Abstract
Increased oxidative stress is associated with perinatal asphyxia and respiratory distress in the newborn period. Induction of nuclear factor erythroid 2 p45-related factor (Nrf2) has been shown to decrease oxidative stress through the regulation of specific gene pathways. We hypothesized that Nrf2 attenuates mortality and alveolar growth inhibition in newborn mice exposed to hyperoxia. Nrf2+/+ and Nrf2−/− newborn mice were exposed to hyperoxia at 24 h. Survival was significantly less in Nrf2−/− mice exposed to 72 h of hyperoxia and returned to room air (P < 0.0001) and in Nrf2−/− mice exposed to hyperoxia for 8 continuous days (P < 0.005). To determine the response of Nrf2 target genes to hyperoxia, glutathione peroxidase 2 (Gpx2) and NAD(P)H:quinone oxidoreductase (NQO1) expression was measured from lung of newborn mice using real-time PCR. In the Nrf2+/+ mice, significant induction of lung Gpx2 and NQO1 above room air controls was found with hyperoxia. In contrast, Nrf2−/− mice had minimal induction of lung Gpx2 and NQO1 with hyperoxia. Expression of p21 and IL-6, genes not regulated by Nrf2, were also measured. IL-6 expression in Nrf2−/− lung was markedly induced by 72 h of hyperoxia in contrast to the Nrf2+/+ mice. p21 was induced in both Nrf2+/+ and Nrf2−/− lung by hyperoxia. Mean linear intercept (MLI) and mean chord length (MCL) were significantly increased in 14-day-old Nrf2−/− mice previously exposed to hyperoxia compared with Nrf2+/+ mice. The percentage of surfactant protein C (Sp-c+) type 2 alveolar cells in 14-day-old Nrf2−/− mice exposed to neonatal hyperoxia was also significantly less than Nrf2+/+ mice (P < 0.02). In summary, these findings indicate that Nrf2 increases survival in newborn mice exposed to hyperoxia and that Nrf2 may help attenuate alveolar growth inhibition caused by hyperoxia exposure.
Keywords: oxidative stress, Gpx2, interleukin-6, developing lung
newborns that develop respiratory distress syndrome often require supplemental oxygen to alleviate air exchange abnormalities and decrease work of breathing. Exposure to hyperoxia, however, can increase oxidative stress in the lung particularly during the critical transitional period from fetal to postnatal life. Furthermore, exposure of newborns to high oxygen concentrations can inhibit alveolar growth leading to decreased pulmonary reserve and altered lung function in later life (10). Premature infants are most susceptible to the development of lung injury from hyperoxia due to a deficiency in lung antioxidants (3). Indeed, hyperoxia-induced oxidant lung injury is considered a major risk factor for the development of bronchopulmonary dysplasia (BPD), a disease in infants characterized by pulmonary inflammation, impaired alveolar growth, and abnormal lung function (16, 22). Unfortunately, limiting oxygen exposure is not always possible in the critically ill infant; therefore, understanding mechanisms that can decrease oxidative stress caused by hyperoxia may help attenuate overall morbidity and mortality in these infants.
Reduction of oxygen molecules leads to the accumulation of oxygen byproducts including highly reactive hydroxyl radicals, superoxides, and hydrogen peroxide. In addition, hyperoxia can also alter the redox status of compounds circulating in the lung leading to alveolar cell damage and death (24). Antioxidant and detoxification pathways exist to combat reactive oxygen byproducts. Induction of nuclear erythroid-related factor 2 (Nrf2) has been shown to attenuate oxidative stress caused by cigarette smoke exposure, LPS, antigen provocation, and hyperoxia (7, 13, 27, 28, 32). Nrf2 regulates the induction of over 50 antioxidant and xenobiotic detoxification genes, genes that contain antioxidant response elements (ARE). Downstream target genes include glutamate cysteine ligase catalytic and modifier subunit, glutathione peroxidase (Gpx), glutathione reductases, glutathione S-transferases, NAD(P)H:quinone oxidoreductase (NQO1), and genes from the thioredoxin and heme oxygenase pathways (17, 27, 30).
Recently, Gpx2 has been shown to be induced in a lung-specific manner in mice exposed to cigarette smoke (30). Gpx2 is a cytosolic selenoenzyme, and selenoenzymes have been shown to have anti-inflammatory and antioxidant properties (26). Selenium also induces NQO1, a phase II enzyme that reduces 2,3,5,6-tetramethyl-1,4-benzoquinone (DQ) (9). NQO1 has been shown to be induced in the lungs of rats during chronic exposure to hyperoxia and is believed to alter the redox status of compounds filtered through the lungs (1).
The simultaneous induction of several Nrf2-regulated antioxidant and detoxification genes in response to oxidant-induced lung injury in the newborn may be more effective than the induction of a single antioxidant pathway. For instance, induction of a single antioxidant pathway may not be enough to attenuate alveolar injury or alternatively may create an antioxidant imbalance in the lung. It remains to be determined whether targeting Nrf2 or downstream genes of Nrf2 can help increase survival and prevent the development of chronic lung disease in the sick newborn. Therefore, elucidating the role and temporal induction of these inducible Nrf2 target genes in the lung of newborn mice exposed to hyperoxia is needed when considering the Nrf2 pathway as a potential therapeutic modality in the newborn period.
METHODS
Nrf2−/− and wild-type (Nrf2+/+) mice on a CD1 background were generated as previously described (14). The animals were maintained on an AIN-76A diet and water ad libitum and housed at a temperature range of 20–23°C under 12:12-h light-dark cycles. All experiments were conducted in accordance with the standards established by the United States Animal Welfare Acts set forth in National Institutes of Health (NIH) guidelines and the Policy and Procedures Manual of the Johns Hopkins University Animal Care and Use Committee. All protocols for this study were submitted and approved by the Johns Hopkins Animal Care and Use Committee.
Hyperoxia.
To increase survival, newborn mice were kept in room air until 24 h of age and then placed in a chamber and exposed to 85–90% hyperoxia. Nursing mothers were rotated every 24 h to prevent injury from acute oxygen toxicity. Excess CO2 was absorbed using anhydrous calcium sulfate (cat. no. 23001; Drierite). One group of newborn mice were exposed to 3 days of continuous hyperoxia and then killed immediately on removal from hyperoxia. A second group of mice were placed in the hyperoxia chamber at 48 h of age and exposed for 3 days and recovered in room air. This later exposure was done to increase overall survival so that lung morphometry and surfactant protein C (Sp-c) staining could be performed in Nrf2−/− and Nrf2+/+ at 2 wk of age.
To assess survival in hyperoxia, Nrf2−/− and Nrf2+/+ newborn mice were placed in hyperoxia at 24 h of age. One group was removed after 3 days of hyperoxia exposure and recovered in room air. A second group of mice was kept in continuous hyperoxia for a total of 8 days.
Lung processing.
Lungs were infused through the trachea with 0.5% low-melting agarose. Lungs were fixed overnight in 10% buffered formalin or 4% paraformaldehyde and paraffin-embedded. Lung was cut into 5-μm sections.
Quantitative RT-PCR analysis.
Reverse transcription was performed using total RNA isolated from oxygen-exposed and control mouse lung from each genotype and processed with the SuperScript first-strand synthesis system for RT-PCR according to the manufacturer's protocol (Invitrogen). Quantitative RT-PCR was performed using the Applied Biosystems (Foster City, CA) TaqMan assay system. All PCR amplifications were carried out on an ABI PRISM 7700 Sequence Detection System using a fluorogenic 5′ nuclease assay (TaqMan probes). Probes and primers were designed and synthesized by Applied Biosystems. Relative gene expressions were calculated by using the 2−ΔΔCt method (19). The GAPDH gene was used as an internal endogenous control.
Western blot.
Lungs were homogenized in ice-cold lysis buffer containing 50 mM Tris, pH 8.0, 150 mM NaCl, 1.0% IGEPAL CA-630, 0.5% sodium deoxycholate, and 0.1% SDS and supplemented with a protease inhibitor cocktail (cat. no. 11836170001; Roche, Indianapolis, IN). After 30 min of incubation at 4°C on a clinical rotator, samples were centrifuged at 14,000 rpm for 10 min to pellet insoluble material. Total protein was measured in supernatants using the Bio-Rad Protein Assay. Thirty micrograms of total protein was resolved on a 12.5% Tris·HCl gel by SDS-PAGE before being transferred to a polyvinylidene difluoride membrane. Nonspecific binding was blocked with 5% nonfat milk. Overnight incubation with an anti-Gpx2 antibody (IMG-3853; Imgenex, San Diego, CA) or anti-β-actin antibody (cat. no. 4967; Cell Signaling Technology, Danvers, MA) diluted by 1:1,000 was followed by a 1-h incubation with a horseradish peroxidase-conjugated secondary antibody and developed with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL). ImageJ software was used to perform densitometry measurements.
Immunohistochemistry.
Fixed lungs were paraffin-embedded, and sections were prepared for subsequent deparaffinization and rehydration with xylenes and a graded ethanol series, respectively. An anti-nitrotyrosine antibody (ab53232; Abcam, Cambridge, MA) diluted by 1:500 was biotinylated and detected as per InnoGenex Mouse-on-Mouse Iso-IHC Kit instructions (BioGenex, San Ramon, CA) and developed with 0.05% diaminobenzidine (DAB; Sigma, St. Louis, MO) in 50 mM Tris, pH 7.6, and 0.006% H2O2.
Lung tissue was analyzed for terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) using the Calbiochem FragEL DNA Fragmentation Detection Kit. Tissue sections were deparaffinized and rehydrated in an ethanol series. Antigen retrieval for 10 min in citrate buffer (Vector Laboratories, Burlingame, CA) and a proteinase K digest for 7 min were performed on the tissue sections followed by a peroxidase block for 5 min at room temperature. After blocking, sections were incubated in equilibration buffer supplied in the kit and then probed with the TdT enzyme labeling reaction for 20 min at room temperature. After incubation, slides were washed and blocked using 4% BSA. After blocking, TUNEL positivity was detected using a peroxidase streptavidin supplied in the kit. For Gpx2 immunohistochemistry, fixed lungs were cryoprotected with 20% sucrose in Sorenson phosphate buffer and snap-frozen in optimal cutting temperature compound. Ten-micrometer sections were rehydrated in PBS, and endogenous peroxidase activity was blocked by immersion in cold methanol containing 1% H2O2 for 15 min at −20°C. Nonspecific antibody activity was blocked with 10% normal rabbit serum (Vector Laboratories) at room temperature for 1 h before sections were incubated overnight at 4°C with a 1:150 dilution of anti-Gpx2 antibody (IMG-3853; Imgenex). After washing with PBS containing 0.1% Tween 20, a horseradish peroxidase-conjugated secondary antibody (Dako, Carpinteria, CA) was applied for 1 h at room temperature. Antibody localization was visualized using aforementioned DAB and counterstained with methyl green (Sigma).
Fluorescent antibodies included rabbit anti-pro-Sp-c (rabbit antibody at 1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), T1α (hamster anti-mouse monoclonal, 1:1,000, clone 8.1.1; University of Iowa Hybridoma Bank), and 4′,6′-diamidino-2-phenylindole (DAPI). Antibodies were visualized using a Nikon E800 fluorescent microscope (Nikon Instruments, Melville, NY). Five fields per lung were obtained from three separate animals, and images were digitally merged to identify dual-positive cells.
Lung morphometry.
Five-micrometer lung sections were stained with hematoxylin and eosin. Each slide contained tissue from the left lobe and represented an individual animal. Fifteen randomly chosen areas from each section were photographed with the ×10 objective of a Nikon Eclipse 80i microscope (Nikon Instruments). Mean linear intercepts (MLI) and mean chord length (MCL) were measured from each image using NIS-Elements AR (Nikon Instruments). The software allowed for manual identification and exclusion of large airways and vessels before MLI and MCL calculations.
Statistics.
Statistical calculations were performed for real-time PCR as described above. Differences in measured variables between experimental and control groups were determined using nonparametric Wilcoxon rank sum test, one-way ANOVA, and Student's t-test (2-tailed, equal variance) using the SPSS 14 statistical software (Chicago, IL). Error bars in figures reflect SD except when otherwise stated. Statistical significance was accepted at P < 0.05.
RESULTS
Decreased survival of Nrf2−/− newborn mice in hyperoxia.
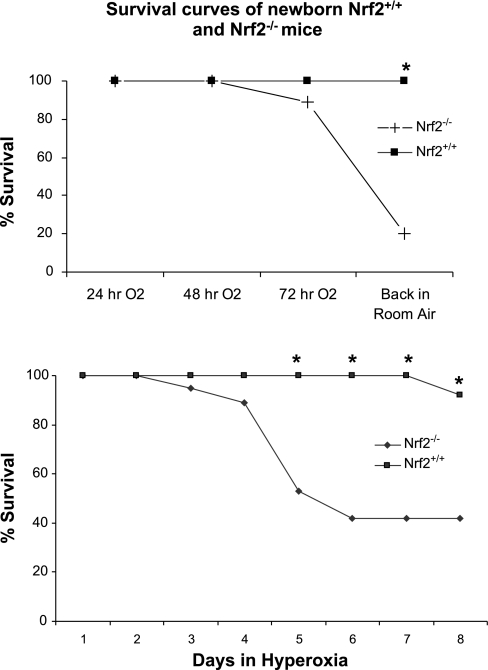
To determine whether differences in survival existed between Nrf2+/+ and Nrf2−/− newborn mice in hyperoxia, mice were placed in hyperoxia at 24 h of age. The first group of mice was exposed to hyperoxia for 3 days and then recovered in room air. In this group, the Nrf2−/− mice had significantly greater mortality when returned to room air (P < 0.0001; Fig. 1, top). This finding indicates that room air recovery following hyperoxia exposure may cause an acute hypoxic stress that increases mortality in newborn mice deficient in Nrf2. A second group of Nrf2+/+ and Nrf2−/− newborn mice was placed in hyperoxia at 24 h of age for 8 continuous days. The Nrf2−/− mice in this group had significantly higher mortality at 5 days of hyperoxia exposure compared with the Nrf2+/+ mice (P < 0.003; Fig. 1, bottom).
Fig. 1.
Decreased survival of nuclear erythroid-related factor 2-deficient (Nrf2−/−) newborn mice in hyperoxia. Top: newborn Nrf2+/+ and Nrf2−/− mice were placed in hyperoxia at 24 h of age, exposed to hyperoxia for 72 h, and returned to room air. Mortality was significantly increased in the Nrf2−/− mice within the first 24 h of room air recovery compared with Nrf2+/+ mice (P < 0.0001). Nrf2+/+ mice, n = 17; Nrf2−/− mice, n = 19 (4 litters per group). Bottom: 24-h-old Nrf2+/+ and Nrf2−/− mice were placed in hyperoxia for 8 continuous days. Mortality was significantly increased in the Nrf2−/− mice at 5 days of continuous oxygen exposure compared with Nrf2+/+ mice (P < 0.003). Nrf2+/+ mice, n = 12; Nrf2−/− mice, n = 19 (2 litters per group). *Significant difference by P value using the Student's t-test.
Increased oxidative stress and cell death in newborn Nrf2−/− lung exposed to 72 h of hyperoxia.
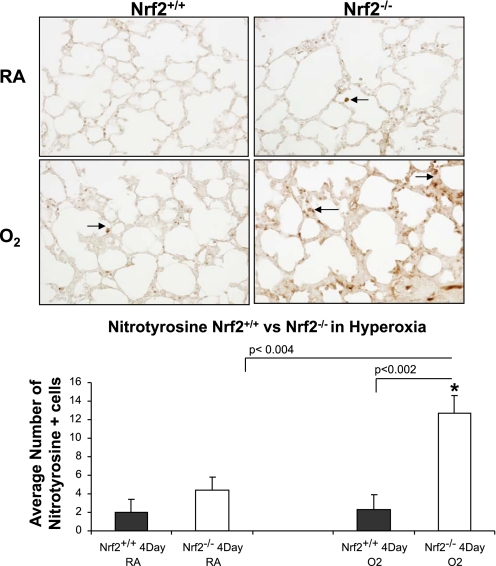
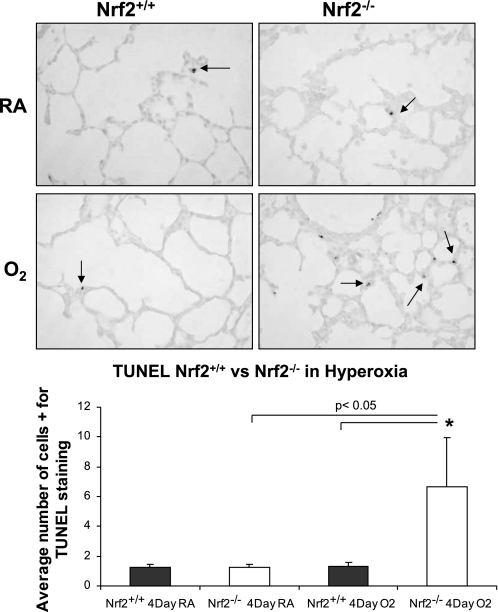
Nitrotyrosine staining and TUNEL were used as markers of oxidative stress and apoptosis in lung of Nrf2+/+ and Nrf2−/− newborn mice exposed to 3 days of hyperoxia. The lungs of Nrf2−/− mice exposed to 72 h of hyperoxia had significantly more nitrotyrosine staining than the lungs of Nrf2+/+ mice exposed to 72 h of hyperoxia (Fig. 2, bottom, black arrows; P < 0.002). TUNEL staining was also significantly increased in the lung of Nrf2−/− mice exposed to hyperoxia compared with lung of Nrf2+/+ mice exposed to hyperoxia (Fig. 3; P < 0.05).
Fig. 2.
Increased nitrotyrosine staining in lung of newborn Nrf2−/− mice exposed to hyperoxia. Top: representative example of nitrotyrosine staining from lung of 4-day-old Nrf2+/+ and 4-day-old Nrf2−/− mice exposed to 72 h of hyperoxia. Arrows point to nitrotyrosine staining. Bottom: significant increase in nitrotyrosine staining in lung of Nrf2−/− oxygen-exposed mice compared with Nrf2+/+ oxygen-exposed mice (P < 0.002) and Nrf2−/− room air mice (P < 0.004), n = 3 for each group. *Significant difference by P value using Student's t-test. RA, room air.
Fig. 3.
Increased terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining in lung of newborn Nrf2−/− mice exposed to hyperoxia. Top: representative example of TUNEL staining from lung of 4-day-old Nrf2+/+ and Nrf2−/− mice exposed to 72 h of hyperoxia. Arrows point to TUNEL staining. Bottom: significant increase in TUNEL staining in lung of Nrf2−/− mice exposed to hyperoxia compared with lung of Nrf2+/+ hyperoxia exposed mice (P < 0.05) and lung of Nrf2−/− mice that were born and raised in room air (P < 0.05), n = 3 for each group. *Significant difference by P value using Student's t-test.
Induction of lung Gpx2 and NQO1 in Nrf2+/+ newborn mice exposed to hyperoxia.
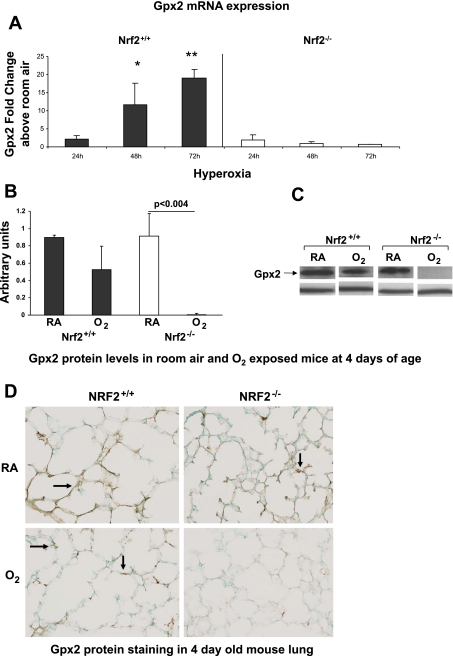
Gpx2 and NQO1 have been shown to be induced by oxidative stress through Nrf2 activation of the ARE promoter region of the gene. We therefore sought to quantify the temporal induction of lung Gpx2 and NQO1 in newborn Nrf2+/+ and Nrf2−/− mice exposed to hyperoxia. Newborn Nrf2+/+ and Nrf2−/− mice were exposed to hyperoxia at 24 h of life. Lung Gpx2 and NQO1 mRNA levels were then measured by real-time PCR at 24, 48, and 72 h of hyperoxia. A marked induction in lung Gpx2 above room air controls was found in Nrf2+/+ mice exposed to hyperoxia at 24 h (2.3 ± 0.9 fold change above room air), 48 h (11.6 ± 6.0 fold change above room air), and 72 h (19.0 ± 2.3 fold change above room air) (Fig. 4A). However, lung Gpx2 levels from Nrf2−/− mice in hyperoxia were minimally induced at 24 h (2.0 ± 1.3 fold change above room air) and not induced at 48 and 72 h (0.9 ± 0.48 and 0.62 ± 0.17 fold change above room air). Nrf2+/+ lung Gpx2 mRNA expression was significantly greater than Nrf2−/− lung at 48 and 72 h of hyperoxia (P < 0.003 and P < 0.0001, respectively). Protein expression of lung Gpx2 in Nrf2+/+ mice exposed to hyperoxia was similar to that of Nrf2+/+ room air mice. In contrast, lung from Nrf2−/− mice exposed to hyperoxia had a marked decrease in protein Gpx2 (Fig. 4, B–D). Although there was a trend toward an increase in lung GSH in the Nrf2+/+ mice at 72 h of hyperoxia, it was not significant compared with room air levels. No increase in GSH was found with hyperoxia in the Nrf2−/− mice (data not shown).
Fig. 4.
Increased expression of lung glutathione peroxidase 2 (Gpx2) in Nrf2+/+ newborn mice exposed to hyperoxia. A: increased expression of lung Gpx2 above room air was found in newborn Nrf2+/+ mice exposed to hyperoxia for 24, 48, and 72 h. Minimal induction of lung Gpx2 expression was found in Nrf2−/− mice exposed to hyperoxia. Nrf2+/+ lung Gpx2 expression was significantly greater than Nrf2−/− at 48 and 72 h of hyperoxia (*P < 0.003 and **P < 0.0001, respectively), n = 3–7 (per group). B: Gpx2 protein was significantly decreased in lung from 4-day-old Nrf2−/− mice exposed to 72 h of hyperoxia (P < 0.004). C: representative example of Gpx2 Western blot. D: decreased Gpx2 staining in 4-day-old Nrf2−/− mice exposed to 72 h of hyperoxia by immunohistochemistry (arrows point to Gpx2 staining). n = 5–6 (per group).
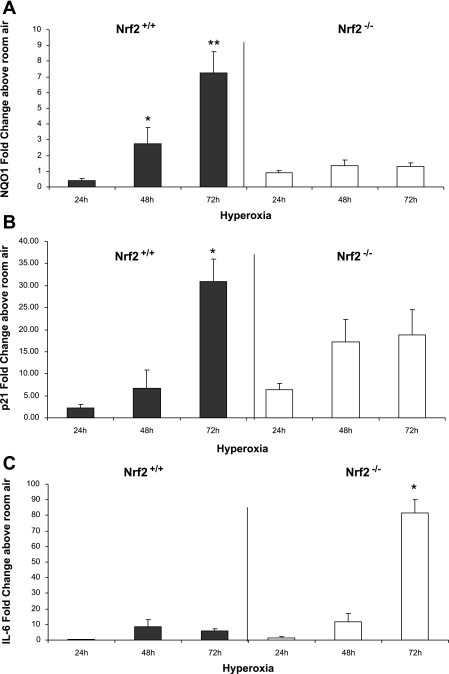
Lung NQO1 mRNA expression from Nrf2+/+ newborn mice was increased at 48 and 72 h of hyperoxia (2.77 ± 1.01 and 7.24 ± 1.37 fold change above room air). In contrast, lung NQO1 levels in newborn Nrf2−/− mice were only minimally increased at 48 and 72 h (1.36 ± 0.35 and 1.32 ± 0.2 fold change). Nrf2+/+ lung NQO1 expression was significantly greater than Nrf2−/− at 48 and 72 h of hyperoxia (P < 0.01 and P < 0.0001, respectively; Fig. 5A).
Fig. 5.
Expression of lung NAD(P)H:quinone oxidoreductase (NQO1), p21, and IL-6 in Nrf2+/+ and Nrf2−/− newborn mice exposed to hyperoxia. A: lung NQO1 was significantly induced in Nrf2+/+ above room air controls but not in lung of Nrf2−/− mice exposed to hyperoxia. Nrf2+/+ lung NQO1 expression was significantly greater than Nrf2−/− at 48 and 72 h of hyperoxia (*P < 0.01 and **P < 0.0001). B: p21 was induced in lung of both Nrf2+/+ and Nrf2−/− mice in response to hyperoxia. At 72 h of Nrf2+/+ lung p21 expression was significantly greater than Nrf2−/− mice in hyperoxia (*P < 0.01). C: IL-6 was significantly induced in Nrf2−/− mice at 72 h of hyperoxia compared with Nrf2+/+ mice in hyperoxia (*P < 0.0001), n = 3–7 (per group).
p21 expression was also measured from lung of Nrf2+/+ and Nrf2−/− mice exposed to hyperoxia. p21, a cyclin-dependent kinase inhibitor that negatively regulates progression of the cell cycle at the G1/S phase, has been shown to be induced by hyperoxia through p53 regulation and is independent of Nrf2 (20, 23). p21 expression was increased in both Nrf2+/+ and Nrf2−/− newborn lung in response to hyperoxia exposure (Fig. 5B). Only at 72 h of hyperoxia was p21 lung expression significantly greater in Nrf2+/+ mice compared with Nrf2−/− mice (30.1 ± 5.0 vs. 17.1 ± 5.1 fold change, respectively; P < 0.01). IL-6 expression was also measured in lung from both Nrf2+/+ and Nrf2−/− mice exposed to hyperoxia. IL-6 is a proinflammatory cytokine associated with decreased survival in newborn mice exposed to hyperoxia (8). Like p21, IL-6 is also independent of Nrf2 regulation. Surprisingly, lung IL-6 mRNA expression was significantly higher in Nrf2−/− newborn mice exposed to hyperoxia for 72 h compared with Nrf2+/+ mice (81.37 ± 8.8 vs. 5.9 ± 1.44 fold change, respectively; P < 0.0001; Fig. 5C).
Basal lung Gpx2 expression in adult Nrf2+/+ mice compared with newborn mice.
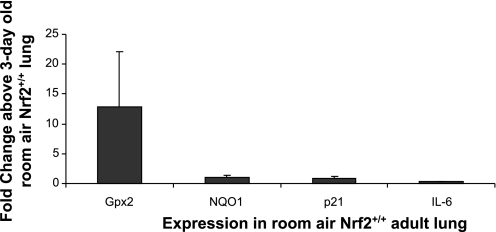
We were interested in determining whether basal levels of lung Gpx2, NQO1, p21, and IL-6 in room air were similar between newborn and adult Nrf2+/+ mice. Lung Gpx2 mRNA expression in adult Nrf2+/+ mice was variable but overall higher in the adult compared with the newborn (12.8 ± 9.3 fold change above neonatal lung; Fig. 6). In contrast, minimal differences in basal levels of lung NQO1, p21, and IL-6 expression were found between adult and newborn Nrf2+/+ mice. These findings suggest that Gpx2 induction in response to oxidative stress may be more critical to the newborn than the adult due to lower basal expression of Gpx2 in the newborn lung.
Fig. 6.
Higher basal Gpx2 expression in lung of adult Nrf2+/+ mice compared with 1-day-old Nrf2+/+ newborn mice. Gpx2 expression at baseline was higher in adult Nrf2+/+ lung compared with newborn Nrf2+/+ lung. No difference in basal NQO1, p21, or IL-6 expression was found between adult Nrf2+/+ lung and newborn Nrf2+/+ lung, n = 3–7; error bars reflect SE of the mean (per group).
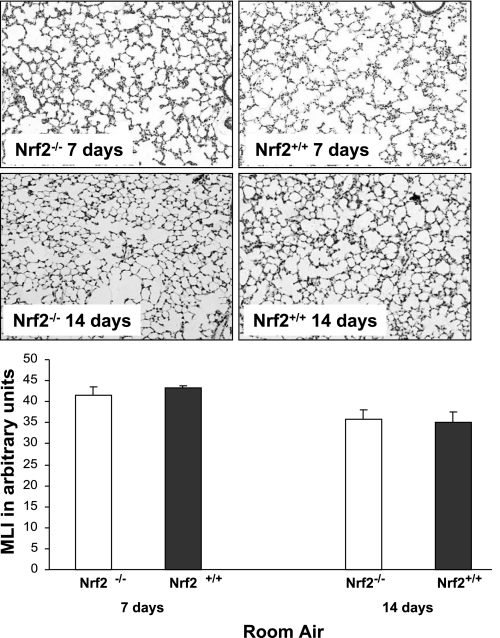
Similar alveolar growth between Nrf2−/− and Nrf2+/+ newborn mice.
To rule out the presence of developmental lung abnormalities in Nrf2−/− mice born and raised in room air conditions, we assessed alveolar growth by MLI in 7- and 14-day-old Nrf2−/− and Nrf2+/+ mice. In 7- and 14-day-old mice, no significant differences were found by MLI between the Nrf2−/− and Nrf2+/+ mice (41.5 ± 2.1 and 43.2 ± 0.5 at 7 days and 35.9 ± 2.1 and 35.1 ± 2.5 at 14 days, respectively; Fig. 7). These findings indicate that alveolar growth is similar between Nrf2+/+ and Nrf2−/− mice maintained in room air.
Fig. 7.
Similar postnatal alveolar growth in Nrf2+/+ and Nrf2−/− mice raised in room air at 7 and 14 days of life. There were no differences in mean linear intercept (MLI) measurements at 7 or 14 days of life between Nrf2+/+ and Nrf2−/− mice born and raised in room air conditions, n = 4–5 (per group).
Alveolar growth inhibition in Nrf2−/− mice exposed to hyperoxia as newborns.
Exposure to hyperoxia has been shown to inhibit alveolar growth in the newborn lung of humans and rodents (4, 6, 11). To determine whether the presence of Nrf2 attenuated alveolar growth inhibition in newborn mice exposed to hyperoxia, we measured MLI and MCL in 2-wk-old Nrf2+/+ and Nrf2−/− mice exposed to hyperoxia in the newborn period. Since Nrf2−/− mice exposed to 72 h of hyperoxia (at 24 h of life) and returned to room air had a high mortality, we waited until mice were 2 days of age before exposing them to 72 h of hyperoxia. Using this protocol, five of eight Nrf2−/− newborn mice and seven of seven Nrf2+/+ newborn mice survived the 72 h of hyperoxia, and all of these mice survived until 2 wk of age. We found that MLI and MCL measurements of 14-day-old Nrf2−/− mice exposed to newborn hyperoxia were modestly but significantly increased compared with the Nrf2+/+ mice exposed to hyperoxia (Fig. 8 and Table 1). These results suggest that hyperoxia exposure in the newborn period leads to more alveolar growth inhibition in Nrf2-deficient mice.
Fig. 8.
Impaired alveolar growth in 2-wk-old Nrf2−/− mice exposed to hyperoxia in the newborn period. A representative example of lung from 2-wk-old Nrf2−/− and Nrf2+/+ mice placed in hyperoxia for 3 days starting at 48 h of life and then recovered in room is shown.
Table 1.
Lung morphometry in 14-day-old mice
| Genotype/Exposure | Mean Chord Length (± SD) | P | Mean Linear Intercept (± SD) | P |
|---|---|---|---|---|
| Nrf2+/+ (neo-O2) | 33.49 (± 2.05) | 37.97 (± 2.48) | ||
| Nrf2−/− (neo-O2) | 37.61 (± 3.21)* | <0.03 | 42.0 (± 3.13)* | <0.04 |
| Nrf2+/+ (room air) | 30.98 (± 2.65) | 35.06 (± 2.5) | ||
| Nrf2−/− (room air) | 31.88 (± 2.05) | <0.57 | 35.92 (± 2.1) | <0.58 |
Forty-eight-hour-old mice were exposed to hyperoxia for 72 h and recovered in room air (neo-O2). At 14 days of life, mean chord length (MCL) and mean linear intercept (MLI) measurements were obtained and compared with age-matched room air controls (room air).
Significant differences in MCL and MLI were found between nuclear erythroid-related factor 2-deficient (Nrf2−/−) (neo-O2) and Nrf2−/− (room air) mice (*P < 0.03 and P < 0.04, respectively) at 14 days of life. There was no significant difference in MCL and MLI between Nrf2+/+ (neo-O2) and Nrf2+/+ (room air) mice (P < 0.57 and P < 0.58, respectively) at 14 days of life.
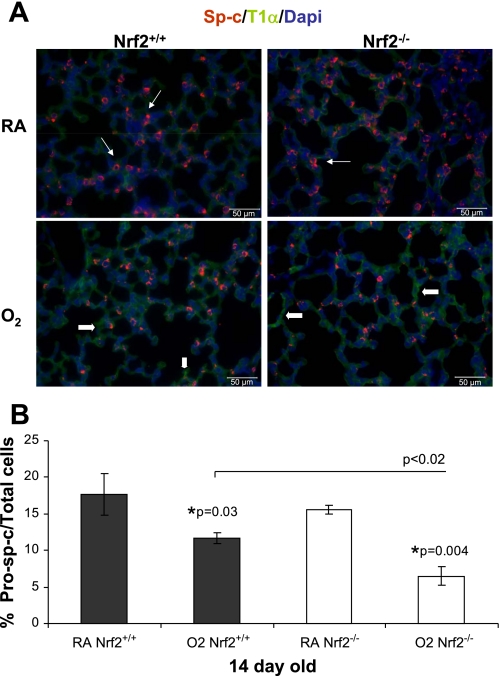
Percentage of type 2 alveolar cells in 14-day-old Nrf2+/+ and Nrf2−/− mice.
It has been previously shown that hyperoxia exposure in the newborn period can cause a decrease in the percentage of type 2 alveolar cells in the adult lung (37), suggesting that exposure to hyperoxia during a critical period of postnatal lung growth can lead to alveolar remodeling through dysregulation of type 2 alveolar cell development. We were interested in determining whether mice deficient in Nrf2 had fewer type 2 alveolar cells following exposure to hyperoxia in the newborn period compared with Nrf2+/+ mice. In mice raised in room air, 2-wk-old Nrf2+/+ and Nrf2−/− mice had similar percentages of Sp-c+ type 2 alveolar cells. When exposed to hyperoxia as newborns, however, 2-wk-old Nrf2+/+ and Nrf2−/− mice had a significantly lower percentage of Sp-c+ type 2 alveolar cells compared with room air controls at 2 wk of age (P < 0.03 and P < 0.004, respectively; Fig. 9, A and B). Furthermore, the Nrf2−/− mice exposed to hyperoxia in the newborn period also had a significantly lower percentage of Sp-c+ type 2 alveolar cells compared with the Nrf2+/+ mice exposed to hyperoxia (P < 0.02). This finding suggests that Nrf2 may modestly protect type 2 alveolar cells exposed to hyperoxia in the newborn period.
Fig. 9.
Decreased percentage of surfactant protein C (Sp-c+) type 2 alveolar cells in lung of 14-day-old Nrf2−/− mice exposed to hyperoxia in the newborn period. A: representative example of Sp-c (red), T1α staining (green) and 4′,6′-diamidino-2-phenylindole (DAPI; blue) in lung from 14-day-old Nrf2+/+ and Nrf2−/− mice raised in room air (top) or exposed to hyperoxia for 72 h in the newborn period (bottom). B: percentage of Sp-c staining cells over total cells was significantly decreased in both Nrf2+/+ and Nrf2−/− mice exposed to hyperoxia in the newborn period (*P < 0.03 and *P < 0.004, respectively). Percentage of Sp-c staining cells over total cells was significantly less in the Nrf2−/− lung exposed to hyperoxia compared with the Nrf2+/+ lung exposed to hyperoxia (P < 0.02), n = 3 (per group). The thin white arrows in the top point to Sp-c+ staining and the thick arrows in the bottom point to T1α staining.
DISCUSSION
The newborn mouse has been shown to survive significantly longer and to sustain less pulmonary injury compared with the adult mouse exposed to similar amounts of hyperoxia (5). The extent to which the Nrf2 and its target genes are responsible for protecting newborn lung from oxidative stress injury is not known. In the adult mouse, induction of Nrf2 has been shown to attenuate injuries caused by sepsis, intracerebral hemorrhage, and mechanical ventilation (25, 32, 34). The goal of this study was to determine whether Nrf2 increases survival in hyperoxia and attenuates hyperoxic-induced alveolar growth inhibition in newborn mice. When Nrf2−/− newborn mice were exposed to 5 days or more of hyperoxia, they had a higher mortality compared with age-matched Nrf2+/+ mice in hyperoxia. Furthermore, when Nrf2−/− newborn mice were exposed to 72 h of hyperoxia and recovered in room air, they had a higher mortality than Nrf2+/+ newborn mice treated similarly. Associated with an increase in survival, the Nrf2+/+ newborn mice exposed to hyperoxia had a marked induction of lung Gpx2 and NQO1, whereas lung from Nrf2−/− mice had minimal or no induction of these genes. Gpx2 and NQO1, downstream target genes of Nrf2, have been shown to be induced in response to different oxidative stresses in the adult mouse (7, 13, 27). In our study, we also found that Nrf2+/+ and Nrf2−/− mice raised in room air had similar alveolar growth. When exposed to hyperoxia in the newborn period, however, the Nrf2−/− mice at 2 wk of age had larger MCL and MLI measurements and fewer Sp-c+ type 2 alveolar cells compared with the 2-wk-old Nrf2+/+ mice exposed to neonatal hyperoxia. This finding indicates that Nrf2 may help protect type 2 alveolar cells exposed to oxidative stress caused by hyperoxia.
Our findings suggest that induction of both lung Gpx2 and NQO1 through the Nrf2 pathway may be beneficial to the newborn exposed to hyperoxia. Audi et al. (1) found that chronic exposure to hyperoxia increased NQO1 activity in the lung leading to enhanced duroquinone reduction. Previous studies have also suggested a beneficial role for glutathione enzyme induction during exposure to oxidative stress (30, 36). White et al. (36) found that preconditioning with hypoxia increased expression of glutathione enzymes such as Gpx, causing an attenuation of lung injury from hyperoxia or H2O2. In mammals that dive, an increase in lung Gpx has been shown to protect the lung against ischemic-reperfusion injury during resurfacing (33). Furthermore, newborns with perinatal asphyxia have been shown to have higher serum levels of Gpx, catalase, and superoxide dismutase, presumably in response to oxidative stress (18). Indeed, it has been shown that preterm infants who develop BPD have lower cellular Gpx activity than preterm infants that do not develop BPD (12). Along these lines, the ability of the newborn to successfully induce Gpx may increase the newborn's tolerance to oxidative stress injuries, whereas the inability to adequately induce Gpx2 may increase morbidity and decrease survival in the newborn exposed to hyperoxia.
Interestingly, we also found that Nrf2−/− newborn mice had an increased induction of lung IL-6 at 72 h of hyperoxia followed by a high mortality during recovery in room air. In contrast, the Nrf2+/+ newborn mice exposed to hyperoxia had relatively low levels of lung IL-6 and, unlike the Nrf2−/− mice, had no increase in mortality following recovery in room air after exposure to 72 h of hyperoxia. Our findings parallel those of previous studies in which newborn mice that overexpressed IL-6 had decreased survival in hyperoxia and transgenic mice that overexpressed surfactant protein D had lower levels of IL-6 associated with increased survival in hyperoxia (8, 15).
Exposure to neonatal hyperoxia has also been associated with impaired alveolar growth in the newborn (21, 35). In our study, we found an increase in TUNEL staining in the lungs of Nrf2−/− newborn mice at 72 h of hyperoxia. At 2 wk of age, a modest but significant increase in MCL and MLI was found in the Nrf2−/− mice exposed to neonatal hyperoxia compared with the Nrf2+/+ mice. These findings suggest a correlation between an inadequate Nrf2 response to hyperoxia and impaired alveolar growth and that early alveolar cell death from hyperoxia may, in part, cause impaired alveolar growth in the Nrf2-deficient mice. Furthermore, 2-wk-old Nrf2−/− mice exposed to neonatal hyperoxia had a decreased percentage of Sp-c+ type 2 alveolar cells compared with Nrf2+/+ mice, suggesting that Nrf2 may differentially protect the type 2 alveolar cells during oxidative stress. A previous study reported that type 2 alveolar cells isolated from adult Nrf2−/− lung had impaired cell proliferation when grown in culture compared with Nrf2+/+ cells (29). Exposure to hyperoxia in the newborn, however, may also interrupt other growth pathways necessary for normal postnatal alveolar development. Balasubramaniam et al. (2) recently found that newborn mice exposed to hyperoxia had reduced endothelial progenitor cells and decreased lung VEGF, VEGFR-2, and endothelial NO. Furthermore, the role of Nrf2 in the lung may be most important immediately after birth in response to significant oxidative stress rather than during recovery from an injury. This will need to be pursued in future studies.
In summary, we found that newborn mice deficient in Nrf2 and exposed to hyperoxia had a higher mortality and more impaired alveolar growth compared with wild-type mice under similar conditions. These findings suggest that the induction of Nrf2 and its target genes are important in the newborn period for both survival and for attenuation of oxidative-induced lung injury. Understanding the mechanisms that allow newborns to cope with oxidative stress may help with the development of strategies that can be used to attenuate morbidity and decrease mortality in sick premature or asphyxiated infants. Treatment strategies that help induce Nrf2-regulated pathway genes before delivery may potentially help improve outcomes of these “at risk” infants.
GRANTS
This work was funded by NIH Grants HL-081205 (S. Biswal), HL-095420, P50-ES-015903, and 5-P30-ES-003819. The project was partially funded by the March of Dimes Birth Defects Foundation Grant 6-FY08-264 (M. O'Reilly).
Acknowledgments
We thank Drs. Thomas Kensler and Masayuki Yamamoto for providing the Nrf2-deficient mice.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Audi SH, Bongard RD, Krenz GS, Rickaby DA, Haworth ST, Eisenhauer J, Roerig DL, Merker MP. Effect of chronic hyperoxic exposure on duroquinone reduction in adult rat lungs. Am J Physiol Lung Cell Mol Physiol 289: L788–L797, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L1073–L1084, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Baydas G, Karatas F, Gursu MF, Bozkurt HA, Ilhan N, Yasar A, Canatan H. Antioxidant vitamin levels in term and preterm infants and their relation to maternal vitamin status. Arch Med Res 33: 276–280, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Blanco LN, Frank L. The formation of alveoli in rat lung during the third and fourth postnatal weeks: effect of hyperoxia, dexamethasone, and deferoxamine. Pediatr Res 34: 334–340, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Bonikos DS, Bensch KG, Northway WH Jr. Oxygen toxicity in the newborn. The effect of chronic continuous 100 percent oxygen exposure on the lungs of newborn mice. Am J Pathol 85: 623–650, 1976. [PMC free article] [PubMed] [Google Scholar]
- 6.Chess PR, D'Angio CT, Pryhuber GS, Maniscalco WM. Pathogenesis of bronchopulmonary dysplasia. Semin Perinatol 30: 171–178, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 26: 175–182, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Choo-Wing R, Nedrelow JH, Homer RJ, Elias JA, Bhandari V. Developmental differences in the responses of IL-6 and IL-13 transgenic mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 293: L142–L150, 2007. [DOI] [PubMed] [Google Scholar]
- 9.El Bayoumy K, Sinha R. Molecular chemoprevention by selenium: a genomic approach. Mutat Res 591: 224–236, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Frank L Developmental aspects of experimental pulmonary oxygen toxicity. Free Radic Biol Med 11: 463–494, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Frank L Protective effect of keratinocyte growth factor against lung abnormalities associated with hyperoxia in prematurely born rats. Biol Neonate 83: 263–272, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Fu RH, Chiu TH, Chiang MC, Lien R, Chou YH, Chiang CC, Cho YH, Yang PH. Lower erythrocyte glutathione peroxidase activity in bronchopulmonary dysplasia in the first week of neonatal life. Neonatology 93: 269–275, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, Sakamoto T, Shimura M, Yoshida A, Yamamoto M, Sekizawa K. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells 10: 1113–1125, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Jain D, Atochina-Vasserman EN, Tomer Y, Kadire H, Beers MF. Surfactant protein D protects against acute hyperoxic lung injury. Am J Respir Crit Care Med 178: 805–813, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev 53: 81–94, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89–116, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Ramakrishna SV, Basu S, Rao GR. Oxidative stress in perinatal asphyxia. Pediatr Neurol 38: 181–185, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 20.McGrath SA Induction of p21WAF/CIP1 during hyperoxia. Am J Respir Cell Mol Biol 18: 179–187, 1998. [DOI] [PubMed] [Google Scholar]
- 21.McGrath-Morrow SA, Cho C, Soutiere S, Mitzner W, Tuder R. The effect of neonatal hyperoxia on the lung of p21Waf1/Cip1/Sdi1-deficient mice. Am J Respir Cell Mol Biol 30: 635–640, 2004. [DOI] [PubMed] [Google Scholar]
- 22.O'Donovan DJ, Fernandes CJ. Mitochondrial glutathione and oxidative stress: implications for pulmonary oxygen toxicity in premature infants. Mol Genet Metab 71: 352–358, 2000. [DOI] [PubMed] [Google Scholar]
- 23.O'Reilly MA, Staversky RJ, Watkins RH, Maniscalco WM. Accumulation of p21(Cip1/WAF1) during hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 19: 777–785, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Pagano A, Barazzone-Argiroffo C. Alveolar cell death in hyperoxia-induced lung injury. Ann N Y Acad Sci 1010: 405–416, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Papaiahgari S, Yerrapureddy A, Reddy SR, Reddy NM, Dodd O, Crow MT, Grigoryev DN, Barnes K, Tuder RM, Yamamoto M, Kensler TW, Biswal S, Mitzner W, Hassoun PM, Reddy SP. Genetic and pharmacologic evidence links oxidative stress to ventilator-induced lung injury in mice. Am J Respir Crit Care Med 176: 1222–1235, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters U, Chatterjee N, Hayes RB, Schoen RE, Wang Y, Chanock SJ, Foster CB. Variation in the selenoenzyme genes and risk of advanced distal colorectal adenoma. Cancer Epidemiol Biomarkers Prev 17: 1144–1154, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114: 1248–1259, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 202: 47–59, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy NM, Kleeberger SR, Cho HY, Yamamoto M, Kensler TW, Biswal S, Reddy SP. Deficiency in Nrf2-GSH signaling impairs type II cell growth and enhances sensitivity to oxidants. Am J Respir Cell Mol Biol 37: 3–8, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh A, Rangasamy T, Thimmulappa RK, Lee H, Osburn WO, Brigelius-Flohe R, Kensler TW, Yamamoto M, Biswal S. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am J Respir Cell Mol Biol 35: 639–650, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun 351: 883–889, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vazquez-Medina JP, Zenteno-Savin T, Elsner R. Antioxidant enzymes in ringed seal tissues: potential protection against dive-associated ischemia/reperfusion. Comp Biochem Physiol C Toxicol Pharmacol 142: 198–204, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Dore S. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med 43: 408–414, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol Lung Cell Mol Physiol 275: L110–L117, 1998. [DOI] [PubMed] [Google Scholar]
- 36.White CW, Jackson JH, McMurtry IF, Repine JE. Hypoxia increases glutathione redox cycle and protects rat lungs against oxidants. J Appl Physiol 65: 2607–2616, 1988. [DOI] [PubMed] [Google Scholar]
- 37.Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN, O'Reilly MA. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol 291: L1101–L1111, 2006. [DOI] [PubMed] [Google Scholar]