Abstract
Human surfactant protein A (hSP-A), a molecule of innate immunity and surfactant-related functions, consists of two functional genes, SP-A1 and SP-A2. SP-A expression is regulated by several factors including environmental stressors. SP-A1 and SP-A2 5′-untranslated region (5′-UTR) splice variants have a differential impact on translation efficiency and mRNA stability. To study whether these variants mediate internal ribosome entry site (IRES) activity (i.e., cap-independent translation), we performed transient transfection experiments in H441 cells with constructs containing one SP-A1 (A′D′, AB′D′, or A′CD′) or SP-A2 (ABD) 5′-UTR splice variant between the Renilla and firefly luciferase genes of a bicistronic reporter vector. We found that 1) variants A′D′, ABD, and AB′D′ exhibit significantly higher IRES activities than negative control (no SP-A 5′-UTR) and A′CD′ has no activity; the order of highest IRES activity was ABD > A′D′ > AB′D; 2) IRES activity of ABD significantly increased in response to diesel particulate matter (20 μg/ml) but not in response to ozone (1 ppm for 1 h); 3) deletion mutants of ABD revealed regulatory elements associated with IRES activity; one at the end of exon A attenuated activity, whereas a region containing a short adenosine-rich motif in the second half of exon B and the start of exon D enhanced activity; 4) elimination of a predicted double-loop structure or increase in free energy significantly reduced IRES activity; 5) elimination of one or both double-loop structures in A′D′ did not affect cap-dependent translation activity. Thus several factors, including cis-elements and secondary structure type and stability, are required for hSP-A 5′-UTR variant-mediated cap-independent translation.
Keywords: circular RNA, internal ribosome entry site, H441 cells, diesel particulate matter, secondary structure of mRNA, surfactant protein A, 5′-untranslated region
initiation of translation in eukaryotes occurs through two distinct mechanisms, cap-dependent and cap-independent initiation (66). Under normal conditions cap-dependent translation is the most common mechanism for cellular mRNA translation, in which specific factors, such as eukaryotic translation initiation factor-4E (eIF-4E), bind to the cap structure of mRNA to start the translation process (17). Cap-independent translation can depend on an internal ribosomal entry site (IRES) in the 5′-untranslated region (5′-UTR) of mRNA (23). Viral IRESs usually share stable secondary structural properties and/or common conserved sequences (23, 30, 62). Although the IRES mechanism of cap-independent translation was first discovered in the expression of encephalomyocarditis virus (32) and poliovirus (58), the first cellular IRES was discovered in 1991 (46), and since then a number of cellular IRES elements have been identified and characterized (3, 23, 57). Cap-independent translation has now been observed in a number of cellular genes, especially in genes that respond to various stressors and environmental modulatory agents and in genes involved in cell apoptosis, such as VEGF (1, 29), hypoxia-inducible factor-1α (HIF-1α) (37), c-myc (7, 67), X-linked inhibitor of apoptosis (XIAP) (24), and heat shock protein 70 (Hsp70) (64). About 3–5% of cellular genes may utilize the alternative IRES translation initiation (3, 26, 57). A “Y” structure in the 5′-UTR of diverse mRNAs was proposed as a model of cellular IRES structure (39), but further study showed that this is not a common characteristic for most cellular IRESs (3). Collectively, the available literature indicates that the cellular IRESs may be more divergent with regard to common conserved sequences or common secondary structures (7, 38). A genomewide analysis showed that cellular IRES activity may depend on short sequence motifs and trans-acting factors required for cellular IRES function (2, 25).
Cap-independent translation is an alternative and transient translation mechanism that produces specific proteins while the cells respond to various stresses/stimuli, such as hypoxia, nutrition deficiency, UV light irradiation, and apoptosis (35). mRNA translation has been shown under certain circumstances to be inhibited and factors involved in cap-dependent translation initiation to be insufficient for translation to proceed (26, 35). However, even under stressful conditions the cells need to produce an increasing amount of certain proteins. In such circumstances an alternative cap-independent translation mechanism can be initiated and utilized (23, 26). Cap-independent translation initiation requires both trans-acting factors and cis-acting element(s), i.e., IRES within the 5′-UTR of mRNA (10, 65). Trans-acting factors and cis-acting elements may depend on the type of host cells and expressing genes. For example, a short poly(A) motif upstream of the start codon (AUG) has recently been identified as a critical IRES cis-acting element for starvation-induced differentiation in yeast (16).
Surfactant protein A (SP-A), a member of the C-type collectin family, plays an important role in innate host defense, modulation of inflammatory processes, and surfactant-related physiological functions (13, 50, 59, 78). The human (h)SP-A locus in chromosome 10 consists of two functional genes, SP-A1 and SP-A2 (27). Both genes are expressed by alveolar epithelial type II cells of the lung (47, 60). Native hSP-A protein is a flowerlike octadecamer consisting of six-trimer subunits. Although it has been suggested that two SP-A1 molecules and one SP-A2 molecule are needed for the formation of the trimer unit (71), in vitro expressed SP-A variants from a single SP-A gene (SP-A1 or SP-A2) are able to produce octadecamers and other oligomeric forms (72, 74). These single gene products are functional, with differences observed in their ability to stimulate cytokine production by the macrophage-like THP-1 cell line (28, 75, 77), enhance bacterial phagocytosis by rat and human alveolar macrophages (53–55, 74), and other functions (14, 72, 76).
hSP-A1 and hSP-A2 expression is differentially regulated during lung development and by various hormones (36, 52). Total SP-A (SP-A1 and SP-A2) levels have been found altered in a variety of lung diseases, such as acute respiratory distress syndrome (ARDS) (18, 19), idiopathic pulmonary fibrosis (IPF) (48, 49, 61), and hypersensitivity pneumonitis (HP) (20, 21), as well as in environmental factor-exposed workers (9, 40). Recently, the ratio of SP-A1 to total SP-A content was shown to vary as a function of aging and pulmonary health (70). Given the differences in function of SP-A1 and SP-A2, the overall functional activity of SP-A may depend on the proportions of SP-A1 and SP-A2 rather than the total SP-A content. Therefore, it is important to understand the mechanisms involved in SP-A1 and SP-A2 expression. Alternations in SP-A expression have been observed in animal models after exposure to various environmental factors such as lipopolysaccharide (LPS)-induced injury (63, 69), corn dust extract (15), and ozone (22, 68). Although SP-A expression is responsive to various stressors and environmental factors, nothing is known about whether cap-independent translation (a transient mechanism that becomes operative in response to various stressful situations) plays a role in its expression under normal and/or changing/stressful conditions.
Previous studies have shown alternative splicing at the 5′-UTR of the hSP-A1 and hSP-A2. The genomic hSP-A contains several exons (A, B, B′, C, C′, D, D′) that splice in different configurations to give rise to a number of 5′-UTR splice variants. These include splice variants AD′ (major), AB′D′, and A′CD′ of SP-A1 and ABD and ABD′ of SP-A2 (33, 51). Although these 5′-UTR splice variants have been shown to be able to be translated both in vitro (33) and in vivo (34), the detailed mechanisms are not well understood. Recently, we studied (73) the functional role of four 5′-UTR splice variants in translation efficiency and mRNA stability, using a monocistronic reporter vector that enables the study of cap-dependent translation. We found significant differences in mRNA stability and translation efficiency among them (73). However, it is unknown whether the SP-A 5′-UTR splice variants mediate cap-independent translation (i.e., exhibit IRES activity) and, if they do, whether differences in activity exist among them.
In this study, we examined the ability of hSP-A 5′-UTR splice variants to mediate cap-independent activity. Using a bicistronic vector with two different reporter genes and hSP-A 5′-UTR sequences, we generated several experimental recombinant constructs and used them in transient transfection experiments of H441 cells. We 1) observed differential cap-independent activities among the hSP-A 5′-UTR variants, 2) identified cis-acting elements through deletion and site-directed mutation, 3) observed a role of type and stability of secondary structure elements in cap-independent translation, 4) showed that a secondary structure (i.e., a double-loop structure) that accounts for approximately half of the cap-independent translation does not play a role in cap-dependent translation, and 5) showed that cap-independent translation activity is operative and specific in response to environmental stressors.
MATERIALS AND METHODS
Cell Line and Cell Culture
The human lung adenocarcinoma cell line (H441) used in this study was purchased from the American Type Culture Collection (ATCC, Manassas, VA). Since H441 cells can express endogenous SP-A protein and generate various SP-A mRNA transcripts that contain several types of SP-A 5′-UTR splice variants (36), the cells should contain all factors for SP-A mRNA transcription, posttranscriptional modification, and protein translation. H441 cells were grown in RPMI 1640 medium (Invitrogen Life Technologies, Carlsbad, CA) with 10% heat-inactivated fetal bovine serum (FBS; Summit Biotechnology, Ft. Collins, CO), 1× antimycotic-antibiotic solution (Sigma, St. Louis, MO), and 1× l-glutamine (Sigma). The cells were cultured at 37°C in a 5% CO2 atmosphere and were subcultured weekly.
Bicistronic Plasmid Constructs
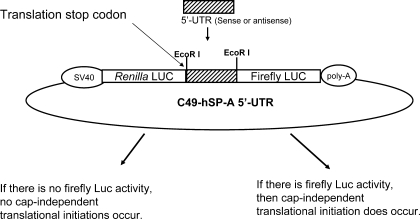
One basic bicistronic vector (defined as C49) was used for the generation of the recombinant construct C49-hSP-A 5′-UTR (see Fig. 1). This bicistronic construct contained two reporter genes (Renilla and firefly luciferase) driven by the SV40 promoter. The 5′-UTR DNA fragment under investigation was cloned into the site between Renilla and firefly luciferase. Therefore, both Renilla and luciferase reporter genes were fused in the transcripts. Renilla luciferase, the first gene in the fused transcript, is translated via cap-dependent translation mechanism with its own 5′-UTR; translation terminates at the stop codon at the end of Renilla luciferase. Thus in the experimental constructs translation of the firefly luciferase is mediated by the experimental DNA variant via cap-independent translation. The sequence for each of the SP-A 5′-UTR splice variants is shown in Fig. 2. In this study, human SP-A 5′-UTR variants (A′D′, ABD, AB′D′, A′CD′) were produced from PCR amplification with human SP-A cDNA as template and appropriate primers (see Table 1). These primers contain one EcoRI restriction site in their 5′ end. The PCR conditions consisted of 1× buffer 2, 0.125 mM of each dNTP, 1 ng/μl of each of the primers, and 3.5 units of expanded high-fidelity PCR system (Roche, Mannheim, Germany) in a 50-μl final volume. Cycling was at 94°C for 2 min, followed by 30 cycles at 94°C for 30 s, 58°C for 30 s, and 72°C for 30 min. The final extension step was at 72°C for 5 min. The PCR products were purified and digested with the EcoRI restriction enzyme and then cloned into the vector (Fig. 1). Transformation in Escherichia coli strain XL1-Blue was performed by standard protocol. Purification of plasmid was performed with the QIAprep spin miniprep kit (Qiagen, Valencia, CA). The DNA sequences and orientation (i.e., sense or antisense of hSP-A 5′-UTR) were verified by DNA sequencing (Molecular Genetics Core Facility at Pennsylvania State University College of Medicine).
Fig. 1.
Schematic representation of the recombinant bicistronic construct used for assessment of internal ribosome entry site (IRES) activity. A basic bicistronic construct C49 contains both Renilla and firefly luciferase (Luc) genes. Renilla and firefly luciferase gene expression is driven by the SV40 promoter upstream of the Renilla luciferase gene. Human surfactant protein A (hSP-A) 5′-untranslated region (5′-UTR) sequence (sense) from hSP-A cDNA clones was inserted at an EcoRI site between the Renilla and firefly luciferase genes. Each Renilla and firefly luciferase gene contains its own stop codon. Therefore, the Renilla reporter gene is consistently translated by a cap-dependent translation mechanism, but the firefly luciferase translation depends on IRES activity of the inserted hSP-A 5′-UTR fragment. IRES activity of the inserted hSP-A 5′-UTR was determined by the ratio of firefly to Renilla luciferase activity.
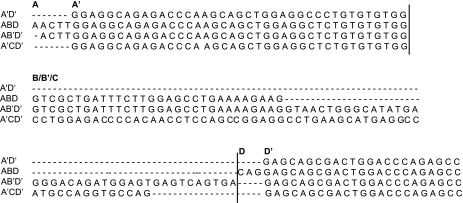
Fig. 2.
Comparison of sequence of hSP-A 5′-UTR splice variants. Exons of SP-A 5′-UTR splice variants are separated by vertical lines. The name of each exon/region is marked at the 1st nucleotide of each exon/region.
Table 1.
Primers used in this study (5′-UTR cap independent)
| Primer Name | Sequence (5′ to 3′) | Comments | |
|---|---|---|---|
| 1472 | GGGgaattcAACTTGGAGGCAGAGACCCA | ABD, AB′D′ variant with EcoRI, sense | |
| 1473 | GGGgaattcGGAGGCAGAGACCCAAGCAG | A′D′, A′CD′ variant with EcoRI, sense | |
| 1474 | CCCGAATTCGGCTCTGGGTCCAGTCGCTG | All 5′-UTR variants with EcoRI, antisense | |
| 1521 | GAACAATAATTCTAGGGGAATTCGTCGCTGATTTCTTGGAGCCTG | Sense | Delete A |
| 1522 | CAGGCTCCAAGAAATCAGCGACGAATTCCCCTAGAATTATTGTTC | Antisense | |
| 1523 | GATTTCTTGGAGCCTGAAAAGAAGGAATTCAGCCACCATGGAAGAC | Sense | Delete D |
| 1524 | GTCTTCCATGGTGGCTGAATTCCTTCTTTTCAGGCTCCAAGAAATC | Antisense | |
| 1525 | CAAGCAGCTGGAGGCTCTGTGGTCGCTGATTTCTTGGAGCCTG | Sense | Deletion 1 |
| 1526 | CAGGCTCCAAGAAATCAGCGACCACAGAGCCTCCAGCTGCTTG | Antisense | |
| 1527 | GCTGGAGGCTCTGTGTGTGGTGATTTCTTGGAGCCTGAAAAG | Sense | Deletion 2 |
| 1528 | CTTTTCAGGCTCCAAGAAATCACCACACACAGAGCCTCCAGC | Antisense | |
| 1529 | GAGGCTCTGTGTGTGGGTCGCTCTTGGAGCCTGAAAAGAAGCAG | Sense | Deletion 3 |
| 1530 | CTGCTTCTTTTCAGGCTCCAAGAGCGACCCACACACAGAGCCTC | Antisense | |
| 1531 | CTGTGTGTGGGTCGCTGATTGAGCCTGAAAAGAAGCAGGAG | Sense | Deletion 4 |
| 1532 | CTCCTGCTTCTTTTCAGGCTCAATCAGCGACCCACACACAG | Antisense | |
| 1533 | GTGTGGGTCGCTGATTTCTTGTGAAAAGAAGCAGGAGCAGCG | Sense | Deletion 5 |
| 1534 | CGCTGCTCCTGCTTCTTTTCACAAGAAATCAGCGACCCACAC | Antisense | |
| 1535 | GGTCGCTGATTTCTTGGAGCCAGAAGCAGGAGCAGCGACTGG | Sense | Deletion 6 |
| 1536 | CCAGTCGCTGCTCCTGCTTCTGGCTCCAAGAAATCAGCGACC | Antisense | |
| 1537 | GCTGATTTCTTGGAGCCTGAAACAGGAGCAGCGACTGGACCC | Sense | Deletion 7 |
| 1538 | GGGTCCAGTCGCTGCTCCTGTTTCAGGCTCCAAGAAATCAGC | Antisense | |
| 1539 | GATTTCTTGGAGCCTGAAAAGAAGGCAGCGACTGGACCCAGAGC | Sense | Deletion 8 |
| 1540 | GCTCTGGGTCCAGTCGCTGCCTTCTTTTCAGGCTCCAAGAAATC | Antisense | |
| 1547 | GTCGCTGATTTCTTGGAGGGAGAAAAGAAGCAGGAGCAG | Sense | To 1 loop |
| 1548 | CTGCTCCTGCTTCTTTTCTCCCTCCAAGAAATCAGCGAC | Antisense of 1547 | |
| 1586 | TGTGTGGGAGCAGCGACACGACCCAGAG | Sense | A′D′ to 1 loop |
| 1587 | CTCTGGGTCGTGTCGCTGCTCCCACACA | Antisense | |
| 1588 | CAAGCAGCTGGAGGCGGTGTGTGTGGGA | Sense | A′D′ to 1 loop |
| 1589 | TCCCACACACACCGCCTCCAGCTGCTTG | Antisense |
5′-UTR, 5′-untranslated region. Lower case indicates the additional sequences present in the given primers. These sequences are restriction enzyme recognition sites (see materials and methods). Boldface letters indicate mutated nucleotides.
Monocistronic Plasmid Constructs
The monocistronic construct (rpCDNA3/5′-UTR/Luc) was used in this study for certain cap-dependent translation experiments. In the monocistronic construct the hSP-A 5′-UTR variant was fused to the coding region of the reporter gene, firefly luciferase, and expression of the reporter gene was driven by the SV40 promoter as described in our previous work (73). Therefore, translation of the firefly luciferase involves primarily the cap-dependent translation mechanism.
Constructs from Deletion and Site-Directed Mutagenesis
A total of 10 deletion mutants were generated from one wild-type (WT) construct (C49-hSP-A 5′-UTR ABD). In these deletion mutants, exon A, exon D, or 5 nucleotides sequentially in exon B of the ABD variant were deleted. Several mutants with nucleotide substitution were generated in this study. Mutation was performed with the Quikchange XL II site-directed mutagenesis kit (Stratagene, La Jolla, CA). The primers, listed in Table 1, were designed by the PrimerSelect program of Lasergene (version 5) and were synthesized in the macromolecular core facility of Pennsylvania State University College of Medicine. The mutation processes were carried out as described previously (74). The DNA sequences of mutants were confirmed by DNA sequencing.
Transient Transfection and Luciferase Activity Assay
The methods of transient transfection and luciferase activity analyses were similar to those described in our previous work (73). In brief, NCI-H441 cells were cultured in RPMI 1640 medium plus 10% FBS to 80–90% confluence in 10-cm dishes. Twenty-four hours before transfection the cells were subcultured into six-well culture plates with ∼1 × 106 cells/well. The conditioned medium was removed 4 h before transfection and replaced with Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) containing neither FBS nor antibiotics.
The transfection procedure was carried out according to manufacturer's instructions with the Lipofectamine Plus reagent kit (Invitrogen). Briefly, for the bicistronic construct containing both firefly and Renilla luciferase reporter genes, 1 μg of plasmid DNA was diluted into 100 μl of DMEM medium without serum. For the monocistronic plasmids, 1 μg of DNA of the experimental construct (containing firefly luciferase gene) plus 0.05 μg of DNA of Renilla plasmid were mixed and then diluted into 100 μl of DMEM medium without serum. The latter was used for normalization of transfection efficiencies. Six microliters of PLUS reagent was added, and the mixture was incubated for 15 min at room temperature. Four microliters of Lipofectamine reagent were diluted into 100 μl of DMEM medium without serum in another tube. The components of the two tubes were then combined, mixed, and incubated for another 15 min at room temperature. The above DNA-Lipofectamine complex was added to 2.5 ml of fresh medium without serum and transferred into one well containing ∼1 × 106 H441 cells. Four hours after transfection, DMEM with 10% FBS was added to normal culture volume (5 ml/well). Transfection was carried out for 36 h or for the indicated time point for the time course of gene expression.
Dual-luciferase assay was performed with the Dual-Luciferase reporter assay system (kit) (Promega, Madison, WI). Briefly, 1) transfected cells were harvested at 36 h after transfection or at the indicated time point; 2) the cells were briefly washed with 1× PBS and lysed in 500 μl of 1× passive lysis buffer (PLB); 3) the culture plates were rocked at room temperature for 15 min, and the lysate was transferred to a tube and centrifuged for 1 min at 4°C to clear the cell lysate; 4) the supernatant was transferred into a new tube for firefly and Renilla luciferase assay according to the protocol of the Dual-Luciferase reporter assay kit (Promega). The ratio of firefly luciferase activity to Renilla luciferase activity is presented as IRES activity in the bicistronic constructs or as cap-dependent translation activity in the monocistronic constructs.
RNA Circularization
A recombinant plasmid carrying either the 5′-UTR ABD variant or a 13-bp sequence (control vector) downstream of the T7 promoter and upstream of the luciferase reporter gene was generated by replacing the SV40 promoter with the T7 promoter in the rpCDNA3/5′-UTR/Luc plasmid (73). The recombinant plasmid DNA was used as a template for RNA preparation. In vitro transcription was carried out with a MEGASCRIPT kit or a mMESSAGE mMACHINE kit (Ambion, Austin, TX) to obtain uncapped RNA or capped RNA, respectively. Briefly, 1 μg of XhoI linearized plasmid was incubated with T7 RNA polymerase, 75 mM nucleotides, and guanosine monophosphate (GMP) in a ratio of 10:1 to GTP (GTP is used only in capped RNA preparation). After template removal by TURBO DNase treatment, RNA was purified by LiCl precipitation. To generate circular RNA, the linear RNA was ligated according to a modified protocol (6, 8). In brief, the uncapped RNA transcript was annealed with a DNA oligodeoxynucleotide (CTCCAAGTTAAGCTTCCCTCGAGGCCTCGG) complementary to the 5′ and 3′ ends of the RNA, heated at 90°C, and cooled to room temperature. Ligation buffer, RNase inhibitor (Invitrogen), and T4 RNA ligase (Promega) were then added, and the mixture was incubated at 37°C for 30 min. After purification with phenol buffer, RNA was denatured in the presence of 50% formamide loading dye, heated at 80°C for 10 min, chilled on ice, and separated on a 1% agarose gel to resolve the linear and circular forms of RNA. Bands of the linear and circular RNAs were gel-purified with the RNaid kit (Bio101) and LiCl precipitation, and purity was confirmed by agarose gel electrophoresis.
In Vitro Translation
A total of 250 ng of linear or circular RNAs was in vitro translated with the Rabbit Reticulocyte Lysate System (Promega) according to the manufacturer's protocol in a final volume of 40 μl. Renilla luciferase RNA (5 ng) was also added to the mixture as a control; the reaction was incubated at 30°C for 90 min. Firefly and Renilla luciferase activities were measured in a 10-μl aliquot as described above, and the ratio of firefly to Renilla luciferase activity was calculated as translational efficiency.
Environmental Stressor (Diesel Particulate Matter or Ozone) Exposure and IRES Activity
H441 cells were transiently transfected with the bicistronic constructs ABD or the vector without SP-A 5′-UTR. After transfection the H441 cells were exposed to the environmental stressors diesel particulate matter (PM) and ozone. In PM-exposed experiments, diesel PM powder 2975 (NIST, Gaithersburg, MD) was first dissolved in dimethyl sulfoxide (DMSO) at a concentration of 25 mg/ml as a stock solution. Before use, PM stock solution for a final concentration of 20 μg/ml, or an equal volume of DMSO (control), was added into DMEM cell culture medium. Twelve hours after transfection the medium of the H441 cells was replaced with medium containing PM at 20 μg/ml or with an equal amount of DMSO. The cells were harvested at 36 h after transfection for luciferase activity assay. In ozone-exposed experiments, after 12 h of transfection the culture medium was removed, 0.5 ml of fresh medium was then added into each well of the six-well plate, and cells were then exposed to 1 ppm of ozone and 5% CO2 or filtered air with 5% CO2 (control) for 1 h. After exposure, 4 ml of culture medium was added to each well and the plate was placed in the cell culture incubator. Both firefly and Renilla luciferase activities were analyzed at the 36 h time point.
Prediction of RNA Secondary Structure
To study differences in secondary structure among 5′-UTRs, we used an online program (Version 3.2) prepared by Dr. M. Zuker (Rensselaer Polytechnic Institute Troy, NY, Refs. 80–82; http://frontend.bioinfo.rpi.edu/applications/mfold/cgi-bin/rna/-form1.cgi). It has been noted that this program more accurately predicts secondary structure. Although our 5′-UTR sequences are well under 1 kb in size, we opted to study the predicted secondary structure of each variant in a 1-kb sequence containing hSP-A 5′-UTR and a partial reporter gene sequence as well as studying the 5′-UTR sequences alone (i.e., without reporter gene sequences). The former could help determine whether flanking sequences can alter the 5′-UTR secondary structure. Similar observations were made in both cases (i.e., with or without reporter gene sequences).
Statistical Analysis
All experiments were repeated three or four times as stated in results, and triplicate assays were performed for each experiment. The data were analyzed by using the standard program software SigmaStat (version 3.5; SPSS). Differences between/among groups were assessed by ANOVA test or multiple-comparison ANOVA (Tukey test). The results are expressed as means ± SE. Statistically significant differences were considered when P < 0.05.
RESULTS
Generation of Bicistronic Constructs with hSP-A 5′-UTR Sequence Variants
The DNA fragments of hSP-A 5′-UTR variants used in this study were amplified from the cDNA of human SP-A splice variants. The PCR products of each hSP-A 5′-UTR variant were cut with restriction enzyme EcoRI and cloned into a basic bicistronic vector, C49. The insert sequences and orientation (sense) of hSP-A 5′-UTR in the recombinant constructs were identified and confirmed by DNA sequencing analysis. Four bicistronic constructs, each containing sense sequences of one hSP-A 5′-UTR variant [A′D′ (62 bp), ABD (100 bp), AB′D′ (137 bp), A′CD′ (122 bp)] were generated. In addition, one bicistronic construct with 5′-UTR sequence of the Hif-1α gene found to exhibit IRES activity in previous studies (37) was used as a positive control. A basic bicistronic vector without 5′-UTR sequence (only a 21-bp linker sequence, TTC TAG GGG AAT TCA GCC ACC, in which an EcoRI restriction enzyme site is included) was used as a negative control. These recombinant bicistronic constructs (Fig. 1) were then used in transient transfections to assess IRES activities of hSP-A 5′-UTR variants in vitro. In these constructs, the SV40 promoter mediates the expression of a fusion transcript containing both the Renilla and firefly reporter genes. The Renilla reporter gene is consistently translated to protein, using its own 5′-UTR for initiation, through cap-dependent translation producing Renilla luciferase activity. In contrast, firefly luciferase activity depends on IRES activity of the hSP-A 5′-UTR fragment. Therefore, the ratio of firefly to Renilla luciferase was used to represent IRES activity of the hSP-A 5′-UTR fragment (see Fig. 1).
Analysis of IRES Activity of Four hSP-A 5′-UTR Splice Variants
Before determining IRES activity of all four hSP-A 5′-UTR variants, we first performed a time-course experiment with the recombinant construct ABD to determine an optimal time point for comparison of the SP-A constructs. The ABD construct was transfected into H441 cells, and the transfected H441 cells were harvested at several time points 12, 24, 30, 36, 42, and 48 h after transfection. Firefly and Renilla luciferase activities were determined with the Dual-Luciferase reporter assay system, and IRES activity was assessed by calculating the ratio of firefly to Renilla luciferase. The firefly luciferase activity gradually increased and approached a plateau at the 36 h time point. The 36 h time point was considered optimal and was used in subsequent experimentation. Furthermore, the time course using the positive control construct with the Hif-1α 5′-UTR region was similar to that of the ABD construct.
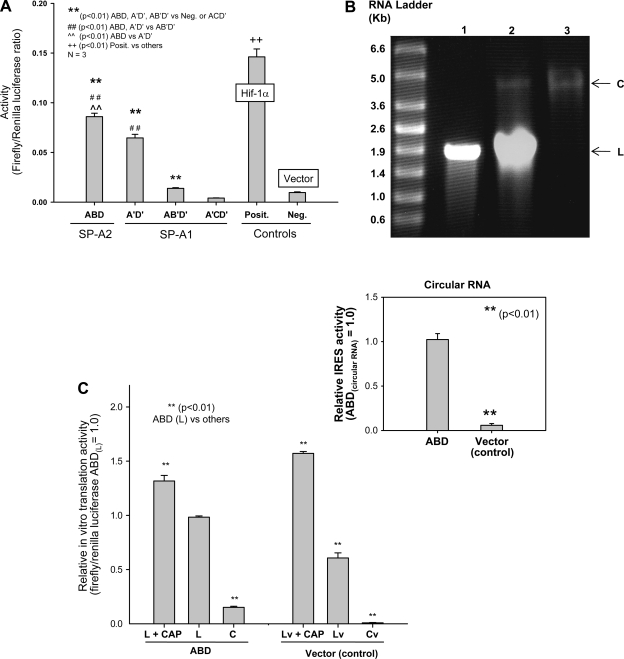
The IRES activities of four hSP-A 5′-UTR splice variants (A′D′, ABD, AB′D′, A′CD′) were examined at 36 h after transfection. The results shown in Fig. 3A indicate that IRES activities (ratio of firefly to Renilla luciferase) differed significantly (P < 0.01) among the 5′-UTR variants. A′D′, ABD, and AB′D′ had significantly higher IRES activities than the negative control (the construct without 5′-UTR), but A′CD′ did not exhibit any activity, indicating that the three hSP-A 5′-UTR variants (A′D′, ABD, AB′D′) are capable of cap-independent translation, but A′CD′ is not. Among the three variants (A′D′, ABD, AB′D′), the order of IRES activities is ABD > A′D′ > AB′D′. IRES activities of the ABD and the A′D′ were about six and five times higher than that of the AB′D′, and the IRES activity of the ABD was significantly higher than that of the A′D′ (P < 0.01). However, compared with the positive control (Hif-1α 5′-UTR), all three (A′D′, ABD, AB′D′) showed lower IRES activity (Fig. 3A).
Fig. 3.
hSP-A 5′-UTR variants exhibit differences in IRES activity. A: IRES activities of hSP-A 5′-UTR variants. H441 cells were transfected with each of the recombinant bicistronic constructs ABD, A′D′, AB′D, and A′CD′ or positive control (Hif-1α) and negative control vector without 5′-UTR. Transfected cells were harvested 36 h after transfection, and firefly and Renilla luciferase activities were measured with the Dual-Luciferase assay as described in materials and methods. The ratio of firefly to Renilla luciferase activities is used to express 5′-UTR-mediated IRES activity. SP-A2 ABD activity was significantly higher (**P < 0.01) than either the SP-A1 (A′D′, AB′D′, A′CD′) constructs tested or the negative control. The A′D′ was significantly higher (##P < 0.01) than the AB′D′, A′CD′, or negative control. The AB′D′ was significantly higher than the A′CD′ or the negative control, but the A′CD′ did not differ significantly from the negative control. In all experiments, the positive control (containing the 5′-UTR sequences of human Hif-1α) exhibited the highest activity. The activity of the negative control that lacked hSP-A 5′-UTR sequence was very low and served as the background activity. Experiments were repeated at least 3 times (n = 3). Bar shows ±SE. B: linear (L) and circular (C) forms of RNAs of the ABD variant in the agarose gel. ABD RNA transcripts were generated through in vitro transcription and then ligated to circular forms by RNA ligase as described in materials and methods. Lane 1, linear RNAs before ligation; lane 2, linear and circular RNAs after ligation; lane 3, circular RNA after gel purification. C: in vitro translation activities of linear and circular forms of RNAs. Linear RNAs with (L+CAP) or without cap structure (L) and circular RNA (C) from the ABD or control vector (Cv) were translated in vitro with the Rabbit Reticulocyte Lysate System. Both firefly and Renilla luciferase activities were determined after analysis of the translation products. The ratio of firefly to Renilla luciferase activity was used to represent the relative in vitro translation activities of linear and circular RNAs. Linear RNA either with cap or without cap exhibits significantly higher in vitro translation activity (P < 0.01) than circular RNA in both the ABD variant and the control vector. Circular RNA of the ABD variant exhibits in vitro translation activity, but the circular RNA of the control does not show any activity. Inset: comparison of IRES activities of circular RNAs of the ABD and control constructs. Experiments were repeated 3 times (n = 3). Bars show ±SE.
The results of recent studies (4, 79) indicate that observations made with linear bicistronic mRNAs should be verified with an independent method because some putative IRES elements can act as cryptic promoters, thereby generating monocistronic products. Therefore, to further confirm IRES activity mediated by SP-A 5′-UTR variants we generated circular RNA molecules (shown in Fig. 3B) as described in materials and methods and then used the linear and circular RNAs to carry out in vitro translation. The 5′-UTR of circular RNA cannot mediate translation in vitro if it lacks an IRES element (6, 8). Thus firefly luciferase activity should be observed only if the circular RNA does indeed contain an IRES element in its 5′-UTR. The results shown in Fig. 3C indicate that 1) the circular ABD-luciferase RNA exhibits IRES activity (C) but a circular RNA lacking the ABD 5′-UTR (control vector; Cv) does not; 2) the translation level (IRES activity) of the ABD circular RNA was ∼15% of that of the ABD linear RNA; 3) capped linear RNA showed higher translation activity than uncapped linear RNA. The translation activity of capped linear ABD (L + CAP) was ∼131% of that of the uncapped linear ABD (L). These results provide further evidence that a cap-independent translation mechanism plays a role in SP-A ABD mRNA translation.
Effect of Environmental Stressors on IRES Activity of 5′-UTR ABD Variant
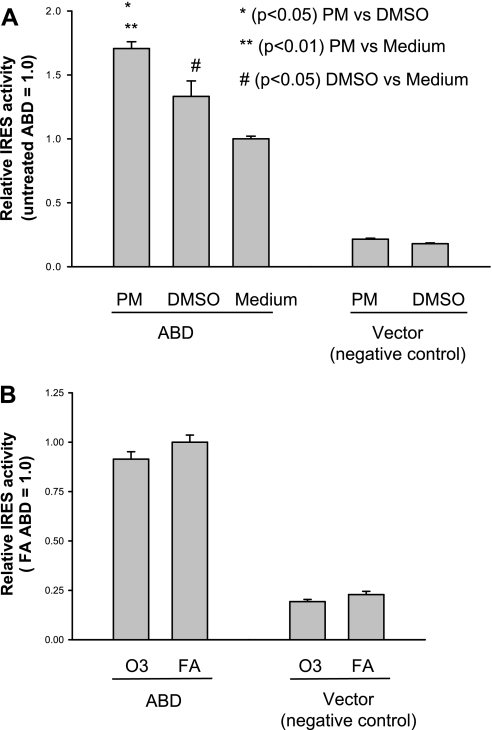
To examine the effect of environmental stressors on the IRES activities of hSP-A 5′-UTR variants, H441 cells were exposed to diesel PM (20 μg/ml) 12 h after transfection with the ABD plasmid, and then firefly and Renilla luciferase activities were determined at 36 h after transfection. The results indicate that IRES activity of the ABD construct was significantly increased in the cells exposed to PM compared with those exposed to DMSO alone (basal level) (Fig. 4A). DMSO treatment also resulted in significantly higher IRES activity compared with no treatment. However, no increase in IRES activity was observed in the cells exposed to ozone at 1 ppm for 1 h (Fig. 4B). These data indicate that not only is the ABD 5′-UTR responsive to environmental stressors but this response is stressor specific, because ozone exposure did not have any effect on IRES activity of the 5′-UTR ABD construct.
Fig. 4.
Effect of environmental stressors [diesel particulate matter (PM) and ozone (O3)] on ABD IRES activity. H441 cells were transfected with the bicistronic construct of SP-A 5′-UTR ABD or vector without SP-A 5′-UTR (control). Cells were then exposed to diesel PM (20 μg/ml of medium; A) or ozone with 1 ppm for 1 h (B) 12 h after transfection. A: IRES activity of the ABD construct significantly increased after exposure to PM compared with either DMSO or medium alone (P < 0.05); the IRES activity after exposure to DMSO was significantly higher compared with medium (P < 0.05). IRES activity of the ABD construct was significantly higher than that of the vector control. B: IRES activity of the ABD and vector constructs after exposure to ozone (1 ppm for 1 h) or filtered air (FA). IRES activity of the ABD construct was significantly higher than that of the control, but no significant difference was observed between ozone and FA exposure. Experiments were repeated twice (n = 2). Bar shows ±SE.
Study of cis-Acting Elements Involved in IRES Activity of SP-A2 ABD Splice Variant via Systematic Deletion Mutation Approach
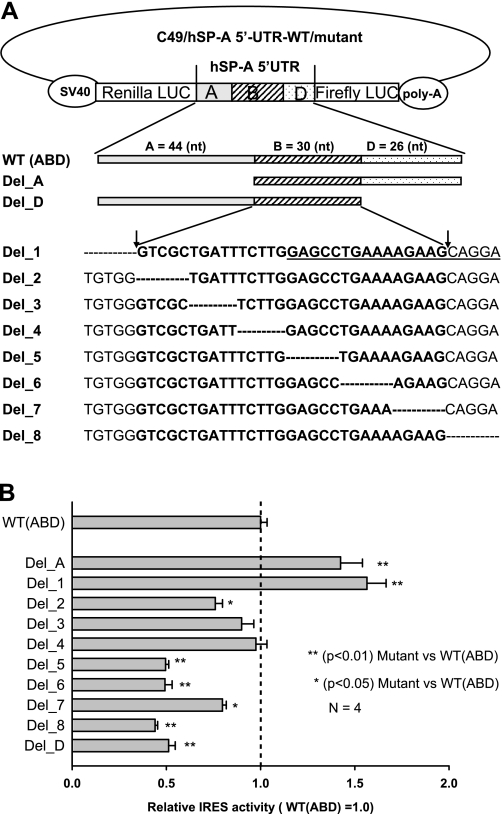
The ABD variant exhibited the highest IRES activity among the four hSP-A 5′-UTR variants. The main difference between the ABD and the A′D′ with the next highest activity is exon B. Therefore, we focused our attention on study of the cis-acting elements in exon B and its boundaries. A series of mutant ABD constructs were generated by deletion mutagenesis as described in materials and methods. First, we generated a set of mutants that lacked exon A or exon D. Two mutants (i.e., constructs Del_A and Del_D) were obtained through deletion mutation in which exon A or exon D of the ABD was removed (Fig. 5A); the A′D′ construct is one of the naturally observed 5′-UTR variants. Subsequently, exon B and its boundary sequences between exons A and B and between exons B and D, were mutated through the sequential deletion of 5 bp. A total of eight constructs (i.e., Del_1 to Del_8) were obtained (Fig. 5A). Ten mutant constructs (Del_1 to Del_8, Del_A, Del_D) and one WT ABD were studied for their IRES activity through transient transfection in the H441 cells.
Fig. 5.
Analysis of cis-acting elements through a series of deletion mutations and IRES activity assay. A: diagram of deletion mutations of the bicistronic ABD construct. hSP-A 5′-UTR ABD fragment, consisting of exons A, B, D, is located between Renilla and firefly reporter genes. First, 2 exon deletion mutants (Del_A and Del_D) were produced through the deletion of the entire exon A or exon D, respectively. Second, 8 deletion mutants (Del_1 to Del_8) were generated, each of which has 5 bp removed within the exon B region or in its boundaries to exon A and D. The wild type (WT) (ABD) is listed above the mutants and used as positive control. An adenosine-rich short motif is underlined in the sequence of Del_1. B: changes of IRES activity in the deletion mutants, compared with the WT (ABD) construct. After transfection with mutants as well as the WT construct, the H441 cells were harvested at 36 h after transfection. Both Renilla and firefly luciferase activities were determined as described in materials and methods. Ratio of firefly to Renilla luciferase activities is used to represent the IRES activity of each mutant. To compare IRES activities of the mutants and the WT, relative IRES activity was calculated. The WT (ABD) IRES activity (ratio of firefly to Renilla luciferase) was set as 1.0, and the relative IRES activity of each mutant was equal to the value of the IRES activity of each mutant divided by the IRES activity of the WT. IRES activities of mutants Del_A and Del_1 were significantly higher (P < 0.01) than that of the WT; IRES activities of Del_D, Del_5, Del_6, and Del_8 were significantly lower (P < 0.01) than that of the WT; mutants Del_2 and Del_7 had lower (P < 0.05) IRES activities compared with the WT but higher IRES activities than most of the other mutants (Del_D, Del_5, Del_6, and Del_8). Experiments were repeated 4 times (n = 4). Bars show ±SE.
The ratio of firefly to Renilla luciferase of these mutants and the WT ABD were obtained at 36 h after transfection, and the results are shown in Fig. 5B. IRES activities of mutants Del_A and Del_1 are significantly higher (P < 0.01) than that of the WT ABD, indicating that repressor element(s) inhibiting IRES activity of the ABD may be present in exon A and specifically in the 3′ end region of exon A. In mutant Del_A the entire exon A was removed, and in mutant Del_1 the last 5 bp of exon A were removed. In contrast, IRES activities of the mutants Del_D, Del_5, Del_6, and Del_8 are significantly lower (P < 0.01) than that of the WT ABD. The Del_2 and Del_7 have lower (P < 0.05) IRES activity than the WT ABD but higher (P < 0.05) than Del_D, Del_5, Del_6 or Del_8. The IRES activity of Del_3 and Del_4 is not significantly different than that of the WT ABD. These results indicate that putative enhancing element(s) of IRES activity exist within exon B and that these for the most part may locate in the second half of exon B and the 5′ end of exon D. In fact, in the nucleotide sequence from Del_5 to Del_8 an adenosine-rich region (45% adenosine in 20 residues) was observed (shown by underline in Del_1 sequence in Fig. 5A). Within this region, the AAAAGAA motif is readily evident and may play a role in the regulation of the ABD IRES activity. Alternatively, or in addition to cis-acting elements, the secondary structure may also play a role in IRES activity.
Study of Effect of Secondary Structure of SP-A2 ABD Splice Variant on IRES Activity by Mutation Analysis
Deletion mutants.
Published literature thus far has indicated that there are no common conserved sequences or structures in the 5′-UTR of cellular genes that associate with IRES activity (2, 25). To gain insight into factors contributing to IRES activity mediated by the SP-A 5′-UTR, we compared the secondary structures of the WT ABD and the deletion mutants shown in Fig. 5A with an online available program (see materials and methods). First, the sequence of ∼1 kb that included the hSP-A 5′-UTR ABD flanked by a partial sequence of the reporter genes firefly or Renilla luciferase was analyzed. The hSP-A 5′-UTR ABD region, being in the middle between the firefly and Renilla luciferase sequences, could form a discrete and readily identifiable secondary structure, which was similar to that formed by the ABD sequence alone (data not shown). Next, similar secondary structure analyses were performed for the 5′-UTR deletion mutant sequences. The presence or absence of the flanking reporter gene sequence similarly did not have any impact on the secondary structure of the SP-A 5′-UTR.
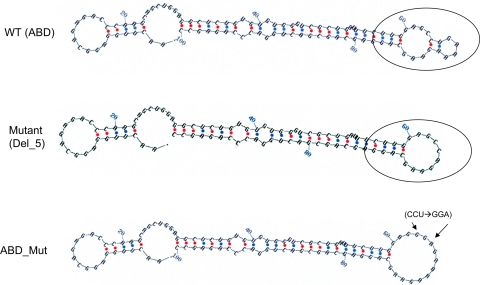
One obvious property of the secondary structure of the WT ABD was the formation of a stable double-loop structure shown in Fig. 6 (WT ABD), where the two loops are separated by two paired nucleotides. Analysis of deletion mutants revealed that the secondary structures of mutants (Del_5, Del_6, Del_8, and Del_D) that showed a 50% reduction in IRES activity had lost this double-loop structure. The single-loop structure of mutant Del_5 is shown as an example in Fig. 6. Mutants (Del_A, Del_1, Del_3, and Del_4) with enhanced activity or no change in their activity maintained the double-loop structure, whereas Del_2 and Del_7, with moderate but yet significant reduction in their activity, either had an altered double-loop structure or had lost the double-loop structure, respectively (not shown).
Fig. 6.
Secondary structures of the WT ABD and 2 of its mutants, a deletion mutant (Del_5) and a mutant generated by site-directed mutagenesis (ABD_Mut). Secondary structures of the WT ABD 5′-UTR and a total of 10 deletion mutants were predicted with the Zuker algorithm of the mRNA folding online program (version 3.2; http://frontend.bioinfo.rpi.edu/applications/mfold/cgi-bin/rna/-form1.cgi). A double-loop structure was identified in the WT (circled) and in some of the mutants (not shown). However, this double-loop structure was changed into a single-loop structure in mutants Del_5 (circled) and mutants Del_6, Del_7, and Del_8 (not shown). A mutant (ABD_Mut) was generated by site-directed mutagenesis, where 3 nucleotides (CCU) in the double-loop region of the WT ABD variant were substituted with GGA by site-directed mutagenesis. With this 3-base change, the double-loop structure (present in WT) was eliminated and 1 larger single-loop structure formed (ABD_Mut). The 2 arrows in ABD_Mut point to the start and the end of the 3 nucleotides altered.
To further gain insight into characteristics of double- versus single-loop structure we evaluated the free energies of each experimental 5′-UTR construct. As shown previously (73), the free energy of the WT ABD is −35.8 kcal/mol (ABD = 100 nt, −0.358 kcal·mol−1·nt−1). The percent change of free energy between the WT ABD structure and that of the serial 5-nt deletion mutants (Fig. 5A), except for Del_2, was minimal with a range of 0–8.1%. This may explain why constructs with a single-loop structure could still exhibit ∼50% of the WT activity, and it also raises the possibility that the structure of the double loop is more favorable (beyond its stability) for cap-independent translation in human SP-A. The Del_2 mutant provides further support that the double-loop structure as well as the stability of the structure are necessary for full IRES activity. The Del_2 mutant maintained an altered form of a double-loop structure, but its stability was considerably reduced [free energy (ΔG) −0.283 kcal·mol−1·nt−1 vs. WT −0.358 kcal·mol−1·nt−1, or 21.1% change]. This in turn translated into a significantly reduced IRES activity compared with WT. Del_A (−0.303 kcal·mol−1·nt−1) and Del_D (−0.312 kcal·mol−1·nt−1) mutants had lower ΔG (more stable) than Del_2 but higher ΔG (less stable) than the remaining Del_mutants, which ranged between −0.335 (Del_3) and −0.387 (Del_5) kcal·mol−1·nt−1.
Together the information from the secondary structure and free energy analyses indicates that both type (double vs. single loop) and stability of secondary structure are necessary for full IRES activity.
Insertion mutant.
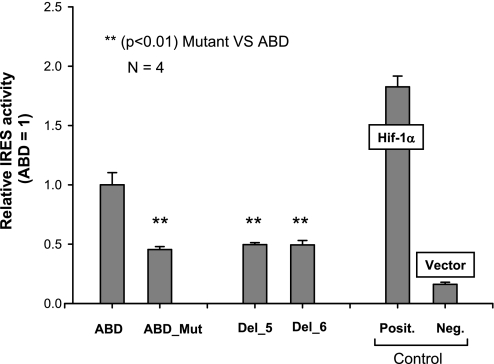
To further test the hypothesis that a double loop is indeed necessary for full cap-independent activity, we changed the double-loop structure of the ABD into a single loop by site-directed mutagenesis. Three nucleotides, CCU, in exon B of the WT ABD were changed to GGA. The secondary structure of this mutant (defined as ABD_Mut) is shown in Fig. 6. Its IRES activity decreased significantly to approximately half of that of the WT ABD (Fig. 7), indicating that the double-loop structure in the ABD variant is necessary for full IRES activity. Furthermore, the decrease of IRES activity of the ABD_Mut was similar to that of the deletion mutants Del_5 and Del_6 (shown in Fig. 7), indicating that for full IRES activity the presence of the double-loop structure is important. Moreover, the IRES activity of these mutants was higher than that of the negative control, indicating that factors other than the secondary structure may also contribute to cap-independent translation.
Fig. 7.
Effect on IRES activity when the double loop is changed into a single loop. The mutant (ABD_Mut) and the WT ABD, as well as positive and negative controls, were assessed for their IRES activity by transfecting each of these constructs into H441 cells. Renilla and firefly luciferase activities were determined at 36 h after transfection, as described in materials and methods. The WT ABD activity was set as 1.0. To compare the effect of the single loop in the insertion mutant (ABD_Mut) activity, the relative IRES activities of 2 deletion mutants (Del_5 and Del_6) are included. Original data of both of these deletion mutants are shown in Fig. 5B. Results showed that the level of IRES activity of the ABD_Mut was significantly lower (**P < 0.01) than that of the WT ABD. However, the level of IRES activity of the ABD_Mut was comparable to that of the 2 deletion mutants (Del_5 and Del_6). Experiments were repeated 4 times (n = 4). Each point shows mean + SE.
Role of Two Double-Loop Structures of SP-A1 A′D′ Splice Variant on IRES Activity
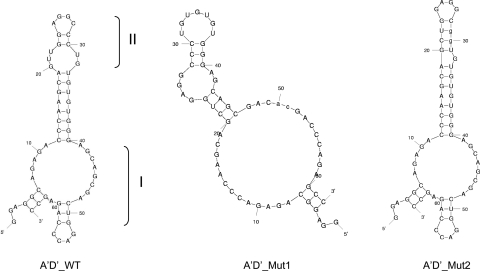
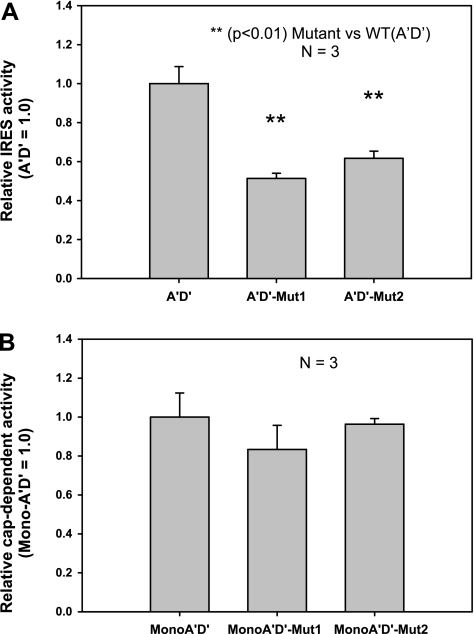
Although the hSP-A 5′-UTR A′D′ variant has only 62 bp, IRES activity was readily detectable as shown in Fig. 3A. This A′D′ splice variant, as assessed by prediction of its mRNA secondary structure, can form two double-loop structures (I and II in Fig. 8). The SP-A1 AB′D′ and A′CD′ splice variants that exhibited low or no IRES activity, respectively, did not show a discrete double-loop structure (data not shown). Since the study of the ABD variant revealed that its double-loop structure is of importance for cap-independent translation, the question was raised as to whether the double-loop structures of the A′D′ are also critical for its cap-independent translation. Two mutants (A′D′_Mut1 and A′D′_Mut2) were generated by site-directed mutagenesis. In A′D′_Mut1 and A′D′_Mut2, double-loops I and II were changed, respectively (see materials and methods), to one loop structure (Fig. 8). We examined the IRES activity of both A′D′ mutants along with the WT A′D′ and found that the IRES activity of each mutant, A′D′_Mut1 and A′D′_Mut2, decreased to ∼50% and 60%, respectively, compared with the WT A′D′ (Fig. 9A). Furthermore, a double mutant (A′D′_Mut1+2), in which both double loops I and II were eliminated (not shown) had IRES activity similar to that of the single double-loop mutants. These data indicate that both double loops are necessary for full IRES activity. Moreover, elimination of either or both double loops reduced IRES activity by ∼50%, indicating that the individual double-loop IRES contribution is not additive and that factors other than secondary structure contribute to the remaining 50% cap-independent translation.
Fig. 8.
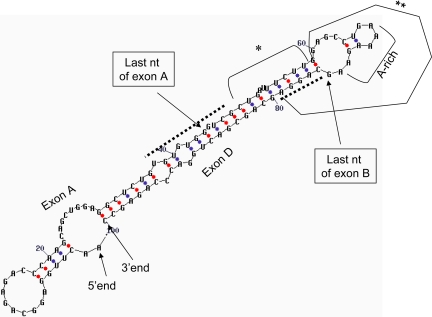
Predicted mRNA secondary structures of the SP-A1 A′D′ WT and 2 of its mutants. The A′D′ WT forms two double-loop structures (double loops I and II). Each of the two double-loop structures of the A′D′ bicistronic construct was eliminated by site-directed mutagenesis generating A′D′_Mut1 and A′D′_Mut2. A′D′_Mut1 was generated through an AC nucleotide substitution of TG at positions 50 and 51, which changed double loop I into a large single loop. Similarly, A′D′_Mut2 was obtained through nucleotide changes from CC to GG at positions 29 and 30; this also changed double loop II into a single loop. After these nucleotide changes, the A′D′_Mut1 and the A′D′_Mut2 each contained only 1 double-loop structure.
Fig. 9.
Effect of each of the double loops of the SP-A1 A′D′ mutants on cap-independent (IRES) and cap-dependent activity. Each of the A′D′ mutants or the WT A′D′ was transfected into H441 cells, and then both firefly and Renilla luciferase activities were analyzed. A: relative IRES (cap independent) activity of the bicistronic construct WT A′D′ and the 2 mutants (A′D′_Mut1, A′D′_Mut2). Results indicate that IRES activities of the 2 A′D′ mutants (A′D′_Mut1, A′D′_Mut2) were significantly lower than that of the WT A′D′ (P < 0.01), and no significant difference was observed between the single mutants (A′D′_Mut1 and A′D′_Mut2). B: relative cap-dependent translation activities of the monocistronic construct WT A′D′ and the 2 mutants (MonoA′D′_mut1 and MonoA′D′_Mut2). No significant difference was observed among these 3 constructs. Experiments in A and B were repeated 3 times (n = 3). Each point shows mean + SE.
Double-Loop Structure of hSP-A1 5′-UTR A′D′ Splice Variant Is Not Required for Cap-Dependent Translation
To determine whether the presence of the double-loop structures is a general phenomenon in SP-A translation, we studied the SP-A1 A′D′ mutants and the WT A′D′ for their ability to carry out cap-dependent translation. For this, a monocistronic vector we have previously described (73) was used to generate constructs each of which contained the mutated (Mut_1 or Mut_2) or the WT A′D′ sequence. The results of the transient transfection experiments of the monocistronic vector constructs are shown in Fig. 9B and indicate that these mutations have no impact on the cap-dependent translation. Thus the double-loop structure in the SP-A1 A′D′ variant, although necessary for full cap-independent translation activity, is not important for cap-dependent translation activity.
DISCUSSION
SP-A is an important lung host defense protein (47, 60). SP-A-deficient knockout mice are more susceptible to infection (41–45) and to exposure of environmental pollutants such as ozone (22). The levels of SP-A are altered in animal models after exposure to ozone (22, 68), LPS (63, 69), and corn dust extract (15). Several human SP-A1 and SP-A2 5′-UTR splice variants exist (33, 51), with differences among them in translation efficiency and mRNA stability (73). Cap-independent translation can become operative in response to various stresses/ stimuli. In the present study, we investigated the hypothesis that SP-A 5′-UTR variants differentially mediate cap-independent translation. Transient transfection experiments of bicistronic constructs each containing a different 5′-UTR variant were carried out with H441 cells. The results showed that 1) under control conditions SP-A1 and SP-A2 5′-UTRs differentially mediate cap-independent translation (ABD > A′D′ > AB′D′; the SP-A1 A′CD′ variant did not exhibit any cap-independent activity); 2) diesel PM exposure significantly increased the cap-independent activity of the 5′-UTR ABD; 3) deletion mutation and site-directed mutagenesis analyses of the SP-A2 ABD variant showed cis-acting elements to associate with cap-independent activity; 4) predicted mRNA structure analysis of SP-A1 and SP-A2 WT and mutants showed that a double-loop structure is necessary for full IRES activity although such a structure did not seem to be necessary for cap-dependent translation; and 5) the stability of the secondary structure is also a contributing factor.
Bioinformatics (57) and genomewide RNA structure search (2) revealed that cellular IRESs may depend on a short sequence motif and trans-acting factors for their function (2, 7, 25), with polypyrimidine (24, 31) and poly(A) (16) tracts identified as playing a role in cap-independent translation. In the present study, cis-acting enhancer and repressor elements were found in the ABD variant (Fig. 10). In a region in which potential enhancer elements reside, an adenosine-rich sequence of ∼20 nt was detected with a core sequence of an AAAAGAA motif. A polyadenosine [poly(A)] tract element (12 of 13 residues) upstream of the start AUG codon in yeast was also shown to play a critical role in IRES activity of yeast genes in response to starvation-induced differentiation (16). However, it is unknown whether the adenosine-rich motif in the ABD variant shares a similar mechanism with yeast, where recruitment of the poly(A) binding protein (Pab1) to the 5′-UTR occurs.
Fig. 10.
Diagrammatic representation of elements involved in cap-independent translation of the SP-A2 5′-UTR ABD splice variant. Dotted line indicates cis-acting elements that when removed increase activity (3′ end of exon A) or decrease activity (5 ′end of exon D). The 5′ end and 3′ end of the ABD variant are noted. *This region does not appear to play a role in cap-independent translation. **Part of exon B and border of exon D sequence is necessary for double loop formation and enhancing activity, and as such may contain important cis-acting elements. A potentially important cis-acting candidate in SP-A regulation is the adenosine or A-rich motif.
The mFOLD program (80–82) predicts secondary structure (12) and is used widely for mRNA secondary structure prediction including IRESs in viral (11) and cellular (3, 56) 5′-UTRs. mFOLD is a powerful “minimum free energy” program based on the principle of mRNA folding into the most thermodynamically stable form. mFOLD analysis of the SP-A2 ABD and SP-A1 A′D′ constructs that exhibited the highest IRES activity showed a stable hairpinlike structure with a double-loop head. Similar stem-loop structures have been observed in other cellular IRES mRNAs, including c-myc (38), FGF-2 (5), and the voltage-gated potassium channel (31), indicating its importance in IRES activity in several genes. Although it is unknown how the double-loop structure of the SP-A 5′-UTR and the cis-acting elements enhance cap-independent translation, published studies indicate that there is a complex interplay between cis-acting and structure elements. For example, in the 5′-UTR mRNA of the kv1.4 (member of voltage-gated potassium channels) a polypyrimidine tract and a stem loop were identified as important for IRES activity, and deletions within the sequence of either one of these elements had opposing effects on activity (31). The elements identified in the present study (Fig. 10) indicate that the integrity of most of exon B and its boundaries with exons A and D are essential for full IRES activity. A substantive sequence integrity requirement of 5′-UTR was shown for cap-independent translation of the Hsp70 mRNA (64). It is possible that in SP-A individual elements positively and negatively impact its expression via a complex interplay of opposing activities, as described for other systems (31).
Of interest, deletions that destroyed or altered the double loop of the WT construct resulted in reduction of IRES activity in each SP-A2 ABD and SP-A1 A′D′ variant, indicating an essential role of such a structure for full IRES activity. The fact that even after elimination of both double-loop structures in the A′D′ the IRES activity remained at 50%, as it was for the constructs containing either loop, indicates that factors yet to be identified may be involved and further points to the complexity of cap-independent translation. Moreover, the lack of negative impact of SP-A1 A′D′ mutants on cap-dependent translation indicates that the double-loop secondary structure, although critical for cap-independent translation, is not necessary for cap-dependent translation of SP-A mRNA. It is possible that putative trans-acting factor(s) involved in cap-independent translation of hSP-A first recognizes/interacts with the double-loop structure in the 5′-UTR IRES site and then recruits other translation factor(s) to the IRES site. Of interest, the A′CD′ variant that did not exhibit cap-independent translation is predicted to lack a double-loop structure, where the putative IRES trans-acting factor(s) binds/interacts to enable cap-independent translation. In addition, the secondary structure stability appears also to contribute to IRES activity. Del_2 mutant with a predicted loop structure (albeit altered), but a reduced stability showed a significantly lower IRES activity compared with WT. On the other hand, no difference in activity was observed between Del_2 and Del_7. The latter had a predicted stability similar to the WT but lacked the double-loop structure.
In summary, the 5′-UTRs of the human SP-A1 and SP-A2 mRNAs differentially mediate cap-independent translation under control conditions and in response to diesel PM (SP-A2, ABD variant). Cis-acting elements, secondary structure, and stability of secondary structure are all contributing factors. The SP-A1 AB′D′ and A′CD′ 5′-UTRs exhibited low and no IRES activity, respectively. The SP-A2 ABD showed the highest activity and the SP-A1 A′D′ the next highest. Both of these UTRs required a predicted secondary structure in the form of a double loop(s) for full IRES activity. The double loop(s), although essential for full IRES activity, was not necessary for cap-dependent translation (A′D′ variant). In the ABD variant most of the B exon and its boundaries with exons A and D were critical for full activity. This region included cis-acting enhancer/ repressor elements, and one of the enhancers contained an adenosine-rich motif residing within a predicted double-loop structure. These findings provide new and important information regarding translational regulation of human SP-A1 and SP-A2 and indicate that cis-acting elements and secondary structure type and stability are playing a role. We postulate that the interplay of these factors along with the interactions of putative trans-acting factors determine the overall IRES activity of hSP-A.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-34788.
Acknowledgments
We thank Susan DiAngelo for her expert technical support and Brett Simmons for his help with some partial experiments, as well as the core facility of Pennsylvania State University College of Medicine for DNA sequencing and oligonucleotide synthesis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akiri G, Nahari D, Finkelstein Y, Le SY, Elroy-Stein O, Levi BZ. Regulation of vascular endothelial growth factor (VEGF) expression is mediated by internal initiation of translation and alternative initiation of transcription. Oncogene 17: 227–236, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Baird SD, Lewis SM, Turcotte M, Holcik M. A search for structurally similar cellular internal ribosome entry sites. Nucleic Acids Res 35: 4664–4677, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird SD, Turcotte M, Korneluk RG, Holcik M. Searching for IRES. RNA 12: 1755–1785, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bert AG, Grepin R, Vadas MA, Goodall GJ. Assessing IRES activity in the HIF-1alpha and other cellular 5′ UTRs. RNA 12: 1074–1083, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnal S, Schaeffer C, Creancier L, Clamens S, Moine H, Prats AC, Vagner S. A single internal ribosome entry site containing a G quartet RNA structure drives fibroblast growth factor 2 gene expression at four alternative translation initiation codons. J Biol Chem 278: 39330–39336, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter PS, Jarquin-Pardo M, De Benedetti A. Differential expression of Myc1 and Myc2 isoforms in cells transformed by eIF4E: evidence for internal ribosome repositioning in the human c-myc 5′UTR. Oncogene 18: 4326–4335, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Cencig S, Nanbru C, Le SY, Gueydan C, Huez G, Kruys V. Mapping and characterization of the minimal internal ribosome entry segment in the human c-myc mRNA 5′ untranslated region. Oncogene 23: 267–277, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science 268: 415–417, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Cormier Y, Israel-Assayag E, Desmeules M, Lesur O. Effect of contact avoidance or treatment with oral prednisolone on bronchoalveolar lavage surfactant protein A levels in subjects with farmer's lung. Thorax 51: 1210–1215, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Breyne S, Yu Y, Pestova TV, Hellen CU. Factor requirements for translation initiation on the simian picornavirus internal ribosomal entry site. RNA 14: 367–380, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Rijk P, Wuyts J, De Wachter R. RnaViz 2: an improved representation of RNA secondary structure. Bioinformatics 19: 299–300, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Dowell RD, Eddy SR. Evaluation of several lightweight stochastic context-free grammars for RNA secondary structure prediction. BMC Bioinformatics 5: 71, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floros J, Phelps DS. Pulmonary surfactant. In: Anesthesia: Biologic Foundations, edited by Yaksh TL, Lynch C III, Zapol WM, Maze M, Biebuyck JF, Saidman LJ. Philadelphia, PA: Lippincott-Raven, 1997, p. 1259–1279.
- 14.Garcia-Verdugo I, Wang G, Floros J, Casals C. Structural analysis and lipid-binding properties of recombinant human surfactant protein A derived from one or both genes. Biochemistry 41: 14041–14053, 2002. [DOI] [PubMed] [Google Scholar]
- 15.George CL, White ML, O'Neill ME, Thorne PS, Schwartz DA, Snyder JM. Altered surfactant protein A gene expression and protein metabolism associated with repeat exposure to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol 285: L1337–L1344, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert WV, Zhou K, Butler TK, Doudna JA. Cap-independent translation is required for starvation-induced differentiation in yeast. Science 317: 1224–1227, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Gray NK, Wickens M. Control of translation initiation in animals. Annu Rev Cell Dev Biol 14: 399–458, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Greene KE, Wright JR, Steinberg KP, Ruzinski JT, Caldwell E, Wong WB, Hull W, Whitsett JA, Akino T, Kuroki Y, Nagae H, Hudson LD, Martin TR. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med 160: 1843–1850, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Gunther A, Siebert C, Schmidt R, Ziegler S, Grimminger F, Yabut M, Temmesfeld B, Walmrath D, Morr H, Seeger W. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med 153: 176–184, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Guzman J, Wang YM, Kalaycioglu O, Schoenfeld B, Hamm H, Bartsch W, Costabel U. Increased surfactant protein A content in human alveolar macrophages in hypersensitivity pneumonitis. Acta Cytol 36: 668–673, 1992. [PubMed] [Google Scholar]
- 21.Hamm H, Luhrs J, Guzman y Rotaeche J, Costabel U, Fabel H, Bartsch W. Elevated surfactant protein A in bronchoalveolar lavage fluids from sarcoidosis and hypersensitivity pneumonitis patients. Chest 106: 1766–1770, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Haque R, Umstead TM, Ponnuru P, Guo X, Hawgood S, Phelps DS, Floros J. Role of surfactant protein-A (SP-A) in lung injury in response to acute ozone exposure of SP-A deficient mice. Toxicol Appl Pharmacol 220: 72–82, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellen CU, Sarnow P. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev 15: 1593–1612, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat Cell Biol 1: 190–192, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Holcik M, Pestova TV. Translation mechanism and regulation: old players, new concepts. Meeting on translational control and non-coding RNA. EMBO Rep 8: 639–643, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6: 318–327, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Hoover RR, Floros J. Organization of the human SP-A and SP-D loci at 10q22-q23. Physical and radiation hybrid mapping reveal gene order and orientation. Am J Respir Cell Mol Biol 18: 353–362, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Huang W, Wang G, Phelps DS, Al-Mondhiry H, Floros J. Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: effect of ozone-induced SP-A oxidation. Am J Physiol Lung Cell Mol Physiol 286: L546–L553, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Huez I, Creancier L, Audigier S, Gensac MC, Prats AC, Prats H. Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol Cell Biol 18: 6178–6190, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson RJ Alternative mechanisms of initiating translation of mammalian mRNAs. Biochem Soc Trans 33: 1231–1241, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Jang GM, Leong LE, Hoang LT, Wang PH, Gutman GA, Semler BL. Structurally distinct elements mediate internal ribosome entry within the 5′-noncoding region of a voltage-gated potassium channel mRNA. J Biol Chem 279: 47419–47430, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Jang SK, Krausslich HG, Nicklin MJ, Duke GM, Palmenberg AC, Wimmer E. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol 62: 2636–2643, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karinch AM, Floros J. 5′ Splicing and allelic variants of the human pulmonary surfactant protein A genes. Am J Respir Cell Mol Biol 12: 77–88, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Karinch AM, Floros J. Translation in vivo of 5′ untranslated-region splice variants of human surfactant protein-A. Biochem J 307: 327–330, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komar AA, Hatzoglou M. Internal ribosome entry sites in cellular mRNAs: mystery of their existence. J Biol Chem 280: 23425–23428, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Kumar AR, Snyder JM. Differential regulation of SP-A1 and SP-A2 genes by cAMP, glucocorticoids, and insulin. Am J Physiol Lung Cell Mol Physiol 274: L177–L185, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Lang KJ, Kappel A, Goodall GJ. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol Biol Cell 13: 1792–1801, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Quesne JP, Stoneley M, Fraser GA, Willis AE. Derivation of a structural model for the c-myc IRES. J Mol Biol 310: 111–126, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Le SY, Maizel JV Jr. A common RNA structural motif involved in the internal initiation of translation of cellular mRNAs. Nucleic Acids Res 25: 362–369, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lesur O, Bernard AM, Begin RO. Clara cell protein (CC-16) and surfactant-associated protein A (SP-A) in asbestos-exposed workers. Chest 109: 467–474, 1996. [DOI] [PubMed] [Google Scholar]
- 41.LeVine AM, Bruno MD, Huelsman KM, Ross GF, Whitsett JA, Korfhagen TR. Surfactant protein A-deficient mice are susceptible to group B streptococcal infection. J Immunol 158: 4336–4340, 1997. [PubMed] [Google Scholar]
- 42.LeVine AM, Gwozdz J, Stark J, Bruno M, Whitsett J, Korfhagen T. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J Clin Invest 103: 1015–1021, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeVine AM, Hartshorn K, Elliott J, Whitsett J, Korfhagen T. Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am J Physiol Lung Cell Mol Physiol 282: L563–L572, 2002. [DOI] [PubMed] [Google Scholar]
- 44.LeVine AM, Kurak KE, Bruno MD, Stark JM, Whitsett JA, Korfhagen TR. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol 19: 700–708, 1998. [DOI] [PubMed] [Google Scholar]
- 45.LeVine AM, Kurak KE, Wright JR, Watford WT, Bruno MD, Ross GF, Whitsett JA, Korfhagen TR. Surfactant protein-A binds group B streptococcus enhancing phagocytosis and clearance from lungs of surfactant protein-A-deficient mice. Am J Respir Cell Mol Biol 20: 279–286, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Macejak DG, Sarnow P. Internal initiation of translation mediated by the 5′ leader of a cellular mRNA. Nature 353: 90–94, 1991. [DOI] [PubMed] [Google Scholar]
- 47.Madsen J, Tornoe I, Nielsen O, Koch C, Steinhilber W, Holmskov U. Expression and localization of lung surfactant protein A in human tissues. Am J Respir Cell Mol Biol 29: 591–597, 2003. [DOI] [PubMed] [Google Scholar]
- 48.McCormack FX, King TE Jr, Bucher BL, Nielsen L, Mason RJ, McCormac FX. Surfactant protein A predicts survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 152: 751–759, 1995. [DOI] [PubMed] [Google Scholar]
- 49.McCormack FX, King TE Jr, Voelker DR, Robinson PC, Mason RJ. Idiopathic pulmonary fibrosis. Abnormalities in the bronchoalveolar lavage content of surfactant protein A. Am Rev Respir Dis 144: 160–166, 1991. [DOI] [PubMed] [Google Scholar]
- 50.McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest 109: 707–712, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCormick SM, Boggaram V, Mendelson CR. Characterization of mRNA transcripts and organization of human SP-A1 and SP-A2 genes. Am J Physiol Lung Cell Mol Physiol 266: L354–L366, 1994. [DOI] [PubMed] [Google Scholar]
- 52.McCormick SM, Mendelson CR. Human SP-A1 and SP-A2 genes are differentially regulated during development and by cAMP and glucocorticoids. Am J Physiol Lung Cell Mol Physiol 266: L367–L374, 1994. [DOI] [PubMed] [Google Scholar]
- 53.Mikerov AN, Umstead TM, Gan X, Huang W, Guo X, Wang G, Phelps DS, Floros J. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol Lung Cell Mol Physiol 294: L121–L130, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mikerov AN, Umstead TM, Huang W, Liu W, Phelps DS, Floros J. SP-A1 and SP-A2 variants differentially enhance association of Pseudomonas aeruginosa with rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 288: L150–L158, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Mikerov AN, Wang G, Umstead TM, Zacharatos M, Thomas NJ, Phelps DS, Floros J. Surfactant protein A2 (SP-A2) variants expressed in CHO cells stimulate phagocytosis of Pseudomonas aeruginosa more than do SP-A1 variants. Infect Immun 75: 1403–1412, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitchell SA, Spriggs KA, Coldwell MJ, Jackson RJ, Willis AE. The Apaf-1 internal ribosome entry segment attains the correct structural conformation for function via interactions with PTB and unr. Mol Cell 11: 757–771, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Mokrejs M, Vopalensky V, Kolenaty O, Masek T, Feketova Z, Sekyrova P, Skaloudova B, Kriz V, Pospisek M. IRESite: the database of experimentally verified IRES structures (www.iresite.org). Nucleic Acids Res 34: D125–D130, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 334: 320–325, 1988. [DOI] [PubMed] [Google Scholar]
- 59.Phelps DS Surfactant regulation of host defense function in the lung: a question of balance. Pediatr Pathol Mol Med 20: 269–292, 2001. [PubMed] [Google Scholar]
- 60.Phelps DS, Floros J. Localization of surfactant protein synthesis in human lung by in situ hybridization. Am Rev Respir Dis 137: 939–942, 1988. [DOI] [PubMed] [Google Scholar]
- 61.Phelps DS, Umstead TM, Mejia M, Carrillo G, Pardo A, Selman M. Increased surfactant protein-A levels in patients with newly diagnosed idiopathic pulmonary fibrosis. Chest 125: 617–625, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Pisarev AV, Chard LS, Kaku Y, Johns HL, Shatsky IN, Belsham GJ. Functional and structural similarities between the internal ribosome entry sites of hepatitis C virus and porcine teschovirus, a picornavirus. J Virol 78: 4487–4497, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quintero OA, Korfhagen TR, Wright JR. Surfactant protein A regulates surfactant phospholipid clearance after LPS-induced injury in vivo. Am J Physiol Lung Cell Mol Physiol 283: L76–L85, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Rubtsova MP, Sizova DV, Dmitriev SE, Ivanov DS, Prassolov VS, Shatsky IN. Distinctive properties of the 5′-untranslated region of human hsp70 mRNA. J Biol Chem 278: 22350–22356, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Sella O, Gerlitz G, Le SY, Elroy-Stein O. Differentiation-induced internal translation of c-sis mRNA: analysis of the cis elements and their differentiation-linked binding to the hnRNP C protein. Mol Cell Biol 19: 5429–5440, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sonenberg N, Dever T. Eukaryotic translation initiation factors and regulators. Curr Opin Struct Biol 13: 56–63, 2003. [DOI] [PubMed] [Google Scholar]
- 67.Stoneley M, Chappell SA, Jopling CL, Dickens M, MacFarlane M, Willis AE. c-Myc protein synthesis is initiated from the internal ribosome entry segment during apoptosis. Mol Cell Biol 20: 1162–1169, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Su WY, Gordon T. Alterations in surfactant protein A after acute exposure to ozone. J Appl Physiol 80: 1560–1567, 1996. [DOI] [PubMed] [Google Scholar]
- 69.Sugahara K, Iyama K, Sano K, Kuroki Y, Akino T, Matsumoto M. Overexpression of surfactant protein SP-A, SP-B, and SP-C mRNA in rat lungs with lipopolysaccharide-induced injury. Lab Invest 74: 209–220, 1996. [PubMed] [Google Scholar]
- 70.Tagaram HR, Wang G, Umstead TM, Mikerov AN, Thomas NJ, Graff GR, Hess JC, Thomassen MJ, Kavuru MS, Phelps DS, Floros J. Characterization of a human surfactant protein A1 (SP-A1) gene-specific antibody; SP-A1 content variation among individuals of varying age and pulmonary health. Am J Physiol Lung Cell Mol Physiol 292: L1052–L1063, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Voss T, Melchers K, Scheirle G, Schafer KP. Structural comparison of recombinant pulmonary surfactant protein SP-A derived from two human coding sequences: implications for the chain composition of natural human SP-A. Am J Respir Cell Mol Biol 4: 88–94, 1991. [DOI] [PubMed] [Google Scholar]
- 72.Wang G, Bates-Kenney SR, Tao JQ, Phelps DS, Floros J. Differences in biochemical properties and in biological function between human SP-A1 and SP-A2 variants, and the impact of ozone-induced oxidation. Biochemistry 43: 4227–4239, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Wang G, Guo X, Floros J. Differences in the translation efficiency and mRNA stability mediated by 5′-UTR splice variants of human SP-A1 and SP-A2 genes. Am J Physiol Lung Cell Mol Physiol 289: L497–L508, 2005. [DOI] [PubMed] [Google Scholar]
- 74.Wang G, Myers C, Mikerov A, Floros J. Effect of cysteine 85 on biochemical properties and biological function of human surfactant protein A variants. Biochemistry 46: 8425–8435, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang G, Phelps DS, Umstead TM, Floros J. Human SP-A protein variants derived from one or both genes stimulate TNF-alpha production in the THP-1 cell line. Am J Physiol Lung Cell Mol Physiol 278: L946–L954, 2000. [DOI] [PubMed] [Google Scholar]
- 76.Wang G, Taneva S, Keough KM, Floros J. Differential effects of human SP-A1 and SP-A2 variants on phospholipid monolayers containing surfactant protein B. Biochim Biophys Acta 1768: 2060–2069, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang G, Umstead TM, Phelps DS, Al-Mondhiry H, Floros J. The effect of ozone exposure on the ability of human surfactant protein A variants to stimulate cytokine production. Environ Health Perspect 110: 79–84, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wright JR Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5: 58–68, 2005. [DOI] [PubMed] [Google Scholar]
- 79.Young RM, Wang SJ, Gordan JD, Ji X, Liebhaber SA, Simon MC. Hypoxia-mediated selective mRNA translation by an internal ribosome entry site-independent mechanism. J Biol Chem 283: 16309–16319, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zuker M Calculating nucleic acid secondary structure. Curr Opin Struct Biol 10: 303–310, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Zuker M Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31: 3406–3415, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zuker M, Jacobson AB. Using reliability information to annotate RNA secondary structures. RNA 4: 669–679, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]