Abstract
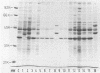
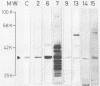
We examined by immunoblot analysis the serum immunoglobulin G antibody response to EDTA-extracted surface proteins of Clostridium difficile in 16 patients with antibiotic-associated diarrhea. For each patient, paired serum samples were tested against proteins of the infecting strain and of a collection strain (C253) known to belong to the electrophoretic group 2 pattern. Eight patients, all harboring group 2 C. difficile strains, exhibited responses to the proteins of the infecting strain; six patients showed increases in the level of antibodies between acute-phase and convalescent-phase sera. A great variability in the antigens recognized was found; however, seven patients possessed antibodies directed against an antigen of about 35 kilodaltons, corresponding to the major protein of group 2 strains. The sera of these seven patients cross-reacted also with the 35-kilodalton and other proteins of strain C253. Our data show that C. difficile proteins other than toxins can elicit an immune response in patients with C. difficile-associated disease; in this group of patients, the major surface protein of the group 2 strains was the antigen most often recognized.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronsson B., Granström M., Möllby R., Nord C. E. Enzyme-linked immunosorbent assay (ELISA) for antibodies to Clostridium difficile toxins in patients with pseudomembranous colitis and antibiotic-associated diarrhoea. J Immunol Methods. 1983 Jun 10;60(3):341–350. doi: 10.1016/0022-1759(83)90291-0. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G. Antibiotic-associated pseudomembranous colitis. Rev Infect Dis. 1979 May-Jun;1(3):530–539. doi: 10.1093/clinids/1.3.530. [DOI] [PubMed] [Google Scholar]
- Borriello S. P., Barclay F. E. Protection of hamsters against Clostridium difficile ileocaecitis by prior colonisation with non-pathogenic strains. J Med Microbiol. 1985 Jun;19(3):339–350. doi: 10.1099/00222615-19-3-339. [DOI] [PubMed] [Google Scholar]
- George W. L., Rolfe R. D., Sutter V. L., Finegold S. M. Diarrhea and colitis associated with antimicrobial therapy in man and animals. Am J Clin Nutr. 1979 Jan;32(1):251–257. doi: 10.1093/ajcn/32.1.251. [DOI] [PubMed] [Google Scholar]
- Gianfrilli P., Luzzi I., Pantosti A., Occhionero M., Gentile G., Panichi G. Cytotoxin and enterotoxin production by Clostridium difficile. Microbiologica. 1984 Oct;7(4):375–379. [PubMed] [Google Scholar]
- Heard S. R., Rasburn B., Matthews R. C., Tabaqchali S. Immunoblotting to demonstrate antigenic and immunogenic differences among nine standard strains of Clostridium difficile. J Clin Microbiol. 1986 Sep;24(3):384–387. doi: 10.1128/jcm.24.3.384-387.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lishman A. H., Al-Jumaili I. J., Record C. O. Antitoxin production in antibiotic-associated colitis? J Clin Pathol. 1981 Apr;34(4):414–415. doi: 10.1136/jcp.34.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Saum K. E., MacDonald D. K., Wilkins T. D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985 Feb;47(2):349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland L. V., Mulligan M. E., Kwok R. Y., Stamm W. E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989 Jan 26;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- Pantosti A., Cerquetti M., Gianfrilli P. M. Electrophoretic characterization of Clostridium difficile strains isolated from antibiotic-associated colitis and other conditions. J Clin Microbiol. 1988 Mar;26(3):540–543. doi: 10.1128/jcm.26.3.540-543.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testore G. P., Pantosti A., Cerquetti M., Babudieri S., Panichi G., Gianfrilli P. M. Evidence for cross-infection in an outbreak of Clostridium difficile-associated diarrhoea in a surgical unit. J Med Microbiol. 1988 Jun;26(2):125–128. doi: 10.1099/00222615-26-2-125. [DOI] [PubMed] [Google Scholar]
- Toma S., Lesiak G., Magus M., Lo H. L., Delmée M. Serotyping of Clostridium difficile. J Clin Microbiol. 1988 Mar;26(3):426–428. doi: 10.1128/jcm.26.3.426-428.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscidi R., Laughon B. E., Yolken R., Bo-Linn P., Moench T., Ryder R. W., Bartlett J. G. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis. 1983 Jul;148(1):93–100. doi: 10.1093/infdis/148.1.93. [DOI] [PubMed] [Google Scholar]