Abstract
Reactive oxygen species (ROS) produced during mitochondrial activity participate in the regulation of intracellular signaling pathways. However, there is only limited information concerning the role that ROS derived from the mitochondrial respiratory chain play in modulating neutrophil activity and participation in acute inflammatory processes. Because mitochondrial complex III is a major site of ROS formation, we examined whether selective complex III inhibition, through exposure of neutrophils to myxothiazol or antimycin A, would affect LPS-induced activation. Culture of neutrophils with antimycin A or myxothiazol resulted in increased intracellular levels of ROS, including superoxide and hydrogen peroxide (H2O2). Inhibition of complex III activity reduced LPS-induced degradation of IκB-α, nuclear accumulation of NF-κB, and proinflammatory cytokine production. The effects of antimycin A or myxothiazol appeared to be dependent on generation of H2O2 since addition of pegylated catalase to neutrophils restored LPS-mediated IκB-α degradation and production of proinflammatory cytokines. Administration of myxothiazol to mice resulted in diminished mitochondrial complex III activity in the lungs and decreased severity of LPS-induced lung injury. These results indicate that inhibition of mitochondrial complex III diminishes Toll-like receptor 4-induced neutrophil activation through a mechanism dependent on H2O2 generation and also reduces the severity of lung injury due to LPS exposure, a pathophysiologic process in which neutrophils play a major role.
Keywords: mitochondria, reactive oxygen species, NF-κB
acute inflammatory processes are associated with enhanced production of reactive oxygen species (ROS) from local cell populations as well as from neutrophils that accumulate in the site of inflammation. Although activated neutrophils produce large quantities of ROS which can then affect adjacent cells, there is only limited information concerning the ability of ROS to modulate the proinflammatory properties of neutrophils. Recent studies have indicated that specific ROS have distinct effects on neutrophil activation. For example, hydrogen peroxide (H2O2), whether derived from extracellular sources or from mitochondria, appears to have anti-inflammatory properties and is able to decrease proinflammatory cytokine production by Toll-like receptor 4 (TLR4)-stimulated neutrophils (37, 53). In contrast, increased intracellular or extracellular levels of superoxide (O2•−) result in increased activation of NF-κB and enhanced production of cytokines in otherwise unstimulated neutrophils as well as in neutrophils stimulated by exposure to LPS (27, 29).
Mitochondria play an essential role in cellular viability and metabolism (20). Within the mitochondrial inner membrane, the transfer of electrons through complexes I to IV generates an electrochemical gradient that is utilized by complex V for ATP production. Under baseline conditions, ∼1–5% of electrons escape from the mitochondrial transport chain to form O2•−. However, this process is enhanced in the presence of mitochondrial electron transport chain inhibitors (24, 25). Studies with isolated mitochondria or in cell culture models demonstrated that inhibition of ubiquinone cytochrome c reductase (complex III) by inactivation of the Q-cycle (Qi site) with the specific agent antimycin A increased ROS production (11, 13, 19). Inhibition of the Q0 site by myxothiazol results in increased reductive capacity upstream of complex III and concomitant inhibition of complex III activity, thereby inducing ROS formation from complexes I and II (11, 36, 47).
A mitochondrial network was recently described to be present in neutrophils (14). Initial studies indicated that modulation of mitochondrial membrane potential influences neutrophil chemotaxis (14). More recently, we demonstrated that inhibition of mitochondrial respiratory complex I in LPS-stimulated neutrophils resulted in enhanced mitochondrial ROS generation, elevated intracellular concentrations of H2O2, and diminished production of cytokines, such as TNF-α (51). However, it is clear that H2O2 does not only have to come from complex I to be inhibitory for neutrophil activation since exposure of neutrophils to extracellular H2O2 also results in suppression of LPS-induced activation of NF-κB and production of cytokines (37, 53). Therefore, modulation of other potential intracellular sources of H2O2, such as mitochondrial complex III, may also affect neutrophil activation. To explore this hypothesis, we examined the effects of mitochondrial complex III inhibition on TLR4-associated neutrophil activation and on the severity of LPS-induced acute lung injury, a pathophysiologic process in which neutrophils play a major role (1, 2, 8).
METHODS
Mice.
Male C57BL/6 or C57BL/6J-Ncf1m1J/J mice, 8–12 wk of age, were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were kept on a 12:12-h light-dark cycle with free access to food and water. All experiments were conducted in accordance with institutional review board-approved protocols.
Reagents.
Escherichia coli 0111:B4 endotoxin (LPS), H2O2, dihydroethidium (DHE), cytochrome c, rotenone, succinate, pegylated catalase, thenoyltrifluoroacetone (TTFA), myxothiazol, and antimycin A were obtained from Sigma (St. Louis, MO). 2′,7′-Dichlorodihydrofluorescein (DCFH-DA) was purchased from Invitrogen (Carlsbad, CA). Cytokine ELISA kits were obtained from R&D Systems (Minneapolis, MN).
Neutrophil isolation and culture.
Bone marrow neutrophils were isolated as described previously (37, 48). Briefly, bone marrow cell suspensions were isolated from the femur and tibia of a mouse by flushing with RPMI 1640 medium with 5% FBS. The cell suspension was passed through a glass wool column and collected by subsequent washing with PBS containing 5% FBS. Negative selection to purify neutrophils was performed by incubation of the cell suspension with biotinylated primary antibodies specific for the cell surface markers F4/80, CD4, CD45R, CD5, and TER119 (StemCell Technologies, Vancouver, BC, Canada, www.stemcell.com/technical/13309-PIS.pdf) for 15 min at 4°C followed by subsequent incubation with anti-biotin tetrameric antibody (100 μl; StemCell Technologies) for 15 min. The complex of anti-tetrameric antibodies and cells was then incubated with colloidal magnetic dextran iron particles (60 μl; StemCell Technologies) for an additional 15 min at 4°C. The T cells, B cells, RBC, monocytes, and macrophages were captured in a column surrounded by a magnet, allowing the neutrophils to pass through. Neutrophil purity, as determined by Wright-Giemsa-stained cytospin preparations, was consistently greater than 97%. The purified neutrophils were cultured in RPMI 1640 medium containing FBS (0.5%) and treated as described in the figure legends. The percentage of cells that were viable and not apoptotic or necrotic, as determined by flow cytometry after staining with annexin V FITC and propidium iodide, was consistently greater than 95%.
Alveolar macrophage isolation and culture.
Alveolar macrophages were isolated from the lungs of C57BL/6 mice by cannulating the trachea with a blunt 20-gauge needle and then lavaging the lungs five times with 1 ml of iced PBS, EDTA (5 mM). Macrophage purity, as determined by Wright-Giemsa-stained cytospin preparations, was consistently greater than 95%. Cells were cultured in RPMI 1640 medium containing FBS (0.5%) and treated as described in the figure legends.
Assays of respiratory complex III and citrate synthase activities.
Respiratory complex III activity in mitochondria-enriched fractions obtained from mouse lungs was measured spectrophotometrically (UV-2501PC Shimadzu; Shimadzu, Japan) and corrected by subtraction of the antimycin A-insensitive rates (5). Briefly, mitochondria-enriched fractions from lungs were lysed in potassium/phosphate buffer by 10 repeated freeze-thaw cycles using liquid nitrogen. Complex III activity was then determined in potassium/phosphate buffer (25 mM, pH 7.2) containing succinate (5 mM), rotenone (5 μM), KCN (1 mM), cytochrome c (50 μM), and equal amounts of protein (20–100 μg) by following complex III-sensitive cytochrome c reduction (λ = 550 nm). Data were acquired every 10 s for 5–7 min after initiation of the reaction, including 5 min after the addition of antimycin A (10 μg/ml). Samples were also incubated in the presence of TTFA (50 μM), applied 5 min after the initiation of cytochrome c reduction. Citrate synthase was measured by using the coupled reaction among oxaloacetate, acetyl-CoA, and 5,5′-dithiobis(2,4-nitrobenzoic acid) (34, 51).
Imaging of DCF and DHE fluorescence.
Intracellular levels of H2O2 or O2•− were determined using the redox-sensitive probes DCFH-DA or DHE in conjunction with fluorescent microscopy (44, 52). Briefly, neutrophils (1.5 × 106/well) were incubated in a four-well chambered coverglass (Nalge, Naperville, IL) and treated with antimycin A or myxothiazol for 60 min. Next, cells cultured in the presence or absence of inhibitors were incubated with DCFH-DA (10 μM) or DHE (10 μM) for an additional 30 min and fluorescent microscopic images were acquired using double bidirectional scans of live neutrophils with a Leica DMIRBE inverted epifluorescence/Nomarski microscope outfitted with Leica TCS NT laser confocal optics. The pinhole setting was 0.2 Airy units and laser excitation was set for 5% to avoid dye photo-oxidation. The levels of fluorescence were averaged using SimplePCI software (Compix, Cranberry Township, PA). Images were processed using IPLab Spectrum and Adobe Photoshop (Adobe Systems, San Jose, CA) software.
Isolation of mitochondria.
Lung mitochondria were isolated according to standard procedures with minor modifications (16, 51). Briefly, liver or lung homogenates were incubated in STE buffer containing sucrose (250 mM), Tris pH 7.4 (10 mM), and EGTA (2 mM), followed by centrifugation (1,000 g) for 3 min at 4°C. Supernatants were then collected and centrifuged (10,000 g) for 10 min at 4°C. The resultant pellets were suspended in STE buffer and centrifuged (10,000 g) for 10 min at 4°C. The pink and light brown pellets surrounding the dark brown center were aspirated and the remaining pellet was suspended in STE buffer (1 ml) and centrifuged (10,000 g) for 15 min at 4°C. Finally, the cell pellet was suspended in potassium/phosphate buffer and stored at −80°C.
Purification of nuclear proteins.
Nuclear proteins were purified from 7 × 106 neutrophils lysed in 100 μl of buffer containing Tris, pH 7.5 (10 mM), NaCl (10 mM), MgCl2 (3 mM), NP 40 (0.02%), EGTA (1 mM), sodium orthovanadate (1 mM), sodium fluoride (50 mM), and the protease inhibitors phenylmethylsulphonyl fluoride (100 μm), leupeptin (10 μg/ml), aprotinin (10 μg/ml), pepstatin A (5 μg/ml), and okadaic acid (1 nm). The lysed cells were centrifuged (2,700 g) for 10 min at 4°C. The supernatant containing the cytosol was collected and then centrifuged (20,800 g) for 15 min at 4°C to obtain the cytosolic fraction. The pellet containing nuclei was washed three times by gentle resuspension in 150 μl wash buffer [PIPES pH 6.8 (10 mm), sucrose (300 mm), MgCl2 (3 mm), EGTA (1 mm), NaCl (25 mm), sodium orthovanadate (1 mm), sodium fluoride (50 mm), and protease inhibitors] and centrifuged (2,700 g) for 5 min at 4°C. Nuclear proteins were obtained using nuclear extraction buffer [Tris, pH 7.4 (50 mM), NaCl (150 mM), NP-40 (0.5% vol/vol), EDTA (1 mM), EGTA (1 mM), okadaic acid (1 nM), and protease inhibitors]. The nuclear lysates were then sonicated and centrifuged (10,000 g) for 15 min at 4°C. The protein concentration of the supernatant was determined using Bradford reagent (Bio-Rad, Hercules, CA) with BSA as a standard.
Electrophoretic mobility shift assay.
Nuclear extracts were obtained from bone marrow neutrophils and electrophoretic mobility shift assays were performed as reported previously (33, 40, 46). In brief, the κB DNA sequence of the Ig gene was used. Synthetic double-strand sequences were filled in and labeled with [32P]dATP (GE Healthcare) using Sequenase DNA polymerase: B sequence, 5′-GCCATGGGGGGATCCCCGAAGTCC-3′ (Geneka Biotechnology).
Western blot analysis.
Briefly, neutrophils (3.5 × 106/well) or macrophages (2 × 105/well) were lysed using buffer containing Tris, pH 7.4 (50 mM), NaCl (150 mM), NP-40 (0.5% vol/vol), EDTA (1 mM), EGTA (1 mM), okadaic acid (1 nM), and protease inhibitors. The cell lysates were sonicated and centrifuged at 10,000 g for 15 min at 4°C. Protein concentration in the supernatants was determined using the Bradford reagent (Bio-Rad) with BSA as a standard. Samples were mixed with Laemmli sample buffer and boiled for 5 min. Equal amounts of proteins were resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred onto PVDF membranes (Immobilon P, Millipore, Billerica, MA). The membranes were probed with specific antibodies to total IκB-α (Cell Signaling, Beverly, MA) or actin (Sigma), followed by detection with horseradish peroxidase-conjugated goat anti-rabbit IgG. Bands were visualized by enhanced chemiluminescence (SuperSignal; Pierce Biotechnology, Rockford, IL) and quantified by AlphaEaseFC Software (Alpha Innotech, San Leandro, CA). Each experiment was carried out two or more times using cell populations obtained from separate groups of mice.
In vivo acute lung injury model.
To induce acute lung injury, E coli 0111:B4 LPS (1 mg/kg) in PBS was administered into the oropharynx as previously performed (15, 50, 51). Briefly, mice were anesthetized with isoflurane, the tongue was gently extended, and LPS in PBS (50 μl) was instilled into the distal part of the oropharynx.
In pretreatment experiments, mice were given myxothiazol (1 mg/kg ip) in saline/DMSO (1:10) 4 h before intratracheal LPS administration (1 mg/kg it). The selected dosage of myxothiazol was below the reported LD50 in mice (17) and no deaths were associated with myxothiazol or myxothiazol and LPS administration.
Harvest of lungs and bronchoalveolar lavage.
Lungs were harvested 24 h after LPS administration. Bronchoalveolar lavage (BAL) was obtained by cannulating the trachea with a blunt 20-gauge needle and then lavaging the lungs three times with 1 ml of iced PBS.
Wet-to-dry lung weight ratios.
The wet-to-dry ratio was determined as reported previously (2, 50, 51). Separate groups of mice were used to measure wet-to-dry ratios and for BAL fluid acquisition. All mice used for lung wet-to-dry weight ratios were of identical ages. Lungs were excised, rinsed briefly in PBS, blotted, and then weighed to obtain the “wet” weight. Lungs were then dried in an oven at 80°C for 7 days to obtain the “dry” weight.
Myeloperoxidase assay.
Myeloperoxidase (MPO) activity was assayed as reported previously with minor modifications (42). In brief, lung tissue was homogenized in 1 ml of potassium phosphate buffer, pH 6.0 (50 mM), containing a reducing agent, N-ethylmaleimide (10 mM), for 30 s on ice. The homogenate was centrifuged (12,000 g) for 30 min at 4°C and washed twice in ice-cold buffer. The pellet was then resuspended and sonicated on ice for 90 s in 10× volume of hexadecyltrimethylammonium bromide (HTAB) buffer [HTAB (0.5%), potassium phosphate, pH 6.0 (50 mM)]. Samples were incubated in a water bath for 2 h at 56°C and then centrifuged (12,000 g) for 10 min. The supernatant was collected for assay of MPO activity as determined by measuring the H2O2-dependent oxidation of 3,3′-dimethoxybenzidine dihydrochloride (λ = 460 nm).
Cytokine ELISA.
ELISA were used to measure cytokines in lung homogenates or in culture media from LPS-stimulated neutrophils. Levels of TNF-α, MIP-2, IL-6, or KC were determined using commercially available ELISA kits (R&D Systems), according to the manufacturer's instructions and as previously described (37, 48).
Statistical analyses.
For each experiment, neutrophils were isolated and pooled from groups of mice (n = 3–6) and all conditions were studied at the same time. One-way ANOVA, the Tukey-Kramer Multiple Comparisons test (for multiple groups), or Student's t-test (for comparisons between 2 groups) were used. P < 0.05 was considered to be statistically significant.
RESULTS
Inhibition of mitochondrial complex III enhances ROS generation in neutrophils.
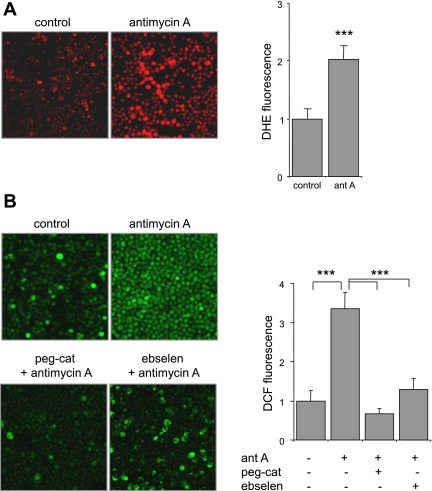
To examine the role of mitochondrial complex III as a source of ROS in neutrophils, we used the specific complex III inhibitor antimycin A. As shown in Fig. 1, neutrophils treated with antimycin A demonstrated significant increases in DHE (∼2-fold) and DCFH (∼3-fold) oxidation compared with untreated neutrophils. Although DHE is a selective probe for detection of intracellular O2•−, DCFH is not specific as it is oxidized in the presence of peroxynitrite or hydroxyl radical (43, 45). Therefore, to confirm the role of complex III in modulating intracellular ROS release, and specifically levels of H2O2, pegylated catalase was added to antimycin A-treated neutrophils. Inclusion of pegylated catalase into the neutrophil cultures effectively diminished antimycin A-dependent increases in DCF fluorescence (Fig. 1B). These results indicate that both O2•− and H2O2 are generated in antimycin A-treated neutrophils. Of note, in a manner similar to pegylated catalase, the glutathione peroxide mimetic ebselen decreased levels of DCFH oxidation induced by antimycin A (Fig. 1B).
Fig. 1.
Antimycin A induces dihydroethidium (DHE) and dichlorodihydrofluorescein (DCFH) oxidation in neutrophils. Representative images and quantitative data show DHE (A) or DCFH (B) fluorescence in neutrophils. Murine bone marrow neutrophils were treated with antimycin A (0 or 10 μg/ml) for 60 min, and then the cells were loaded with DHE (10 μM) or DCFH (10 μM) for 30 min and images were acquired using a laser-scanning confocal microscope. Pegylated catalase (peg-cat; 0 or 125 U/ml) or ebselen (0 or 100 μM) was included in the culture for 90 min before addition of antimycin A as indicated. Mean DHE or DCF fluorescence was obtained from 3 or more randomly chosen fields of neutrophils and expressed as pixel intensity/cell (means ± SD; ***P < 0.001).
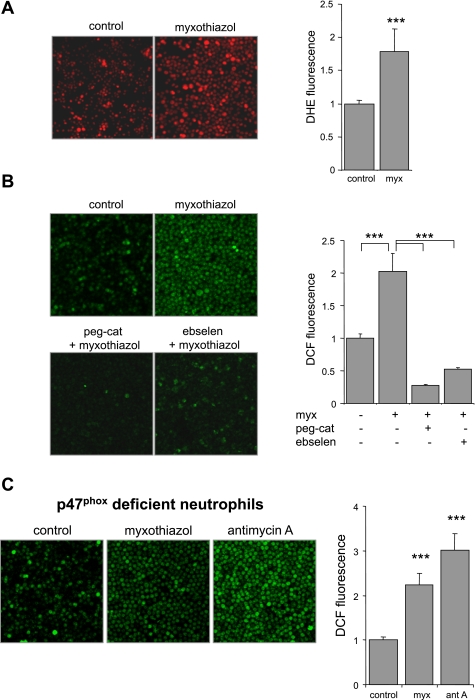
Exposure of neutrophils to a second complex III inhibitor, myxothiazol, also resulted in increase in ROS formation, as detected by DHE and DCFH oxidation (Fig. 2, A and B). As was the case with antimycin A, addition of pegylated catalase or ebselen to the neutrophils effectively diminished myxothiazol-induced ROS generation.
Fig. 2.
Effects of myxothiazol on DHE or DCFH oxidation in neutrophils. A and B: representative images and average of DHE or DCF fluorescence in neutrophils incubated with myxothiazol (0 or 10 μM) for 60 min. Neutrophils were incubated with probes for 30 min after being treated with myxothiazol (10 μM) for 60 min. In the indicated experiments, peg-cat (0 or 125 U/ml) or ebselen (0 or 100 μM) was included in the cultures for 90 min before addition of myxothiazol. Quantitative data are shown as fold change of averaged pixel intensity in untreated cells (means ± SD; ***P < 0.001 for control compared with myxothiazol or for myxothiazol alone compared with peg-cat plus myxothiazol or ebselen plus myxothiazol-treated neutrophils). C: myxothiazol or antimycin A induces DCFH oxidation in NADPH oxidase-inactive neutrophils. Representative images and quantitative data show DCF fluorescence in bone marrow neutrophils isolated from C57BL/6J-Ncf1m1J/J mice. Cells were either left untreated or were cultured with myxothiazol (10 μM) or antimycin A (10 μg/ml) for 60 min and then incubated with DCFH for an additional 30 min followed by image acquisition (n = 4, means ± SD; ***P < 0.001).
As shown in Fig. 2C, myxothiazol and antimycin A significantly increased DCFH oxidation in neutrophils isolated from C57BL/6J-Ncf1m1J/J mice that have inactive NADPH oxidase as a result of lacking the functional p47phox subunit. Although basal levels of DCF fluorescence were lower in C57BL/6J-Ncf1m1J/J neutrophils compared with those from control mice (data not shown), inhibition of complex III resulted in similar fold increases in DCF fluorescence in both populations of neutrophils (Fig. 2C). These experiments indicate that NADPH oxidase is not critical for the enhancement of ROS generation found in myxothiazol- or antimycin A-exposed neutrophils and also suggest that the increased intracellular ROS levels present under such conditions are a direct result of inhibition of mitochondrial complex III.
Effect of mitochondrial complex III inhibitors on LPS-induced neutrophil activation.
Mitochondrially generated reactive oxygen and nitrogen species participate in the regulation of intracellular signal transduction pathways (10, 44, 49, 54), including NF-κB activation (9, 51). Because treatment with antimycin A and myxothiazol resulted in increased intracellular ROS levels, we examined the effects of complex III inhibition in neutrophils on LPS-induced nuclear translocation of NF-κB and cytokine production.
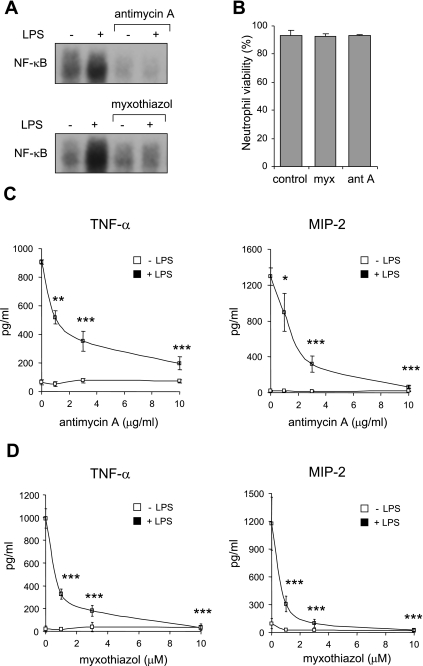
Exposure of LPS-stimulated neutrophils to antimycin A or myxothiazol was associated with diminished nuclear translocation of NF-κB compared with that found in neutrophils cultured with LPS alone (Fig. 3A). Consistent with this finding, antimycin A and myxothiazol dose dependently diminished production of the cytokines TNF-α and MIP-2, whose transcription is primarily regulated by NF-κB activation (18) (Fig. 3, C and D). Addition of antimycin A (10 μg/ml) to nuclear extracts obtained from control or LPS-treated neutrophils had no effect on NF-κB-DNA complex formation (data not shown).
Fig. 3.
Mitochondrial respiratory complex III modulates LPS-induced nuclear translocation of NF-κB and cytokine production by neutrophils. A: representative EMSA shows the effects of complex III inhibitors on LPS-induced nuclear accumulation of NF-κB. Nuclear fractions were obtained from neutrophils treated with antimycin A (0 or 10 μg/ml) or myxothiazol (0 or 10 μM) for 60 min and then stimulated with LPS (0 or 100 ng/ml) for 1 h. Viability of control, myxothiazol (10 μM)-, or antimycin A (10 μg/ml)-treated neutrophils was examined after incubation for 4 h at 37°C. The viability of the cells was determined by flow cytometry after staining with Annexin V-FITC and propidium iodide (PI). Means ± SD, n = 3 independent experiments, P = nonsignificant. C and D: TNF-α or MIP-2 levels were measured in culture supernatants after exposure of neutrophils to antimycin A (0–10 μg/ml) or myxothiazol (0–10 μM) for 1 h followed by LPS (0 or 100 ng/ml) stimulation for 5 h. Means ± SD, n = 3. *P < 0.05, **P < 0.01, or ***P < 0.001 compared with cells treated with LPS alone.
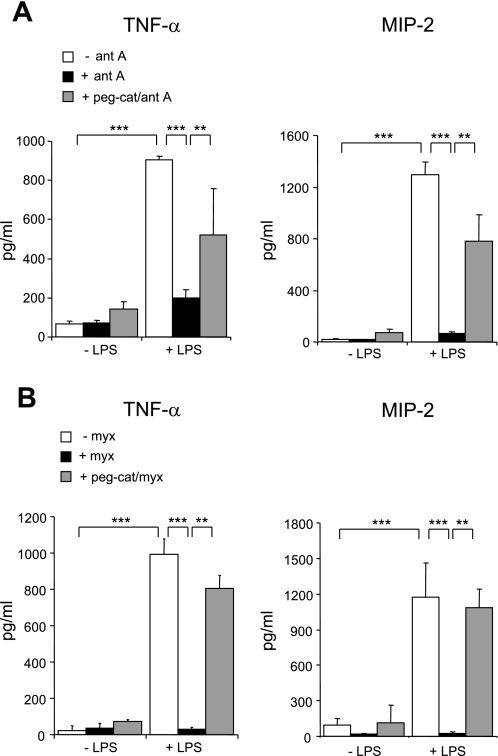
To determine whether the inhibitory effects of antimycin A or myxothiazol on LPS-induced cytokine production were H2O2 dependent, pegylated catalase was included in the cultures. As shown in Fig. 4, A and B, addition of pegylated catalase to LPS-stimulated neutrophils reversed the inhibitory effects of antimycin A or myxothiazol on cytokine production. Coculture of neutrophils with the cell-permeable superoxide dismutase mimetic MnTBAP had no effect on LPS-induced cytokine expression and also had no effect on antimycin A- or myxothiazol-dependent inhibition of LPS-mediated proinflammatory cytokine production (data not shown).
Fig. 4.
Peg-cat restores LPS-induced cytokine production in antimycin A- or myxothiazol-treated neutrophils. Neutrophils were either left untreated or were cultured with peg-cat for 90 min followed by addition of antimycin A (10 μg/ml) or myxothiazol (10 μM) to the cultures for 60 min. The neutrophils were then stimulated with LPS (0 or 100 ng/ml) for 5 h and TNF-α and MIP-2 concentrations were measured in supernatants. Means ± SD, n = 3. ***P < 0.001 compared with cells treated with LPS alone or **P < 0.01 comparing neutrophils cultured with LPS and antimycin A or myxothiazol to those cultured with LPS, antimycin A, or myxothiazol, and peg-cat.
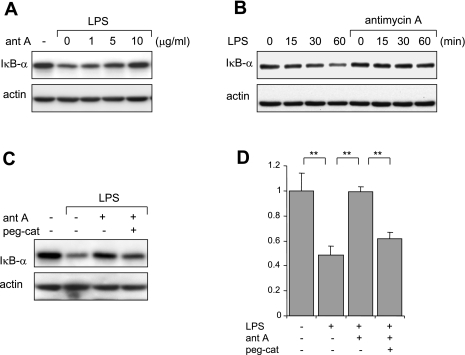
Phosphorylation, ubiquitination, and subsequent degradation of IκB-α occur after TLR4 engagement and lead to nuclear translocation of NF-κB (12). As shown in Fig. 5A, antimycin A dose dependently inhibited LPS-associated IκB-α degradation (Fig. 5B).
Fig. 5.
Effects of antimycin A on LPS-induced IκB-α degradation in neutrophils. Representative Western blots show dose-dependent effects of antimycin A on LPS-induced IκB-α degradation (A) and time-dependent effects of antimycin A on LPS-induced degradation of IκB-α (B). A: neutrophils were either left untreated or were incubated with antimycin A (0–10 μg/ml) for 60 min and then were stimulated with LPS (0 or 100 ng/ml) for an additional 60 min. B: neutrophils were pretreated with antimycin A (0 or 10 mg/ml) for 60 min followed by treatment with LPS (0 or 100 ng/ml) for the indicated time periods. Cell lysates were subjected to SDS-PAGE and probed with IκB-α or actin-specific antibodies. A second experiment provided similar results. C and D: catalase reverses the inhibitory effects of antimycin A on LPS-induced IκB-α degradation. A representative Western blot and average band optical density analysis show levels of IκB-α obtained from neutrophils treated with peg-cat (0 or 125 μg/ml) for 90 min, followed by antimycin A (0 or 10 μg/ml) for 60 min and then LPS (0 or 100 ng/ml) for an additional 60 min. Means ± SD optical densitometry from 3 independent experiments. Data are expressed as IκB-α fold changes by comparing untreated cells to treated (**P < 0.01).
We previously demonstrated that exposure of neutrophils to H2O2, either through addition of H2O2 to the cultures or by using glucose/glucose oxidase to generate H2O2, diminished LPS-induced degradation of IκB-α (37, 53). To determine whether increases in intracellular H2O2 resulting from inhibition of mitochondrial complex III contributed to the suppression of IκB-α degradation in LPS-treated neutrophils, pegylated catalase was included in the cultures. As shown in Fig. 5C, coculture of LPS-stimulated neutrophils with antimycin A and pegylated catalase reversed the inhibitory effects of antimycin A on IκB-α degradation (Fig. 5, C and D).
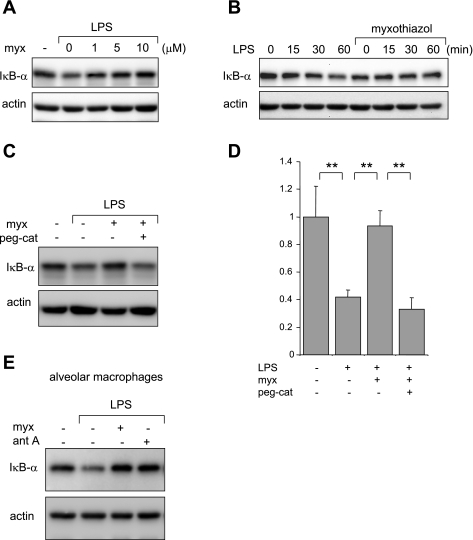
Similar to antimycin A, myxothiazol inhibited IκB-α degradation in LPS-stimulated neutrophils (Fig. 6, A and B). This effect of antimycin A was reversed by the inclusion of pegylated catalase in the cultures (Fig. 6, C and D). The inhibitory effects of myxothiazol on LPS-induced degradation of IκB-α as well as those of antimycin A were not limited to neutrophils, since both agents had similar actions in LPS-stimulated alveolar macrophages (Fig. 6E).
Fig. 6.
Myxothiazol inhibits LPS-induced IκB-α degradation. A representative Western blot shows levels of IκB-α and actin obtained from neutrophils incubated with myxothiazol (0, 1, 3, or 10 μM; A) for 60 min followed by LPS (0 or 1 μg/ml) for 60 min and neutrophils treated with myxothiazol (0 or 10 μM; B) for 60 min followed by LPS (0 or 100 ng/ml) for the indicated time periods. An additional experiment demonstrated similar inhibitory effects of myxothiazol on IκB-α degradation. C and D: representative Western blot analysis and averages of IκB-α band optical density obtained from neutrophils treated with peg-cat (0 or 125 μg/ml) for 90 min, then myxothiazol (0 or 10 μM) for 60 min, and LPS (0 or 100 ng/ml) for an additional 60 min. Data are expressed as IκB-α fold changes of control (n = 3, means ± SD, **P < 0.01 comparing control to treated cells). E: complex III inhibition diminishes LPS-induced IκB-α degradation in alveolar macrophages. Cells were incubated with myxothiazol (0 or 10 μM) or antimycin A (10 μg/ml) for 60 min followed by LPS (0 or 100 ng/ml) for 60 min. Representative blots of IκB-α and actin are shown. A second experiment provided similar results.
Incubation of neutrophils with antimycin A or myxothiazol alone had no effect on nuclear accumulation of NF-κB or expression of TNF-α or MIP-2 (Fig. 3, A, C, and D). In addition, staining with annexin V and propidium iodide showed no changes in neutrophil viability or progression to apoptosis after exposure to antimycin A (10 μg/ml) or myxothiazol (10 μM) for 4 h compared with neutrophils cultured under control conditions (Fig. 3B). Similarly, no decrease in cell viability was found after pretreatment of neutrophils with antimycin A or myxothiazol for 60 min followed by culture with LPS (100 ng/ml) for an additional 4 h (data not shown).
Effects of mitochondrial complex III inhibition on LPS-induced acute lung injury.
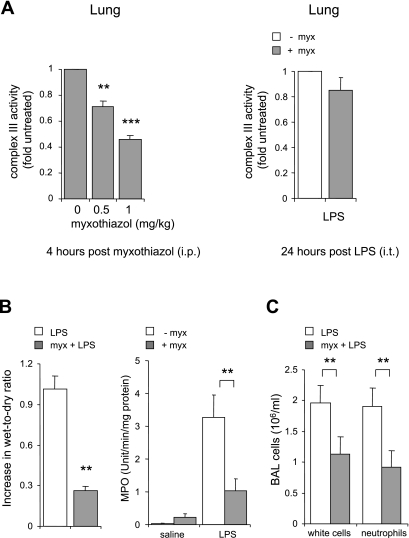
Because inhibitors of complex III diminished TLR4-induced activation of neutrophils in vitro, we hypothesized that this approach would also decrease the severity of LPS-induced acute lung injury, an inflammatory condition in which neutrophils play a major role (1, 2, 8). Significant decreases in complex III activity were found in lung homogenates obtained 4 h after myxothiazol administration (Fig. 7A). These inhibitory effects of myxothiazol on complex III activity appeared to have almost completely dissipated by 24 h after administration (Fig. 7A).
Fig. 7.
Effects of myxothiazol on LPS-induced acute lung injury. A: mitochondrial complex III activity was measured in mitochondria-enriched fractions obtained from the lungs of mice given myxothiazol (0, 0.5, or 1 mg/kg ip) 4 h previously or myxothiazol (0 or 1 mg/kg ip) 24 h previously (n = 4 mice, means ± SE; **P < 0.01 and ***P < 0.001 compared with control, i.e., animals not given myxothiazol). B: myxothiazol (1 mg/kg) was administered ip in saline or saline alone was administered ip and then 4 h later the mice were given LPS (1 mg/kg it). Lungs were harvested and bronchoalveolar lavages (BAL) were obtained 24 h post-LPS administration. The fold increase in lung wet-to-dry ratios was compared with that in mice given saline alone. Lung homogenate myeloperoxidase concentrations (MPO) and total cell as well as neutrophil numbers in BAL fluid are shown (n = 6 mice per group; C).
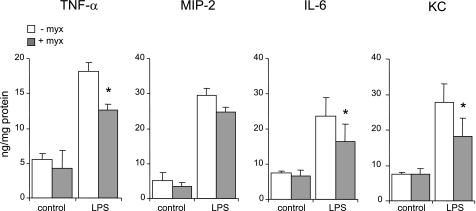
As shown in Fig. 7, B and C, mice treated with myxothiazol before LPS administration demonstrated diminished interstitial edema (as measured by wet-to-dry ratio) as well as decreased neutrophil accumulation into the pulmonary interstitium and airspaces (as determined by lung homogenate MPO and neutrophil numbers in BAL fluid) compared with mice given LPS alone. LPS-induced levels of proinflammatory cytokines and chemokines, including TNF-α, KC, and IL-6, in lung homogenates were significantly diminished, compared with controls, in mice treated with myxothiazol (Fig. 8).
Fig. 8.
Effects of myxothiazol on LPS-induced pulmonary cytokine production in vivo. Mice were given myxothiazol (1 mg/kg ip) in saline or saline alone followed 4 h later by LPS (0 or 1 mg/kg it). Lung homogenates for cytokine measurement were obtained 24 h after LPS administration; n = 5 mice for each condition. Means ± SE are shown. *P < 0.05 comparing LPS-treated mice given saline alone with mice given myxothiazol.
In mice not exposed to LPS, there were no significant differences between those given myxothiazol in saline or saline alone in terms of wet-to-dry ratio, lung MPO, or BAL cytokine levels (Figs. 7, B and C, and 8). There were no noticeable adverse events associated with myxothiazol administration.
DISCUSSION
In the present studies, we found that inhibition of mitochondrial complex III with antimycin A or myxothiazol resulted in increased intracellular levels of ROS, including H2O2, diminished activation of LPS-stimulated neutrophils, and reduced severity of LPS-induced acute lung injury in mice. Previous studies with isolated mitochondria, cells in culture, or in mice showed that antimycin A and myxothiazol are specific and potent inhibitors of mitochondrial complex III (11, 13, 19). In particular, antimycin A blocks electron transfer at the Qi site at a step necessary for semiquinone radical reduction to quinol. Such inhibition of specific Q cycle-related events leads to subsequent semiquinone radical accumulation and O2•− formation within the mitochondria (11), as confirmed in these studies in antimycin A-treated and DHE-loaded neutrophils. Myxothiazol inhibits mitochondrial complex III through its activity at the F0 site bc1, a mechanism distinct from that of antimycin A (6, 39, 41).
Using DCFH as a probe of oxidative activity, we found an increase in ROS formation in antimycin A- and in myxothiazol-treated neutrophils. Although DCFH oxidation is not a selective measure of the presence of H2O2, both antimycin A- and myxothiazol-associated increases in DCF fluorescence diminished when neutrophils were cultured with the glutathione peroxidase mimetic ebselen or the more specific H2O2 scavenger pegylated catalase. The findings from our studies therefore indicate that inhibition of mitochondrial complex III in neutrophils results in enhanced production of both O2•− and H2O2, similar to previously reported data in other cell populations (38, 47).
The role of mitochondria in the regulation of neutrophil proinflammatory functions is not well characterized. Although decrease of mitochondrial membrane potential was recently shown to result in alteration of cell shape and chemotaxis (14), treatment of neutrophils with carbonyl cyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP), an uncoupler of mitochondrial respiration that results in inhibition of mitochondrial ATP synthesis, had no effect on LPS-induced IκB-α degradation or NF-κB-dependent cytokine production (51). In contrast to FCCP, inhibition of mitochondrial complex I with rotenone (51) or of complex III with antimycin A or myxothiazol, as undertaken in these experiments, induced increases in intracellular levels of H2O2 that were associated with inhibition of activation of NF-κB and reduced production of proinflammatory cytokines in LPS-stimulated neutrophils. The central role that mitochondrially derived H2O2 occupies in diminishing LPS-induced inflammatory responses in neutrophils was shown by the reversal of the anti-inflammatory effects of antimycin A and myxothiazol by pegylated catalase. Pegylated catalase, unlike native catalase, is cell permeable and accumulates intracellularly (3, 38).
Although a number of studies found that oxidants, and particularly H2O2, can enhance NF-κB activation, these findings were primarily obtained in macrophages (23, 28) and have not been a universally observed effect (7, 30, 31). In several cell populations, including neutrophils, increased intracellular levels of H2O2 appear to inhibit inflammatory responses (21, 22, 32). We recently demonstrated that inhibition of mitochondrial complex I by metformin in LPS-stimulated neutrophils resulted in increased intracellular concentrations of H2O2, stabilization of IκB-α, diminished nuclear translocation of NF-κB, reduced production of proinflammatory cytokines, and decreased severity of LPS-induced acute lung injury (51). Metformin was also previously shown to prevent liver damage in a model of postsurgical sepsis (4). In the present study, inhibition of mitochondrial complex III produced similar inhibitory effects in LPS-stimulated neutrophils. Of note, the decrease in proinflammatory cytokine production and degradation of IκB-α that were present in LPS-stimulated neutrophils coincubated with antimycin A or myxothiazol were reversed by inclusion of pegylated catalase in the cultures, consistent with the anti-inflammatory effects of complex III inhibition being directly due to increased intracellular concentrations of H2O2. These results are concordant with previous studies showing that H2O2 has potent anti-inflammatory effects in LPS-stimulated neutrophils through mechanisms involving inhibition of 26S proteasome activity that result in stabilization of cytoplasmic concentrations of IκB-α and prevention of nuclear translocation of NF-κB (53).
Administration of myxothiazol to mice resulted in significantly decreased mitochondrial complex III activity in the lungs and reduced severity of LPS-induced acute lung injury. Of note, the inhibition of complex III activity produced by myxothiazol at 4 h after LPS administration was no longer significant at the 24-h time point when parameters of lung injury were assessed. These findings suggest that inhibition of mitochondrial complex III at early stages after TLR4 engagement as well as associated increases in intracellular concentrations of H2O2 resulting from complex III inhibition have prolonged effects on inflammatory responses of neutrophils and resident pulmonary cell populations that translate into reduced severity of acute lung injury. An important question for future studies is whether treatment with mitochondrial complex III inhibitors at later points in the septic process, when neutrophils and other cellular populations are activated to produce proinflammatory mediators and mitochondrial dysfunction is present (26, 35), will also be effective in diminishing injury to the lungs and other organs.
The present results, showing that inhibition of mitochondrial complex III reduced TLR4-induced neutrophil activation and lung injury, coupled with our previous study (51), which found similar effects with inhibition of mitochondrial complex I, highlight a novel anti-inflammatory role of mitochondria. In particular, enhanced production of mitochondrially derived H2O2 appears to diminish neutrophil activation and lung injury produced by TLR4 engagement. These studies also suggest that antioxidant approaches to acute inflammatory conditions in which neutrophils play a major role should be carefully considered. In particular, therapies that increase intracellular concentrations of H2O2, while minimizing those of O2•−, would be predicted to have greater anti-inflammatory properties than more general antioxidant agents.
GRANTS
This work was supported in part by National Institutes of Health Grants HL-62221, HL-76206, and HL-068743 to E. Abraham and by the Société Française d'Anesthésie et de Réanimation and the University Hospital of Amiens (France) to E. Lorne.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abraham E Neutrophils and acute lung injury. Crit Care Med 31: S195–S199, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 279: L1137–L1145, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Beckman JS, Minor RL Jr, White CW, Repine JE, Rosen GM, Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem 263: 6884–6892, 1988. [PubMed] [Google Scholar]
- 4.Bergheim I, Luyendyk JP, Steele C, Russell GK, Guo L, Roth RA, Arteel GE. Metformin prevents endotoxin-induced liver injury after partial hepatectomy. J Pharmacol Exp Ther 316: 1053–1061, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Birch-Machin MA, Turnbull DM. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol 65: 97–117, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Bolgunas S, Clark DA, Hanna WS, Mauvais PA, Pember SO. Potent inhibitors of the Qi site of the mitochondrial respiration complex III. J Med Chem 49: 4762–4766, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Brennan P, O'Neill LA. Effects of oxidants and antioxidants on nuclear factor kappa B activation in three different cell lines: evidence against a universal hypothesis involving oxygen radicals. Biochim Biophys Acta 1260: 167–175, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet 368: 157–169, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol 165: 1013–1021, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Chen K, Thomas SR, Albano A, Murphy MP, Keaney JF Jr. Mitochondrial function is required for hydrogen peroxide-induced growth factor receptor transactivation and downstream signaling. J Biol Chem 279: 35079–35086, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278: 36027–36031, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZJ Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol 7: 758–765, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crofts AR The cytochrome bc1 complex: function in the context of structure. Annu Rev Physiol 66: 689–733, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Fossati G, Moulding DA, Spiller DG, Moots RJ, White MR, Edwards SW. The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J Immunol 170: 1964–1972, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Foster WM, Walters DM, Longphre M, Macri K, Miller LM. Methodology for the measurement of mucociliary function in the mouse by scintigraphy. J Appl Physiol 90: 1111–1117, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2: 287–295, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Gerth K, Irschik H, Reichenbach H, Trowitzsch W. Myxothiazol, an antibiotic from Myxococcus fulvus (myxobacterales). I. Cultivation, isolation, physico-chemical and biological properties. J Antibiot (Tokyo) 33: 1474–1479, 1980. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 109, Suppl: S81–S96, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Gille L, Nohl H. The ubiquinol/bc1 redox couple regulates mitochondrial oxygen radical formation. Arch Biochem Biophys 388: 34–38, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Green DR, Reed JC. Mitochondria and apoptosis. Science 281: 1309–1312, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Jaspers I, Zhang W, Fraser A, Samet JM, Reed W. Hydrogen peroxide has opposing effects on IKK activity and IkappaBalpha breakdown in airway epithelial cells. Am J Respir Cell Mol Biol 24: 769–777, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Kanayama A, Inoue J, Sugita-Konishi Y, Shimizu M, Miyamoto Y. Oxidation of Ikappa Balpha at methionine 45 is one cause of taurine chloramine-induced inhibition of NF-kappa B activation. J Biol Chem 277: 24049–24056, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kaul N, Forman HJ. Activation of NF kappa B by the respiratory burst of macrophages. Free Radic Biol Med 21: 401–405, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J Biol Chem 279: 39414–39420, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Lenaz G, Fato R, Genova ML, Bergamini C, Bianchi C, Biondi A. Mitochondrial complex I: structural and functional aspects. Biochim Biophys Acta 1757: 1406–1420, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Levy RJ Mitochondrial dysfunction, bioenergetic impairment, and metabolic downregulation in sepsis. Shock 28: 24–28, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Lorne E, Zmijewski JW, Zhao X, Liu G, Tsuruta Y, Park YJ, Dupont H, Abraham E. Role of extracellular superoxide in neutrophil activation: interactions between xanthine oxidase and TLR4 induce proinflammatory cytokine production. Am J Physiol Cell Physiol 294: C985–C993, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, Wahl LM. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-kappa B activity in lipopolysaccharide-activated human primary monocytes. J Immunol 175: 5423–5429, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Mitra S, Abraham E. Participation of superoxide in neutrophil activation and cytokine production. Biochim Biophys Acta 1762: 732–741, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal 8: 1791–1806, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal 7: 42–59, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci USA 103: 13086–13091, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shenkar R, Schwartz MD, Terada LS, Repine JE, McCord J, Abraham E. Hemorrhage activates NF-kappa B in murine lung mononuclear cells in vivo. Am J Physiol Lung Cell Mol Physiol 270: L729–L735, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Shepherd D, Garland PB. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J 114: 597–610, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer M Mitochondrial function in sepsis: acute phase versus multiple organ failure. Crit Care Med 35: S441–S448, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Starkov AA, Fiskum G. Myxothiazol induces H2O2 production from mitochondrial respiratory chain. Biochem Biophys Res Commun 281: 645–650, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Strassheim D, Asehnoune K, Park JS, Kim JY, He Q, Richter D, Mitra S, Arcaroli J, Kuhn K, Abraham E. Modulation of bone marrow-derived neutrophil signaling by H2O2: disparate effects on kinases, NF-κB, and cytokine expression. Am J Physiol Cell Physiol 286: C683–C692, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Teoh ML, Sun W, Smith BJ, Oberley LW, Cullen JJ. Modulation of reactive oxygen species in pancreatic cancer. Clin Cancer Res 13: 7441–7450, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Thierbach G, Reichenbach H. Myxothiazol, a new inhibitor of the cytochrome b-c1 segment of th respiratory chain. Biochim Biophys Acta 638: 282–289, 1981. [DOI] [PubMed] [Google Scholar]
- 40.Tsuruta Y, Park YJ, Siegal GP, Liu G, Abraham E. Involvement of vitronectin in lipopolysaccaride-induced acute lung injury. J Immunol 179: 7079–7086, 2007. [DOI] [PubMed] [Google Scholar]
- 41.von Jagow G, Ljungdahl PO, Graf P, Ohnishi T, Trumpower BL. An inhibitor of mitochondrial respiration which binds to cytochrome b and displaces quinone from the iron-sulfur protein of the cytochrome bc1 complex. J Biol Chem 259: 6318–6326, 1984. [PubMed] [Google Scholar]
- 42.Wang XQ, Bdeir K, Yarovoi S, Cines DB, Fang W, Abraham E. Involvement of the urokinase kringle domain in lipopolysaccharide-induced acute lung injury. J Immunol 177: 5550–5557, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Wardman P Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med 43: 995–1022, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe N, Zmijewski JW, Takabe W, Umezu-Goto M, Le Goffe C, Sekine A, Landar A, Watanabe A, Aoki J, Arai H, Kodama T, Murphy MP, Kalyanaraman R, Darley-Usmar VM, Noguchi N. Activation of mitogen-activated protein kinases by lysophosphatidylcholine-induced mitochondrial reactive oxygen species generation in endothelial cells. Am J Pathol 168: 1737–1748, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wrona M, Patel K, Wardman P. Reactivity of 2′,7′-dichlorodihydrofluorescein and dihydrorhodamine 123 and their oxidized forms toward carbonate, nitrogen dioxide, and hydroxyl radicals. Free Radic Biol Med 38: 262–270, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Yang KY, Arcaroli JJ, Abraham E. Early alterations in neutrophil activation are associated with outcome in acute lung injury. Am J Respir Crit Care Med 167: 1567–1574, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Young TA, Cunningham CC, Bailey SM. Reactive oxygen species production by the mitochondrial respiratory chain in isolated rat hepatocytes and liver mitochondria: studies using myxothiazol. Arch Biochem Biophys 405: 65–72, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Yum HK, Arcaroli J, Kupfner J, Shenkar R, Penninger JM, Sasaki T, Yang KY, Park JS, Abraham E. Involvement of phosphoinositide 3-kinases in neutrophil activation and the development of acute lung injury. J Immunol 167: 6601–6608, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292: H2023–H2031, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L497–L504, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, Siegal GP, Abraham E. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am J Respir Crit Care Med 178: 168–179, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zmijewski JW, Moellering DR, Le Goffe C, Landar A, Ramachandran A, Darley-Usmar VM. Oxidized LDL induces mitochondrially associated reactive oxygen/nitrogen species formation in endothelial cells. Am J Physiol Heart Circ Physiol 289: H852–H861, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Zmijewski JW, Zhao X, Xu Z, Abraham E. Exposure to hydrogen peroxide diminishes NF-κB activation, IκB-α degradation, and proteasome activity in neutrophils. Am J Physiol Cell Physiol 293: C255–C266, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WGt Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem 279: 43940–43951, 2004. [DOI] [PubMed] [Google Scholar]