Abstract
Thromboxane (TxA2) and nitric oxide (NO) are potent vasoactive autocoids that modulate tubuloglomerular feedback (TGF). Each is produced in the macula densa (MD) by cyclooxygenase-2 (COX-2) and neuronal nitric oxide synthase (nNOS), respectively. Both enzymes are similarly regulated in the MD and their interaction may be an important factor in the regulation of TGF and glomerular filtration rate. We tested the hypothesis that TGF is modified by the balance between MD nNOS-dependent NO and MD COX-2-dependent TxA2. We measured maximal TGF during perfusion of the loop of Henle (LH) by continuous recording of the proximal tubule stopped flow pressure response to LH perfusion of artificial tubular fluid (ATF) at 0 and 40 nl/min. The response to inhibitors of COX-1 (SC-560), COX-2 [parecoxib (Pxb)], and nNOS (l-NPA) added to the ATF solution was measured in separate nephrons. COX-2 inhibition with Pxb reduced TGF by 46% (ATF + vehicle vs. ATF + Pxb), whereas COX-1 inhibition with SC-560 reduced TGF by only 23%. Pretreatment with intravenous infusion of SQ-29,548, a selective thromboxone/PGH2 receptor (TPR) antagonist, blocked all of the SC-560 effect on TGF, suggesting that this effect was due to activation of TPR. However, SQ-29,548 only partially diminished the effect of Pxb (−66%). Specific inhibition of nNOS with l-NPA increased TGF, as expected. However, the ability of Pxb to reduce TGF was significantly impaired with comicroperfusion of l-NPA. These data suggest that COX-2 modulates TGF by two proconstrictive actions: generation of TxA2 acting on TPR and by simultaneous reduction of NO.
Keywords: blood flow, systemic volume, electrolyte homeostasis
the kidney's ability to regulate its blood flow is a major factor in maintaining systemic volume and electrolyte homeostasis. Renal autoregulation is regulated by two distinct, but possibly interactive, systems that maintain stable renal blood flow (RBF): the vascular myogenic reflex and tubuloglomerular feedback (TGF). Renal myogenic control reflects changes in pressure that generate compensatory changes in tone to maintain consistent flow and is similar to extrarenal vascular regulation. TGF, however, is unique to the kidney and maintains vascular tone in response to the changes in solute delivery sensed at the macula densa (MD) segment of each nephron. TGF acts to stabilize glomerular filtration rate (GFR) by regulating RBF during acute challenges, such as volume, flow, or pressure changes. TGF is mediated by locally generated adenosine acting on receptors in the vessels that alters microvascular tone. However, multiple autocoids and hormones modify TGF; including ATP, ANG II, nitric oxide (NO), prostaglandins (PG), and thromboxane (TxA2) all of which are generated in the juxtaglomerular apparatus.
PGs and TxA2 are produced by specific enzymes acting on products of cyclooxygenase (COX). COX is a family of fatty acid derivatives that regulate vascular tone (16) and also modify TGF (25, 35, 36). The profile of COX-1 and COX-2 products differs, yet both enzymes generate vasodilators (PGE2, PGI2) and vasoconstrictors (TxA2) (24). The expression and location of these enzymes appear to be the major rate-limiting factor. COX-1 is expressed primarily in the distal tubule, connecting tubule, and collecting duct (29). COX-2 is constitutively expressed in the MD and adjacent cortical thick ascending limb of Henle (cTAL) cells (7, 8, 12, 21, 31). The neuronal isoform of NO synthase (nNOS) is also abundantly expressed in MD cells (18, 22, 23, 39). Expression of nNOS and COX-2 in the MD is increased by high-protein intake (43), renovascular hypertension (30), low-salt intake (6, 10), and diabetes (42), all of which alter TGF. However, the interaction of the products of these enzymes, NO and PGs, in acute regulation of TGF has not been clearly established. The aim of the present study is to examine the separate and combined effects of MD COX-2 and nNOS inhibition on TGF and to explore possible interactions. We tested the hypothesis that the balance of products of the MD enzymes nNOS and COX-2 modifies TGF. We measured the maximal TGF response during loop of Henle (LH) perfusion of inhibitors to COX-1, COX-2, and nNOS in anesthetized rats to identify the interaction of these enzymes.
MATERIALS AND METHODS
Male Sprague-Dawley rats weighing 250–300 g were maintained on a standard rat chow (0.3 g/100 g Na+ content) with free access to food and water until the day of the study. The use of animals for this study was approved by the Georgetown University Animal Care and Use Committee and performed according to the National Institutes of Health guidelines for the conduct of experiments in animals.
On the day of the experiment, animals were anesthetized with thiobarbital (100 mg/kg body wt ip inactin; Research Biochemicals, Natick, MA). The trachea was cannulated to allow spontaneous breathing. Cannulas were placed in the left jugular vein for fluid infusion and in the right femoral artery for recording of mean arterial pressure (MAP) from the electrically damped output of a pressure transducer. The left kidney was exposed by a flank incision, cleaned of connective tissue, and stabilized in a Lucite cup. The kidney was bathed in 0.154 M NaCl solution maintained at 37°C. The left ureter and the urinary bladder were cannulated to measure urine volume from both kidneys. After completion of surgery, rats were infused with 0.154 M NaCl solution at 1.5 ml/h to maintain euvolemia. Micropuncture studies were initiated after 30–45 min stabilization and performed as preciously described (36, 37). Briefly, a micropipette (8-μm OD) containing artificial tubular fluid (ATF; in mM: 123 NaCl, 4 NaHCO3, 5 KCl, 2 CaCl2, 7 urea, 2 MgCl2) stained with FD&C #2 dye was inserted into a late proximal tubule (PT) to identify the nephron and the direction of the flow. Subsequently, grease (T grade, Apiezon, Manchester, UK) was inserted into the micropuncture site with a micropipette (8-μm OD) connected to a hydraulic drive (Trent Wells, La Jolla, CA) to halt tubular flow. A perfusion pipette containing ATF with testing compounds or vehicle was inserted into the late PT downstream from the grease block and connected to a nanoliter microperfusion pump (Vestavia Scientific, Birmingham, AL). A pressure pipette (2- to 3-μm OD) was inserted into the PT upstream from the grease block to measure proximal stop-flow pressure (PSF). The micropressure system (model 900A, World Precision Instruments, Sarasota, FL) was connected to a Powerlab (AD Instruments, Colorado Springs, CO) to record MAP and PSF. Maximal TGF for each nephron was assessed by the difference in PSF at zero loop perfusion and during perfusion at 40 nl/min (for 2 min). Although the drugs were delivered via microperfusion into the late PT, other unrecognized cells expressing COX-2 and nNOS may have been affected in this study and had an effect on vascular tone. However, both enzymes are nearly exclusively expressed in the MD and the inhibitory effects on TGF are ascribed to these cells.
Study Design
Series 1.
The acute effects of local COX-2 inhibition on TGF were measured in control rats (n = 6) and in rats treated systemically with the thromboxone/PGH2 receptor (TPR) antagonist SQ-29,548 (SQ; n = 5). a) To determine the effect of the inhibition of MD-COX-2, we microperfused ATF + vehicle (Veh) or parecoxib (Pxb; 10−5 M) into the same blocked, late PT and measured maximal TGF. This dose of Pxb was maximally effective based on pilot dose-response studies. b) To confirm the effect of Pxb was due to the loss of TxA2 production by COX-2, we repeated series 1a in rats systemically pretreated with SQ (8 mg/kg body wt bolus plus 8 mg·kg body wt−1·h−1), a dose that abolished the vasoconstriction generated by U-46619 (U) in a previous study (35) and confirmed in this study.
Series 2.
The effects of local COX-1 inhibition on TGF were measured in control rats (n = 5) and in rats treated systemically with SQ (n = 4). a) To determine the effect of nephron COX-1, we microperfused SC-560 (SC; 10−8 M), a selective COX-1 inhibitor or Veh into the same late PT and measured maximal TGF. b) To determine whether the effects of SC were mediated by TxA2, we microperfused SC or Veh into the late PT and measured TGF in rats pretreated with systemic infusion of SQ as in series 1b.
Series 3.
The effects of local nNOS inhibition with and without simultaneous local COX-2 inhibition on TGF were measured in control rats (n = 9). a) To determine the effect of MD-NO inhibition, TGF was measured in tubules microperfused with Nω-propyl l-arginine (l-NPA; 10−6 M), a highly specific nNOS inhibitor, or Veh. l-NPA is 149-fold more selective for nNOS than endothelial (e)NOS (44). b) To determine the possible interaction of local NO and COX-2, we microperfused Pxb alone or Pxb + l-NPA at maximal doses used in series 1a and series 3a in the same PT and measured maximal TGF.
Drugs
Pxb is a selective inhibitor of COX-2 and is converted to valdecoxib by the enzyme CYP3A4, which is expressed in renal tubular cells (19). The drug was provided by Pharmacia Europe EEIG, SQ and U were purchased from Cayman Chemical (Ann Harbor, MI), l-NPA was purchased from Tocris Bioscience (Ellisville, MO), and SC was kindly provided by Pfizer (Cambridge, MA).
Statistics
The data were analyzed by 2 × 2 repeated-measures ANOVA comparing the effects of microperfused COX inhibitors on TGF in the same nephron during systemic infusion of Veh or SQ, in series 1 and 2. Similar analysis was used in series 3 to compare microperfused Veh or l-NPA with microperfused COX-2 (Pxb) inhibitor in series 3.
RESULTS
Urine flow, MAP, heart rates, and body weights were similar in all groups (Table 1).
Table 1.
BW, V, and MAP of the rats used in the micropuncture studies
| BW, g | V, μl/min |
MAP, mmHg | ||
|---|---|---|---|---|
| Right | Left | |||
| Series 1 (n = 11) | 288±10 | 2.26±0.17 | 2.28±0.19 | 108±1 |
| Series 2 (n = 9) | 261±11 | 2.37±0.13 | 2.39±0.13 | 112±2 |
| Series 3 (n = 9) | 283±9 | 2.65±0.19 | 2.50±0.18 | 105±3 |
Values are means ± SE. BW, body weight; V, urine output; MAP, mean arterial pressure.
Series One
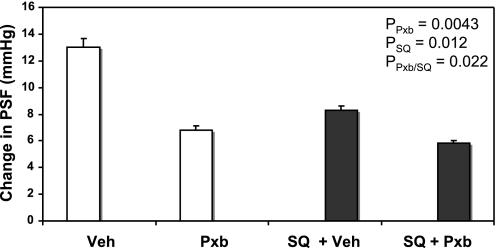
There was an overall inhibitory effect of Pxb (P = 0.0043) and SQ (P = 0.012), with a significant interaction (P = 0.022). Pxb microperfused into the late PT and reduced maximal TGF by 48% compared with Veh (Veh: 13.0 ± 0.7 vs. Pxb: 6.8 ± 0.6 mmHg, P < 0.001, n = 12 tubules; Fig. 1). When rats were treated systemically with SQ, the maximal TGF response was reduced by 36% compared with normal rats (Veh: 13.0 ± 0.7 vs. Veh + SQ: 8.3 ± 0.3 mmHg, P < 0.001, n = 12 tubules), similar to the levels we previously showed (28). In the presence of SQ, the inhibition of TGF by Pxb was 29% compared with Veh (Veh + SQ: 8.3 ± 0.3 vs. Pxb + SQ: 5.8 ± 0.2 mmHg, P < 0.01, n = 12 tubules), suggesting that more than a third (from 46 to 29%) of the inhibitory effect of Pxb is due to the loss of TxA2 activation of TP-R.
Fig. 1.
Maximal tubuloglomerular feedback (TGF) responses [change in proximal stop-flow pressure (PSF) in loop of Henle (LH) perfusion of 0 and 40 nl/min] during perfusion of late proximal tubules with artificial tubular fluid (ATF) + vehicle (Veh), ATF + cyclooxygenase (COX)-2 inhibitor Parecoxib (Pxb; 10−5 M), with (open bars) or without systemic infusion of SQ-29,548 (SQ; closed bars).
Series Two
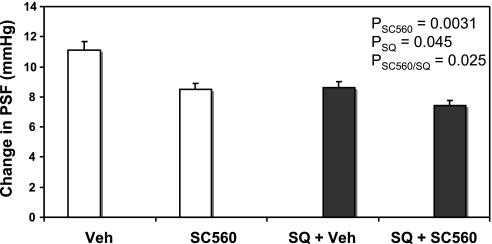
There was a significant effect of SC (P = 0.0031) and SQ (P = 0.045) and interaction of the two (P = 0.025), suggesting that in the presence of SQ, SC was less effective. Post hoc testing showed that the inhibition of local COX-1 by SC decreased TGF by 23% compared with Veh (Veh: 11.1 ± 0.3 vs. SC: 8.5 ± 0.4 mmHg, P < 0.001, n = 9 tubules; Fig. 2). In the presence of SQ, SC reduced TGF by 12%, which was not different from SQ alone (Veh + SQ: 8.6 ± 0.4 vs. SC + SQ: 7.5 ± 0.5 mmHg, n = 8 tubules; Fig. 2). This shows that COX-1 has less impact on TGF than COX-2, but all of its effect is mediated by TxA2 activation of TPR.
Fig. 2.
Maximal TGF responses (change in PSF in LH perfusion of 0 and 40 nl/min) during perfusion of late proximal tubules with ATF + Veh, ATF + COX-1 inhibitor SC-560 (SC; 10−8 M), with (open bars) or without systemic infusion of SQ (closed bars).
To confirm that our dose of SQ sufficiently blocked TPR, we perfused several tubules with the TxA2 agonist U (10−6 M) with and without systemic SQ. U increased TGF by 32% compared with Veh (Veh: 11.1 ± 0.3 vs. U: 14.7 ± 0.7 mmHg, P < 0.001, n = 8 tubules), and this was completely abolished in the presence of systemic SQ (SQ + V: 8.4 ± 0.4 vs. SQ + U: 9.0 ± 0.5 mmHg, P < 0.001, n = 12 tubules).
Series Three
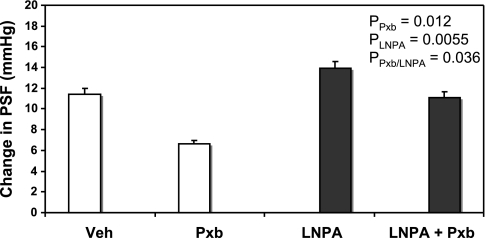
Figure 3 shows that the ability of Pxb to reduce TGF was ameliorated by coperfusion with the selective nNOS inhibitor l-NPA. l-NPA increased TGF compared with Veh (Veh: 11.2 ± 0.5 mmHg vs. l-NPA: 14.1 ± 0.8, P < 0.001, n = 9 tubules), confirming our previous study that MD NO modulates TGF (32). However, during simultaneous microperfusion the effect of Pxb was significantly impaired. ANOVA showed a significant interaction (P = 0.036) between the effects of PxB and l-NPA. When nNOS is blocked by l-NPA, Pxb is less effective.
Fig. 3.
Maximal TGF responses (change in PSF in LH perfusion of 0 and 40 nl/min) during perfusion of late proximal tubules with ATF + Veh, ATF + Pxb (10−5 M; open bars), and ATF + neuronal nitric oxide synthase inhibitor Nω-propyl l-arginine (LNPA; 10−6 M) and ATF + LNPA + Pxb (closed bars).
DISCUSSION
The new information in this study shows that COX-2, which is expressed in the MD, regulates TGF, at least partly via two mechanisms: generation of TxA2 or related agonists of TPR and reduction of nNOS-dependent NO. This is based on our results that show inhibition of MD COX-2 by microperfusion of Pxb into the later PT reduced the maximal TGF control of GFR. This effect was partially dependent on TPR and partially independent of TPR, since the effect of COX-2 inhibition was reduced by blockade of nNOS, which is expressed exclusively in the MD.
TP receptors are located in glomeruli, arterioles, vasa recta, and in the nephron (5, 21, 28, 29, 37) and are modified by salt intake (40) and ANG II (41). The mechanisms involved in TP receptor-induced TGF responses are not completely elucidated. Direct microperfusion of the LH with the TPR agonist U increases TGF and microperfusion of TPR antagonists reduces TGF (35, 36). However, TGF is not different in TPR knockout mice compared with wild-type mice (26). This may be due to compensation by other systems that regulate TGF following deletion of TPR. Blockade of TPR by SQ reduced TGF, as we previously showed (35). However, since TPR are activated by endoperoxide (PGH2) and other ligands, it remains possible that other related agents may regulate TGF.
COX-1 inhibition reduced TGF and this was completely blocked by SQ. This suggests that the modest effect of SC was entirely due to actions on TPR. Since COX-1 is primarily expressed in platelets, these results suggest that plasma levels of TxA2 are sufficient to increase vascular tone, especially on the afferent arteriole. The effect of COX-1 therefore may not be due to TGF, but directly on the microvasculature. However, acute COX-2 inhibition has a greater effect than COX-1 inhibition and its effect was only partially reduced by systemic blockade of TPR. These results suggest that in normal conditions COX-2 affects other vasoactive agents, either a TPR-independent constrictor or an agent that inhibits vasodilation.
One possibility is the production of other vasoconstrictive PGs such as PGE2. PGE2 constricts the afferent arteriole in rats, acting on EP1 or EP3 receptors (1, 2, 16). EP1 receptors are primarily expressed in the collecting duct but are present also in glomeruli (3). EP3 receptors are highly expressed in the thick ascending limb of Henle's loop (TAL) and collecting duct (2, 3). PGE2 has been shown to induce renin release in isolated preglomerular JGA cells (1) and therefore may induce greater vasoconstriction via the renin-angiotensin system. Conversely, PGE2 also inhibits TAL transport through EP3 receptor, which could lead to less TGF (3, 43). Therefore, it remains a possibility that COX-2 could have additional effects on TGF via PGE2. Further studies need to be performed to elucidate this possible pathway.
We tested the alternate possibility that COX-2 suppresses the vasodilator NO. We confirmed that selective inhibition of MD-nNOS, by microperfusing a highly selective inhibitor directly into the late PT, increases TGF (Fig. 3). We further showed that during NO inhibition that Pxb is less effective in reducing TGF, suggesting that NO is normally inhibited by COX-2 and acts to enhance the effects of TxA2. Inhibition of nNOS decreases afferent arteriolar diameter (13, 14) consistent with its effects on TGF (32, 38). Our new data show that during simultaneous COX-2 and nNOS inhibition TGF responses are normalized. This result confirms that these two powerful, yet opposing, vasoactive systems respond to acute changes in solute delivery to the MD. The exact mechanism is not clear.
Several reports suggest that COX and NO interact and that nNOS remains upstream from the COX-2 signal transduction sequence (9, 11, 13, 14, 17). Ichihara et al. (14) showed that COX-2 and nNOS modulate afferent arteriole autoregulatory responses in juxtamedullary nephrons and that COX-2 activity is dependent on nNOS activity. However, the most relevant interaction of these two systems may be related to the generation of superoxide (O2−) by COX-2. O2− is generated by constitutive COX-2 in neural cells (15), in the gastric mucosa (33), in cerebral arteries under normal conditions (4), in the aorta in endotoxic shock (34), and in the diabetic kidney (20). Thus, O2− could react with NO to form ONOO−, reducing the bioavailable NO. This could provide an alternate explanation for our results, as well. Under normal conditions, O2− generated by MD COX-2 reacts with NO to reduce NO. During COX-2 inhibition, there is more NO available, which enhances the Pxb effect on TGF. Thus, without MD-NO, Pxb has less effect on TGF.
These data are the first to show that COX-2-generated TxA2 and nNOS-dependent NO are involved in acute regulation of TGF, but the long-term interaction of these enzymes is less clear. Theilig et al. (30) demonstrated that the coexpression of nNOS in the MD did not modify stimulation or inhibition of COX-2 expression in kidneys of rats and mice. More recently, Paliege et al. (23) using in vivo and in vitro studies showed that COX-2 knockout mice have decreased nNOS expression and activity. They also showed that MD cells incubated with PGE2 had a reduction in nNOS mRNA due to increased intracellular cAMP. Since we demonstrated in this study that TxA2 and NO modulate TGF and enzymes that generate each are colocalized in the MD, further studies in TPR, COX-2, or nNOS knockout mice may elucidate possible long-term interaction of these modulators on TGF.
In conclusion, our data demonstrate that acute inhibition of MD COX-2 suppresses TGF via reduction of the vasoconstrictor TxA2 acting on TP receptors and also by stimulation of nNOS.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol 279: F12–F23, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Breyer M, Davis L, Jacobson H, Breyer RM. Differential localization of prostaglandin E receptor subtypes in human kidney. Am J Physiol Renal Fluid Electrolyte Physiol 270: F912–F918, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Breyer MD, Jacobson HR, Davis LS, Breyer RM. In situ hybridization and localization of mRNA for the rabbit prostaglandin EP receptor. Kidney Int 43: 1372–1378, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Brian JE, Faraci FM, Moore SA. COX-2-dependent delayed dilatation of cerebral arterioles in response to bradykinin. Am J Physiol Heart Circ Physiol 280: H2023–H2029, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Brown GP, Venuto RC. Thromboxane receptors in human kidney tissues. Prostaglandins Other Lipid Mediat 57: 179–188, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Cheng HF, Wang JL, Zhang MZ, McKanna JA, Harris RC. Role of p38 in the regulation of renal cortical cyclooxygenase-2 expression by extracellular chloride. J Clin Invest 106: 681–688, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng HF, Zhang NZ, Harris RC. Nitric oxide stimulates cyclooxygenase-2 in cultured cTAL cells through a p38 dependent pathway. Am J Physiol Renal Physiol 290: F1391–F1397, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cheng HF, Wang JL, Zhang MZ, McKanna JA, Harris RC. Nitric oxide regulates renal cortical cyclooxygenase-2 expression. Am J Physiol Renal Physiol 279: F122–F129, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Deng A, Wead LM, Blantz RC. Temporal adaptation of tubuloglomerular feedback: effects of COX-2. Kidney Int 66: 2348–2353, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94: 2504–2510, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris RC COX-2 and the kidney. J Cardiovasc Pharmacol 47, Suppl 1: S37–S42, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Hartner A, Cordasic N, Goppelt-Struebe M, Veelken R, Hilgers KF. Role of macula densa cyclooxygenase-2 in renovascular hypertension. Am J Physiol Renal Physiol 284: F498–F502, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Ichihara A, Imig JD, Navar LG. Cyclooxygenase-2 modulates afferent arteriolar responses to increases in pressure. Hypertension 34: 843–847, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Ichihara A, Imig JD, Inscho EW, Navar LG. Cyclooxygenase-2 participates in tubular flow-dependent afferent arteriolar tone: interaction with neuronal NOS. Am J Physiol Renal Physiol 275: F605–F612, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Im JY, Kim D, Paik SG, Han PL. Cyclooxygenase-2-dependent neuronal death proceeds via superoxide anion generation. Free Radic Biol Med 41: 960–972, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Inscho E, Carmines P, Navar L. Prostaglandin influences on afferent arteriolar responses to vasoconstrictor agonists. Am J Physiol Renal Fluid Electrolyte Physiol 259: F157–F163, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Kammerl MC, Richthammer W, Kurtz A, Kramer BK. Angiotensin II feedback is a regulator of renocortical renin, COX-2, and nNOS expression. Am J Physiol Regul Integr Comp Physiol 282: R1613–R1617, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs G, Komlosi P, Fuson A, Peti-Peterdi J, Rosivall L, Bell PD. Neuronal nitric oxide synthase: its role and regulation in macula densa cells. J Am Soc Nephrol 14: 2475–2483, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Lash LH, Putt DA, Cai H. Drug metabolism enzyme expression and activity in primary cultures of human proximal tubular cells. Toxicology 244: 56–65, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Chen YJ, Quilley J. Effect of tempol on renal cyclooxygenase expression and activity in experimental diabetes in the rat. J Pharmacol Exp Ther 314: 818–824, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Mannon RB, Coffman TM, Mannon PJ. Distribution of binding sites for thromboxane A2 in the mouse kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F1131–F1138, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Ollerstam A, Perrson EG. Macula densa neuronal nitric oxide synthase. Cardiovasc Res 56: 189–196, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Paliege A, Mizel D, Medina C, Pasumarthy A, Huang YG, Bachmann S, Briggs JP, Schnermann JB, Yang T. Inhibition of nNOS expression in the macula densa by COX-2-derived prostaglandin E2. Am J Physiol Renal Physiol 287: F152–F159, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Qi Z, Cai H, Morrow JD, Breyer MD. Differentiation of cyclooxygenase 1- and 2-derived prostanoids in mouse kidney and aorta. Hypertension 48: 323–328, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Schnermann J, Weber PC. Reversal of indomethacin-induced inhibition of tubuloglomerular feedback by prostaglandin infusion. Prostaglandins 24: 351–361, 1982. [DOI] [PubMed] [Google Scholar]
- 26.Schnermann J, Traynor T, Pohl H, Thomas DW, Coffman TM, Briggs JP. Vasoconstrictor responses in thromboxane receptor knockout mice: tubuloglomerular feedback and uretheral obstruction. Acta Physiol Scand 168: 201–207, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Schweda F, Kammerl M, Wagner C, Kramer BK, Kurtz A. Upregulation of macula densa cyclooxygenase-2 expression is not dependent on glomerular filtration. Am J Physiol Renal Physiol 287: F95–F101, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Silldorff EP, Hilbun LR, Pallone TL. Angiotensin II constriction of rat vasa recta is partially thromboxane dependent. Hypertension 40: 541–546, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi N, Takeuchi K, Abe T, Sugawara A, Abe K. Immunolocalization of rats thromboxane receptor in the kidney. Endocrinology 137: 5170–5173, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Theilig F, Debiec H, Nafz B, Ronco P, Nusing R, Seyberth HW, Pavenstadt H, Bouby N, Bachmann S. Renal cortical regulation of COX-1 and functionally related products in early renovascular hypertension rat. Am J Physiol Renal Physiol 291: F987–F994, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Theilig F, Campean V, Paliege A, Breyer M, Briggs JP, Schnermann J, Bachmann S. Epithelial COX-2 expression is not regulated by nitric oxide in rodent cortex. Hypertension 39: 848–853, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Thorup C, Sundler F, Ekblad E, Persson EG. Resetting of the tubuloglomerular feedback mechanism by blockade of NO synthase. Acta Physiol Scand 148: 359–360, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Villegas I, Martin MJ, La Casa C, Motilva V, De La Lastra CA. Effects of oxicam inhibitors of cyclooxygenase on oxidative stress generation in rat gastric mucosa. A comparative study. Free Radic Res 36: 769–777, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Virdis A, Colucci R, Fornai M, Blandizzi C, Duranti E, Pinto S, Bernardini N, Segnani C, Antonioli L, Taddei S, Salvetti A, Del Tacca M. Cyclooxygenase-2 inhibition improves vascular endothelial dysfunction in a rat model of endotoxic shock: role of inducible nitric oxide synthase and oxidative stress. J Pharmacol Exp Ther 312: 945–953, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Welch WJ Effects of isoprostanes on tubuloglomerular feedback: roles for TP receptors, NOS and salt intake. Am J Physiol Renal Physiol 288: F757–F762, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Welch WJ, Wilcox CS. Potentiation of tubuloglomerular feedback in the rat by thromboxane mimetic: role of macula densa. J Clin Invest 89: 1857–1865, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welch WJ, Peng B, Takeuchi K, Abe K, Wilcox CS. Salt loading enhances rat renal TxA2/PGH2 receptor expression and TGF response to U-46,619. Am J Physiol Renal Physiol 273: F976–F983, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Wilcox CS, Welch WJ, Schmidt HHHW, Murad F, Gross SS, Taylor G, Levi R. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA 89: 11993–11997, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilcox CS, Welch WJ, Snellen H. Thromboxane mediates renal hemodynamic response to infused angiotensin II. Kidney Int 40: 1090–1097, 1991. [DOI] [PubMed] [Google Scholar]
- 40.Wilcox CS, Welch WJ. Thromboxane synthase and TP receptor mRNA in rat kidney and brain: effects of salt intake and ANG II. Am J Physiol Renal Physiol 284: F525–F531, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Winters CJ, Reeves WB, Andreoli TE. A survey of transport properties of the thick ascending limb. Semin Nephrol 11: 236–247, 1991. [PubMed] [Google Scholar]
- 42.Yabuki A, Tahara T, Taniguchi K, Matsumoto M, Suzuki S. Neuronal nitric oxide synthase and cyclooxygenase-2 in diabetic nephropathy of type 2 diabetic OLETF rats. Exp Anim 55: 17–25, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Yao B, Xu J, Qi Z, Harris RC, Zhang MZ. Role of renal cortical cyclooxygenase-2 expression in hyperfiltration in rats with high-protein intake. Am J Physiol Renal Physiol 291: F368–F373, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Zhang HQ, Fast W, Marletta MA, Martasek P, Silverman RB. Potent and selective inhibition of neuronal nitric oxide synthase by N-ω-propyl l-arginine. J Med Chem 40: 3869–3870, 1997. [DOI] [PubMed] [Google Scholar]