Abstract
Objective quantification of epidermal melanin concentration (EMC) should be useful in laser dermatology to determine the individual maximum safe radiant exposure (IMSRE). We propose a single-wavelength remittance measurement at 390 nm as an alternative optical method to determine EMC and IMSRE. Remittance spectra (360 to 740 nm), melanin index (MI) measurements and the transient radiometric temperature increase, ΔT(t), upon skin irradiation with an Alexandrite laser (755 nm, 3-ms pulse duration, 6 J/cm2) were measured on 749 skin spots (arm and calf) on 23 volunteers (skin phototypes I to IV). Due to the shallow penetration depth and independence of blood oxygen saturation (isosbestic point), remittance at 390 nm appears to provide better estimates for EMC and IMSRE than MI.
Keywords: melanin concentration, diffuse remittance, isosbestic point, oxygen saturation, penetration depth
1 Introduction
The individual maximum safe radiant exposure (IMSRE) in many dermatological laser therapies depends mainly on epidermal melanin concentration (EMC), which varies on an individual patient basis. For the clinical purpose of EMC/IMSRE determination, the Fitzpatrick scale is of relatively little use, since it provides insufficient precision (only six categories), is subjectively assessed, and was designed to express response to UV irradiation instead of quantifying EMC. Most commercially available EMC meters [e.g., mexameter (Courage and Khazaka), erythema-melanin meter (Diastron), dermaspectrometer (Cortex)] use a variation of a method first proposed by Dawson et al.1 In this approach, remittance at two (infra-) red wavelengths (where absorption by oxyhemoglobin is small compared to that of melanin) is used to derive a metric for EMC, often referred to as melanin index (MI). An acknowledged2,3 limitation of this approach is that due to deep penetration of red light (> 1 mm) into human skin, variations in blood oxygenation or dermal blood volume fraction can significantly affect the EMC estimate. This inherent limitation may be why laser therapists do not commonly use these instruments to determine IMSRE. Guidelines to translate MI into IMSRE values are absent in the literature, which may be related to the lack of standardization: different commercial implementations use different sets of (infra-) red wavelengths, algorithms, and MI scales. Validation studies correlating MI or an alternative expression for optically estimated EMC with independent, not purely optical, measurements of EMC are rare.3–6
We recently demonstrated that a photothermal (PT) measurement can be used as an independent estimate for EMC and IMSRE.7 The PT method has intuitive appeal because its fundamental measurement (laser-induced temperature increase ΔT) relates directly to the laser impact during a therapeutic laser pulse.
2 Methods
For this study, three basic measurements were performed on the forearm and calf of volunteers: remittance spectroscopy, MI, and a PT measurement. Remittance spectroscopy was performed with a handheld spectrometer (CM-2600d, Konica Minolta, Japan, 360 to 740 nm, 10-nm increments at 15-nm bandwidth). An 8-mm aperture was used. MI measurements were performed with a mexameter, which uses wavelengths 566, 659, and 873 nm. The latter two wavelengths are presumably used to determine MI using a proprietary algorithm (but likely similar to Dawson's1) and 566 nm to determine an additional erythema index (EI). PT measurements were performed with a custom-built handheld probe consisting of an uncooled photovoltaic IR photodetector (PVI-5, spectral bandwidth 2.5 to 5 μm, HgCdZnTe, Boston Electronics, Boston) aligned with the hand piece of an Alexandrite laser (755 nm, 3-ms pulse duration, Gentlelase, Candela, Wayland, Massachussetts). The PT probe was calibrated prior to each measurement with a blackbody (BB701, Omega) and measured ΔT(t) signals with 1-ms temporal resolution up to 1 s after laser exposure. Alignment of the laser hand piece and IR detector was such that the laser irradiated (at 6 J/cm2) a slightly elliptical skin spot (18 mm diameter) and the IR detector measured the radiometric temperature increase in a slightly smaller area. PT measurements are essentially the same as those measured with an IR camera after spatial averaging.7 Remittance spectroscopy and MI measurements were always prior to the PT measurement, repeated three times on the same skin spot and averaged to reduce measurement error (standard deviation for n = 3 approximately 2%).
Twenty-three healthy adult volunteers enrolled in the study and gave witnessed informed consent (IRB #1999-2250). Enrollment was not guided by skin phototype. However, the full spectrum of phototypes I to IV was reasonably represented. Per skin area (arm or calf), 15 skin spots were identified for testing. Overall, measurements were on 50 skin areas and 749 skin spots. (One data point was eliminated due to movement artifact during the PT measurement.) Preparation of the skin sites prior to measurements was the same as that described in a recent publication.7
3 Results
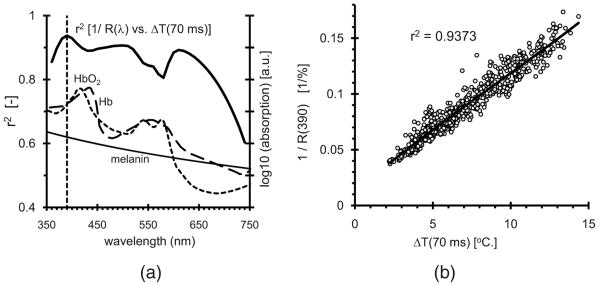
We analyzed our data using the very simple rationale that remittance is inversely proportional with EMC (disregarding influence of scattering and absorption by blood) and ΔT(t) is proportional to EMC. We computed the coefficients of determination r2 for linear regressions between 1/Ri(λ) and ΔTi(t) (with i an index for 749 skin spots) for each combination of λ and t. The highest r2 value (0.9372) was for λ = 390 nm and t = 70 ms. The uppermost curve in Fig. 1(a) represents the r2 for 1/R(λ) versus ΔT(70 ms), clearly featuring a peak at 390 nm. It should be noted that the choice of 70 ms is not critical; all r2 values for 20 ms<t<450 ms are higher than 0.921 for λ = 390 nm. Regardless of t, the maximum r2 was always at 390 nm. The individual data points for λ = 390 nm and t = 70 ms are shown in Fig. 1(b) and display a surprisingly linear relationship between 1/R (390 nm) and ΔT (70 ms).
Fig. 1.
(a) Coefficients of determination for 1/R (λ) versus ΔT (70 ms). Absorption curves (right axis) for Hb, HbO2, and melanin. The vertical line at 390 nm represents an isosbestic wavelength coinciding with the peak correlation. (b) 1/R (390 nm) versus ΔT (70 ms).
MI data were similarly analyzed. Computing r2 values for MIi versus ΔTi(t) for all t revealed that the highest correlation (r2=0.9354) also occurred at t=70 ms. Temporal dependence of r2 is very similar to that for 1/R (390 nm) versus ΔT(t), although the r2 are slightly lower for all t. The relationship between MIi and ΔTi (70 ms) is very similar to the data in Fig. 1(b) (equally linear, slightly lower r2), with the exception that data for subjects with lighter skin phototypes (low ΔT) deviate significantly more from the linear fit (not shown) than the 1/R (390 nm) data.
For the purpose of predicting IMSRE, the reciprocal value of EMC is of interest.7 Therefore, we also calculated r2 for the reciprocal values: Ri(λ) versus 1/ΔTi(t) and 1/MIi versus 1/ΔTi(t). Whereas for the R (390 nm) method, r2 values are still high (maximum r2 = 0.934 at 140 ms, and r2 > 0.91 for 24 ms < t < 800 ms), for 1/MI versus ΔT(t), the maximum r2 is much lower (0.864) at t = 35 ms. The lower value is mostly due to the relatively high weighing of the data from subjects with light skin phototypes, for which MI data were found to have relatively large deviations from the best linear fit (not shown). Figures 2(a) and 2(b) show R (390 nm) and 1/MI data, respectively, plotted versus 1/ΔT (35 ms). These data illustrate how either R (390 nm) or 1/MI measurements can be used to determine IMSRE [assuming that IMSRE is proportional to 1/ΔT for short times t (Ref. 7)]. Lines in Figs. 2(a) and 2(b) are second- and third-order, respectively, polynomial best fits with corresponding r2 values as indicated. Increasing the polynomial order from 2 to 3 for the R (390 nm) data did not produce a higher r2. To estimate the precision with which 1/ΔT (35 ms) and, by implication, IMSRE may be predicted with either R (390 nm) or 1/MI, we show the relative deviations from the best fits in Figs. 2(c) and 2(d). Although for subjects with relatively dark skin (1/ΔT < 0.15), the median absolute error for 1/MI is smaller (5.1%) than for R (390 nm) (5.6%), the number of outlying data points for 1/MI is larger. For subjects with lighter skin, the R (390 nm) method is clearly more accurate than the 1/MI estimates. The overall median absolute errors are 5.2% and 5.6% for R (390 nm) and 1/MI, respectively. Note that the choice of 35 ms favors the MI method since the maximum correlation was found at this time. For other times (e.g., t = 20 ms, which was used to predict IMSRE recently7), the same analysis shows R (390 nm) to be more accurate in predicting 1/ΔT for the full range of phototypes considered here. The 15 data points with the highest 1/MI values [Fig. 2(b)] are all for one individual (skin phototype I, many freckles) and heavily determine the fit shape. The fit, therefore, would likely change significantly if data for more individuals with similar pigmentation were available. This means that the estimated MI errors for fair skin are likely to be larger.
Fig. 2.
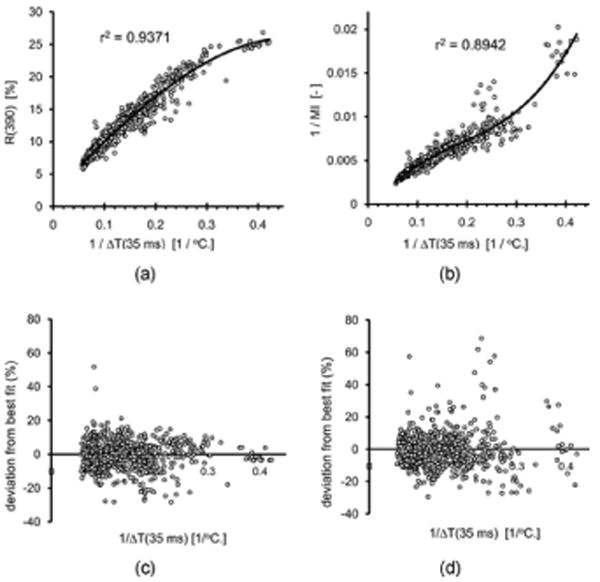
(a) Data of Fig. 1(b) plotted as reciprocal values (axes proportional to IMSRE). (b) Similarly plot for the mexameter. Relative deviations from fit lines are shown in (c) and (d), respectively, providing a measure for the IMSRE prediction accuracy.
4 Discussion and Conclusions
Three explanations for the very surprising robustness of 1/R (390 nm) to predict EMC can be identified. First, intra- and inter-individual variations in oxygenation are of minimal influence on the remittance at 390 nm since this wavelength represents an isosbestic point for Hb and HbO2 [Fig. 1(a)]. Second, due to high scattering of 390-nm light in human skin, this wavelength probes predominantly the epidermis and is therefore only moderately sensitive for inter- and intra-individual variations in dermal blood volume (see the following). Third, using a skin remittance model,8 we found that the two wavelength methods are sensitive for skin surface melanin concentration (integrated over depth) measuring equal MI regardless of whether the same amount of melanin is distributed over a thick or thin epidermis. This is because the penetration depth of red light is at least an order of magnitude larger than the epidermal thickness, and thus, the remittance is virtually independent of how the melanin is distributed. In contrast, due to its much shallower penetration depth (in the same order of magnitude as the epidermal thickness), R (390 nm) is more sensitive for volumetric melanin concentration (proportional to absorption coefficient). At short times t, ΔT(t) is also more proportional to the volumetric melanin concentration.
The rationale for relating 1/R(λ) to ΔT ignores inter- and intra-individual variations in scattering, which are, obviously, of strong influence on remittance. Our data suggest that among the 23 subjects, variations in scattering are of less concern than variations in blood volume (see the following). A possible explanation is that the main source of scattering at 390 nm is melanin itself; coupling absorption and scattering into a single EMC-dependent parameter.9
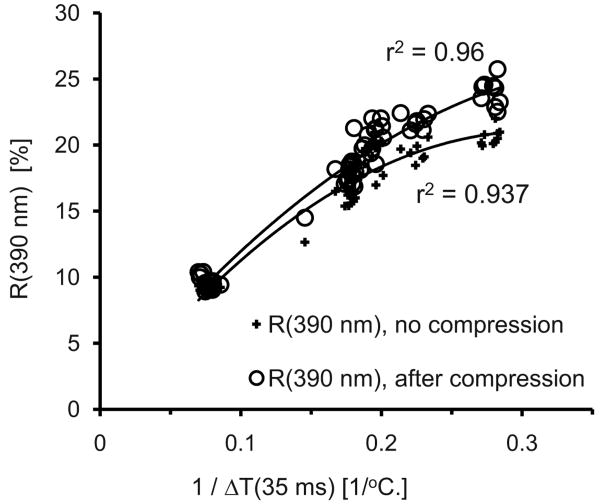
The single-wavelength method at 390 nm may be improved in at least two ways. First, the specified FWHM bandwidth of a channel in the spectrometer is approximately 15 nm. A narrower bandwidth would further reduce the perturbing effect of variations in oxygen saturation and narrow the relationship between 1/R (390 nm) and ΔT. Second, absorption by Hb or HbO2 relative to that of melanin is approximately 10 times greater at 390 nm [Fig. 1(a)] than at 755 nm (used for the PT measurements). Therefore, skin with a relatively high dermal blood volume fraction will have R (390 nm) decreased by a larger amount than its corresponding 1/ΔT value. Deviations from the trend line in Fig. 2(a) seem to confirm this: deviations are somewhat skewed toward the area below the fit. We hypothesize that these data points are for skin with relatively high dermal blood volume fractions. We performed additional measurements on four subjects, 15 spots each, in which we compressed the skin for approximately 2 s and measured R(λ) within approximately 0.5 s after the pressure was released. Compression always increased R (390 nm) compared to R (390 nm) measured without compression. PT measurements were also performed. The R (390 nm) measured after compression correlate better than the R (390 nm) data obtained without compression (Fig. 3), supporting our hypothesis. In other words, the “noise” in Fig. 2(a) may have been smaller had we used compression. Possibly, a more controlled and reproducible compression could be done with a probe that measures R (390 nm) through a UV transparent window that simultaneously compresses the skin, also known as diascopy.10 Diascopy may be useful to enhance MI accuracy as well, although the deeper penetration of red wavelengths would require us to empty blood vessels to much greater depths than for the 390-nm method.
Fig. 3.
Preliminary results of the compression technique. R (390 nm) increases more for lighter skin, rendering a higher correlation with 1/ΔT than presented in Figs. 1 and 2.
Even without the compression technique, we believe that the results are both surprising and promising. In addition to a potentially higher accuracy of estimating EMC, particularly for patients with lighter skin phototypes, the proposed single-wavelength remittance method at 390 nm has a number of potential advantages over existing methods. First, it may be applied on skin with high dermal chromophore concentrations (e.g., tattoos, vascular lesions), where methods using wavelengths with deep penetration into human skin likely give inaccurate results. Second, a probe measuring R (390 nm) may be miniaturized, as opposed to probes using red light, which have to collect light remitted from a relatively large lateral area. Miniaturization would allow for evaluation of small pigmented lesions (without the need to resort to hyperspectral imaging such as the SIAscope11) or of hard to access areas such as between the toes or in the ear.
To the best of our knowledge, a single wavelength in the UV at an isosbestic point is a novel method to quantify pigmentation. A single-wavelength or single-band method to estimate EMC was proposed12,13 but referred to red wavelengths. The UV or near UV to quantify EMC has been explored but always involved algorithms using multiple wavelengths,5,14,15 which might provide better correlation than the single-wavelength R (390 nm) method. To date, however, we have not been able to identify such an algorithm with our current set of data. We evaluated an existing algorithm using two UV wavelengths5—420 and 400 nm—and found a poor correlation with ΔT. Similarly, the two-wavelength method was evaluated for 700 to 650 nm,1 720 to 620 nm,16,17 and 710 to 650 nm.18 All revealed much better correlation than the 420- 400-nm method but performed poorer than the R (390 nm) and MI methods, in particular for lighter skin. We also tried, unsuccessfully, to use the EI (measured simultaneously with the MI), to improve the correlation with ΔT. Other commercial melanin meters have not been evaluated yet. Last, just like many proposed optical methods to quantify EMC, our method should be calibrated and validated with EMC values obtained with histochemical methods, to express 1/R (390 nm) in units of melanin mass per skin volume.
With these reservations in mind, and to the extent that ΔT for early times t (e.g., <50 ms) can be assumed a good measure of EMC, we conclude that R (390 nm) is a promising method to quantify EMC and associated IMSRE for the range of phototypes studied.
Acknowledgments
We greatly appreciate the Candela Corporation for the use of the Gentlelase Alexandrite laser. This work was made possible through the funding of the following. NIH: K24 IRT and NIBIB: EB-02495-02.
Contributor Information
Wim Verkruysse, University of California, Irvine Beckman Laser Institute, 1002 Health Sciences Road, Irvine, California 92612.
Lars O. Svaasand, Norwegian University of Science and Technology, Department of Electronics and Telecommunications, Trondheim 7006, Norway
Walfre Franco, Cutera, Inc., 3240 Bayshore Boulevard, Brisbane, California 94005.
J. Stuart Nelson, University of California, Irvine Beckman Laser Institute, 1002 Health Sciences Road, Irvine, California 92612.
References
- 1.Dawson JB, Barker DJ, Ellis DJ, Grassam E, Cotterill JA, Fisher GW, Feather JW. A theoretical and experimental-study of light-absorption and scattering by in vivo skin. Phys Med Biol. 1980;25:695–709. doi: 10.1088/0031-9155/25/4/008. [DOI] [PubMed] [Google Scholar]
- 2.Stamatas GN, Zmudzka BZ, Kollias N, Beer JZ. Noninvasive measurements of skin pigmentation in situ. Pigment Cell Res. 2004;17:618–626. doi: 10.1111/j.1600-0749.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 3.Matts PJ, Dykes PJ, Marks R. The distribution of melanin in skin determined in vivo. Br J Dermatol. 2007;156:620–628. doi: 10.1111/j.1365-2133.2006.07706.x. [DOI] [PubMed] [Google Scholar]
- 4.Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A. The impact of epidermal melanin on objective measurements of human skin colour. Pigment Cell Res. 2002;15:119–126. doi: 10.1034/j.1600-0749.2002.1o072.x. [DOI] [PubMed] [Google Scholar]
- 5.Dwyer T, Blizzard L, Ashbolt R, Plumb J, Berwick M, Stankovich JM. Cutaneous melanin density of Caucasians measured by spectrophotometry and risk of malignant melanoma, basal cell carcinoma, and squamous cell carcinoma of the skin. Am J Epidemiol. 2002;155:614–621. doi: 10.1093/aje/155.7.614. [DOI] [PubMed] [Google Scholar]
- 6.Dwyer T, Muller HK, Blizzard L, Ashbolt R, Phillips G. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203–206. [PubMed] [Google Scholar]
- 7.Verkruysse W, Jia W, Franco W, Milner TE, Nelson JS. Infrared measurement of human skin temperature to predict the individual maximum safe radiant exposure (IMSRE) Lasers Surg Med. 2007;39:757–766. doi: 10.1002/lsm.20581. [DOI] [PubMed] [Google Scholar]
- 8.Svaasand LO, Norvang LT, Fiskerstrand EJ, Stopps EKS, Berns MW, Nelson JS. Tissue parameters determining the visual appearance of normal skin and port-wine stains. Lasers Med Sci. 1995;10:55–65. [Google Scholar]
- 9.Wolbarsht ML, Walsh AW, George G. Melanin, a unique biological absorber. Appl Opt. 1981;20:2184–2186. doi: 10.1364/AO.20.002184. [DOI] [PubMed] [Google Scholar]
- 10.Kollias N, Stamatas GN. Optical non-invasive approaches to diagnosis of skin diseases. J Investig Dermatol Symp Proc. 2002;7:64–75. doi: 10.1046/j.1523-1747.2002.19635.x. [DOI] [PubMed] [Google Scholar]
- 11.Moncrieff M, Cotton S, Claridge E, Hall P. Spectrophotometric intracutaneous analysis: a new technique for imaging pigmented skin lesions. Br J Dermatol. 2002;146:448–457. doi: 10.1046/j.1365-2133.2002.04569.x. [DOI] [PubMed] [Google Scholar]
- 12.Buckley WR, Grum F. Reflection spectrophotometry—Use in evaluation of skin pigmentary disturbances. Arch Dermatol. 1961;83:249–261. [Google Scholar]
- 13.Takiwaki H, Shirai S, Kanno Y, Watanabe Y, Arase S. Quantification of erythema and pigmentation using a videomicroscope and a computer. Br J Dermatol. 1994;131:85–92. doi: 10.1111/j.1365-2133.1994.tb08462.x. [DOI] [PubMed] [Google Scholar]
- 14.Andersen PH, Bjerring P. Noninvasive computerized analysis of skin chromophores in vivo by reflectance spectroscopy. Photodermatol Photoimmunol Photomed. 1990;7:249–257. [PubMed] [Google Scholar]
- 15.Andersen PH, Bjerring P. Spectral reflectance of human skin in vivo. Photodermatol Photoimmunol Photomed. 1990;7:5–12. [PubMed] [Google Scholar]
- 16.Kollias N, Baqer A. On the assessment of melanin in human-skin in vivo. Photochem Photobiol. 1986;43:49–54. doi: 10.1111/j.1751-1097.1986.tb05590.x. [DOI] [PubMed] [Google Scholar]
- 17.Kollias N, Baqer A. Spectroscopic characteristics of human melanin in vivo. J Invest Dermatol. 1985;85:38–42. doi: 10.1111/1523-1747.ep12275011. [DOI] [PubMed] [Google Scholar]
- 18.Dolotov LE, Sinichkin YP, Tuchin VV, Utz SR, Altshuler GB, Yaroslavsky IV. Design and evaluation of a novel portable erythema-melanin-meter. Lasers Surg Med. 2004;34:127–135. doi: 10.1002/lsm.10233. [DOI] [PubMed] [Google Scholar]