Abstract
In this preliminary study, 16 psychotropic-naïve pediatric OCD patients were studied using magnetic resonance spectroscopy (MRS) and genotyped for six candidate polymorphisms in two glutamate system genes. A significant association was identified between the rs1019385 polymorphism of glutamate receptor, ionotropic, N-methyl-d-aspartate 2B (GRIN2B) and decreased anterior cingulate cortex (ACC) glutamatergic concentration (Glx, p=0.02) but not with occipital Glx. These results suggest that GRIN2B may be associated with Glx in ACC, a region consistently implicated in OCD.
Keywords: glutamate transporter, magnetic resonance spectroscopy, N-methyl-d-aspartate
1. Introduction
OCD is a complex genetic disorder, likely involving multiple genes of small effect and environmental factors (Pauls 2008). C andidate gene studies have been conducted based on a priori etiological hypotheses, one of which postulates altered glutamate neurotransmission (MacMaster et al., 2008). Strong support for this hypothesis comes from proton magnetic resonance spectroscopy (1H-MRS) studies revealing greater glutamatergic concentrations (Glx) in caudate (Rosenberg et al., 2000) and lower Glx in anterior cingulate cortex (ACC) (Rosenberg et al., 2004) in pediatric OCD patients. This finding was replicated in adults with OCD (Yucel et al., 2008). Recently, we reported associations between OCD and two glutamate system genes: the glutamate subunit receptor GRIN2B (glutamate receptor, ionotropic, N-methy1-D-aspartate 2B), (Arnold et al., 2004), and the glutamate transporter SLC1A1 (solute linked carrier, family 1, member 1) (Arnold et al., 2006; Dickel et al., 2006). These associations have been replicated in independent samples (Arnold, 2007; Stewart et al., 2007).
Intermediate phenotypes derived from neuroimaging may increase power to identify genetic effects (closer link between genotype and phenotype) and delineate pathways linking risk genes to disorders (Meyer-Lindenberg et al., 2006). To our knowledge, no previous genetic studies of OCD have utilized neuroimaging phenotypes. Therefore, we set out to conduct a proof-of-principle study of an imaging genetics paradigm in which we test associations between glutamate system genes and ACC Glx as a proposed intermediate phenotype for OCD. We hypothesized that putative risk alleles identified in previous candidate gene studies of GRIN2B and SLC1A1 would be associated with reduced ACC Glx in pediatric OCD patients.
2. Methods
2.1. Subjects
Participants included 16 (11 male, 5 female) medication-naïve pediatric OCD patients 7 to 18 years (mean = 11.0 years, S.D. = 3.1). Eight patients overlap with the sample from a previous investigation measuring ACC Glx, with detailed assessment procedures and inclusion/exclusion criteria described previously (Rosenberg et al., 2004). Written informed consent was obtained from parents/guardians, and written assent from pediatric participants. Patients were administered the Schedule for Affective Disorders and Schizophrenia – School-Age Children (Kaufman et al., 1997) and the following clinician-administered instruments (mean ± S.D. in parentheses): Children’s Yale-Brown Obsessive Compulsive Scale (CYBOCS, Scahill et al., 1997) (24.0 ± 7.8),, Hamilton Anxiety Rating Scale (HAM-A, (Hamilton, 1959)) (6.76 ± 3.90), Hamilton Depression Rating Scale (HAM-D; (Hamilton, 1967)) (6.47 ± 4.47), and the Yale Global Tic Severity Scale (YGTSS, Leckman et al, 1989) (3.69 ± 8.72).
2.2. Procedures
2.2.1. Imaging Procedures
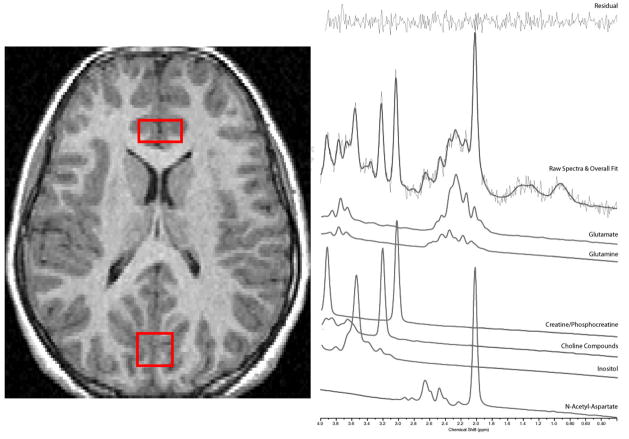
Imaging data were collected using a Sigma 1.5-Tesla unit (Horizon LX software, General Electric Medical Systems, Milwaukee, WI). MRS scanning, image acquisition and analysis procedures are described elsewhere (Rosenberg et al., 2004). Briefly, 1H-MRS spectra were acquired from a 2 × 1.5 × 1cm (3cc) voxel centered in ACC and a 2 × 2 × 2cm (8cc) voxel centered in OCC (see figure 1) with a short-echo single voxel double spin-echo point resolved spectroscopy (PRESS) pulse sequence (Bottomley, 1987). The anterior cingulate voxel contained predominantly bilateral anterior cingulate gray matter (Brodmann areas 32 and 12) with minor contributions from midline CSF and cingulum white matter. Using LCModel (Provencher, 2001), we acquired data for: Glx (includes glutamate and glutamine), N-acetyl-aspartate (NAA), choline compounds (Cho), creatine/phosphocreatine (Cr), and myo-inositol (mI). We measured ACC volume with boundaries used previously (Szeszko et al., 2004).
Figure 1.
1H-MRS voxel placement (A) and Sample Spectra from the Anterior Cingulate and Occipital Lobe
2.2.2. Genotyping procedures
Genomic DNA was extracted from blood using standard methods. Polymorphisms were selected based on our Toronto family-based association study (Arnold, 2007), from GRIN2B (rs1019385, rs890, rs1805476 and rs1805502) and SLC1A1 (rs3087879 and rs3056). Another polymorphism in SLC1A1, rs301434 was genotyped but excluded from analysis since there were less than 4 individuals in the homozygote groups. Genotyping was performed using Applied Biosystems Inc. (Foster City, CA) Taqman® assays described elsewhere (Arnold, 2007).
2.3. Statistical Analysis
ANCOVA analyses were performed (Statistical Package for Social Sciences (SPSS), version 15) with genotypes as independent variables and ACC metabolites as dependent variables. We hypothesized a priori that candidate gene associations would be specific to Glx. Covariates included age and ACC volume (see Rosenberg & Keshavan, 1998). As a control, we analyzed for associations with occipital Glx, with age and gray matter volume as covariates. To directly contrast effects of brain region, two-way repeated measures ANCOVA was performed with brain region (ACC or OCC) as the within-subjects factor, genotype as the between-subjects factor, and age as a covariate. When there were less than five individuals per genotype group these subjects were grouped together with heterozygotes (see Papassotiropoulos et al., 2006). Normality and homogeneity of variance between groups were checked through boxplots and Levene’s test.
3. Results
The mean absolute concentration of Glx was 9.94 millimoles (mM) for ACC and 10.38 mM for OCC. The mean SD for Glx was 6.53 mM (range 5 to 31 mM) for ACC and 7.20 mM (range 4 to 31 mM) for OCC. The only statistically significant association was between GRIN2B-rs1019385 and ACC Glx. Decreased ACC Glx was seen with G/G genotype compared with T/G or T/T (F=6.2 (1, 13), P=0.03). ACC Glx values for rs1019385 genotype groups were normally distributed. Repeated analysis after removing a single outlier (Glx level =13.4) maintained a strong trend for association (F=4.26 (1, 12), P=0.06). Data for ACC and OCC Glx by genotype are illustrated in Table 1. Adding ACC volume to age as a covariate did not change results. Since Glx may change with menstrual cycle (Batra et al., 2008) we repeated analyses after removing the only post-menarchal female, with no effect on results.
Table 1.
Glutamatergic Concentration (Glx) and Genotype
| F value1(df) | P value | F value (df) | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Region-Gene Association | Polymorphism | Genotype | N | Mean Glx in mM (S.D.)2 | Controlled for Age | Controlled for Age, Volume3 | ||
| ACC – GRIN2B | rs1019385 | G/G | 6 | 9.60 (1.39) | 6.20(1,13) | 0.03 | 5.86(1,12) | 0.03 |
| G/T or T/T | 10 | 10.44 (1.19) | ||||||
| rs890 | T/T | 4 | 10.41 (0.36) | 0.06 (1,12) | 0.81 | 0.07 (1,11) | 0.80 | |
| T/G or G/G | 11 | 10.07 (1.55) | ||||||
| rs1805476 | A/A or A/C | 8 | 10.11 (1.75)4 | 1.70 (1.12) | 0.22 | 2.76 (1,11) | 0.12 | |
| C/C | 7 | 10.00 (0.65) | ||||||
| rs18055025 | T/T | 9 | 10.03 (1.72) | 0.25 (1,12) | 0.63 | 0.23 (1,11) | 0.64 | |
| T/C | 6 | 10.36 (0.40) | ||||||
| ACC – SLC1A14 | rs3087879 | G/G | 6 | 9.66 (1.17) | 0.70 (1,13) | 0.42 | 0.67 (1,12) | 0.43 |
| G/C or C/C | 10 | 10.40 (1.34) | ||||||
| rs3056 | G/A | 5 | 10.26 (0.44) | 0.25 (1,12) | 0.62 | 0.21 (1,11) | 0.66 | |
| A/A | 10 | 10.08 (1.64) | ||||||
| OCC – GRIN2B | rs1019385 | G/G | 6 | 8.45 (1.46) | 0.89 (1,13) | 0.36 | 0.94 (1,12) | 0.35 |
| G/T or T/T | 10 | 9.22 (1.81) | ||||||
| rs890 | T/T | 4 | 9.29 (1.09) | 0.10 (1,12) | 0.75 | 0.11 (1,11) | 0.74 | |
| T/G or G/G | 11 | 8.75 (1.94) | ||||||
| rs1805476 | A/A or A/C | 8 | 9.53 (0.91)4 | 3.05 (1.12) | 0.11 | 2.80 (1,11) | 0.12 | |
| C/C | 7 | 7.95 (1.93) | ||||||
| rs18055025 | T/T | 9 | 9.07 (1.92) | 0.54 (1,12) | 0.48 | 0.34 (1,11) | 0.57 | |
| T/C | 6 | 8.65 (1.54) | ||||||
| OCC – SLC1A1 | rs3087879 | G/G | 6 | 8.65 (1.60) | 0.12 (1,13) | 0.73 | 0.08 (1,12) | 0.79 |
| G/C or C/C | 10 | 9.10 (1.79) | ||||||
| rs3056 | G/A | 5 | 8.74 (1.64) | 0.16 (1,12) | 0.69 | 0.15 (1,11) | 0.70 | |
| A/A | 10 | 8.90 (1.82) | ||||||
ACC = Anterior Cingulate Cortex; OCC = Occipital Cortex
F and p values are for tests of between-subjects effects.
Mean Glx = absolute concentration in millimoles (mM) with standard deviation in parentheses.
Volume = Total ACC volume for ACC, Total grey matter volume for OCC.
Levene’s test violated, indicating that error variances are not equal across genotype groups.
None of the subjects had the least common C/C genotype
Significant p values (p<0.05) are indicated in bold text.
N.B. Trend for significance (F=4.26, p=0.06) for GRIN2B-rs1019385 after removal of outlier
CYBOCS was not different between GRIN2B-rs1019385 genotype groups (F=0.31 (1,13), P=0.59). The association betweenGRIN2B rs1019385 and ACC Glx remained statistically significant when controlling for CYBOCS score (F=5.37 (1, 12), P=0.046), HAM-D score (F=6.38 (1, 12), P=0.03) or HAM-A score (F=10.38 (1, 12), P=0.008).
No associations were found with occipital Glx. No region by genotype interaction was identified using two-way repeated measures ANCOVA for GRIN2B-rs1019385 (F=0.28 (1, 13), P=.87). The only significant region by genotype interaction was with GRIN2B-rs1805476 (F=5.66 (1, 12), P=0.04) due to a greater difference between genotype groups for OCC (Table 1) compared with ACC. No associations were found with ACC NAA, Cho, Cr, or mI).
4. Discussion
In this preliminary study, we found a significant association between ACC but not OCC Glx and the GRIN2B-rs1019385 polymorphism, with GG individuals exhibiting decreased Glx compared with carriers of the T allele. Our family-based studies have found the G allele (GG genotype specifically) to be associated with OCD (Arnold, 2007). The G allele of rs1019385 is a promoter region variant that leads to reduced transcription (Miyatake et al., 2002), which could affect glutamate neurotransmission. Association between the risk G/G genotype and ACC Glx is consistent with previous studies implicating ACC in OCD (Szeszko et al., 2004) and is in the expected direction (lower Glx) (Rosenberg et al., 2004). The same pattern was observed in the other 5 polymorphisms, in that the putative risk genotype (e.g. the G/G genotype for rs890, Arnold et al., 2004) was correlated with lower Glx, although these associations were not statistically significant and may not have been detected due to low power.
Strengths of this study include minimizing confounding of 1H-MRS results due to chronic illness and pharmacotherapy, but limitations exist. Small sample size resulted in limited power to detect between-group differences (Type II error), while there was a risk of Type I error from multiple comparisons. The problem of Type I error was mitigated by strong a priori hypotheses regarding the SNPs, which have all been associated with OCD in previous studies, however we acknowledge that our results would not hold up to correction for multiple comparisons,. Nonetheless, we believe our results represent a novel proof-of-principle experiment of an imaging genetics paradigm in OCD and therefore are of sufficient interest to be reported. We hope that these findings stimulate further research including replication in independent samples, which is required to confirm genetic association results (Gorroochurn et al., 2007).
Using 1.5 T MRS enabled measurement of Glx, a multi-peak signal including glutamate, glutamine, and gamma-amino butyric acid (GABA) rather than glutamate alone. Although Glx may be a reasonable proxy for glutamate (Provencher, 1993; Rosenberg et al., 2005) there is consensus in the field that the subcomponents of Glx are best resolved at higher field. Although we controlled for intracranial volume, ideally one would control for proportions of grey and white matter and cerebrospinal fluid. Using tissue segmentation to better account for voxel water content allows for the more accurate calculation of absolute concentration. Not employing this technique may contribute to measurement error (Stanley et al., 1995). We have initiated studies at high field (3T) for better resolution of Glx subcomponents and are controlling for grey matter, white matter, and CSF in order to ensure the most accurate possible calculations of absolute concentration.
Future studies should explore other regions implicated in OCD (e.g. caudate and orbital-frontal cortex) and/or employ more comprehensive candidate gene or genome-wide association approaches. As approximately 30% of Caucasians are G/G and most don’t have OCD, and most individuals with OCD do not have G/G, variation in rs1019385 is neither necessary nor sufficient for the disorder. This situation is expected with complex genetic traits, in which risk factors are probabilistic rather than deterministic in nature (Page et al., 2003). We did not detect any difference in symptom severity between rs1019385 genotype groups, however other possible genotype-phenotype associations (e.g. family history, drug response, symptoms subtype) should be further explored.
In summary, ACC, but not occipital, Glx was associated with a GRIN2B variant that may confer increased risk for OCD. Findings were in the expected direction, associated with decreased ACC Glx. This is the first published report to examine the relationship between genetic variation and a neurochemical phenotype in OCD, and therefore represents a novel proof-of-principle experiment. We hope that our findings will stimulate further research on the genetic basis of OCD, ultimately leading to improved diagnosis and treatment.
Acknowledgments
We thank Ms. Tamara Arenovich of the Biostatistics Unit of the Centre for Addiction and Mental Health for statistical support and consultation. We also thank Mr. S.M. Shaheen for editorial assistance.
Support was provided by the Ontario Mental Health Foundation through a Type B grant (PDA, MAR, JLK), the Canadian Institutes for Health Research through an operating grant (MOP-38077) (PDA, EM, JLK, MAR) and Fellowship to Dr. Arnold, the National Alliance on Research in Schizophrenia and Depression (Young Investigator Award, PDA), an Obsessive-Compulsive Foundation Research Award (PDA, MAR, JLK), Joe F. Young Sr. Psychiatric Research and Training Program, the Miriam Hamburger Endowed Chair of Child Psychiatry at Children’s Hospital of Michigan and Wayne State University and the National Institute of Mental Health (R01MH59299, K24MH02037).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold PD. PhD Dissertation. University of Toronto; Toronto: 2007. Glutamate genes in obsessive-compulsive disorder including childhood onset cases and neuroimaging. [Google Scholar]
- Arnold PD, Rosenberg DR, Mundo E, Tharmalingam S, Kennedy JL, Richter MA. Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: a preliminary study. Psychopharmacology (Berl) 2004b;174(4):530–538. doi: 10.1007/s00213-004-1847-1. [DOI] [PubMed] [Google Scholar]
- Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63(7):769–776. doi: 10.1001/archpsyc.63.7.769. [DOI] [PubMed] [Google Scholar]
- Batra NA, Seres-Mailo J, Hanstock C, Seres P, Khudabux J, Bellavance F, Baker G, Allen P, Tibbo P, Hui E, Le Melledo JM. Proton magnetic resonance spectroscopy measurement of brain glutamate levels in premenstrual dysphoric disorder. Biol Psychiatry. 2008;63:1178–1184. doi: 10.1016/j.biopsych.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH, Jr, Hanna GL. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63(7):778–785. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- Gorroochurn P, Hodge SE, Heiman GA, Greenberg DA. A unified approach for quantifying, testing and correcting population stratification in case-control association studies. Hum Hered. 2007;64(3):149–59. doi: 10.1159/000102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ. The yale global tic severity scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Macmaster FP, O’Neill J, Rosenberg DR. Brain imaging in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2008 doi: 10.1097/CHI.0b013e318185d2be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7(10):818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Miyatake R, Furukawa A, Suwaki H. Identification of a novel variant of the human NR2B gene promoter region and its possible association with schizophrenia. Mol Psychiatry. 2002;7(10):1101–1106. doi: 10.1038/sj.mp.4001152. [DOI] [PubMed] [Google Scholar]
- Page GP, George V, Go RC, Page PZ, Allison DB. “Are we there yet?”: Deciding when one has demonstrated specific genetic causation in complex diseases and quantitative traits. Am J Hum Genet. 2003;73:711–9. doi: 10.1086/378900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papassotiropoulos A, Stephan DA, Huentelman MJ, Hoerndli FJ, Craig DW, Pearson JV, Huynh KD, Brunner F, Corneveaux J, Osborne D, Wollmer MA, Aerni A, Coluccia D, Hanggi J, Mondadori CR, Buchmann A, Reiman EM, Caselli RJ, Henke K, de Quervain DJ. Common Kibra alleles are associated with human memory performance. Science. 2006;314(5798):475–478. doi: 10.1126/science.1129837. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. T he cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20(1):91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Pauls DL. The genetics of obsessive compulsive disorder: a review of the evidence. Am J Med Genet C Semin Med Genet. 2008 May 15;148(2):133–9. doi: 10.1002/ajmg.c.30168. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Rosenberg D, Keshavan M. Toward a neurodevelopmental model of obsessive-compulsive disorder. Biol Psychiatry. 1998;43:623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg D, MacMaster F, Keshavan M, Fitzgerald K, Stewart C, Moore G. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry. 2000;39(9):1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, Bhandari R, Rose M, Ivey J, Boyd C, Moore GJ. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatry. 2004;43(9):1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, MacMaster FP, Mirza Y, Smith JM, Easter PC, Banerjee SP, Bhandari R, Boyd C, Lynch M, Rose M, Ivey J, Villafuerte RA, Moore GJ, Renshaw P. Reduced anterior cingulate glutamate in pediatric major depression: a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58(9):700–704. doi: 10.1016/j.biopsych.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Stanley JA, Drost DJ, Williamson PC, Thompson RT. The use of a priori knowledge to quantify short echo in vivo 1H MR spectra. Magnetic Resonance in Medicine. 1995;34(1):17–24. doi: 10.1002/mrm.1910340105. [DOI] [PubMed] [Google Scholar]
- Stewart SE, Fagerness JA, Platko J, Smoller JW, Scharf JM, Illmann C, Jenike E, Chabane N, Leboyer M, Delorme R, Jenike MA, Pauls DL. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144(8):1027–1033. doi: 10.1002/ajmg.b.30533. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Chen S, Baribault K, Lim KO, Ivey J, Rose M, Banerjee SP, Bhandari R, Moore GJ, Rosenberg DR. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. Am J Psychiatry. 2004;161(6):1049–1056. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- Yücel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J, Velakoulis D, Pantelis C. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Aust N Z J Psychiatry. 2008;42(6):467–77. doi: 10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]