Abstract
Two mammalian sphingosine kinase (SphK) isoforms, SphK1 and SphK2, possess identical kinase domains but have distinct kinetic properties and subcellular localizations, suggesting each has one or more specific roles in sphingosine-1-phosphate (S1P) generation. Although both kinases use sphingosine as a substrate to generate S1P, the mechanisms controlling SphK activation and subsequent S1P generation during lung injury are not fully understood. In this study, we established a murine lung injury model to investigate LPS-induced lung injury in SphK1 knockout (SphK1−/−) and wild-type (WT) mice. We found that SphK1−/− mice were much more susceptible to LPS-induced lung injury compared with their WT counterparts, quantified by multiple parameters including cytokine induction. Intriguingly, overexpression of WT SphK1 delivered by adenoviral vector to the lungs protected SphK1−/− mice from lung injury and attenuated the severity of the response to LPS. However, adenoviral overexpression of a SphK1 kinase-dead mutant (SphKKD) in SphK1−/− mouse lungs further exacerbated the response to LPS as well as the extent of lung injury. WT SphK2 adenoviral overexpression also failed to provide protection and, in fact, augmented the degree of LPS-induced lung injury. This suggested that, in vascular injury, S1P generated by SphK2 activation plays a distinctly separate role compared with SphK1-dependent S1P generation and survival signaling. Microarray and real-time RT-PCR analysis of SphK1 and SphK2 expression levels during lung injury revealed that, in WT mice, LPS treatment caused significantly enhanced SphK1 expression (∼5×) levels within 6 h, which declined back to baseline levels by 24 h posttreatment. In contrast, expression of SphK2 was gradually induced following LPS treatment and was elevated within 24 h. Collectively, our results for the first time demonstrate distinct functional roles of the two SphK isoforms in the regulation of LPS-induced lung injury.
Keywords: lipopolysaccharide, SphK1
sphingosine kinases (SphKs) 1 and 2, which are highly conserved, regulate generation of sphingosine-1-phosphate (S1P) from sphingosine in mammalian cells (14, 32, 36). The human SphK1 and SphK2 genes are located on chromosomes 17q25.2 and 19q13.2, respectively, and both forms have five conserved domains (C1–C5) with the catalytic domain located within the C1–C3 domain (15). SphK1 is a ∼43-kDa protein for which the highest expression levels are found in the lungs, spleen, and liver, whereas SphK2 is a ∼66-kDa protein that is most abundantly expressed in the liver and heart (11). These two isoforms of SphK each possess distinct kinetic properties and temporal gene expression patterns during development and are involved in specific cellular functions regulated by independent mechanisms. Although several studies have shown that cytokines and growth factors rapidly activate SphK1 (9, 30), less is known about the stimulation of SphK2 activation. Although the mechanisms by which SphK activity is regulated are not fully defined, recent studies have indicated the possible involvement of calcium, MAPK, protein kinase C, phospholipase D, and protein tyrosine kinases in agonist-induced activation of SphK1 (8, 13, 18).
S1P acts as an intracellular second messenger as well as extracellularly as a ligand for a family of five G protein-coupled receptors (33). S1P, generated by either SphK1 and/or SphK2, is implicated in several biological processes including angiogenesis, inflammation, and cancer (7, 41). In the vessel wall, extracellular S1P is a potent stimulator of angiogenesis (23) and is a major chemotactic factor for endothelial cells (44). In addition to its extracellular action via G protein-coupled receptor S1P (1–5), S1P functions as an intracellular second messenger in the regulation of Ca2+ mobilization and the suppression of apoptosis (45). In the pulmonary endothelium, S1P increases transendothelial electrical resistance via lipid rafts by increasing the cortical actin content and decreasing actin stress fibers (12, 35). Furthermore, in vivo administration of S1P significantly decreased pulmonary/renal vascular leakage and inflammation in a murine model of LPS-induced lung injury (31), and pretreatment with S1P dramatically reduced edema formation in a canine model of lung injury (26). In the murine and canine models, S1P administration also drastically reduced neutrophil accumulation in the lungs compared with vehicle-treated animals (26, 31). Although S1P-induced modulation of vascular permeability is effected by cytoskeletal reorganization via cell surface S1P receptors and downstream Rho/Rac1/phosphatidylinositol 3-kinase (PI3K)/MAPK, it is not clear how the exogenously administered S1P increases endothelial barrier stability and blocks LPS-induced injury in animal models (5, 25). These key questions lead us to examine SphK function and regulation in lung injury.
The protection of endothelial barrier disruption by S1P in endotoxin-induced inflammatory lung injury implies a role for SphKs in the repair mechanism. Overexpression of a SphK1 kinase-dead (SphKKD) mutant partially blocked S1P-induced increases in electrical resistance of human lung endothelial cells (V. Natarajan, unpublished observations), whereas activated protein C protected the endothelial barrier by activating SphK1 via protease-activated receptor-1, enhancing S1P production and S1P1 receptor activation (10). Inhibition of SphK1 expression using antisense or pharmacological inhibitors such as dimethylsphingosine attenuated neutrophil activation, chemotaxis, and lung permeability (38). However, disruption of the SphK1 gene in mice had no effect on the recruitment of inflammatory cells during thioglycolate-induced peritonitis (1), suggesting that SphK2 can compensate for a deficiency in SphK1 activity (27, 43). Although simultaneous deletion of both SphK1 and SphK2 resulted in mice with undetectable levels of S1P, these mice were ultimately nonviable due to lethal congenital defects in neuronal and vascular development. Although increasing evidences suggest the involvement of both SphKs in immunity and inflammation in vitro (6), very little is known regarding the role of SphKs as protective enzymes in animal models of pulmonary edema, inflammation, and lung injury. To understand the role of SphKs in lung injury, we studied the expression patterns of SphK1 and SphK2 in a murine model of LPS-induced lung injury. In wild-type (WT) LPS-treated mice, SphK1 expression was significantly increased at early time points, returning to normal levels within 24 h following treatment. However, SphK2 expression exhibited delayed induction and remained upregulated through 24 h. Furthermore, native SphK1 levels were detected in bronchoalveolar lavage (BAL) fluids, and treatment with LPS stimulated a severalfold increase of SphK1 protein in BAL fluid. In SphK1 knockout (SphK1−/−) mice, LPS-induced injury was markedly increased, suggesting a functional role for SphK1 in the regulation of lung injury. In SphK1−/− mice, the endotoxin-induced inflammatory response was further augmented by overexpression of SphK2, whereas overexpression of WT SphK1 protein attenuated lung injury. This suggested that SphK2 activation plays a distinctly separate role in the regulation of vascular injury compared with SphK1 activation. These results for the first time identify the involvement of SphK activation in lung injury and suggest the differential roles of both SphK isoforms in S1P synthesis during lung injury. This work was previously reported, in part, at American Thoracic Society (ATS; Ref. 40).
METHODS
Animal preparation and treatment.
All animal protocols were approved by the Institutional Animal Care and Use Committees of the Veterans Affairs (VA) Medical Center, State University of New York (SUNY) Downstate Medical Center, and, initially, Johns Hopkins University. SphK1−/− mice and their counterpart C57BL/6 WT controls were selected for all surgeries. SphK1−/− transgenic mice were obtained from Dr. Proia's laboratory at National Institute of Diabetes and Digestive and Kidney Diseases (National Institutes of Health). Before all procedures, mice were anesthetized with a 0.03-ml intraperitoneal injection of a 10:1 ketamine (100 mg/kg)-xylazine (10 mg/kg) solution with additional anesthesia administered as necessary.
Murine model of ALI and assessment of vascular permeability.
LPS was used to induce acute lung injury (ALI) in animals as described previously (4, 31). SphK1−/− and WT mice were divided into 3 groups: 1) untreated mice (sham surgery); 2) LPS-injected mice; and 3) PBS-injected mice. Up to 12 animals per group were studied unless statistical significance could be achieved with fewer numbers. Mice were administered either a control vehicle or LPS (2 μg/g) intratracheally. After intratracheal instillation of PBS or LPS, total mouse weight was determined. Twenty-four hours following treatment, the lungs were excised separately, and each lung was immediately weighed to determine wet lung weight. The samples were then dried in an oven (65°C; Fisher Scientific Isotemp) for 72–96 h and weighed daily to establish the dry lung weight. As a complementary measure of pulmonary edema formation, wet-to-dry lung weight ratios were determined for both left and right lungs. The results were then compared with those from the sham-operated mice. Wet-to-dry lung weight ratio was expected to increase with an increase in extravascular lung water, increased blood volume, or both.
Lung lavage protein concentration.
In the next set of experiments, lung BAL protein levels were determined following induction of lung injury. Pulmonary vascular permeability was first evaluated by measurement of the protein concentration in BAL fluid, which is indicative of protein leak from the vascular to the alveolar space. Lavage was performed by intratracheal instillation of 0.5 ml of saline immediately following anesthetization. In a separate group of mice, lungs were lavaged with Hanks’ balanced salt solution. Fluid was then aspirated to the lungs via the endotracheal tube, and the amount of fluid recovered carefully was recorded. Protein concentrations were measured in undiluted lavage fluid (Bio-Rad, Hercules, CA).
Assessment of lung injury.
After 4–6 h of observation following LPS or PBS treatment, mice were exsanguinated via transection of the abdominal aorta. The pulmonary artery was cannulated, the left atrial appendage excised, and 0.5–0.75 ml of PBS perfused through the pulmonary circulation to remove blood-borne elements. The left lung was then tied off, and the right lung was lavaged via three sequential intratracheal injections of Hanks’ balanced salt solution. The left lung was then excised en bloc, blotted dry, weighed, and snap-frozen in liquid nitrogen. Lungs from sham-operated and from PBS- or LPS-instilled mice were inflated to 20 cm with 0.2% of low melting agarose and fixed in 3.8% formaldehyde for histological staining and analysis by hematoxylin and eosin.
MPO activity assay.
BAL and lung lysate MPO activity, an indicator of neutrophil extravasation, was measured by kinetic readings over 20 min using a reaction buffer containing potassium phosphate buffer, 0.5% hexadecyltrimethylammonium bromide (HTAB), 0.167 mg/ml o-dianisidine dihydrochloride, and 0.0006% H2O2. The rate of change in absorbance was measured at a wavelength of 405 nm on a VMax kinetic microplate reader, with the results adjusted for total lung weight and presented as MPO units per lung.
Recombinant adenoviral delivery in the lungs.
For intratracheal delivery of adenoviruses (AdV), 8-wk-old WT control and SphK1−/− mice weighing between 20 and 25 g were deeply anesthetized as described earlier by Peng et al. (31). Recombinant adenoviral vectors, prepared as described previously (44), at a concentration of AdV 1010 plaque-forming units (PFU) in 50 μl of PBS (containing 3% sucrose, 0.1% bovine serum albumin) were delivered to the animals intratracheally. Ten days after delivery of the adenoviral vectors to the lungs, the mice were administered LPS intratracheally. After a subsequent 24 h following LPS treatment, mice were euthanized with pentobarbital (150 mg ip), and lungs were used for biochemical assays or biophysical measurements.
Statistical analysis.
Differences in lavage protein concentrations and wet-to-dry lung weight ratios between groups were compared using two-way ANOVA. Differences between WT and SphK1−/− mice were compared using one-way ANOVA (37). When significant variance ratios were obtained, the least significant differences were calculated to allow comparison of individual group means. Differences were considered significant at P < 0.05.
RNA isolation.
After the set period of exposure, the lungs were excised and immediately flash-frozen in liquid nitrogen. The frozen lung tissue was homogenized, and MPO activity was measured. For isolation of RNA from tissue and cultured human pulmonary artery endothelial (HPAE) cells, samples were initially suspended in 1 ml of TRIzol solution (Invitrogen-GIBCO BRL, Life Technologies). Chloroform (0.2 ml) was added, and samples were agitated and then centrifuged at 11,500× relative centrifugal force (RCF) for 15 min at 4°C. The supernatant containing the RNA was then preserved, and 0.5 ml of isopropanol was added followed by repeat agitation and centrifugation. The supernatant was then preserved once again, and 100 μl of diethyl pyrocarbonate (DEPC) water and 200 μl of cold ethanol were added. Before cDNA synthesis, RNA was purified using the RNeasy Mini Kit according to the manufacturer's protocol (Qiagen). RNA quality and quantity could then be quantified by spectrophotometric analysis.
Microarray methods.
All expression profiling experiments were performed using either the Affymetrix Human U133 or Mouse Expression Set 430 GeneChip systems in the Expression Profiling Core from the Department of Medicine, Proteomics and Genomics Facilities, Division of Pulmonary and Critical Care Medicine at the Johns Hopkins Asthma and Allergy Center. Total pooled RNA from duplicate control and LPS-challenged mice was hybridized to an Affymetrix mouse chip for microarray, and RNA expression values from each chip were normalized using the Affymetrix program MAS. The complete gene expression profiles for all 12 chips were then evaluated using significance analysis of microarrays (SAM) algorithm analysis to avoid false-positive errors, which can result from the large number of statistical analyses being performed as described earlier (17).
Data analysis.
Data analysis was performed using GeneSpring software (Silicon Genetics). This software incorporates filtering tools as wells as multiple clustering algorithms, including hierarchical, K-means, and self-organizing maps.
Antibodies, expression plasmids, and viral vector preparation.
SphK1 and SphK2 antibodies were purchased from Abgent (San Diego, CA), whereas SphK deletion mutants in adenoviral expression vectors, including internal deletion mutants, were generated with AdEasy Adenoviral Vector System and purified as described previously (44). All DNA manipulations were performed according to standard protocols. Oligonucleotide synthesis and DNA sequencing were performed on site by the Downstate Medical Center Core Facility.
Real-time and RT-PCR.
Total RNA from control and experimental lung samples were isolated using TRIzol (Invitrogen-GIBCO BRL, Life Technologies) solution. Purified RNA (1 μg) from each lung sample was used in a RT reaction to produce cDNA using the SuperScript First-Strand Synthesis system. Primers for mouse SphK1 and SphK2 to perform PCR amplification by TaqMan Gene Expression Assay were used (Applied Biosystems assay ID for primers: SphK1, Mm20944; SphK2, Mm24222). As an internal control, 18S rRNA primers and probes were used (Sigma-Genosys). For mouse and human SphK1 and SphK2, RT-PCR primers were synthesized as described previously (44).
RESULTS
Differential expression of SphKs and S1P phosphatase in LPS-induced lung injury.
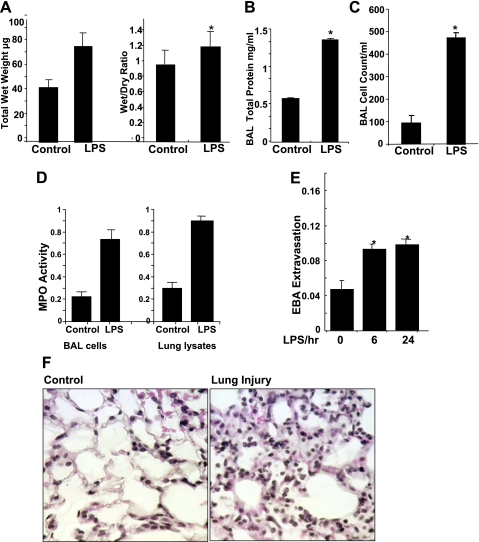
Intratracheal instillation of LPS (2 μg/g in 50 μl of sterile PBS) produced significant lung injury when assessed at 6 and 24 h by histological analysis and measurement of BAL protein levels as well as Evans blue dye albumin leakage from the intravascular space into the lung parenchyma. LPS treatment caused an increase in total wet lung weight and in the wet-to-dry lung weight ratio (Fig. 1A) as well as significantly elevated total protein levels in BAL fluid (∼3-fold increase; Fig. 1B) compared with in PBS-challenged mice. Furthermore, intratracheal administration of LPS caused a significant increase in polymorphonuclear neutrophil (PMN) accumulation in BAL fluid compared with treatment with PBS (Fig. 1C). MPO activity was also significantly greater in the BAL fluid as well as the lung tissue in LPS-treated mice compared with mice treated with only PBS (Fig. 1D). Within 6 h, LPS treatment increased tissue Evans blue concentration >60% (Fig. 1E) and substantial infiltration of inflammatory cells into the capillary and extracapillary spaces (Fig. 1F).
Fig. 1.
LPS-induced lung injury in C57BL/6 mice. A: total wet weight of lungs compared between control and LPS-treated mice. The wet-to-dry lung weight ratio was then calculated and compared in control and LPS-treated mice. B: right lung were lavaged using normal saline, cells collected from bronchoalveolar lavage (BAL) fluid were lysed, and total protein concentration was determined. C: total number of cells in the lavage fluid was measured. D: MPO activity was determined in lavage fluid and lung lysates. E: pulmonary vascular leakage was assessed by the extravasation of Evans blue albumin (EBA) into lung parenchyma. Concentration in the lung homogenates from LPS-treated and control mice standardized to total body weight were compared. Results were compared at 0, 6, and 24 h after LPS treatment. *Significant differences in LPS vs. saline-treated groups. F: hematoxylin and eosin staining of lung tissue was performed following intratracheal LPS administration for 24 h. Statistical significance was calculated by 1-way ANOVA. *Values significantly different between the LPS- and saline-treated groups (P < 0.05).
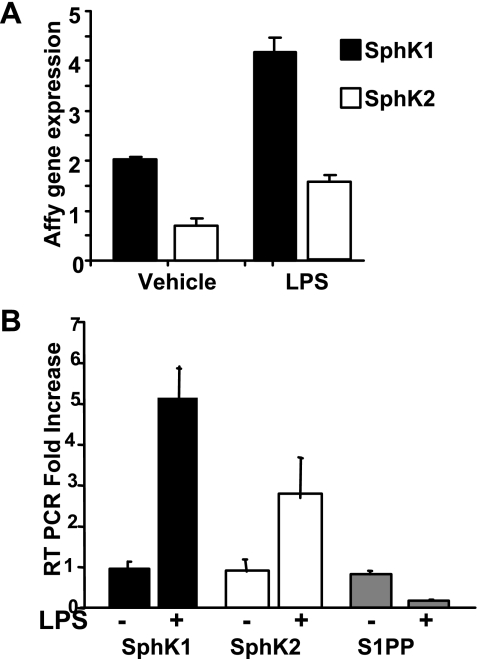
Using microarray analysis in control and LPS-treated mice, we evaluated the diversity of the transcriptional effects of LPS on enzymes regulating S1P metabolism in acute lung inflammatory responses (Fig. 2A; Ref. 3). To further understand the role of SphKs in lung injury, in vivo SphK1 and SphK2 expression levels in LPS-treated and untreated mice were determined by semiquantitative RT-PCR using primers specific for SphK1, SphK2, and sphingosine-1-phosphate phosphatase (S1PP). RT-PCR analysis revealed that the mRNA expressions of SphK1, normalized with GAPDH as a control, increased ∼5-fold following LPS treatment, whereas the expression of SphK2 was increased by ∼3-fold after LPS challenge. However, under similar experimental conditions, the expression of S1PP, a lipid phosphate phosphatase that degrades S1P to sphingosine, was downregulated following LPS challenge (Fig. 2B).
Fig. 2.
Differential regulation of sphingosine kinase (SphK) isoforms SphK1 and SphK2 following LPS-induced lung injury. Mice were treated with either a vehicle, PBS, or LPS, and lungs were separated for RNA analysis. Selected gene expression in the sphingolipid pathways were analyzed by GeneSpring software. A: SphK1 and SphK2 expression in response to LPS treatment compared between vehicle- and LPS-treated mice. B: fold change in mRNA expression levels of SphK1 and SphK2 and sphingosine-1-phosphate (S1P) phosphatases (S1PP) measured by microarray and analyzed by RT-PCR using GAPDH as an internal control.
Increased protein expression of SphK1 by LPS in C57BL/6 mice.
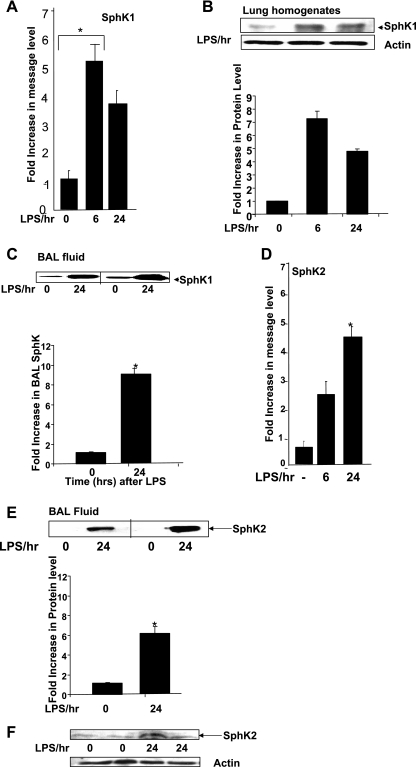
Given that intratracheal administration of LPS increased mRNA expression of SphK1 in the murine lung, we next characterized SphK1 gene and protein expression changes at 6- and 24-h time points following LPS administration. In LPS-treated mice, the expression of SphK1 mRNA, as determined by real-time RT-PCR normalized to 18S RNA as a control, was increased ∼5-fold within 6 h of treatment and then fell back to a ∼3-fold increase above baseline levels by 24 h (Fig. 3A). The increase in SphK1 mRNA levels after LPS exposure correlated with a significant upregulation of SphK1 protein expression at the same time points, as determined by Western blotting using a SphK1-specific antibody with actin as a loading control (Fig. 3B). Earlier studies have shown that overexpression of WT SphK1, but not of WT SphK2, in endothelial cells results in secretion of SphK1 protein into the culture medium (2). However, in vivo it is unclear whether the native SphK1 protein was secreted from cells or from tissues into the surroundings. Therefore, we analyzed BAL fluid from control and LPS-treated mice for the presence of SphK1. After separating the cells from BAL fluid, total protein concentration in the supernatant was determined and analyzed by Western blotting, which revealed that LPS treatment increased the levels of native SphK1 protein in BAL fluid (Fig. 3C). However, BAL fluid collected from PBS-treated mice also contained SphK1, which suggested the possibility that damaged cells present in the BAL fluid might be the source of SphK1 enzyme secretion in the fluid supernatant. In LPS-treated mice, SphK2 mRNA expression was determined by real-time PCR normalized to 18S RNA. At basal levels, SphK2 expression was very low in total lung tissue; however, following LPS treatment, SphK2 expression was increased 2- to 3-fold within 6 h and >4-fold after 24 h (Fig. 3D). The increases in SphK2 expression levels in BAL fluid (Fig. 3E) and lung homogenates (Fig. 3F) were significant, considering at basal levels no SphK2 was detected (Fig. 3, E and F). These results show for the first time that LPS upregulates SphK1 and SphK2 mRNA and protein expression in murine lungs as well as increases the protein levels in the BAL fluid.
Fig. 3.
Time-dependent increase of SphK1 following LPS treatment. A: equal amounts of mRNA from LPS-treated and PBS-treated lungs were analyzed and compared by real-time PCR using SphK1-specific primers. Fold increase in expression was normalized to 18S RNA. B: SphK1 expression levels in PBS-treated and 6- and 24-h LPS-treated lung tissue homogenates as observed by Western blotting of lung homogenates. SphK1 expression increased nearly 8-fold in the first 6 h following LPS treatment and then decreased significantly by 24 h posttreatment. C: expression of SphK1 in lung lavage fluid, shown by Western blotting of BAL fluid from LPS-treated wild-type (WT) mice. Nearly 5-fold increase in SphK1 expression was observed in BAL fluid 24 h after LPS treatment. D: SphK2 mRNA levels were analyzed by real-time PCR using SphK2-specific primers. *Significant increase in SphK1 expression from 3 independent experiments. P < 0.05 is considered significant. E and F: SphK2 protein expression levels in lung lavage fluid and in lung tissue, respectively, were analyzed by Western blotting and immunoblotted for SphK2-specific antibody.
Genetic deletion of SphK1 exacerbates LPS-induced pulmonary edema, inflammation, and lung injury.
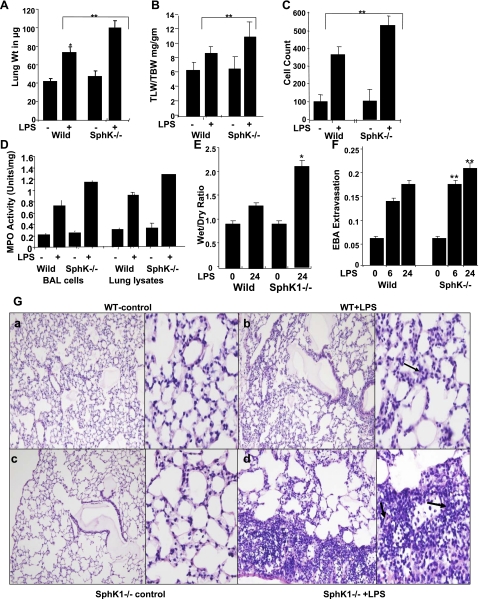
Earlier studies on SphK1−/− mice have shown that these mice generate significantly lower circulating plasma levels of S1P and that their platelets exhibit reduced S1P synthesis; however, these mice display no noticeable vascular phenotype (10, 38). Therefore, we examined the role of SphK1 in LPS-induced lung injury using SphK1−/− and WT littermates. In WT mice, LPS treatment resulted in increases in total lung weight (Fig. 4A), lung wet weight-to-total body weight ratio (Fig. 4B), inflammatory cell numbers in BAL fluid (Fig. 4C), and MPO activity (Fig. 4D). However, in SphK1−/− mice, LPS treatment caused significantly greater increases in total wet lung weight, inflammatory cells in BAL fluid, and MPO activity compared with similarly treated WT mice (Fig. 4, A–D). Furthermore, there was significant increase in the wet-to-dry lung weight ratio compared with WT mice (Fig. 4E). Additionally, histological analysis of lung tissues from SphK1−/− mice stained with hematoxylin and eosin showed increased neutrophil infiltration in capillary and extracapillary spaces after LPS treatment compared with WT mice (Fig. 4G). Evans blue albumin leakage also significantly increased in SphK1−/− mice (Fig. 4F). These results indicate that genetic deletion of SphK1 significantly augments LPS-induced pulmonary edema, infiltration of inflammatory cells, and MPO activity in the lungs.
Fig. 4.
Effects of SphK1 deficiency on pulmonary vascular permeability in LPS-treated lungs. Comparison of LPS-induced lung injury in SphK1−/− and WT control mice. A: total wet lung weight was measured. *Significant increase in lung weight in WT and knockout mice. B: total lung weight to body weight (TLW/TBW) compared between WT and knockout mice. *Significant increase. C: right lungs were lavaged using normal saline, and total number of cells in the lavage fluid was measured. D: MPO activity was measured from lung lysates and cells from lavage samples. Average of 3 independent lung lysates was plotted. E: the wet-to-dry lung weight ratio was compared in the WT and SphK−/− mice after LPS-treatment. F: pulmonary vascular leakage was assessed by EBA extravasation assay at 6 and 24 h after LPS was delivered. **Comparison between WT and knockout mice and significant differences. G, a–d: histological comparison of LPS-induced lung injury in WT and SphK1−/− mice. In SphK1−/− mice, LPS produced prominent neutrophil accumulation compared with WT mice (shown by black arrows).
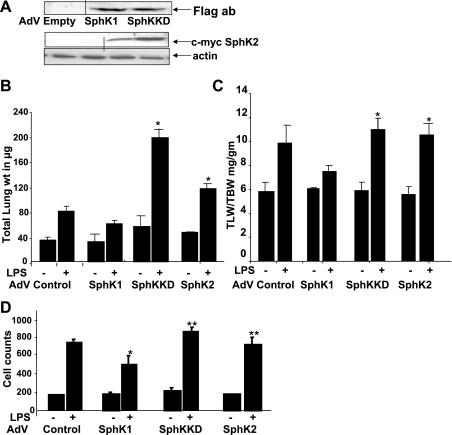
Adenoviral delivery of SphK1 attenuated LPS-induced lung injury.
Earlier in our in vitro studies, we showed that TNFα-induced S1P synthesis in HPAE cells is blocked by overexpression of SphKKD and enhanced by WT SphK1 overexpression (44). This suggested that S1P synthesis is regulated directly by SphK1 activation and that SphKKD acts like a dominant negative to inhibit S1P synthesis. To better understand the role of SphKKD-dependent inhibition in a lung injury model in vivo, we next compared the effects of adenoviral SphK1, SphKKD, and SphK2 expression on LPS-induced lung injury in SphK1−/− mice. Intratracheal administration of replication-deficient adenoviral vectors expressing one of the three AdV SphKs, at an inoculum of 108 PFU per mouse, led to increased expression of Flag-tagged SphK1 and SphKKD, and c-myc-tagged SphK2 in the lungs 8 days following infection (Fig. 5A). In SphK1−/− mice, overexpression of WT SphK1, but not of SphKKD, partly attenuated LPS-induced lung injury, as determined by total lung weight (Fig. 5B), total lung-to-body weight ratio (Fig. 5C), and infiltration of PMNs into the lungs (BAL fluid) (Fig. 5D). Next, we overexpressed SphK2 in SphK1−/− mice and studied the LPS response. Compared with SphK1-expressing mice, SphK2 overexpression did not confer any protection from lung injury but, in fact, significantly exacerbated the LPS response (Fig. 5, B–D). These results illustrated the utility of adenoviral WT SphK1 delivery, specifically in the lung tissue. Furthermore, our data suggested that, in SphK1−/− mouse lungs, adenoviral expression of WT SphK1, but not of SphKKD or WT SphK2, reduced LPS-induced lung injury and inflammation.
Fig. 5.
Attenuation of LPS-induced injury by SphK1 overexpression. Recombinant adenovirus (AdV) vectors were produced as described in methods and intratracheally delivered into lungs. SphK1−/− mice were infected with 1010 plaque-forming units (PFU) of empty vector AdV, WT SphK1, or SphK1 kinase-dead mutant (SphKKD)-expressing AdV for 10 days, and LPS-induced lung injury was compared. A: SphK1 and SphKKD expression following adenoviral infection was detected by Flag antibody (ab) immunostaining. Lung homogenate from 2 control and 2 SphK2-infected lungs were analyzed for c-myc tag SphK2 expression using c-myc-specific antibody. B–D: lung injury was measured in LPS and saline-treated control mice. B: total lung weight. C: ratio of total lung weight to total body weight. *Significant increase in lung weight and ratio of total lung weight to total body weight in AdV SphKKD and AdV SphK2-infected lungs compared with AdV SphK1-expressing lungs. D: cell count to compare relative injury between SphK1 AdV-infected, SphKKD mutant AdV-infected, and uninfected SphK1−/− control mice. *Significant decrease in cell count after SphK1 overexpression. **Highly significant increase in cell count after SphKKD and SphK2 expression and LPS treatment. * And ** represent values with P < 0.05 considered significant.
Adenoviral expression of WT SphK1 but not of WT SphK2, attenuates LPS-induced TNFα generation and S1P synthesis in the lungs of SphK1−/− mice.
To understand the effects of SphK overexpression on LPS-induced inflammation in the lungs, we measured TNFα expression and S1P synthesis in control and SphK1 or SphK2 AdV-overexpressing SphK1−/− mice lungs challenged with either PBS or LPS. Following LPS challenge for 24 h, a significant increase in expression of TNFα was observed in SphKKD- and SphK2-infected lungs. However, overexpression of SphK1 in SphK−/− mice inhibited TNFα expression (Fig. 6A). Additionally, we examined the inflammatory response in adenoviral SphK-infected mice by examining the levels of macrophage inflammatory protein (MIP) expression following LPS treatment (Fig. 6B). We found that adenoviral delivery of SphK1 and SphK2 significantly reduced LPS-induced MIP levels in SphK1−/− mice, whereas SphKKD expression did not elicit any response. Since TNFα activates SphK in endothelial cells (13), we next examined the effects of TNFα induction on S1P synthesis in SphK1−/− mice lungs overexpressing either SphK1, SphK2, or SphK1 mutant. As shown in Fig. 6C, lung lysates had the ability to convert sphingosine to S1P, and overexpression of SphK1 or SphK2 WT in SphK1−/− mice enhanced S1P formation before and after LPS treatment.
Fig. 6.
Regulation of TNFα expression and S1P synthesis in SphK-overexpressing lungs. SphK1−/− mice intratracheally infected with AdV-containing SphK1, SphK2, and SphKKD were treated with vehicle alone or LPS for 24 h, and total lung lysates were prepared for protein analysis. A: quantitation of TNFα expression by ELISA assay. Fold increase in TNFα expression was averaged from 3 independent experiments. *Significant increase in TNFα expression after LPS treatment in AdV SphK2- and AdV SphKKD-infected lungs. **Significant decrease in TNF level in AdV SphK1-infected lungs. B: macrophage inflammatory protein (MIP) ELISA assay. Equal protein concentration from lung homogenates was analyzed for MIP level using MIP-specific monoclonal antibody and sandwich ELISA technique. Comparative fold increase in concentration in protein level is shown. AdV SphK1 expression significantly decreased MIP level compared with control, SphK2-, or SphKKD-infected lungs (P < 0.05 is considered significant). *Significant increase in MIP levels after SphK2 overexpression. **Significant decrease in LPS-induced MIP levels after SphK1 overexpression. C: SphK activity was measured in total cell lysates in presence of 1% Triton X-100 as described in methods and earlier reports (1, 44). Lung tissue lysates were solubilized in 1.0% Triton X-100, and equal amounts of protein were extracted using 1 ml of chloroform-25 mM HCl methanol (1:1) as described in Refs. 1 and 44, and extracted lipids were separated on TLC plates.
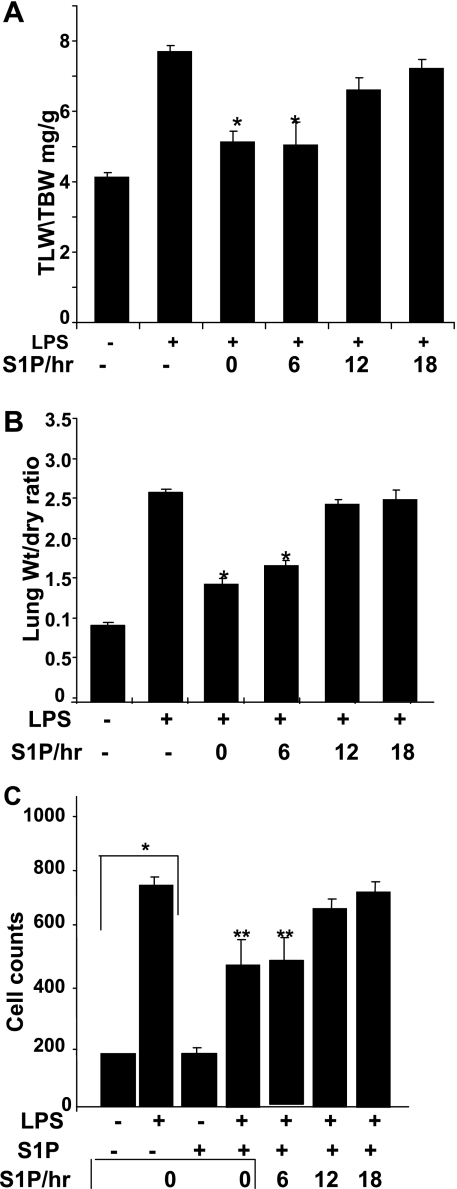
Time-dependent inhibition of LPS-induced lung injury by S1P administration.
We observed that overexpression of SphK1 as well as SphK2 induced S1P synthesis; however, SphK2 alone was unable to protect mice from lung injury. To further understand the mechanism of SphK1-induced protection, we administered S1P to SphK1−/− mice in a time-dependent manner. LPS-induced lung injury, as measured by total lung weight-to-total body weight and wet-to-dry lung weight ratios, was attenuated by S1P administration in a time-dependent manner (Fig. 7, A and B). Within the first 6 h following LPS treatment, S1P administration dramatically decreased lung injury. However, at later time points (6–24 h), S1P did not provide the lungs any significant protection from further injury (Fig. 7C). This suggested a direct role of S1P generation in attenuation of lung injury. Although SphK2 expression was induced in lung tissue following LPS treatment, its activity alone was not sufficient to protect mice from injury. Furthermore, our observations suggest that differential activation of both SphK isotypes, and subsequent S1P synthesis, plays a distinct functional role in the regulation of lung injury in a time-dependent manner.
Fig. 7.
Time-dependent effect of S1P administration on LPS-induced lung injury and inflammation. A: after intratracheal instillation of LPS, S1P was administered at 0-, 6-, 12-, and 18-h time points, and lung injury was assessed 24 h after LPS exposure. Lung injury in LPS- and saline-treated control mice were assessed 24 h after treatment as described in methods. A: ratio of total lung wet weight to total body weight. B: ratio of total lung wet-to-dry weight. C: effect of S1P on BAL cell count. Relative injury was compared between S1P-treated and untreated mice after LPS challenge. BAL fluid was assessed at 24 h after intratracheal saline or LPS challenge and analyzed for total and differential cell counts. *Significant decrease in TLW/TBW ratio after S1P treatment (A) and lung wt/dry ratio (B) or total increase in cell count (C). **Significant decrease in BAL cell counts after S1P treatment compared with LPS-treated mice BAL cell count. Values with P < 0.05 are considered significant.
DISCUSSION
Sphingosine and its metabolic precursor, ceramide, are both growth-inhibiting lipids involved in the processes of differentiation and apoptosis. Activation of SphK is the final, rate-limiting enzymatic step in the conversion of sphingosine to S1P. S1P acts as both an intracellular and extracellular signaling molecule and is implicated in the regulation of many important cellular processes including growth, survival, differentiation, cytoskeletal rearrangement, motility, angiogenesis, and Ca2+ mobilization (31, 32, 35, 36). In this study, we hypothesized that within the sphingolipid-rich environment of the lung vasculature, the activation of SphKs and the subsequent generation of S1P plays a barrier-protective role by controlling inflammatory responses. Therefore, we examined the expression and functions of both SphK isoenzymes, SphK1 and SphK2, and regulation of their expression during LPS-induced lung injury. In this study, we present for the first time a protective function for SphK1, but not SphK2, in LPS-induced lung injury.
Current understanding of the role(s) of SphKs and S1P in inflammation and lung injury is limited. Earlier studies by Peng et al. (31) and McVerry et al. (26) showed that, in a murine model of LPS-mediated ALI, infusion of S1P significantly decreased pulmonary and renal vascular leakage and inflammation. These findings may represent a novel therapeutic strategy for vascular barrier dysfunction (31, 35). Furthermore, addition of exogenous S1P to cultured neonatal rat ventricular myocytes conferred protection from hypoxia-induced cell death (20). Similarly, in an ex vivo mouse model, S1P administration via aortic cannula before ischemia-reperfusion injury lead to improved hemodynamics, reduced creatine kinase release, and diminished infarct size (19). Although S1P plays a barrier-protective role in LPS-induced lung injury, the involvement of SphK, its enzymatic activation, and its transcriptional regulation during different phases of lung injury are not clear. Our studies show that SphK1−/− mice, compared with WT controls, are much more susceptible to LPS-induced inflammation and lung injury. In parallel studies, overexpression of a SphK1 kinase-dead mutant in SphK1−/− mice further exacerbated the LPS response and lung injury. Conversely, overexpression of WT SphK1 protected SphK1−/− mice from LPS-induced lung injury. However, WT SphK2 overexpression in SphK1−/− mice failed to provide protection and, in fact, augmented LPS-induced lung injury. Based on these results, the present study clearly establishes a function of SphK1 in protection against LPS-induced lung injury in a murine model.
Although SphK1 and SphK2 catalyze phosphorylation of sphingosine to S1P in mammalian cells, our results suggest a differential role of these isoenzymes in LPS-induced lung injury. Furthermore, earlier studies indicate that enzyme activity of SphK1 may be higher in conversion of sphingosine into S1P compared with SphK2 in vitro. Our earlier studies (40, 44) have characterized the infection and expression of adenoviral SphK1 and SphK2 WT and SphK1 mutant in HPAE cells and, further, have optimized the conditions for their expression in lung tissues. In contrast to WT SphK1 or SphK2, the kinase-dead mutant acted like a dominant-negative kinase, blocking intracellular generation of S1P from exogenously added sphingosine in HPAE cells (44). Furthermore, in human lung endothelial cells, overexpression of SphK1, compared with SphK2, significantly enhanced the conversion of sphingosine to S1P (44), suggesting a major role for SphK1 in S1P formation. As SphK1 and SphK2 double-knockout mice are not viable, blocking S1P synthesis via SphKKD in the lungs could generate a phenotype to better understand the function of the two intracellular SphKs. Infection of adenoviral SphKKD mutant in mouse lungs did not alter basal level BAL cell count or total protein concentration but significantly enhanced LPS-induced lung injury and protein levels in BAL. In contrast to SphKKD, overexpression of SphK1 WT kinase significantly attenuated the LPS response, and all of these responses were highly pronounced in SphK−/− mice compared with WT controls. However, overexpression of SphK2 WT showed very little effect on LPS-induced lung injury in SphK1−/− mice. This clearly suggests an in vivo role for SphK1 plays in the regulation of LPS-induced lung injury. In contrast to a protective role for SphKs in the endothelium (44), SphKs can promote a proinflammatory response in epithelial and immune cells (46). In epithelial cells, activation of SphK1 by TNFα, IL-1α, or LPS mediates enhanced expression of cyclooxygenase-2 (COX-2) and monocyte chemoattractant protein-1 (MCP-1) (6), whereas inhibition of SphK attenuated shock-induced lung injury (43). Thus SphKs can promote inflammation or protect against injury in the lung pointing to differential action of SphKs and S1P in lung function.
Although SphK1−/− mice are viable and fertile and showed no phenotypic differences, the total S1P synthesis and SphK activity was substantially, but not completely, reduced in SphK1−/− null mice. These results raise the possibility that SphK1 and SphK2 might have redundant functions in mice and that SphK2 could compensate for a deficiency in SphK1 activity (27, 43). To investigate the physiological functions of both isoforms, Mizugishi, et al. (27) and Kharel et al. (21) generated SphK2 knockout mice, which were viable and fertile like SphK1−/− (21). Simultaneous deletion of both enzymes resulted in nonviable mice with undetectable levels of S1P with defects in neuronal and vascular development, suggesting a necessary role of SphK activity and S1P generation for survival (1, 27). Although significant protein expressions of SphK1 and SphK2 were identified in HPAE cells (44), relatively lower level of SphK2 was detected in total murine lung tissue as measured by real-time PCR and immunostaining. However, following LPS-induced injury, in addition to SphK1, we also observed an increase in SphK2 transcripts as well as increased secretion in BAL fluid after LPS treatment, representing either a potential compensation for diminished S1P synthesis via SphKs or possible involvement of SphK2 in a second phase LPS response required for SphK2-induced apoptosis.
Our observations on the function(s) of SphKs in barrier regulation and protection from vascular injury are further supported in a recent paper by Li et al. (22) from Drs. Vadas and Gamble's group. This paper clearly shows that vascular leakage is increased in SphK1−/− mice and that transgenic overexpression of SphK1 attenuates the barrier leakiness. Their paper proposes that the mechanism controlling angiopoietin-induced barrier protection involves SphK1 activation. In this study, not only do we demonstrate a protective effect of SphK1 overexpression in lung injury, but also we show that SphK1−/− are more prone to LPS-induced lung injury. We further demonstrate that SphK2 activation and S1P synthesis are not protective and may contributing factors to the worsening of lung injury. We believe that these observations lead us more toward understanding the mechanism of SphK activation and S1P synthesis. We demonstrated that the earlier phase of S1P production or treatment attenuates the severity of lung injury. However, we found that later phase treatment with S1P did not protect animals from lung injury. This finding is consistent and correlates with our observations that although overexpression of both SphK isoforms results in S1P production, the outcome of each kinase activation differs and that SphK2 induction and S1P synthesis do not protect animals from injury. It is not clear whether altered levels of S1P are causing more injury or protection, however, our data suggest that the timing of S1P production is significant. At basal levels, SphK2 expression is minimal in lung tissue, however, within 6 h of LPS treatment SphK2 levels were upregulated. Therefore, S1P production by SphK2 overexpression did not occur in the early phase and did not confer any protection from lung injury. In AdV SphK2-infected lungs, S1P was synthesized but did not protect against lung injury. This observation may be associated with the location of S1P synthesis and duration of SphK2 activation, which plays a role in regulation of lung inflammation. Alternatively, S1P production may not have any role in regulation of inflammatory responses in SphK1- or SphK2-overexpressing mice, and the enzymatic or structural roles of these kinases may be different and may depend on environment or agonist treatment. Although overexpression of SphK1 promotes cell survival and protects cells from apoptotic insults such as serum withdrawal (39, 42), overexpression of SphK2 suppresses cell growth and enhances apoptosis in cultured cells (24, 29). Thus generation of S1P may function as an internal sensor that can decide the fate of cells to undergo apoptosis or to survive.
In summary, we have demonstrated a novel role for intracellular SphK1 activation in the enhancement of vascular endothelial barrier protection in LPS-mediated pulmonary inflammation in vivo. A major finding of this study is that the intracellular function of SphK1, independent of the extracellular signaling of its product S1P, is critical in the regulation of inflammatory responses and the severity of lung injury. This is the first study to show that adenoviral delivery of genes, in this case SphK1, in the lungs significantly decreases vascular leakage and inflammation in a murine model of LPS-mediated ALI. Our data further suggest that SphK1 is important for the maintenance of pulmonary vascular permeability in vivo, and therefore its deficiency may have a significant impact on the development of ALI under pathophysiological conditions such as sepsis.
GRANTS
This work was supported by a VA Merit Award for R. Wadgaonkar and National Heart, Lung, and Blood Institute Grant R01-HL-79396 to V. Natarajan.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, Van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem 279: 52487–52492, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem 277: 6667–6675, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Barnard J, Wadgaonkar R, Sammani S, Garcia JG. Targeted deletion of gelsolin potentiates endotoxin-induced murine lung vascular leak (Abstract). FASEB J 18: 2004.
- 4.Becker PM, Kazi AA, Wadgaonkar R, Pearse DB, Kwiatkowski D, Garcia JG. Pulmonary vascular permeability and ischemic injury in gelsolin-deficient mice. Am J Respir Cell Mol Biol 28: 478–484, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya J Lung injury: sphingosine-1-phosphate to the rescue. Am J Respir Crit Care Med 170: 928–929, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal 17: 1203–1217, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann V Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 115: 84–105, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Bu S, Yamanaka M, Pei H, Bielawska A, Bielawski J, Hannun YA, Obeid L, Trojanowska M. Dihydrosphingosine 1-phosphate stimulates MMP1 gene expression via activation of ERK1/2-Ets1 pathway in human fibroblasts. FASEB J 20: 184–186, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381: 800–803, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 105: 3178–3184, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda Y, Kihara A, Igarashi Y. Distribution of sphingosine kinase activity in mouse tissues: contribution of SPHK1. Biochem Biophys Res Commun 12: 155–160, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh TK, Bian J, Gill DL. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science 248: 1653–1656, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Hla T Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol 15: 513–520, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Igarashi Y Functional roles of sphingosine, sphingosine 1-phosphate, and methylsphingosines: in regard to membrane sphingolipid signaling pathways. J Biochem 122: 1080–1087, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem 278: 46832–46839, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 288: L1026–L1032, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Jin ZQ, Karliner JS. Low dose N, N-dimethylsphingosine is cardioprotective and activates cytosolic sphingosine kinase by a PKCepsilon dependent mechanism. Cardiovasc Res 71: 725–734, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, Goetzl EJ, Karliner JS, Gray MO. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol 282: H1970–H1977, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol 33: 1713–1717, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Kharel Y, Lee S, Snyder AH, Sheasley-O'neill SL, Morris MA, Setiady Y, Zhu R, Zigler MA, Burcin TL, Ley K, Tung KS, Engelhard VH, Macdonald TL, Pearson-White S, Lynch KR. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem 280: 36865–36872, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Stankovic M, Bonder CS, Hahn CN, Parsons M, Pitson SM, Xia P, Proia RL, Vadas MA, Gamble JR. Basal and angiopoietin-1-mediated endothelial permeability is regulated by sphingosine kinase-1. Blood 7: 3489–3497, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limaye V, Li X, Hahn C, Xia P, Berndt MC, Vadas MA, Gamble JR. Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood 105: 3169–3177, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem 278: 40330–40336, 2003. [DOI] [PubMed] [Google Scholar]
- 25.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem 92: 1075–1085, 2004. [DOI] [PubMed] [Google Scholar]
- 26.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med 170: 987–993, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 25: 11113–11121, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, Gao S, Miwa N, Jahangeer S, Nakamura S. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem 280: 36318–36325, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Osawa Y, Banno Y, Nagaki M, Brenner DA, Naiki T, Nozawa Y, Nakashima S, Moriwaki H. TNF-alpha-induced sphingosine 1-phosphate inhibits apoptosis through a phosphatidylinositol 3-kinase/Akt pathway in human hepatocytes. J Immunol 167: 173–180, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 169: 1245–1251, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J 349: 385–402, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem 92: 913–922, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Savov JD, Gavett SH, Brass DM, Costa DL, Schwartz DA. Neutrophils play a critical role in development of LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol 283: L952–L962, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Singleton PA, Dudek SM, Chiang ET, Garcia JG. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J 19: 1646–1656, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4: 397–407, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Steele RG, Torie JH. Principles and Procedures of Statistics: A Biometrical Approach (2nd ed.). New York: McGraw-Hill, 1999.
- 38.Vlasenko LP, Melendez AJ. A critical role for sphingosine kinase in anaphylatoxin-induced neutropenia, peritonitis, and cytokine production in vivo. J Immunol 174: 6456–6461, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Wadgaonkar R, Garcia JG, Natarajan V. Competitive interaction of TRAF2 with SphK1 and ASK1: theme in endothelial apoptosis and survival (Abstract). ATS Meeting 2005.
- 40.Wadgaonkar R, Patel V, Butnariu D, Natajan V, Garcia JG. Differential regulation of sphingosine kinase in lung injury (Abstract). ATS Meeting 2006.
- 41.Xia P, Gamble JR, Wang L, Pitson SM, Moretti PA, Wattenberg BW, D'Andrea RJ, Vadas MA. An oncogenic role of sphingosine kinase. Curr Biol 23: 1527–1530, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Xia P, Wang L, Gamble JR, Vadas MA. Activation of sphingosine kinase by TNFα inhibits apoptosis in human endothelial cells. J Biol Chem 274: 34499–34505, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Zemann B, Urtz N, Reuschel R, Mechtcheriakova D, Bornancin F, Badegruber R, Baumruker T, Billich A. Normal neutrophil functions in sphingosine kinase type 1 and 2 knockout mice. Immunol Lett 109: 56–63, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y, Kalari S, Usatyuk PV, Gorshkova I, He DH, Watkins T, Brindley DN, Chaode S, Bittman R, Garcia JG, Evgeni Berdyshev V, Natarajan V. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem 282: 14165–14177, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng DM, Kitamura T, Ikejima K, Enomoto N, Yamashina S, Suzuki S, Takei Y, Sato N. Sphingosine 1-phosphate protects rat liver sinusoidal endothelial cells from ethanol-induced apoptosis: role of intracellular calcium and nitric oxide. Hepatology 44: 1278–1287, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Zhi L, Leung BP, Melendez AJ. Sphingosine kinase 1 regulates pro-inflammatory responses triggered by TNFα in primary human monocytes. J Cell Physiol 208: 109–115, 2006. [DOI] [PubMed] [Google Scholar]