Abstract
Neuroinflammatory processes have been implicated in the progressive loss of ventral midbrain dopaminergic neurons that give rise to Parkinson’s disease, a late-onset movement disorder that affects 2% of the population over age 70. Previously, we demonstrated that inhibition of the pro-inflammatory cytokine Tumor Necrosis Factor through nigral infusion of dominant-negative Tumor Necrosis Factor protein (XENP345) in two rat models of Parkinson’s disease attenuates dopaminergic neuron loss. The objective of this study was to develop a constitutive lentiviral vector encoding dominate-negative Tumor Necrosis Factor and to determine if a gene therapy approach to deliver dominant-negative TNF directly into the rodent substantia nigra could prevent or attenuate neurotoxin-induced dopaminergic neuron loss and associated behavioral deficits. Here we demonstrate that a single injection of lentivirus expressing dominant-negative TNF into rat substantia nigra administered concomitant with a striatal 6- hydroxydopamine lesion resulted in sufficiently high expression of inhibitor in vivo to attenuate both dopaminergic neuron loss and behavioral deficits resulting from striatal dopamine depletion. Our findings demonstrate the feasibility and efficacy of dominant negative Tumor Necrosis Factor gene transfer as a novel neuroprotective strategy to prevent or delay nigrostriatal pathway degeneration with potential future therapeutic applications in the treatment of Parkinson’s disease.
Introduction
Parkinson’s disease (PD) is a chronic, progressive neurodegenerative disorder characterized by the loss of dopaminergic neurons (DA) in the substantia nigra pars compacta (SNpc) that project to the striatum. This selective loss of dopaminergic neurons results in striatal dopamine depletion which is responsible for the clinical manifestations of the disease. Although the critical mechanisms responsible for dopaminergic neuron loss are not fully understood, many studies implicate pro-inflammatory processes in contributing to disease progression 1–3, and prospective studies have shown that chronic use of non-steroidal anti-inflammatory drugs (NSAIDS) can lower the incidence of PD by 46% 4.
The pro-inflammatory cytokine Tumor Necrosis Factor (TNF) is elevated in postmortem brains and cerebrospinal fluid of PD patients 5, and TNF mRNA and protein levels are increased in many experimental models of PD including 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) 6 and 6-hydroxydopamine (6-OHDA) 7. We have shown previously that chronic infusion of dominant-negative TNF (DN-TNF) protein, a selective inhibitor of soluble TNF, into the substantia nigra via an osmotic pump attenuates dopaminergic neuron loss resulting from a striatal 6-OHDA lesion or from chronic intranigral lipopolysaccharide (LPS) infusion 8. DN-TNF-dependent nigrostriatal protection in the 6-OHDA model correlates with preservation of striatal dopamine as measured by amelioration of amphetamine-induced rotational behavior 8. To circumvent the inherent limitations associated with chronic delivery of such biologics through an invasive infusion device, in this report we investigate the feasibility and efficacy of lentiviral delivery of DN-TNF to achieve nigrostriatal protection in the rat 6-OHDA model of PD.
Our neurohistological and behavioral results demonstrate that a single intranigral injection of lentivirus expressing DN-TNF (lenti-DN-TNF) can significantly attenuate dopaminergic neuron loss and striatal dopamine depletion induced by the oxidative neurotoxin 6-OHDA. These findings provide further validation of soluble TNF as a therapeutic target in PD and suggest that anti-TNF gene transfer of a dominant negative TNF biologic into the substantia nigra may be an effective and novel therapeutic strategy to delay or prevent the progressive loss of dopaminergic neurons in humans with early stage PD.
Results
We previously demonstrated that the soluble TNF-selective dominant negative TNF inhibitor XENP345 9, 10 was efficacious in attenuating 6-OHDA-induced DA neuron loss 8 when delivered directly into the midbrain substantia nigra through a chronically implanted cannula connected to an osmotic pump. Given the neuroprotective effects of infusion of recombinant DN-TNF observed in these previous studies, we investigated the extent to which intranigral delivery of lenti-DN-TNF could protect against DA neuron loss and behavioral deficits in vivo in a unilateral 6-OHDA lesion model of PD. The sequence of green fluorescent protein (GFP) (Supplementary Figure S1a) or the DN-TNF variant A145R/I97T (Supplementary Figure S1b) was subcloned into a self-inactivating, vesicular stomatitis virus (VSV) pseudotyped lentiviral vector downstream from the cytomegalovirus/β-actin hybrid(CAG) promoter. The DN-TNF vector included the TNF-alpha converting enzyme (TACE) cleavage site necessary for the conversion of the transmembrane form of the protein to soluble TNF 11 as well as an internal ribosome entry site (IRES)-driven GFP sequence to monitor in vivo gene expression.
Efficient in vitro transduction of dopaminergic neuron-like cells with lenti-DN-TNF
To measure the steady-state levels of DN-TNF protein following transduction, we first evaluated transduction efficiency, protein expression, and inhibition of TNF signaling by lentivirus derived DN-TNF in the MN9D dopaminergic cell line 12. Infection of MN9D dopaminergic cells with lentivirus expressing GFP (lenti-GFP) or lenti-DN-TNF resulted in transduction efficiencies of greater than 50% (Supplementary Figure S1c) and production of DN-TNF protein (> 30ng/mL) was detectable in the conditioned medium as early as 24 hours (Figure 1a) after infection and remained at this steady state level for up to 72 hours, as measured by a human TNF quantitative ELISA which recognizes the human DN-TNF (h-DN-TNF) sequence but does not detect rodent-derived TNF.
Figure 1.
In vitro transduction of MN9D dopaminergic cell cultures with lenti-DN-TNF results in the production of DN-TNF and prevents TNF-induced signaling. (a) MN9D cultures were mock-, lenti-GFP-, or lenti-DN-TNF- transduced and DN-TNF production was measured by hTNF ELISA analysis of the culture supernatants 24, 48, and 72hr after infection. Values were analyzed by two-way ANOVA followed by Tukey’s post hoc test, n = 3 per condition, values shown as mean + SEM, * significantly different from mock-infected cultures at p < 0.0005. Interaction: F4,18=1.38, p=0.28, Lenti-infection: F2,28=888.15, p< 0.0001, Time after infection: F2,18 =0.61, p=0.553. (b) Transduction with lenti-DN-TNF but not lenti-GFP prevents nuclear translocation and enrichment of the p65RelA subunit required for activation of NFκB-dependent gene transcription in response to stimulation with soluble TNF. MN9Ds were mock-, lenti-GFP-, or lenti-DN-TNF-infected 24hr after plating. At 24hr after infection cultures were switched to 0.5% FBS containing media; 24hr later cultures were stimulated with 2ng/mL TNF for 15 minutes. After fixation cells were immunolabeled for the localization of NFκB p65RelA to assess cytoplasmic to nuclear translocation. Scale bar = 10μM.
Lenti-DN-TNF blocks TNF-induced NFκB pathway activation
Next, we tested the ability of lentivirus-derived DN-TNF to prevent activation of the nuclear factor-kappa B (NFκB) signaling pathway by exogenously added soluble murine TNF. Transduction of MN9D cultures with lenti-DN-TNF, but not lenti-GFP or mock transduction, attenuated TNF-dependent nuclear enrichment of the p65 RelA subunit of NFκB that normally occurs within 15 minutes of stimulation with soluble TNF (Figure 1b) 13. These data demonstrate that transduction of dopaminergic cells with lenti-DN-TNF results in efficient production of functionally active DN-TNF protein that can inactivate signaling by exogenously added soluble TNF.
Inhibition of microglia activation and neuroprotection by lenti-DN-TNF in rat primary ventral midbrain cultures
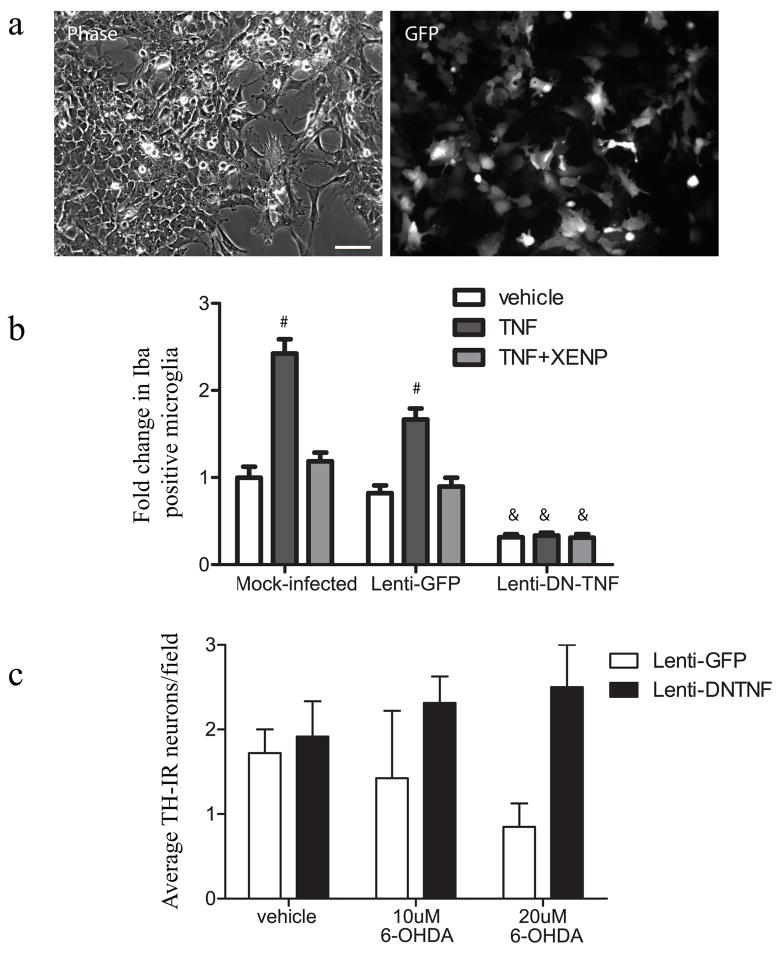
To validate the transduction efficiency and DN-TNF production attained by lenti-DN-TNF transduction of primary cells, we used mixed neuron/glia cultures obtained from embryonic (E14) rat ventral mesencephalon (EVM). Based on GFP fluorescence driven by the IRES, we estimated that between 30 – 50% of cells were transduced in primary cultures (Figure 2a) depending on the plating density. Lenti-GFP infection of primary cultures resulted in transduction of microtubule-associated protein 2 (MAP2)-positive neurons, CD11b/c (OX-42)-positive microglia, and to a lesser degree glial fibrillary acidic protein (GFAP)-positive astrocytes (Supplementary Figure S2). Also as seen in rat substantia nigra, tyrosine hydroxylase (TH)-immunoreactive dopaminergic neurons appeared to be relatively resistant to lentiviral infection compared to other neurons. This infection pattern is not unexpected as substantia nigra DA neurons have been shown to be resistant to infection by VSV pseudotyped vectors and CMV promoters have been demonstrated to have poor activity in this population 14,15. Importantly lentiviral-derived DN-TNF is secreted from infected cells and therefore specific infection of DA neurons is not necessary. Furthermore, limiting infection of these neurons may be beneficial if degenerative processes are ongoing. DN-TNF protein expression in primary cultures was detectable in lenti-DN-TNF, but not lenti-GFP-infected cultures as early as 24 hours after transduction with secreted levels of DN-TNF peaking between 35 and 50ng/mL (data not shown). To determine the ability of lentivirus-derived DN-TNF to prevent TNF signaling, we performed microglial activation assays. After 5 days in vitro, rat EVM cultures were transduced with lenti-GFP, lenti-DN-TNF, or received no virus, and 48 hours after transduction they were stimulated with 5ng/mL recombinant murine TNF with or without co-addition of the recombinant DN-TNF biologic XENP345 (as a positive control for TNF inhibition). Cultures were fixed after a 24-hour stimulation and immunostained for the microglial activation marker ionized calcium-binding adaptor molecule 1 (Iba-1). Mock- and lenti-GFP infected cultures exhibited TNF-induced microglial activation that could be blocked by the dominant negative protein XENP345. In contrast, lenti-DN-TNF cultures displayed a lower basal level of microglial activation and showed no increase in microglial activation upon addition of soluble TNF (Figure 2b). DN-TNF-mediated neuroprotection was further examined in lentivirus-transduced rat EVM cultures in the presence of 6-OHDA, a known neurotoxin which induces DA neuron death 16. Cultures transduced with lenti-GFP and exposed to 6-OHDA displayed dose-dependent 50 – 75% loss of TH-immunoreactive neurons compared to vehicle-treated cultures (Figure 2c). In contrast, cultures transduced with lenti-DN-TNF were resistant to 6-OHDA-induced death consistent with our previous observations that soluble TNF is a critical mediator of 6-OHDA-induced dopaminergic death [8]. On the basis of these cell culture data we predicted that lentiviral transduction of neurons and/or glia in the midbrain would result in secretion of sufficient amounts of bioactive DN-TNF protein to antagonize midbrain TNF production elicited by 6-OHDA and its death-inducing effects on ventral midbrain dopaminergic neurons.
Figure 2.
Lentiviral-DN-TNF transduction of rat embryonic ventral midbrain results in production of bioactive DN-TNF as measured by reduced microglial activation resulting from TNF treatment and increased DA neuron survival after 6-OHDA exposure. (a) Phase and GFP fluorescence images of rat EVM cultures 72hr after lenti-GFP infection. Scale bar = 50μm. (b) EVM cultures were infected with lenti-GFP or lenti-DN-TNF. 48 hours after infection 5ng/mL TNF was added +/− 200ng/mL XENP345. 24 hours after TNF stimulation the cultures were fixed and labeled with anti-Iba1. Iba1-immunoreactive microglia were counted manually in mock-infected, lenti-GFP-infected, or lenti-DN-TNF-infected cultures. Basal microglia activation in mock-infected cultures treated with vehicle was set to 1 and other values were expressed as fold change relative to this condition. Values shown are mean Iba-immunoreactive microglia per field + SEM. Values were analyzed by two-way ANOVA followed by Tukey’s post-hoc test; # denotes significant difference from mock-infected, vehicle treatment a p< 0.0005. & denotes significant difference from mock-infected, TNF+XENP treatment at p<0.0005. Interaction: F4,345=14.88, p< 0.0001, Lenti-infection: F2,345 =116.90, p< 0.0001, Time after infection: F2,345=53.13, p< 0.0001. (c) The number of tyrosine hydroxylase positive dopaminergic neurons was counted to obtain an average number per well in mock-infected, lenti-GFP-infected, or lenti-DN-TNF-infected cultures treated with 6-OHDA on an average of 8 fields per well. Two wells per treatment condition were counted. Values shown are average TH immunoreactive neurons per well + SEM. Data was analyzed by two-way ANOVA. Interaction: F2,6=1.38, p=0.36, Lenti-infection: F1,2 =5.70, p=.054, Treatment: F1,2=0.09, p=0.913.
In vivo neuroprotection of the nigrostriatal pathway by nigral delivery of lenti-DN-TNF
Pilot studies established that a single intranigral stereotaxic injection of 2μL lenti-DN-TNF (100μg/mL p24) resulted in production of DN-TNF protein (0.6–2.0 ng per midbrain hemisphere, n = 4), as detected by hTNF ELISA (which specifically detects the hDN-TNF sequence and does not cross react with endogenous rat TNF) in homogenates of microdissected midbrain two weeks after injection. We investigated the transduction of specific cell types after lenti-DN-TNF infection of rat mibrain, and found that the majority of lentivirus-transduced cells were microglia as assesed by immunofluorescence detection of co-expressed GFP or hDN-TNF with cell type specific markers (Supplementary Figure S3). Transduction of predominantly microglial cells in vivo, and neuronal cells to a lesser extent, is consistent with in vitro infection results as DA neurons do not appear to be transduced efficiently either in vitro or in vivo; GFAP-immunoreactive astrocytes were present at much lower density in the substantia nigra relative to that of microglia. Next, lenti-DN-TNF or lenti-GFP (as a negative control) was injected into the substantia nigra immediately following intrastriatal 6-OHDA- or mock- lesion to investigate the neuroprotective effects of DN-TNF gene transfer. Using unbiased stereologic methods to estimate the number of nigral DA neurons, we found that 6-OHDA-lesioned lenti-DN-TNF-injected animals displayed significantly less nigral degeneration compared to 6-OHDA-lesioned lenti-GFP injected animals. Mock-lesioned lenti-DN-TNF-injected animals displayed a slight increase in the number of nigral dopaminergic neurons that was not significantly different from that in mock-lesioned lenti-GFP-injected animals (Figure 3a). On average, 42% of TH positive dopaminergic neurons remained in the lesioned substantia nigra in lenti-DN-TNF injected animals, whereas in lenti-GFP animals only 24% of nigral DA neurons survived compared to animals which received a striatal saline infection, constituting an approximately 50% reduction in DA neuron loss. Neither lenti-GFP nor lenti-DN-TNF transduction caused a significant change in DA neuron number in unlesioned control animals. These neurohistological findings indicate that delivery of DN-TNF into the nigrostriatal pathway by gene transfer affords significant neuroprotection that is comparable to chronic infusion of DN-TNF protein [8]. Consistent with our previous report in which chronic treatment of primary rat EVM cultures with the recombinant DN-TNF biologic XENP345 attenuated DA neuron loss without preventing microglial activation 8, we did not see a reduction in microglial activation in lenti-DN-TNF infected animals compared to lenti-GFP infected animals after saline or 6-OHDA injection into the striatum (Supplementary Figures S4 and S5).
Figure 3.
Transduction of rat midbrain with lenti-DN-TNF attenuates 6-OHDA-induced DA neuron loss. A unilateral striatal lesion was induced by injecting 6-OHDA into the caudate-putamen (CPu) of rats. Animals were stereotaxically injected with lentivirus expressing GFP or DN-TNF into the substantia nigra. Animals were perfused three weeks after the lesion and brains were harvested for immunohistology. (a) Stereological estimate of DA neuron number (TH/NeuN-IR cells) in SNpc after a striatal 6-OHDA lesion and transduction of the substantia nigra with lentiviral GFP or DN-TNF. 6-OHDA induced significant (p < 0.05) loss of DA neurons. Inhibition of TNF with lentivirus-derived DN-TNF significantly (p < 0.05) rescued DA neurons. The number of animals in each group are as follows: control (n=3), saline/saline (n=3), saline/GFP (n=3), saline/DNTNF (n=4), 6-OHDA/saline (n=4), 6-OHDA/GFP (n=5), 6-OHDA/DNTNF (n=5). For comparison of groups, data were analyzed by two-way ANOVA followed by Tukey’s post hoc test for significance. Values are expressed as means ± SEM; * denotes significant difference (p < 0.05) from control lesioned brain, # denotes significant difference (p < 0.05) from both control lesioned brain and 6-OHDA lesioned, mock, or GFP infection. Interaction: F6,40=13.92, p< 0.0001, Contralateral/Ipsilateral: F1,40 =50.26, p< 0.0001, Treatment: F6,40=16.98, p< 0.0001. (b) TH-IR in nigral sections from 6-OHDA lesioned rats. Scale bar = 400μM.
To investigate whether neuroprotection by lenti-DN-TNF was accompanied by amelioration of 6-OHDA-induced behavioral deficits, we performed two routine behavioral tests to estimate the degree of striatal DA depletion: amphetamine-induced rotational behavior and vibrissae-evoked forelimb placing. Both behavioral tests were performed at weekly intervals after the induction of 6-OHDA lesion and lentivirus injection. To test vibrissae-evoked forelimb placement we measured forelimb placing (out of 5 trials) for both forelimbs of the animal upon stimulation of the whiskers on each side of the head. Measuring forelimb placement by both same-side and cross-midline vibrassae stimulation on each forelimb allows the differentiation between deficits in sensorimotor integration (as would occur in conditions such as stroke) and motor function, as occurs in parkinsonism 17. In unilateral 6-OHDA-lesioned animals, placement of the forelimb contralateral to the lesion is expected to be impaired upon stimulation of whiskers on either side of the head; while the forelimb ipsilateral to the lesion should be unaffected with either same-side or cross-midline limb placement. We found there was no difference between mock lesion and 6-OHDA-lesioned groups in placement of the forelimb ipsilateral to the 6-OHDA lesion when whiskers on either side of the head were stimulated (data not shown). However, 6-OHDA lesioned groups displayed a decrease in successful forelimb placement for the forelimb contralateral to the lesion upon stimulation of both ipsilateral and contralateral vibrissae, indicative of motor initiation deficits characteristic of parkinsonian akinesia (Figure 4b and data not shown). The deficit in forelimb placement of lesioned animals which received lenti-DN-TNF was reduced but was not significantly different from unlesioned lenti-GFP infected rats at any time post lesion. In contrast, 6-OHDA-lesioned animals that received lenti-GFP had a statistically significant deficit in forelimb placement after the lesion. The deficits in forelimb placement were consistent when vibrissae on either side of the head were stimulated, indicating a lack of significant deficits in sensorimotor integration.
Figure 4.
Transduction of rat midbrain with lenti-DN-TNF attenuates 6-OHDA-induced behavioral deficits. (a) Measurement of amphetamine-induced rotations indicated significant attenuation of ipsiversive behavior. Values shown are mean (n = 3 for striatal saline with lenti-GFP, n = 4 for saline/DN-TNF, and n = 5 for both 6-OHDA lesioned groups), +/− SEM. Values were analyzed by repeated measures two-way ANOVA followed by Tukey’s post hoc test, * significantly different from vehicle lesioned, lenti-GFP infected controls at p < 0.05. Interaction: F6,26=1.75, p=0.1501, Treatment: F3,26 =11.39, p= 0.0006, Time: F2,26=1.76, p=0.1923, Matching: F13,26=2.39, p=0.0.287. (b) The vibrissae-evoked forelimb placing test was performed weekly after rats were lesioned with 6-OHDA and virus injection. 6-OHDA lesioned animals that received lenti-DN-TNF never develop any significant deficits in forelimb placement whereas lenti-GFP injected animals display significant deficits 3 weeks after lesions. Values are plotted as contralateral limb placing out of 5 trials at three weeks after 6-OHDA lesioning; with whiskers on the right (ipsilateral) of the head being stimulated. Values plotted are means + SEM (n = 3 for saline/GFP, n = 4 for saline/DN-TNF, and n = 6 for both 6-OHDA lesioned groups) Data was analyzed by repeated measures two-way ANOVA followed by Tukey’s post hoc test. * significantly different vehicle lesioned, lenti-GFP infected controls at p < 0.05., # no significant difference from either vehicle lesioned lenti-GFP infected controls, or 6-OHDA/GFP. Interaction: F6,30=0.24, p=0.9578, Treatment: F3,30 =3.53, p= 0.0408, Time: F2,30 =0.42, p=0.6595, Matching: F15,30=6.19, p<0.0001.
In addition to vibrissae-evoked forelimb placing, we measured amphetamine-induced rotational behavior as an indirect measure of striatal DA depletion. Consistent with results of forelimb placing tests, 6-OHDA-lesioned/lenti-GFP-injected animals displayed progressive amphetamine-induced rotational behavior that was significantly greater than that in unlesioned animals at all time-points (Repeated measures two-way ANOVA, p<0.05). In contrast, 6-OHDA/lenti-DN-TNF injected animals displayed reduced rotational behavior which was not significantly different from unlesioned control animals (Figure 4a). The improvements in both forelimb placement and amphetamine-induced rotational behavior suggest that lenti-DN-TNF injections may have prevented striatal DA depletion, thus resulting in functional neuroprotection of the nigrostriatal pathway in the 6-OHDA model.
Discussion
Our results demonstrate the feasibility and efficacy of DN-TNF gene transfer as a potential therapeutic strategy to achieve nigrostriatal neuroprotection in PD. Specifically, here we show that lentiviral DN-TNF infection results in detectable secretion of DN-TNF both in vitro in a dopaminergic cell line and in primary mixed cultures, as well as in vivo in the SNpc. Lentivirus-derived DN-TNF was efficacious in blocking TNF-induced signaling and microglial activation in vitro and attenuated neurotoxin-induced DA neuron loss in vitro and in vivo. Results from this study in which nigral lenti-DN-TNF infection led to an approximate doubling of remaining nigral dopaminergic neurons (42% of nigral DA neurons remaining in lenti-DN-TNF infected animals compared to 24% remaining in lenti-GFP infected animals) are consistent with our previous report of nigral DA neuron protection mediated by the chronic infusion of the recombinant DN-TNF biologic XENP345 (31% of nigral neurons remained with vehicle infusion compared to 62% with DN-TNF infusion) 8. There are a number of possible explanations for the greater variability in DA neuron survival obtained with lenti-DN-TNF delivery as opposed to infusion of the DN-TNF protein XENP345 [8]. One possibility is that the amount of DN-TNF production in the lentivirus-infected SNpc may have been more variable compared to the constant amount of XENP345 protein delivered by chronic infusion through an osmotic pump; alternatively, the spread of lentivirus-derived DN-TNF protein may have been more restricted. Another difference in experimental design that may account for the greater variability in DA neuron rescue with lenti-DN-TNF versus infusion of DN-TNF protein is the inherent lag time (typically 3–4 days) required for gene expression following transduction with the lentivirus; this is not the case with infusion of DN-TNF inhibitor which is available immediately to block the potent neurotoxic effects of TNF. Thus, injection of the lentivirus days or weeks prior to 6-OHDA lesions (as done in several glial-derived neurotrophic factor (GDNF) studies 18–20) might have led to an even greater rescue of DA neurons and larger behavioral effect. In addition to doubling the number of remaining nigral DA neurons after a striatal 6-OHDA lesion, lentiviral delivery of DN-TNF also attenuated behavioral deficits both in forelimb placement and drug-induced rotation resulting from 6-OHDA lesions. We did not investigate the neuroprotective effects of intrastriatal delivery of lenti-DN-TNF in these studies because previous experience with intrastriatal delivery of XENP345 afforded no neuroprotection 8. Taken together, our studies provide proof of concept for the feasibility and efficacy of DN-TNF gene delivery as a means to administer TNF inhibitors into specific regions of the CNS without the need for an invasive chronic infusion device. In the future, it will be of interest to determine the extent to which significantly delayed TNF signaling inhibition still affords neuroprotective effects, a question of therapeutic relevance when one considers a clinical diagnosis of Parkinson’s disease means significant dopamine neuron loss has already occurred.
Although anti-TNF drugs may be effective disease-modifying therapies in other conditions characterized by chronic inflammation 21, the currently FDA-approved systemic administration of anti-TNF biologics (i.e., large pegylated Fc-fused TNF decoy receptors or TNF antibodies) to treat peripheral autoimmune diseases such as rheumatoid arthritis and Crohn’s disease, is unlikely to provide adequate brain penetration to affect TNF signaling in the brain 22–24. Therefore, CNS applications may require use of chronic infusion devices or alternative delivery methods such as the one reported here. Gene therapy approaches are attractive for use in PD treatment for several reasons including the spatially-defined and cell type-specific pathology of the disease, the requirement for consistent drug administration to minimize or prevent dose fluctuations, and the difficulty in chronically administering drugs which can not cross the blood brain barrier. Pending questions about the usefulness of a DN-TNF gene-based therapy in the clinic include the need to identify when and where it would need to be administered to achieve efficacy in patients. Because the disease process begins well before degeneration of nigral DA neurons gives rise to clinical symptoms, use of anti-inflammatory therapy is likely to be most effective in the early stages of disease. In addition, it may also be necessary to target several nuclei in order to achieve robust rescue and prevent progression of the disease. Spatial restriction of TNF signaling inhibition may be important as TNF signaling has been demonstrated to regulate synaptic scaling in hippocampal neurons 25.
In summary, although the exact mechanisms responsible for degeneration of nigral DA neurons in PD have not been fully delineated, the wealth of data implicating inflammatory processes in the progressive loss of these neurons coupled with the protective effects of NSAIDs against idiopathic PD, provide compelling reasons to accelerate research approaches to selectively target inflammatory factors with demonstrated neurotoxic effects on DA neurons. Our studies demonstrate the feasibility and efficacy of dominant negative Tumor Necrosis Factor gene transfer as a novel neuroprotective strategy to prevent or delay nigrostriatal pathway degeneration in a pre-clinical model of PD.
Materials and Methods
Cloning of DN-TNF and GFP sequences into lentiviral vectors and preparation and purification of lentivirus stocks
The human full length DN-TNF DNA sequence required to generate XENP345 protein (TNF variant A145R/I97T) in mammalian cells 10, provided to us by David E. Szymkowski (Xencor, Inc., Monrovia, CA), included the signal peptide sequence required for membrane insertion and TACE recognition sequence required for natural cleavage and extracellular secretion. The DN-TNF sequence was subcloned into a constitutive self-inactivating lentiviral vector based on the plasmid pLV 26 5′ of an internal ribosome entry site (IRES) followed by the GFP coding sequence. The GFP-expressing lentiviral plasmid has been described previously 26, 27. Lentiviral vectors were VSV pseudotyped and DN-TNF or GFP expression was driven by the CMV/β-actin hybrid promoter (CAG). Lentivirus stocks were produced and purified according to a previously published protocol 27. The final titer was 125μg/mL p24 and 1.6 × 109 infectious units (IU)/mL for the negative control lentivirus-GFP and 980μg/mL p24 and 8 × 108 IU/mL for lentivirus-DN-TNF. Vector stocks were diluted in Hanks balanced salt solution (HBSS) (Invitrogen, Carlsbad CA) as indicated.
Measurement of p65Rel A cytoplasmic-to-nuclear translocation
MN9D dopaminergic cells were infected at an MOI of 10 in a total of 250μL per well. At 24 hr post transduction, cells were switched to DMEM containing 0.5% FBS. At 48 hr post transduction, cells were stimulated for 15 min with 2ng/mL recombinant mouse TNF (R&D systems, Minneapolis MN), fixed, and assessed for p65Rel A nuclear translocation by NFκBp65 immunocytochemistry (Santa Cruz Biotechnology, Santa Cruz CA; 1:200 dilution). Supernatants from the transduced cells were collected to measure DN-TNF production by hTNF ELISA (Invitrogen).
Animal studies
Young adult Sprague Dawley SASCO and timed-pregnant young adult CDF/Fischer 344 rats were purchased from Charles River Laboratories (Wilmington MA) and housed in pathogen-free climate-controlled facilities at the UT Southwestern Medical Center. All animal studies were approved by the Institutional Animal Care and Use Committee at UT Southwestern Medical Center at Dallas.
Expression of lenti-GFP and lenti-DN-TNF in E14 rat ventral mesencephalon (EVM) neuron/glia cultures
Primary rat EVM cultures were prepared by modification of a published protocol (see supplemental methods) 8, 28. To determine the cell types that were infected by lentivirus, cells were transduced after 5 days in vitro (DIV) with lenti-GFP using 250,000 IU in 200μL DMEM/F12 containing 2.5% FBS and lacking bFGF. Twenty-four hours after infection, 150μL per well of DMEM/F12 with 2.5% FBS was added, 72 hours after infection cultures were fixed, and analysis was performed using rabbit anti-tyrosine hydroxylase (TH) (1:250), mouse anti-MAP2 (1:400), mouse anti-complement type 3 receptor (Ox-42) (1:60) (Chemicon, Temecula, CA), or rabbit anti-GFAP (Dako, Carpinteria CA; 1:1000 dilution), and the appropriate Alexa 594-conjugated secondary antibodies (Invitrogen) diluted to 1:1000. To determine DN-TNF production of lentivirus infected cultures, at 5 DIV cells were infected with lenti-GFP or lenti-DN-TNF at 250,000 IU in 200μL DMEM/F12 with 2.5% FBS lacking bFGF. 24 hr after infection, 150μL per well of DMEM/F12 with 2.5% FBS was added and hDN-TNF expression was measured by hTNF ELISA (Invitrogen) 24 and 48hr after viral transduction.
Measurement of TNF-induced microglial activation and 6-OHDA induced dopaminergic neuron loss in EVM cultures
Microislands (2, 25-microliter volumes) of a neuron/glia single-cell suspension were plated at a density of 1×106 cells/mL onto poly-D-lysineand laminin coated 4-well chamber slides (Fisher Scientific, Waltham MA). At 5 DIV, the cells were infected with 250,000 IU of lenti-GFP or lenti-DN-TNF in a 300μL volume. To assess microglial activation, forty-eight hours post-transduction, the volume was brought up to 500μL with media containing the treatment (vehicle, TNF 5ng/mL, or TNF+ XENP345 at 200ng/mL as a positive control) cells were fixed 24 hours after the addition of TNF or TNF+ XENP345, and analysis was performed using goat anti-Iba-1 (Abcam, Cambridge MA; 1:150 dilution) and Donkey Anti Goat-Alexa 594 (Invitrogen; 1:1000 dilution). The total number of activated microglia per well was counted manually (an average of 13 fields per well, each condition in triplicate) in mock-infected, lenti-GFP-infected, or lenti-DN-TNF-infected cultures. To assess 6-OHDA-induced DA neuron loss, forty-eight hours post-transduction, the culture volume was brought up to 500μL with media containing the treatment (saline vehicle, 10μM 6-OHDA (Sigma), or 20μM 6-OHDA) cells were fixed 48 hours after 6-OHDA treatment and immunocytochemical analysis was performed using rabbit anti-TH (and DAG-Alexa 594 diluted to 1:1000. The total number of TH- positive DAneurons per well were counted in mock-, lenti-GFP-, or lenti-DN-TNF-infected cultures on an average of 8 fields per well. Two wells per treatment condition were counted.
Substantia nigra lentivirus infection to measure DN-TNF production
Briefly, 2 young adult female (200–250g) and 2 young adult male (340–400g) Sprague Dawley SASCO rats were anesthetized with halothane (2%) and placed in a stereotaxic frame. Burr holes were drilled to permit unilateral stereotaxic injection of 2uL of lenti-DN-TNF (diluted in HBSS to a concentration of 100ug/mL p24) at a rate of 0.5uL/min into the substantia nigra pars compacta (stereotaxic coordinates from bregma: AP: −5.3mm from bregma, ML: −2.3mm, and DV: −7.3mm below surface of dura). Two weeks after lenti-DN-TNF injection, rats were deeply anesthetized, and brains were quickly harvested, midbrain was dissected, divided into left and right hemispheres, and flash-frozen in liquid nitrogen. hTNF ELISA was performed on brain homogenates and DN-TNF production was determined for each animal.
Intrastriatal 6-OHDA injection and substantia nigra lentivirus infection
6-OHDA lesions were performed as previously described (see supplemental methods) 8, 16. Vehicle (sterile HBSS), 2uL of Lenti-DN-TNF or GFP (diluted 1:10 in HBSS) was stereotaxically injected at a rate of 0.5uL/min through a burr hole into the SNpc (coordinates from bregma: AP: −5.3mm, ML: −2.3mm, and DV: −7.3mm below surface of dura) immediately following intrastriatal 6-OHDA delivery.
Vibrissae-evoked forelimb placing and amphetamine-induced rotation
At 1, 2 and 3 weeks post 6-OHDA or sham lesions the vibrissae-evoked forelimb test was performed as previously described 17, and amphetamine-induced rotational behavior was measured. Vibrissae-evoked forelimb placing was measured for both same-side forelimb placing and cross-midline placing in 5 trials per condition (see supplemental methods). Amphetamine-induced rotational behavior was monitored in a glass cylinder (diameter 24.5cm). Animals received 2.5mg/kg D-amphetamine (Sigma) i.p. and 60 min after the injection, rotational asymmetry was monitored for 20 min. Rotation towards the lesion (ipsilateral) was scored as positive and net rotational asymmetry score was expressed as full body turns/min.
Brightfield immunohistochemistry of brain sections
Nigral sections on glass slides were labeled with rabbit anti-TH (1:750) and secondary antibody incubations performed with biotinylated goat anti-rabbit (1:400) (Vector Laboratories, Burlingame CA) followed by incubation of peroxidase labeled neutravidin at 1:5000 (Vector Laboratories). The signal was detected by using 0.6mg/ml diaminobenzidine with 6.0mg/mL nickel ammonium sulfate and 0.006% hydrogen peroxide as substrate in Tris buffer. Following immunohistochemistry for tyrosine hydroxylase, the same protocol followed using mouse anti-NeuN (Chemicon; 1:300 dilution) as the primary antibody, and biotinylated horse anti-mouse secondary antibody. The NeuN signal was detected with diaminobenzide lacking nickel ammonium sulfate (see supplemental methods).
Nigral DA neuron counts
StereoInvestigator analyses software (Micro Bright Field Inc., Williston VT) was used to perform unbiased stereological counts of NeuN/TH-immunoreactive (NeuN/TH-IR) cell bodies in the SNpc using the optical fractionator method 29. The boundary of SNpc was defined according to previous anatomical demarcation in the rat 30. (see supplemental methods).
Fluorescence immunohistochemistry
Immunohistochemisty was performed as described previously 8. Antibody dilutions were as follows: MAP2 (Chemicon) 1:400, TH (Chemicon) 1:250, Ox-42 (BD Pharmingen) 1:60, GFAP (Dako) 1:1000, hTNF (R &D systems) 1:500, or GFP (Chemicon or Rockland, Gilbertsville PA) 1:1000. The appropriateInvitrogen Alexa-conjugated secondary antibodies were used at a dilution of 1:1000 in cell culture and 1:500 in tissue. Imageswere captured with a CoolSnap CCD ES monochromatic camera and analyzed with MetaMorph software (UniversalImaging Systems, West Chester, PA)
Supplementary Material
Acknowledgments
We thank Barry Botterman (UT Southwestern) and Keith Tansey (UT Southwestern) for use of their surgical suite. We thank Jonathan Zalevsky and David Szymkowski (Xencor) for providing plasmids with DN-TNF sequences. The authors declare no conflicting interests. Funding support for this work was from The Michael J. Fox Foundation for Parkinson’s Research and the National Institutes of Health NINDS 1R01NS049433 (MGT).
References
- 1.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson’s disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Experimental Neurology. 2007;208(1):1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150(8):963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60(8):1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- 5.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165(1–2):208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 6.Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson’s disease. Faseb J. 2002;16(11):1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- 7.Mogi M, Togari A, Tanaka K, Ogawa N, Ichinose H, Nagatsu T. Increase in level of tumor necrosis factor (TNF)-alpha in 6-hydroxydopamine-lesioned striatum in rats without influence of systemic L-DOPA on the TNF-alpha induction. Neurosci Lett. 1999;268(2):101–104. doi: 10.1016/s0304-3940(99)00388-2. [DOI] [PubMed] [Google Scholar]
- 8.McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, et al. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J Neurosci. 2006;26(37):9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steed PM, Tansey MG, Zalevsky J, Zhukovsky EA, Desjarlais JR, Szymkowski DE, et al. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science. 2003;301(5641):1895–1898. doi: 10.1126/science.1081297. [DOI] [PubMed] [Google Scholar]
- 10.Zalevsky J, Secher T, Ezhevsky SA, Janot L, Steed PM, O’Brien C, et al. Dominant-negative inhibitors of soluble TNF attenuate experimental arthritis without suppressing innate immunity to infection. J Immunol. 2007;179(3):1872–1883. doi: 10.4049/jimmunol.179.3.1872. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal BB, Samanta A, Feldmann M. TNFα Oppenheim. In: JJaF M, editor. Cytokine Reference. Academic Press; London: 2000. pp. 414–434. [Google Scholar]
- 12.Choi HK, Won LA, Kontur PJ, Hammond DN, Fox AP, Wainer BH, et al. Immortalization of embryonic mesencephalic dopaminergic neurons by somatic cell fusion. Brain Res. 1991;552(1):67–76. doi: 10.1016/0006-8993(91)90661-e. [DOI] [PubMed] [Google Scholar]
- 13.Hohmann HP, Remy R, Poschl B, van Loon AP. Tumor necrosis factors-alpha and -beta bind to the same two types of tumor necrosis factor receptors and maximally activate the transcription factor NF-kappa B at low receptor occupancy and within minutes after receptor binding. J Biol Chem. 1990;265(25):15183–15188. [PubMed] [Google Scholar]
- 14.Rosenblad C, Gronborg M, Hansen C, Blom N, Meyer M, Johansen J, et al. In Vivo Protection of Nigral Dopamine Neurons by Lentiviral Gene Transfer of the Novel GDNF-Family Member Neublastin/Artemin. Molecular and Cellular Neuroscience. 2000;15(2):199–214. doi: 10.1006/mcne.1999.0817. [DOI] [PubMed] [Google Scholar]
- 15.Georgievska B, Kirik D, Bjorklund A. Overexpression of Glial Cell Line-Derived Neurotrophic Factor Using a Lentiviral Vector Induces Time- and Dose-Dependent Downregulation of Tyrosine Hydroxylase in the Intact Nigrostriatal Dopamine System. J Neurosci. 2004;24(29):6437–6445. doi: 10.1523/JNEUROSCI.1122-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152(2):259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- 17.Woodlee MT, Asseo-Garcia AM, Zhao X, Liu SJ, Jones TA, Schallert T. Testing forelimb placing “across the midline” reveals distinct, lesion-dependent patterns of recovery in rats. Exp Neurol. 2005;191(2):310–317. doi: 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Bensadoun J-C, Deglon N, Tseng JL, Ridet J-L, Zurn AD, Aebischer P. Lentiviral Vectors as a Gene Delivery System in the Mouse Midbrain: Cellular and Behavioral Improvements in a 6-OHDA Model of Parkinson’s Disease Using GDNF. Experimental Neurology. 2000;164(1):15–24. doi: 10.1006/exnr.2000.7409. [DOI] [PubMed] [Google Scholar]
- 19.Georgievska B, Kirik D, Rosenblad C, Lundberg C, Bjorklund A. Neuroprotection in the rat Parkinson model by intrastriatal GDNF gene transfer using a lentiviral vector. Neuroreport. 2002;13(1):75–82. doi: 10.1097/00001756-200201210-00019. [DOI] [PubMed] [Google Scholar]
- 20.Dowd E, Monville C, Torres EM, Wong LF, Azzouz M, Mazarakis ND, et al. Lentivector-mediated delivery of GDNF protects complex motor functions relevant to human Parkinsonism in a rat lesion model. Eur J Neurosci. 2005;22(10):2587–2595. doi: 10.1111/j.1460-9568.2005.04414.x. [DOI] [PubMed] [Google Scholar]
- 21.Scheinfeld N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J Dermatolog Treat. 2004;15(5):280–294. doi: 10.1080/09546630410017275. [DOI] [PubMed] [Google Scholar]
- 22.Tweedie D, Sambamurti K, Greig NH. TNF-alpha inhibition as a treatment strategy for neurodegenerative disorders: new drug candidates and targets. Curr Alzheimer Res. 2007;4(4):378–385. doi: 10.2174/156720507781788873. [DOI] [PubMed] [Google Scholar]
- 23.Robinson William H., MCGLWM Demyelinating and neurologic events reported in association with tumor necrosis factor ? antagonism: By what mechanisms could tumor necrosis factor ? antagonists improve rheumatoid arthritis but exacerbate multiple sclerosis? Arthritis & Rheumatism. 2001;44(9):1977–1983. doi: 10.1002/1529-0131(200109)44:9<1977::AID-ART345>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 24.van Oosten BW, Barkhof F, Truyen L, Boringa JB, Bertelsmann FW, von Blomberg BM, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996;47(6):1531–1534. doi: 10.1212/wnl.47.6.1531. [DOI] [PubMed] [Google Scholar]
- 25.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-[alpha] Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 26.Pfeifer A, Ikawa M, Dayn Y, Verma IM. Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc Natl Acad Sci U S A. 2002;99(4):2140–2145. doi: 10.1073/pnas.251682798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26(38):9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther. 2000;293(2):607–617. [PubMed] [Google Scholar]
- 29.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 30.German DC, Manaye KF. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. J Comp Neurol. 1993;331(3):297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.