Abstract
Pulmonary hypertension (PH) is a serious disease of multiple etiologies mediated by hypoxia, immune stimuli, and elevated pulmonary pressure that leads to vascular thickening and eventual right heart failure. In a chronic hypoxia model of PH, we previously reported the induction of a novel pleiotropic cytokine, hypoxia-induced mitogenic factor (HIMF), that exhibits mitogenic, vasculogenic, contractile, and chemokine properties during PH-associated vascular remodeling. To examine the role of HIMF in hypoxia-induced vascular remodeling, we performed in vivo knockdown of HIMF using short hairpin RNA directed at rat HIMF in the chronic hypoxia model of PH. Knockdown of HIMF partially blocked increases in mean pulmonary artery pressure, pulmonary vascular resistance, right heart hypertrophy, and vascular remodeling caused by chronic hypoxia. To demonstrate a direct role for HIMF in the mechanism of PH development, we performed HIMF-gene transfer into the lungs of rats using a HIMF-expressing adeno-associated virus (AAV). AAV-HIMF alone caused development of PH similar to that of chronic hypoxia with increased mean pulmonary artery pressure and pulmonary vascular resistance, right heart hypertrophy, and neomuscularization and thickening of small pulmonary arterioles. The findings suggest that HIMF represents a critical cytokine-like growth factor in the development of PH.
Keywords: vascular remodeling, hypoxia, T-helper 2
pulmonary hypertension (PH) is a complex disease with multiple etiologies, including hypoxia, immune stimuli, and elevated pulmonary pressure. It can occur as pulmonary arterial hypertension, without a specific cause, or more commonly, secondary to other disease processes (12, 29). Pulmonary inflammation has been increasingly associated with many forms of PH. Despite the complexity of this disease, both clinical and experimental studies reveal common pathological features, the most important of which is pulmonary vascular remodeling (13, 37). This process involves endothelial dysfunction and proliferation as well as smooth muscle cell proliferation. Proliferation of these cell types thickens pulmonary vessels and narrows the lumen, particularly of small resistance vessels, resulting in increased pulmonary vascular resistance (PVR). Over time, this increased resistance leads to progressive right ventricular (RV) hypertrophy ending with right heart failure and death (12, 28). Currently, the mechanism(s) of pulmonary vascular remodeling in PH is poorly understood, and therapy remains inadequate.
We previously identified a novel gene product that is upregulated in the remodeling lung vasculature in a murine model of hypoxia-induced PH (34). This molecule, which we named hypoxia-induced mitogenic factor (HIMF), was also named “found in inflammatory zone 1” (FIZZ1) for its prominence in experimentally induced allergic asthma (11), where it is T-helper 2 (Th2) activated, and resistin-like molecule α (RELMα) for its role in insulin resistance (30). In our early studies with animal models of PH, we demonstrated that HIMF has mitogenic, angiogenic, vasoconstrictive, and chemokine-like properties, all of which are associated with vascular remodeling (31, 34, 41). In the hypoxia model, HIMF was expressed de novo in remodeling proliferating cell nuclear antigen (PCNA)-positive vascular smooth muscle and endothelium. HIMF is also highly expressed in the lung following bleomycin-induced injury and was shown to induce the transformation of fibroblasts to myofibroblasts that is essential for the observed lung fibrosis (17, 18). In addition, it was shown to be upregulated in the lung during compensatory growth following pneumonectomy (16). The HIMF expression pattern in these models was consistent with those of our previous study (34). In vivo, the addition of recombinant HIMF protein directly into the lung increases PCNA staining of several types of pulmonary cells (16) and recruitment of CD68- and CD34-positive-staining cells (41). HIMF is known to be induced by hypoxia and by activation of Th2-related immune pathways (IL-4 and IL-13); both stimuli have been implicated in the etiology of many forms of human PH and in animal models (6, 7, 12, 32, 34). All of these data suggest a role for HIMF in the vascular remodeling and hemodynamic changes of PH, but, until now, actual proof of a role for HIMF in the pathophysiology of PH has been lacking.
In this study, we tested the hypothesis that HIMF could induce the vascular and hemodynamic changes similar to those stimulated by PH and that blockade of HIMF could ameliorate the vascular remodeling and hemodynamic changes associated with the classic chronic hypoxia-induced rodent model of PH. We found that in vivo knockdown of HIMF in the chronic hypoxia model of PH partially blocked the vascular remodeling and hemodynamic changes of PH, including increased mean pulmonary artery pressure (mPAP) and PVR, thickening and muscularization of small arterioles, and RV hypertrophy. Also, in vivo pulmonary gene transfer of HIMF increased these same markers. This evidence implicates HIMF as a novel hypoxia- and Th2-activated pleiotropic cytokine in the pulmonary vascular remodeling and hemodynamic changes of PH.
MATERIALS AND METHODS
Experimental animals.
Adult male Sprague-Dawley rats weighing 200–250 g (Hilltop Lab Animals, Scottsdale, PA) were used for all experiments. Animal housing and experimental protocols were approved by the Animal Care and Use Committee of the Johns Hopkins University.
Viral vectors.
To induce controlled expression of HIMF in the intact rat lung, we used a recombinant adeno-associated virus (AAV) vector that selectively expresses murine HIMF. This AAV vector was designed with the ubiquitous CB promoter. It was prepared by the vector core lab located at the University of Florida (Dr. Terry Flotte). We used a similar empty AAV vector (AAV-null) to control for the possibility of viral-associated effects. To achieve suppression of HIMF in the intact rat lung, we used a recombinant, replication-defective adenovirus (Ad) that expresses green fluorescent protein (GFP) and an artificial miRNA engineered to selectively suppress HIMF expression (Ad-EmGFP-HIMF-shRNAmiR). Construction of the artificial miRNA was based on an endogenous murine miRNA (miR-155) (14) whose short hairpin (sh) RNA sequence was replaced by shRNA sequences targeting HIMF. This was then incorporated into a commercial vector in the GATEWAY recombination cloning system (pcDNA6.2-DEST; Invitrogen, Carlsbad, CA). In this vector, the shRNA containing the antisense to the target sequence in rat HIMF (NM_053333) was placed downstream of a cDNA encoding a modified GFP (EmGFP) so that it was transcribed along with the EmGFP. The target sequence for rat HIMF (5′-gataactatccctctgctgca-3′; bp 156–176) used in this study was chosen from among four different sequences generated by web-based selection programs in a screen in which individual shRNAmiRs were cotransfected into HEK-293 cells along with a plasmid expressing rat HIMF. Lysates were prepared 2 days later and probed for HIMF protein expression. The region containing EmGFP linked to the artificial miRNA was then transferred into pAd.CMV-DEST by recombinase cloning (Invitrogen). We also prepared an Ad containing EmGFP along with a negative control shRNAmiR (Ad-EmGFP-Neg-shRNAmiR), predicted not to target any known vertebrate gene. Large-scale amplification and purification of virus was performed by Vector Biolabs (Philadelphia, PA) or in our laboratory using Adenopure LS adenovirus purification kit (Pursyn, Malvern, PA) according to the manufacturer's instructions.
In vivo pulmonary gene transfer.
Rats were anesthetized with an intraperitoneal (ip) injection of a cocktail of ketamine-hydrochloride (100 mg/kg), xylazine (10 mg/kg), and acepromazine (100 mg/kg) and then placed in a supine position at a 45° angle. A MicroSprayer (model IA-1B) aerosolizer (Penn-Century, Philadelphia, PA) was primed with 200 μl of solution that contained either 5 × 1010 viral particles (VP) AAV-HIMF [with 5 μl Lipofectamine 2000 (Invitrogen)], 5 × 1010 VP AAV-null (with 5 μl Lipofectamine 2000), or Lipofectamine 2000 alone and was inserted into the trachea just above the carina. The solution was expelled under constant pressure, and the MicroSprayer tip was then carefully removed from the trachea; the rat was maintained at a 45° angle for 1 min. Rats were killed 1, 2, or 5 wk after the intratracheal instillation of the AAV-HIMF, and the efficiency of instillation was evaluated by Western blot for murine HIMF.
In vivo knockdown of HIMF in the chronic hypoxia model of PH.
For hypoxia studies, animals were given either 0.5–1.0 × 109 pfu Ad-EmGFP-HIMF-shRNAmiR or Ad-EmGFP-Neg-shRNAmiR intratracheally and allowed to recover for 48 h before being placed into Plexiglass chambers for exposure to hypoxia or normoxia. The two groups of rats (Ad-EmGFP-HIMF-shRNAmiR and Ad-EmGFP-Neg-shRNAmiR) were exposed to either normal room air (20.8% O2) or 10% O2 for 4 or 14 days as we have described previously (8, 9, 34, 40). To achieve the desired internal Po2, oxygen concentration was regulated by solenoid-controlled nitrogen flow linked to a Clark electrode (Pro-Ox controller; BioSpherix, Redfield, NY). The chambers were continuously scavenged for CO2 and ammonia.
Quantitative real-time PCR.
Rats were given 0.5–1.0 × 109 pfu Ad-EmGFP-HIMF-shRNAmiR intratracheally and killed 72 h later. Total RNA was isolated from lung, heart, liver, and spleen via the TRIzol extraction method (Invitrogen). It was then treated to remove genomic DNA contamination and further purified with the RNeasy kit (Qiagen, Valencia, CA). A small amount of the purified total RNA (1–5 μg) resulting from these purification steps was reverse transcribed with random hexamers using a commercially available kit (First Strand Synthesis Kit, Qiagen). Real-time PCR was performed on 25 ng cDNA/sample using the iQ SYBR Green Supermix and the iCycler Real Time PCR Detection System (Bio-Rad). Annealing temperatures were optimized by gradient PCR. The fold change in expression in GFP relative to 18S rRNA was calculated based on the threshold cycle (Ct) as 2−Δ(ΔCt), where ΔCt = Cttarget-Ct18S and Δ(ΔCt) = ACtsample-vCtcontrol. GFP- and 18S-rRNA-specific primers were selected in silico with Beacon Designer Software (Bio-Rad, Hercules, CA). The sequences of these primers were GFP-F, catcctggtcagactggac; GFP-R, gacttgaagaagtcgtgc; 18S rRNA-F, agttccagcacattttgcgag; and 18S rRNA-R, tgctcctccgtgagttctc. All PCR products were initially separated by agarose gel electrophoresis (1% in TAE buffer) to verify product formation and expected size. To confirm the identity of the PCR product, DNA was extracted from the band (GeneClean II; MP Biomedical, Solon, OH), cloned into a TA cloning vector (Invitrogen), and the cloned insert was then sequenced.
Visualization of GFP.
The left lobe of the lung was tied off while the right lobes of the lung were perfused with cold 4% paraformaldehyde for 10 min. The right lobes were then inflated through the trachea with 4% paraformaldehyde, tied off, and immersed in 4% parafromaldehyde overnight. The next day, the lungs were washed with PBS and kept in cold (4°C) PBS for up to 2 wk. The day before sectioning, the lungs were placed in 18% sucrose in PBS. Ten-micrometer sections were cut on a cryostat and then directly imaged on a Nikon Eclipse Model TE2000-S fluorescent microscope. Images were captured with a Retiga 2000R camera and imported into Image ProPlus (version 5.1). All fluorescent images were collected using an identical exposure time.
In vivo hemodynamic analysis.
To obtain in vivo hemodynamic measurements, rats were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). The rats were then ventilated by the insertion of a tracheal cannula attached to a rodent ventilator (Harvard Apparatus, South Natick, MA) set to 90 breaths/min with a tidal volume of 8 ml/kg body wt. The chest was opened with a midline incision, and then a four-electrode pressure-volume catheter (Millar Instruments, Houston, TX) was placed through the right ventricular apex to record chamber volume by impedance and pressure micromanometry as described previously (33, 42).
Histology and laboratory testing.
Once appropriate hemodynamic measurements were made, 1 ml of blood was taken from the left ventricle for hematocrit analysis. Rats were then euthanized by exsanguination, and the heart and lungs were removed en bloc. The bronchus leading to the right lung was tied off, and the left lung was inflated with 1% low-melt agarose in PBS with constant pressure (25 cmH2O) and then placed on ice for 5–10 min. The inflated left lung was then placed in 4% paraformaldehyde at 4°C for 48 h, cut into four saggital sections, and subsequently processed for histology as described previously (8, 9). The right lung was then removed, snap frozen in liquid N2, and stored at −80°C for use in Western analysis. The heart was then bisected into the RV and left ventricle (LV) plus septum (S). Each portion of the heart was weighed, and the RV and LV+S ratio was determined [RV/(LV+S)].
Immunohistochemistry for HIMF.
Paraffin blocks of lungs from rats treated with AAV-HIMF (5 × 1010 VP), AAV-null (5 × 1010 VP), and vehicle were cut into 6-μm sections and placed on clean glass slides. The slides were heated to 60°C for 15 min and then subjected to deparaffinization [100% xylene, 3 × 15 min at room temperature (RT)] and rehydration by washes in decreasing concentrations of ethanol (2 × 100%, 1 × 95%, 1 × 70%, and 1 × 50% for 10 min each) followed by one 10-min wash in double-distilled H2O and one 10-min wash in PBS. Rehydrated slides were placed in antigen unmasking solution (Vector Laboratories, Burlingame, CA) at 95°C for 20 min. Then endogenous peroxidase was blocked with 3% H2O2 for 10 min. Endogenous avidin and biotin were blocked for 15 min each using the Avidin/Biotin Blocking Kit (Vector Laboratories). Nonspecific protein binding was blocked by treatment of the slides with normal rabbit serum for 30 min. After the final blocking step, the sections were treated with polyclonal goat anti-mouse RELMα (1:200; R&D Systems, Minneapolis, MN) or antibody diluent alone for 120 min at RT. The slides were washed with PBS (3×) and treated with a rabbit anti-goat biotinylated secondary antibody (Vector Laboratories) and then an ABC horseradish peroxidase (HRP) reagent for 30 min each at RT (Vectastain Elite ABC Kit, Vector Laboratories). The lung sections were then treated with the Peroxidase Substrate Kit DAB (Vector Laboratories). The sections were then counterstained with Hematoxylin Solution, Gill No. 1 (Sigma, St. Louis, MO), and blue color was developed with a saturated solution of lithium carbonate (Sigma). Finally, the sections were dehydrated with ethanol (1 × 50%, 1 × 70%, 1 × 95%, 2 × 100% for 10 min each), treated with xylene (3 × 10 min), and mounted with Cytoseal60 (Richard-Allan Scientific, Kalamazoo, MI). The sections were visualized with an Olympus-BHS microscope attached to a QImaging Retiga 4000RV digital camera and captured by ImagePro Plus (version 5.1) software.
Evaluation of vascular remodeling.
Pulmonary vascular remodeling of the rats was assessed as we have previously published (8, 9, 40). For initial analysis, sections were stained with hematoxylin and eosin. Additional lung sections were dual labeled with von Willebrand factor to stain endothelium and α-smooth muscle actin for vascular smooth muscle, as we have described (40). To examine cellular proliferation, lung sections were stained for PCNA as we have described (39) and then counterstained with hematoxylin. To assess remodeling of the lung arteries and arterioles, 100 arteries per lung section were randomly examined by an investigator blinded to the treatment group using an Olympus-BHS microscope attached to a QImaging Retiga 4000RV digital camera. Small arteries with an internal diameter of <80 μm were then classified as non-muscular (NM), partially muscular (PM), or fully muscular (FM), according to α-smooth muscle actin staining. PM vessels contained at least one cell that was positive for α-smooth muscle actin but lacked a continuous layer. FM vessels had a continuous α-smooth muscle actin band. These vessels were then analyzed as described (25, 40). The percent medial thickness (%MT) was determined in sections receiving a Movat stain as described (8) and was calculated as %MT = [(external diameter − internal diameter)/external diameter] × 100. Only vessels with a circular appearance and external diameter between 25 and 200 μm were used for this study. Measurements were made by using MetaMorph software (Molecular Devices, Downingtown, PA). Approximately 25 arteries in 25 consecutive fields were evaluated per lung.
Western blot analysis.
Frozen rat lung tissue was homogenized on ice with a Brinkman Homogenizer (Polytron, Westbury, NY) in homogenization/lysis buffer containing 50 mM Tris·HCl (pH 7.4), 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM vanadate, 1 mM NaF, 10 mM pyrophosphate, 1 mg/ml pepstatin A (all purchased from Sigma), and 1 tablet of Complete Protease Inhibitor Cocktail (Roche Molecular Biochemicals, Indianapolis, IN) per 20 ml. The homogenates were centrifuged, and the supernatants were assayed for protein concentration with a standard Bio-Rad protein assay kit (Bio-Rad Chemical Division). The samples were resolved by a 4–20% SDS-PAGE (Bio-Rad) gel and transferred onto nitrocellulose (Bio-Rad) membranes. The membrane blots were blocked with 5% milk in Tris-buffered saline with 0.1% Tween 20 for 1 h and then incubated with rabbit anti-HIMF (1:500) followed by HRP-conjugated anti-rabbit IgG (1:5,000, Bio-Rad). The blots were developed with enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ) and exposed to X-ray film (Denville Scientific, Metuchen, NJ). To confirm equal protein loading and transfer, blots were stripped with Blot Restore Membrane Rejuvenation Kit (Chemicon International) and reprobed with murine anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Bands of interest were quantified by laser densitometry (Molecular Dynamics, Sunnydale, CA) and normalized to β-actin.
Statistical analysis.
Experimental values are expressed as means ± SE. A paired Student's t-test was used to compare the mean responses of two groups. ANOVA was used to compare the mean responses among experimental and control groups for experiments containing multiple groups. The Tukey multiple comparison test was used to determine between which groups significant differences existed. A P value of <0.05 was considered statistically significant.
RESULTS
Efficiency of in vivo knockdown of HIMF in the chronic hypoxia model.
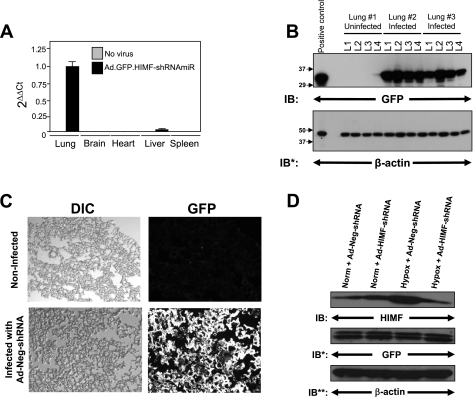
Figure 1A shows that 4 days after tracheal instillation of the adenovirus Ad-EmGFP-HIMF-shRNAmiR (Ad-HIMF-shRNA), engineered to suppress HIMF expression and produce GFP, the overwhelming majority of GFP was detected in the lung. A very small amount of GFP was detected in the liver, but it was <0.1% of that in the lung after normalization by 18S rRNA. No GFP was detected in the heart, spleen, or brain. To better evaluate the viral distribution, we collected the left lung 4 days after the intratracheal instillation of Ad-EmGFP-Neg-shRNAmiR (Ad-Neg-shRNA) and divided it into four equal sections. The lung sections were then processed for Western blot analysis. Equal amounts of GFP protein were seen in each section of the left lung, suggesting an even distribution of the adenoviral vector (Fig. 1B). We also visualized the viral-induced GFP expression. Frozen lung sections from Ad-Neg-shRNA vector displayed intense GFP signal that appeared to be evenly distributed throughout the lungs (Fig. 1C).
Fig. 1.
In vivo knockdown of hypoxia-induced mitogenic factor (HIMF) in the chronic hypoxia model of pulmonary hypertension. A: real-time PCR for green fluorescent protein (GFP) from RNA isolated from the lung, heart, liver, and spleen 72 h after intratracheal instillation of Ad-HIMF-shRNA. B: equally divided left lungs from uninfected rats or rats infected with Ad-Neg-shRNA 4 days earlier were homogenized and evaluated for GFP expression. The homogenates were resolved by 4–20% SDS-PAGE and transferred to nitrocellulose. The blots were probed with rabbit polyclonal anti-GFP antibodies and developed with ECL. To confirm equal protein loading and transfer, the blots were stripped and reprobed with monoclonal anti-β-actin antibodies. IB, immunoblot; IB*, immunoblot after stripping. C: visualization of GFP in control rat lungs or lungs infected with Ad-Neg-shRNA. DIC, differential interference contrast. D: lung homogenates from animals exposed to 4 days of normoxia (20.8% O2) or hypoxia (10.0% O2) were evaluated for HIMF expression. The homogenates were resolved by 4–20% SDS-PAGE and transferred to nitrocellulose. The blots were probed with polyclonal anti-HIMF antibodies and developed by ECL. To confirm introduction of the viral vectors, the blot was stripped and reprobed with polyclonal anti-GFP antibodies. To confirm equal protein loading, the blots were stripped and reprobed with monoclonal anti-β-actin antibodies. IB**, immunoblot after second stripping.
We have previously demonstrated that peak HIMF expression occurs 4 days following chronic hypoxia exposure (34). Therefore, we compared the efficiency of HIMF suppression by the Ad-HIMF-shRNA to that resulting from a vector expressing a nonspecific shRNAmiR (Ad-Neg-shRNA) at this time point. Lungs from animals treated for 4 days with Ad-HIMF-shRNA or Ad-Neg-shRNA in either hypoxia (10% O2) or normoxia (20.8% O2) were excised and processed for Western blot analysis. Immunoblotting revealed increased HIMF expression in the lungs of rats exposed to 4 days of hypoxia, but not in the lungs of normoxic animals. Introduction of Ad-HIMF-shRNA reduced hypoxia-induced HIMF expression (Fig. 1D), whereas the Ad-Neg-shRNA had no effect. HIMF expression in untreated normoxic/hypoxic rats was similar to that of normoxic/hypoxic animals treated with Ad-Neg-shRNA (data not shown).
Effect of HIMF knockdown on vascular remodeling in the chronic hypoxia model of PH.
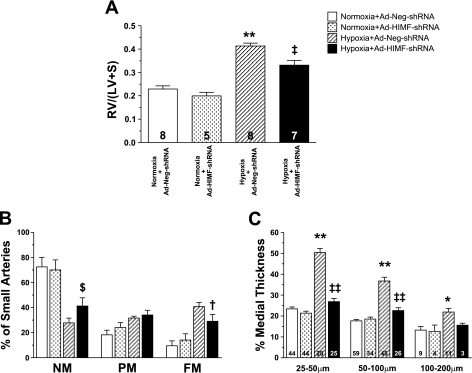
Rats were treated with Ad-Neg-shRNA or Ad-HIMF-shRNA and exposed to normoxia or hypoxia for 14 days. Among the animals exposed to hypoxia, those pretreated with Ad-HIMF-shRNA exhibited ∼45% less RV hypertrophy (0.33 ± 0.02) than control animals exposed to Ad-Neg-shRNA (0.41 ± 0.01; Fig. 2A). No differences were observed in RV hypertrophy between the normoxia+Ad-Neg-shRNA (0.23 ± 0.01) and the normoxia+Ad-HIMF-shRNA (0.20 ± 0.01) groups in terms of vascular remodeling (Fig. 2A).
Fig. 2.
In vivo knockdown of HIMF reduces hypoxia-induced vascular remodeling. A: the histogram shows means ± SE of right ventricular weight/(left ventricular + septal weight) [RV/(LV+S)] of rats exposed to 14 days of normoxia (20.8% O2) or hypoxia (10.0% O2) following intratracheal instillation of Ad-Neg-shRNA or Ad-HIMF-shRNA. The number of animals studied is indicated within each bar. B: muscularization of small pulmonary arteries is indicated as non-muscular (NM), partially muscular (PM), or fully muscular (FM) in rat lungs from the 4 different groups of rats as stated. C: comparison of percent medial wall thickness of pulmonary arteries 25–50 μm, 50–100 μm, and 100–200 μm among the 4 groups of rats. The number of vessels counted is indicated within each bar. *Significantly increased vs. normoxia+Ad-Neg-shRNA control at P < 0.05. **Significantly increased vs. normoxia+Ad-Neg-shRNA control at P < 0.001. $Significantly increased vs. hypoxia+Ad-Neg-shRNA control at P < 0.05. †Significantly decreased compared with hypoxia+Ad-Neg-shRNA control at P < 0.05. ‡Significantly decreased compared with hypoxia+Ad-Neg-shRNA control at P < 0.01. ‡‡Significantly decreased compared with hypoxia+Ad-Neg-shRNA control at P < 0.001.
After being exposed to hypoxia, control animals pretreated with Ad-Neg-shRNA developed significant increases in muscularization of the small pulmonary vessels (see Figs. 2B and 3C), but these increases were significantly attenuated in rats pretreated with Ad-HIMF-shRNA (see Figs. 2B and 3D). The percentage of FM small vessels dropped from 40.80 ± 3.17% to 29.00 ± 5.49% (P < 0.05) in response to Ad-HIMF-shRNA pretreatment, and the percentage of NM small vessels increased from 27.80 ± 3.76% to 41.25 ± 6.51% (P < 0.05) (Fig. 2B). Histological representation of the muscularization in these small vessels is shown in Fig. 3, A–D. After 14 days of hypoxia, the number of α-smooth muscle actin-positive cells was increased in rats treated with Ad-Neg-shRNA, and many vessels had a continuous band of positive staining (Fig. 3C). This increased α-smooth muscle actin staining was greatly reduced by knockdown of hypoxia-induced HIMF expression (Fig. 3D).
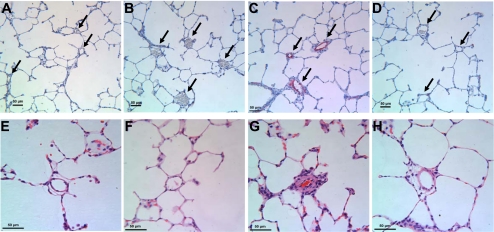
Fig. 3.
In vivo knockdown of HIMF reduces hypoxia-induced histological changes. A–D: lung sections were double-stained with antibodies to α-smooth muscle actin (red) and von Willebrand factor (black). Cell nuclei were counterstained with hematoxylin (blue). Arrows indicate small pulmonary vessels. A: normoxia+Ad-Neg-shRNA. B: normoxia+Ad-HIMF-shRNA. C: hypoxia+Ad-Neg-shRNA. D: hypoxia+Ad-HIMF-shRNA. E–H: hematoxylin and eosin-stained lung sections from rats exposed to normoxia+Ad-Neg-shRNA (E), normoxia+Ad-HIMF-shRNA (F), hypoxia+Ad-Neg-shRNA (G), or hypoxia+Ad-HIMF-shRNA (H) for 14 days. Scale bars = 50 μm.
The %MT of pulmonary vessels was decreased in rats exposed to hypoxia+Ad-HIMF-shRNA compared with rats exposed to hypoxia+Ad-Neg-shRNA (Fig. 2C). The %MT of vessels from hypoxic rats that were 25–50 μm in external diameter decreased from 50.41 ± 1.97% (hypoxia+Ad-Neg-shRNA) to 26.87 ± 1.49% (hypoxia+Ad-HIMF-shRNA) (P < 0.001; Fig. 2C). The hypoxia-induced increase in %MT also was reduced in vessels 50–100 μm in external diameter from 36.76 ± 1.81% in hypoxia+Ad-Neg-shRNA rats to 22.61 ± 1.34% in hypoxia+Ad-HIMF-shRNA-treated animals (P < 0.001; Fig. 2C). There were no statistically significant changes in %MT of vessels 100–200 μm in external diameter, but there was a similar trend in reduction of the %MT. Histological representation of changes in %MT is shown in Fig. 3, E–H, for vessels of ∼50 μm in external diameter. Hypoxia led to increases in the thickness of the vessel wall and intima (Fig. 3G), but these changes were absent in lung vessels of rats treated with Ad-HIMF-shRNA (Fig. 3H).
Effect of HIMF knockdown on hemodynamic parameters in the chronic hypoxia model of PH.
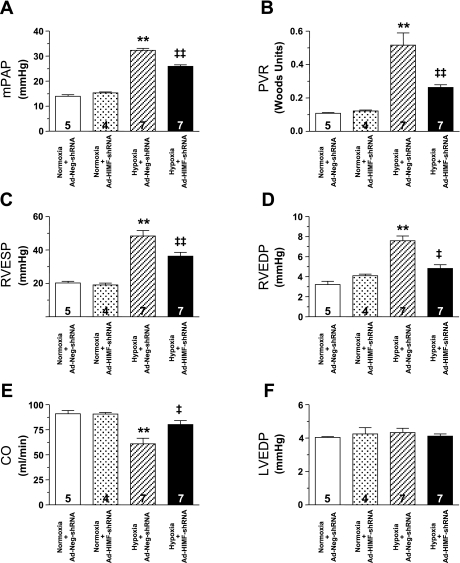
Exposure to 14 days of hypoxia created a PH condition in the Ad-Neg-shRNA-treated rats that resulted in significantly increased mPAP, PVR, RV end systolic pressure (RVESP), and RV end diastolic pressure (RVEDP) and significantly decreased cardiac output (CO) (Fig. 4). Treatment with Ad-HIMF-shRNA reduced the hypoxia-induced increase in mPAP by ∼35% (hypoxia+Ad-Neg-shRNA: 33.46 ± 1.13 mmHg vs. hypoxia+Ad-HIMF-shRNA: 24.99 ± 0.98 mmHg; P < 0.001; Fig. 4A) and blocked >50% of the observable increases in PVR (hypoxia+Ad-Neg-shRNA: 0.517 ± 0.073 Woods units vs. hypoxia+Ad-HIMF-shRNA: 0.263 ± 0.016 Woods units; P < 0.001; Fig. 4B). Ad-HIMF-shRNA treatment led to a reduction in RVESP from 48.36 ± 3.27 mmHg to 36.29 ± 2.28 mmHg (P < 0.01; Fig. 4C) and in RVEDP from 7.59 ± 0.47 mmHg to 4.81 ± 0.38 mmHg (P < 0.01; Fig. 4D). Finally, CO was brought back nearly to normoxic baseline levels by Ad-HIMF-shRNA treatment (hypoxia+Ad-Neg-shRNA: 60.91 ± 5.63 ml/min vs. hypoxia+HIMF-shRNA: 80.20 ± 3.83 ml/min; P < 0.05; Fig. 4E). Hematocrit was equally increased in the two hypoxic groups compared with the normoxia controls (normoxia+Ad-Neg-shRNA: 39.71 ± 1.54%; normoxia+Ad-HIMF-shRNA: 40.00 ± 0.71%; hypoxia+Ad-Neg-shRNA: 55.38 ± 1.16%; hypoxia+Ad-HIMF-shRNA: 54.71 ± 1.61%). No differences were apparent in any of the hemodynamic parameters between the normoxia+Ad-Neg-shRNA and normoxia+Ad-HIMF-shRNA groups of rats.
Fig. 4.
In vivo knockdown of HIMF reduces hypoxia-induced changes in hemodynamics. Vertical bars represent means ± SE of mean pulmonary artery pressure (mPAP; A), pulmonary vascular resistance (PVR; B), right ventricular end systolic pressure (RVESP; C), right ventricular end diastolic pressure (RVEDP; D), cardiac output (CO; E), and left ventricular end diastolic pressure (LVEDP; F) in rats pretreated with Ad-Neg-shRNA or Ad-HIMF-shRNA and exposed to either normoxia or hypoxia for 14 days. The number of rats studied is indicated within each bar. **P < 0.001 compared with normoxia+Ad-miNeg-shRNA control; ‡P < 0.01 compared with hypoxia+Ad-Neg-shRNA control. ‡‡P < 0.001 compared with hypoxia+Ad-Neg-shRNA control.
Efficiency of pulmonary HIMF gene transfer.
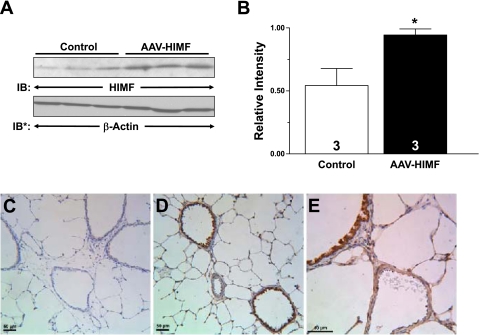
The effectiveness of HIMF gene transfer into the lungs of rats 14 days following intratracheal instillation with murine HIMF-expressing AAV (5 × 1010 VP) is illustrated in Fig. 5. Western blot analysis revealed that HIMF expression was substantially higher in the lungs of rats that received AAV-HIMF than in those of controls (Fig. 5B). To localize the expression of the AAV-HIMF, we performed immunochemistry for murine HIMF on rat lungs that were intratracheally given equal concentrations of either AAV-null or AAV-HIMF (5 × 1010 VP) (Fig. 5). HIMF was undetectable in the lungs of rats that were given the AAV-null (Fig. 5C). The antibody that was used for these immunohistochemistry experiments detects only murine HIMF and does not cross-react with rat HIMF; therefore, it only detected viral-induced HIMF. Evaluation of the AAV-HIMF-treated lungs revealed intense staining for HIMF, particularly in the large airways (Fig. 5D). Alveolar epithelial cells as well as the vasculature stained positive for murine HIMF (Fig. 5D). Figure 5E shows a detailed view of an airway and vessel that stained positive for murine HIMF. It is possible, but not likely, that the introduction of AAV into the lungs can induce pulmonary inflammation (2, 43). We therefore examined the AAV-null slides for an inflammatory response. There was no noticeable pulmonary inflammation associated with the introduction of AAV-null compared with simultaneously prepared vehicle controls (data not shown).
Fig. 5.
Effectiveness of pulmonary HIMF gene transfer. A: 2 wk after rats were intratracheally instilled with AAV-HIMF, the lungs were homogenized, resolved by 4–20% SDS-PAGE, and transferred to nitrocellulose. The blots were probed with rabbit anti-HIMF antibodies and developed by ECL. To confirm equal loading and transfer, the blot was stripped and reprobed with anti-β-actin monoclonal antibodies. B: laser densitometry was used to quantify HIMF levels in the lung samples. Data were normalized to β-actin expression and expressed as relative intensity (means ± SE). The number of lungs studied is indicated within each bar. *P < 0.05 compared with the control lung. C–E: paraffin-embedded lung sections from AAV-null-treated (C) and AAV-HIMF-treated (D and E) mice were rehydrated and stained with goat anti-mouse HIMF polyclonal antibodies. Scale bar = 50 μm.
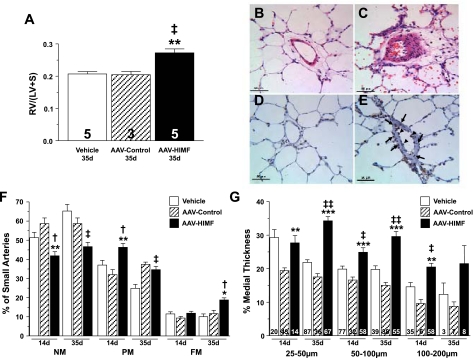
Effect of pulmonary HIMF gene transfer on vascular remodeling.
Thirty-five days after rats were instilled with AAV-HIMF, the RV/LV+S ratio was ∼32% greater than that in both vehicle-treated and AAV-null controls (P < 0.01; Fig. 6A). The muscularization of small pulmonary vessels was also markedly increased (Fig. 6, C and F). Layering of smooth muscle cells within the intima of blood vessels from the lungs of rats treated with AAV-HIMF was substantially increased compared with that in similarly sized vessels from control animals (Fig. 6, B and C). There were significant increases in the number of PM (34.60 ± 2.08% vs. 24.80 ± 1.75%; P < 0.05) and FM (18.80 ± 1.07% vs. 10.00 ± 1.48%; P < 0.05) vessels with concomitant decreases in NM vessels (46.60 ± 2.25%; Fig. 6F) compared with simultaneously treated vehicle controls (65.20 ± 3.48%; P < 0.05). There were also significant increases in the number of FM vessels (18.80 ± 1.07% vs. 11.67 ± 1.76%) compared with simultaneously treated AAV-null controls. The %MT of pulmonary vessels was also increased in these animals (Fig. 6G). Vessels that were 25–50 μm in external diameter increased from 21.86 ± 0.77% in vehicle controls and 17.51 ± 0.99% in AAV-null to 34.27 ± 1.23%MT 35 days after AAV-HIMF-treatment (P < 0.0001; Fig. 6G). The %MT also was increased in vessels 50–100 μm in external diameter from 19.77 ± 1.05% in vehicle and 15.02 ± 0.85% in AAV-null control rats to 29.59 ± 1.50% in AAV-HIMF-treated animals (P < 0.001; Fig. 6G). There were no significant changes in %MT of vessels 100–200 μm in external diameter, but there was a trend toward increased %MT (Fig. 6G).
Fig. 6.
Pulmonary gene transfer of HIMF induces vascular remodeling. A: vertical bars represent means ± SE of right ventricular weight/(left ventricular + septal weight) [RV/(LV+S)] in rats 35 days after intratracheal instillation with vehicle, AAV-null (5 × 1010 VP), or AAV-HIMF (5 × 1010 VP). The number of animals studied is indicated within each bar. B and C: hematoxylin and eosin-stained lung sections from rats 35 days after being treated with vehicle (B) or AAV-HIMF (5 × 1010 VP) (C). D and E: lung sections from rats 14 days after being treated with vehicle (D) or AAV-HIMF (5 × 1010 VP) (E) were probed with antibodies against PCNA (brown) and then counterstained with hematoxylin (blue). F: muscularization of small pulmonary arteries is indicated as non-muscular (NM), partially muscular (PM), or fully muscular (FM) in rat lungs 14 or 35 days after initial intratracheal instillation of vehicle, AAV-null (5 × 1010 VP), or AAV-HIMF (5 × 1010 VP). G: comparison of percent medial wall thickness of pulmonary arteries 25–50 μm, 50–100 μm, and 100–200 μm among rats 14 or 35 days after intratracheal instillation of vehicle, AAV-null (5 × 1010 VP), or AAV-HIMF (5 × 1010 VP). The number of vessels counted is indicated within each bar. †P < 0.05, ‡P < 0.01, ‡‡P < 0.001 compared with simultaneous vehicle controls. *P < 0.05, **P < 0.01, ***P < 0.001 compared with simultaneous AAV-null.
To determine if cellular proliferation was occurring in the vasculature of rats treated with AAV-HIMF, we performed immunohistochemistry for the cell proliferation marker PCNA (Fig. 6, D and E). Pulmonary vessels from rats treated with AAV-HIMF for 14 days displayed an increased number of PCNA-positive cells. These cells were particularly prominent in the smooth muscle layer as well as in the endothelial cell layer (Fig. 6E). Vessels from control animals did not display PCNA-positive cells in either of these layers (Fig. 6D). Analysis of right heart hypertrophy as determined by the RV/(LV+S) ratio 14 days after AAV-HIMF treatment revealed no significant changes (data not shown); however, mild but significant changes in vascular muscularization were noted (Fig. 6F). The mean number of PM vessels was significantly greater than that of controls [46.29 ± 2.02% vs. 32.00 ± 4.58% (AAV-null), 37.00 ± 2.46% (vehicle); P < 0.05], and the number of NM vessels was decreased [41.86 ± 2.15% vs. 58.67 ± 5.03 (AAV-null), 51.33 ± 2.77% (vehicle); P < 0.05]; there were no distinguishable differences in the number of FM vessels (Fig. 6F). There were no differences in the %MT of vessels with external diameters of 25–50 μm between the experimental and vehicle control group, but there was a significant difference between AAV-HIMF (27.68 ± 2.16%) and AAV-null (19.44 ± 0.77%; P < 0.01) (Fig. 6G). The %MT of vessels 50–100 μm in external diameter was significantly greater in AAV-HIMF-treated rats (24.86 ± 1.35%) than in either vehicle-treated (19.85 ± 0.89%; P < 0.01) or AAV-null-treated (16.59 ± 0.91%; P < 0.001) control animals (Fig. 6G). The %MT in 100–200 μm vessels also was greater in AAV-HIMF-treated animals (20.28 ± 1.84%) than in controls (vehicle 11.00 ± 1.57%; P < 0.01; AAV-null 9.59 ± 1.22%; P < 0.01). Rats examined 7 days after treatment with AAV-HIMF displayed no changes in RV hypertrophy, muscularization, or %MT of the pulmonary arteries (data not shown).
In vivo hemodynamics after pulmonary gene transfer of HIMF.
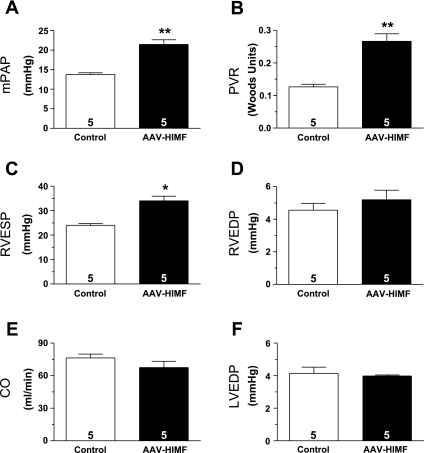
Rats were examined for changes in hemodynamic parameters 35 days after pulmonary gene transfer of HIMF. Animals were anesthetized, and the pulmonary vascular and right ventricular hemodynamics were assessed as described in materials and methods. The mPAP increased from 13.80 ± 0.40 mmHg in vehicle-treated rats to 21.47 ± 1.18 mmHg in rats that received pulmonary HIMF gene transfer (P < 0.01; Fig. 7A). PVR also increased from 0.127 ± 0.005 Woods units in control animals to 0.266 ± 0.023 Woods units in AAV-HIMF-treated animals (P < 0.01; Fig. 7B). In addition, the RVESP was significantly higher in the AAV-HIMF-treated rats (34.00 ± 0.71 mmHg) than in the control rats (24 ± 1.92 mmHg; P < 0.05; Fig. 7C). There were no differences between the two groups in RVEDP (Fig. 7D), CO (Fig. 7E), or LVEDP (Fig. 7F). As an additional control, we evaluated mPAP and RVESP in rats treated with AAV-null 35 days prior. The mPAP of these animals was 15.07 ± 1.20 mmHg, and the RVESP was 22.33 ± 1.86 mmHg. No statistical significance between these values and the values obtained from the vehicle-control animals was seen. However, the values of mPAP and RVESP in these rats were statistically lower than those in the rats treated with AAV-HIMF (P < 0.01).
Fig. 7.
Pulmonary gene transfer of HIMF alters in vivo hemodynamics. Vertical bars represent means ± SE of mPAP (A), PVR (B), RVESP (C), RVEDP (D), CO (E), and LVEDP (F) in rats 35 days after treatment with vehicle or AAV-HIMF (5 × 1010 VP). The number of rats studied is indicated within each bar. *P < 0.05, **P < 0.01 compared to simultaneous control.
DISCUSSION
There has been much discussion, speculation, and indirect evidence regarding the involvement of HIMF in pulmonary vascular remodeling and PH, but this report is the first to provide definitive proof that HIMF is directly involved. We initially focused our research on HIMF because of its prominence in the chronic hypoxia model of PH as shown through microarray analysis (34). We found that HIMF has biological properties that make it an intriguing candidate for pathological signaling in the development of PH. These include its colocalization in PCNA-positive hyperplastic vascular smooth muscle cells and endothelial cells in remodeling lung, where we have found HIMF to have promitogenic, proangiogenic, antiapoptotic, vasoconstrictive, and chemokine-like functions (31, 34, 39, 41).
In the current study, we used two approaches, blockade of HIMF in a chronic hypoxia model of PH and overexpression of HIMF in the lungs of previously normal rats, to show a direct involvement of HIMF in the vascular remodeling and hemodynamic changes that result in PH. First, we showed that in vivo knockdown of HIMF in the chronic hypoxia model of PH inhibits the vascular remodeling and hemodynamic changes of PH. Second, we performed pulmonary gene transfer of HIMF using HIMF-expressing AAV and demonstrated that HIMF induces vascular remodeling and hemodynamic changes identical to those associated with chronic hypoxia and other forms of PH.
HIMF could be involved in the development of PH through several possible mechanisms, including induction of vasoconstriction and regulation of cellular proliferation. HIMF is the most potent endogenous vasoconstrictor of the pulmonary circulation that we have tested in our rodent studies (34). It possesses vasoconstrictive properties that are more potent and more efficacious than serotonin, endothelin-1, or angiotensin II (34). In addition, HIMF also induces vascular smooth muscle mitogenesis and hyperplasia. We observed that the addition of recombinant HIMF to pulmonary microvascular smooth muscle cells in culture significantly increases [3H]thymidine incorporation in a dose-dependent manner (34). Proliferation of vascular smooth muscle cells is perhaps the most critical element in the vascular remodeling that occurs in PH.
Neo-muscularization of small, previously non-muscular arterioles and hyperplasia and hypertrophy of vascular smooth muscle in these and larger muscular pulmonary arterioles and arteries are hallmarks of PH (4, 28). Medial thickening and neointima formation require a dysregulation of cellular proliferation, particularly of vascular smooth muscle. Here, we showed that HIMF can induce medial thickening as well as vascular smooth muscle proliferation through pulmonary HIMF gene transfer (Fig. 6). We also showed that knocking down HIMF protein expression in the chronic hypoxia model of PH reduces these effects (Fig. 2). Our laboratory has demonstrated that HIMF is expressed in and colocalizes with PCNA in developing murine lung, particular in the saccular and alveolar stages during times of intense cell proliferation (39). In the current study, vessels from rats that were intratracheally treated with AAV-HIMF displayed increased PCNA-positive staining in the smooth muscle layer (Fig. 6E). Li et al. (16) also have demonstrated that HIMF is intensely expressed in the lung during compensatory growth following pneumonectomy in the same cells and is temporally coincident with the cell proliferation marker PCNA. It is interesting to note that a recent study established that HIMF is expressed in the remodeling pulmonary arteries of mice with antigen-induced asthma (6). As stated above, we have previously published that the addition of recombinant murine HIMF to cultured rat pulmonary microvascular smooth muscle cells increases the proliferative response in a dose-dependent manner (34). Together, these data suggest that HIMF induces direct cellular proliferation that could mediate the vascular remodeling of PH, consistent with our current observations that HIMF induces lung vascular remodeling identical to that which occurs following chronic hypoxia.
Alterations in the normal levels of apoptosis may result in excess cellular proliferation during vascular remodeling associated with PH. In earlier studies, we and others (5, 35, 39) demonstrated that HIMF has antiapoptotic properties. For example, we showed that mouse embryonic lung explants cultured in the presence of recombinant murine HIMF exhibit fewer TUNEL-positive apoptotic cells and greater lung density than media-control explants (39). In addition, treatment of mouse lung epithelial cells with recombinant HIMF protected against lipopolysaccharide-induced apoptosis (35). Finally, Chung et al. (5) demonstrated that mouse lung fibroblasts treated with recombinant HIMF were resistant to tumor necrosis factor-α/cyclohexamide-induced apoptosis. This reduced apoptosis was associated with reductions in caspase-3 and caspase-8 activity. The effect of HIMF on fibroblasts is interesting because the fibroblast may play a significant role in the vascular remodeling associated with the development of PH.
The increased medial thickness and muscularization during the remodeling of small pulmonary arteries may be due to the actions of myofibroblasts; HIMF has been shown to be a key mediator of the fibroblast-to-myofibroblast transition. Liu et al. (17) demonstrated that transfection of normal fibroblasts with a HIMF-expressing construct increases collagen I and α-smooth muscle actin in these cultured cells in a time-dependent manner. These two proteins are markers of fibroblast-to-myofibroblast transformation. The results indicate that HIMF can convert fibroblasts to myofibroblasts and suggest the intriguing possibility that HIMF may do so in vivo to contribute to vascular remodeling associated with PH (4).
Inflammatory processes increasingly are thought to play a central role in the development of many forms of human and animal models of PH, including the chronic hypoxia model used in the current study (6, 7, 19, 21, 32, 41). In our initial report of HIMF (Ref. 34, online supplement), we noted that the HIMF gene has transcription binding sites for inflammatory stimuli (STAT6 and NF-κB) and for hypoxia (HIF-1 and CEBP-β). Furthermore, we have demonstrated that HIMF expression increases in the lung in response to chronic hypoxia (Fig. 1D) (34) and that intravenous injection of recombinant HIMF increases the amount of inflammation in the lungs (41). In addition, Holcomb et al. (11) reported that HIMF was markedly increased in the bronchial epithelium of acute pulmonary inflammation induced by allergic asthma as well as in the macrophages; in fact, HIMF is used as a marker for alternatively (Th2) activated macrophages (26). These alternatively activated macrophages are indicative of a Th2 immune response. In a recent report, investigators suggested a role for the Th2 immune response in pulmonary vascular remodeling (6). They reported that vascular remodeling occurred in Th2-dependent allergic models of asthma and that HIMF was expressed in the cells (macrophages and pulmonary epithelial cells) surrounding the remodeling vessels, but not in the vascular tissue itself; hemodynamic indicators of PH were not present. HIMF is also increased in the remodeling vasculature of animals exposed to chronic hypoxia (34). It is possible that although Th2 stimulus can induce HIMF in inflammatory cells, hypoxic stimulus is necessary for HIMF induction in vascular cells. It is conceivable that the vascular remodeling associated with these two pathological responses is related to HIMF and that the vascular remodeling in the Th2 model is dependent on the secretion of HIMF from perivascular inflammatory cells. Strong evidence indicates that the pulmonary fibrosis associated with bleomycin-induced lung injury is driven by an HIMF-dependent Th2-mediated process (18). Bleomycin-induced HIMF expression and fibrosis are reduced in both IL-4 and IL-13 knockout mice. In dual IL-4/IL-13 knockout mice as well as in mice lacking the Th2-dependent transcription factor STAT6, these effects are completely ablated. These findings are notable because pulmonary fibrosis and Th2 immune responses are each associated with secondary forms of PH related to scleroderma and HIV infection. In fact, two separate studies of HIV-related pulmonary disease have demonstrated HIMF expression in pulmonary macrophages (22, 32).
Currently, little is understood regarding the signaling pathways associated with HIMF. We have previously ascertained that HIMF increases rodent vascular smooth muscle cell proliferation in vitro and that this proliferation is inhibited by pharmacological inhibition of phosphoinositide-3 kinase (PI3K) (34). Further investigation revealed that recombinant HIMF induced phosphorylation of the protein kinase Akt, a downstream target of PI3K, in a time-dependent manner. We have also demonstrated that pharmacological inhibition of PI3K inhibited HIMF-induced proliferation of primary-cultured rat pulmonary microvascular endothelial cells (41). Inhibition of VEGF receptor 2 with a neutralizing antibody also blocked HIMF-induced proliferation of these cells. Tong et al. (36) reported that the addition of recombinant HIMF to the endothelial cell line SVEC4–10 increased phosphorylation of Akt, as well as the signaling molecules p38 and ERK1/2. Only Akt inhibition blocked endothelial cell proliferation in that study. In murine fibroblasts, recombinant HIMF induced ERK1/2 phosphorylation and induced Akt phosphorylation only in tumor necrosis factor-α/cyclohexamide-pretreated cells (5).
No membrane receptor(s) for HIMF or its related molecules has yet been identified, but our laboratory has identified a HIMF binding partner. We have recently demonstrated through GST-HIMF pull-down studies and mass spectroscopy that Bruton's tyrosine kinase (BTK) is a functional binding partner for HIMF (31). Also, HIMF stimulation of myeloid cells induces autophosphorylation of BTK and its rapid activation and cellular redistribution to the forward process associated with cell migration. HIMF is chemotactic for these cells, and pharmacological inhibition of BTK abolishes this HIMF-stimulated effect. HIMF and BTK also colocalize in the remodeling lung vasculature of chronically hypoxic rats (unpublished data). It is conceivable that BTK signaling is responsible for some of the inflammatory response and recruitment and migration of cells to the lung.
Other aspects of vascular remodeling could be attributable to HIMF action. For example, endothelial cell dysfunction is a key element in the pathogenesis of PH (4, 28). Plexiform lesions seen in severe cases of PH may be due to unregulated angiogenesis and/or prevention of apoptosis in endothelial cells (38). Clinical studies have demonstrated that there is increased angiogenesis in the lungs of patients with idiopathic PH (15, 20). In fact, cultured endothelial cells from these patients have hyperproliferative and antiapoptotic properties (20). It is interesting to note that PCNA-positive cells are present in the endothelium of rats treated with AAV-HIMF (Fig. 6E). This finding suggests some type of endothelial proliferation, but the exact role that this endothelial cell proliferation is playing in HIMF-induced vascular remodeling needs further examination. Our laboratory and others have shown that HIMF has a direct effect on endothelial cells in vivo as well as in vitro. We have reported that HIMF induces endothelial cell migration and increases vascular tube formation in an in vivo matrigel plug assay (34). Also, mouse aortic rings that are cultured in the presence of recombinant HIMF undergo more vascular sprouting than those grown in vehicle (36). In vitro and in vivo studies revealed that HIMF can induce both proliferation and migration of rat pulmonary microvascular endothelial cells as well as stimulate recruitment of CD34-positive cells to the lung (41). Beyond its mitogenic effects, our lab has shown that HIMF can induce production of proangiogenic factors such as VEGF, VEGF receptor 2, stromal cell-derived factor-1, and monocyte chemoattractant protein-1 in both endothelial cell and whole lung organ culture (41). This activity has potential significance because plexiform lesions express several angiogenesis-related molecules, including VEGF (38). These results suggest that HIMF has both direct and indirect effects on endothelial cells involved in vascular remodeling. HIMF can directly stimulate mitogenesis, migration, and endothelial cell tube formation while inducing other factors that are important to this process of vascular remodeling.
Several pathological conditions that involve the Th2 immune response or chronic hypoxia are associated with the development of PH in humans, including collagen vascular diseases (e.g., scleroderma), HIV infection, sleep apnea, and chronic obstructive pulmonary disease. The human homolog to HIMF, RELMβ, is expressed in lung and is inducible by hypoxia in primary cultured pulmonary vascular smooth muscle cells and in the non-small cell lung cancer cell line A459 (27). Furthermore, RELMβ is mitogenic for pulmonary vascular smooth muscle cells. RELMβ expression is induced in the human colon cancer cell line LS174T by treatment with recombinant human IL-4 or IL-13 (1, 10), suggesting a potential role in the Th2 immune response. It is interesting to note that an alternative splice variant to a related molecule, resistin, is expressed in human lung (24). One previous study demonstrated that resistin can induce both mitogenesis and migration of human lung microvascular endothelial cells in vitro (23), and another showed that resistin induces proliferation of cultured human vascular smooth muscle cells (3). We have found RELMβ to be upregulated in lungs from patients with scleroderma-associated PH. It is prominent in the endothelium and smooth muscle of small vessels, CD68-positive monocytes, CD3-positive T cells, and throughout the plexiform lesions (unpublished data). It is still too early to know the exact role that RELMβ and resistin play in the development of PH, but these data are highly suggestive of a potentially pathological function.
The current study demonstrates direct involvement of HIMF in the development of PH. Pulmonary HIMF gene transfer mimics the beginning stages of this disorder, and knocking down HIMF production during chronic hypoxia protects against these effects. Our study indicates that HIMF is involved, at least in part, in the vascular remodeling and hemodynamic changes associated with the development of PH. These findings could potentially lead to more effective therapies against this disease and possible new biomarkers to predict severity and progression of PH.
GRANTS
The research performed in this study was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-39706 (R. A. Johns), SCCOR-P50-084946 (R. A. Johns), and F32-HL-086236 (D. J. Angelini).
Acknowledgments
We thank Claire Levine for critical review and assistance with the manuscript preparation.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA 101: 13596–13600, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle MP, Enke RA, Adams RJ, Guggino WB, Zeitlin PL. In utero AAV-mediated gene transfer to rabbit pulmonary epithelium. Mol Ther 4: 115–121, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation 110: 3335–3340, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol 44: 14–30, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung MJ, Liu T, Ullenbruch M, Phan SH. Antiapoptotic effect of found in inflammatory zone (FIZZ)1 on mouse lung fibroblasts. J Pathol 212: 180–187, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Daley E, Emson C, Guignabert C, de Waal MR, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, Grunig E, Grunig G. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med 205: 361–372, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J 22: 358–363, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Girgis RE, Li D, Zhan X, Garcia JG, Tuder RM, Hassoun PM, Johns RA. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Heart Circ Physiol 285: H938–H945, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Girgis RE, Mozammel S, Champion HC, Li D, Peng X, Shimoda L, Tuder RM, Johns RA, Hassoun PM. Regression of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Lung Cell Mol Physiol 292: L1105–L1110, 2007. [DOI] [PubMed] [Google Scholar]
- 10.He W, Wang ML, Jiang HQ, Steppan CM, Shin ME, Thurnheer MC, Cebra JJ, Lazar MA, Wu GD. Bacterial colonization leads to the colonic secretion of RELMbeta/FIZZ2, a novel goblet cell-specific protein. Gastroenterology 125: 1388–1397, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV Jr, Shelton DL, Hebert CC. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J 19: 4046–4055, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 351: 1425–1436, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Jeffery TK, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis 45: 173–202, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol 12: 735–739, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Levy M, Maurey C, Celermajer DS, Vouhe PR, Danel C, Bonnet D, Israel-Biet D. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol 49: 803–810, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Fernandez LG, Dodd-o J, Langer J, Wang D, Laubach VE. Upregulation of hypoxia-induced mitogenic factor in compensatory lung growth after pneumonectomy. Am J Respir Cell Mol Biol 32: 185–191, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Liu T, Dhanasekaran SM, Jin H, Hu B, Tomlins SA, Chinnaiyan AM, Phan SH. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol 164: 1315–1326, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T, Jin H, Ullenbruch M, Hu B, Hashimoto N, Moore B, McKenzie A, Lukacs NW, Phan SH. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: role of IL-4/IL-13 and mediation via STAT-6. J Immunol 173: 3425–3431, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Madjdpour C, Jewell UR, Kneller S, Ziegler U, Schwendener R, Booy C, Klausli L, Pasch T, Schimmer RC, Beck-Schimmer B. Decreased alveolar oxygen induces lung inflammation. Am J Physiol Lung Cell Mol Physiol 284: L360–L367, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L548–L554, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA 98: 8798–8803, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mora AL, Torres-Gonzalez E, Rojas M, Corredor C, Ritzenthaler J, Xu J, Roman J, Brigham K, Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol 35: 466–473, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mu H, Ohashi R, Yan S, Chai H, Yang H, Lin P, Yao Q, Chen C. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res 70: 146–157, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Nohira T, Nagao K, Kameyama K, Nakai H, Fukumine N, Okabe K, Kitano S, Hisatomi H. Identification of an alternative splicing transcript for the resistin gene and distribution of its mRNA in human tissue. Eur J Endocrinol 151: 151–154, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Quinlan TR, Li D, Laubach VE, Shesely EG, Zhou N, Johns RA. eNOS-deficient mice show reduced pulmonary vascular proliferation and remodeling to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 279: L641–L650, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh GG. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol 71: 597–602, 2002. [PubMed] [Google Scholar]
- 27.Renigunta A, Hild C, Rose F, Klepetko W, Grimminger F, Seeger W, Hanze J. Human RELMbeta is a mitogenic factor in lung cells and induced in hypoxia. FEBS Lett 580: 900–903, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Rubin LJ Primary pulmonary hypertension. N Engl J Med 336: 111–117, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 43: 5S–12S, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, Lazar MA. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA 98: 502–506, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su Q, Zhou Y, Johns RA. Bruton's tyrosine kinase (BTK) is a binding partner for hypoxia induced mitogenic factor (HIMF/FIZZ1) and mediates myeloid cell chemotaxis. FASEB J 7: 1376–1382, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Swain SD, Han S, Harmsen A, Shampeny K, Harmsen AG. Pulmonary hypertension can be a sequela of prior pneumocystis pneumonia. Am J Pathol 171: 790–799, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 11: 214–222, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELMalpha, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res 92: 1065–1067, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Tong Q, Zheng L, Kang Q, Dodd O, Langer J, Li B, Wang D, Li D. Upregulation of hypoxia-induced mitogenic factor in bacterial lipopolysaccharide-induced acute lung injury. FEBS Lett 580: 2207–2215, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Tong Q, Zheng L, Li B, Wang D, Huang C, Matuschak GM, Li D. Hypoxia-induced mitogenic factor enhances angiogenesis by promoting proliferation and migration of endothelial cells. Exp Cell Res 312: 3559–3569, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 28: 23–42, vii, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuder RM, Voelkel NF. Plexiform lesion in severe pulmonary hypertension: association with glomeruloid lesion. Am J Pathol 159: 382–383, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner KF, Hellberg AK, Balenger S, Depping R, Dodd-o, Johns RA, Li D. Hypoxia-induced mitogenic factor has antiapoptotic action and is upregulated in the developing lung: coexpression with hypoxia-inducible factor-2alpha. Am J Respir Cell Mol Biol 31: 276–282, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Xue C, Johns RA. Upregulation of nitric oxide synthase correlates temporally with onset of pulmonary vascular remodeling in the hypoxic rat. Hypertension 28: 743–753, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Yamaji-Kegan K, Su Q, Angelini DJ, Champion HC, Johns RA. Hypoxia-induced mitogenic factor has proangiogenic and proinflammatory effects in the lung via VEGF and VEGF receptor-2. Am J Physiol Lung Cell Mol Physiol 291: L1159–L1168, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Zaiman AL, Podowski M, Medicherla S, Gordy K, Xu F, Zhen L, Shimoda LA, Neptune E, Higgins L, Murphy A, Chakravarty S, Protter A, Sehgal PB, Champion HC, Tuder RM. Role of TGF-β/ALK5 kinase in monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med 177: 896–905, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol 76: 4580–4590, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]