Google Scholar]
- 29.Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. Patterns of cannabis use among patients with multiple sclerosis. Neurology. 2004;62:2098–100. doi: 10.1212/01.wnl.0000127707.07621.72. [DOI] [PubMed] [Google Scholar]
- 30.Orlewska E, Mierzejewski P, Zaborski J, et al. A prospective study of the financial costs of multiple sclerosis at different stages of the disease. Eur J Neurol. 2005;12:31–9. doi: 10.1111/j.1468-1331.2004.00950.x. [DOI] [PubMed] [Google Scholar]