Abstract
Objective
To assess the effect of HIV coreceptor tropism (CRT) on the relative risk of progression to a composite outcome of CD4+ count ≤350 cells/μL, treatment initiation or death.
Methods
CRT assays were performed after study closure in baseline samples obtained from enrollees in a prospectively monitored cohort of treatment-naïve adults with ≥450 CD4+ cells/μL and ≥1,000 HIV-1 RNA copies/mL.
Results
Dual/mixed (D/M) and R5 CRT was detected in 32 and 282 patients, respectively. The baseline CD4+ count (617 vs 694 cells/μL; p=0.05) differed in patients with D/M versus R5 CRT. Otherwise, baseline laboratory characteristics were similar.
The relative risk of progression to the composite endpoint was 2.15 (p=0.002) for D/M versus R5 CRT, 2.07 per 1.0 log10 higher viral load (p<0.001) and 0.87 per 50 cell/μL higher CD4+ cell count (p<0.001). The effect of D/M CRT was also significant in separate analyses of time to initiation of antiretroviral therapy or CD4+ cell count ≤350.
Conclusion
Untreated patients with D/M rather than R5 CRT had a faster rate of disease progression, whether assessed by a composite outcome of time to CD4+ count ≤350, treatment initiation or death, or by separate analyses of time to CD4+ count ≤350 or treatment initiation.
Keywords: Tropism, HIV receptors, Natural history, Progression, Prognosis
Introduction
Recommendations regarding the timing of therapy for HIV-1 – infected patients are based primarily on CD4+ cell count measurements. Present guidelines recommend that therapy be started when the CD4+ cell count is below 350 CD4+ cells/μL. Other considerations include the rate of CD4+ cell loss, the magnitude of the viral load, and symptoms of HIV disease progression1.
The loss of CD4+ cells is largely determined by the plasma HIV-1 RNA concentration2. Other factors that influence the rate of HIV disease progression and CD4+ loss include patient age, HIV-directed immune responses, diminished cellular activation, host HLA genotype and chemokine receptor polymorphisms and characteristics of viral isolates such as deletions in nef, syncitium formation (or the related property of co-receptor tropism) and replicative capacity1;3–13.
Viral isolates from persons in the early stages of HIV-1 infection are usually non-syncytium inducing (NSI) in MT-2 cell cultures whereas isolates from persons with more advanced disease are often syncytium inducing (SI)14–16. Furthermore, emergence of the SI phenotype has been associated with acceleration in the rate of CD4+ cell loss14;17. Importantly, HIV-1 isolates that utilize the CCR5 coreceptor predominantly express the NSI phenotype whereas isolates that utilize the CXCR4 coreceptor largely express the SI phenotype18. These observations and technical considerations have largely led to the replacement of assessment of the NSI/SI phenotype by recombinant virus assays for HIV co-receptor use19–21 or by predictions of co-receptor tropism based on the sequence of the V3 loop of the HIV envelope22–26.
Although several studies have assessed the relationship between HIV-1 co-receptor tropism (CRT) and the rate of HIV disease progression27–30, there are few data regarding the prognostic significance of infection by CCR5 or CXCR4 tropic HIV-1 isolates in a diverse population of chronically infected, treatment naïve patients with relatively preserved CD4+ cell counts30. In this study, we evaluated the relationship between CRT and HIV disease progression in such a cohort, namely the treatment-naïve participants who were enrolled in the Long-Term Monitoring Protocol (LTM) sponsored by the Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA).
Methods
Patient population
Inclusion criteria for enrollment in the CPCRA LTM treatment naïve cohort required that patients be HIV-infected, be ≥ 13 years old, provide written informed consent, and be either antiretroviral-naïve, defined as no previous use of protease inhibitor or non-nucleoside reverse transcriptase inhibitors, or having received ≤1 week of treatment with lamivudine and ≤4 weeks of treatment with other nucleoside reverse transcriptase inhibitors. Patients were also required to have a life expectancy of at least 6 months. There were no exclusion criteria. The first participant was enrolled in April 1999. All participants were followed to a common closing date of July 1, 2006.
The association between baseline CRT and HIV disease progression was determined in LTM treatment-naïve patients who met the following additional eligibility criteria: a minimum of 4 months of follow-up during which antiretroviral therapy was not initiated, a baseline CD4+ cell count of ≥450 cells/μL, a baseline viral load ≥1,000 HIV-1 RNA copies/mL and the availability of sufficient baseline plasma for laboratory analyses.
Study procedures
Baseline CD4+ cell count and viral loads were determined within 120 days prior to study enrollment. Plasma obtained from within 120 days prior to baseline was centrally stored for future CPCRA-approved, HIV-related research. Subsequent data collection visits occurred every 4 months after enrollment. At each visit, information was collected regarding the occurrence of new HIV-related diagnoses, current antiretroviral therapy, and CD4+ cell count and plasma HIV-1 RNA level. Clinical management decisions, i.e., whether to initiate antiretroviral therapy, were not specified by the study protocol but were instead left to the discretion of the treating physician. After closure of the study to follow-up, all assays of CRT were performed using the Trofile™ Assay (Monogram Biosciences, South San Francisco, CA) as previously described21.
The primary objectives were to determine the prevalence of R5, mixtures of singly or dual R5 and X4 tropic virus (hereafter referred to as dual/mixed or D/M-tropic virus), and X4 tropic virus at baseline and the relationship between the baseline CRT and the time to progression to the composite outcome of ≤350 CD4+ cells/μL, initiation of therapy or death (whichever came first). All time-to-event endpoints were censored for death, loss-to-follow-up or study end. In addition, time to CD4 ≤350 as a secondary endpoint was censored for initiation of therapy.
Statistical analyses
Event rates by CRT category were estimated per 100 person-years of follow-up. Survival methods, including life tables (Kaplan-Meier graph) and Log Rank test (stratified by tertile of baseline CD4+ cell count), were used to compare time-to-event data between CRT groups. Relative risks were estimated using proportional hazards models stratified by clinical center, adjusting for baseline CD4+ cell count and baseline log10 viral load. Additional proportional hazards models, stratified by clinical center, further adjusted for possible confounding by other variables, including gender, race, age, transmission risk category (same sex contact or injection drug use), hepatitis B and C co-infection, duration of known HIV infection, and presence of prior AIDS-defining illness. To explore the effect of early drop-outs or events on these analyses, subgroup analyses were conducted on those participants remaining antiretroviral-naïve through month 8 and month 12 of follow-up.
Changes from baseline laboratory values (CD4+ cell count, log10 viral load) were compared between tropism groups using linear regression. Lab values were censored following the initiation of antiretroviral therapy. For viral load, change was estimated from baseline to month 12 and month 24. To attempt to address bias due to drop-out, initiation of therapy, or loss-to-follow-up, linear regression was also used to estimate change in viral load from baseline to the average of all available follow-up measures through month 24. All such comparisons were adjusted for baseline log10 viral load. Secondary analyses included the addition of other baseline factors as covariates.
Because participants were selected to have baseline CD4+ ≥450, baseline CD4+ cell counts were discarded for this analysis to avoid introduction of bias from regression to the mean. Therefore, change in CD4+ cell count was measured from Month 4 through Month 16 and Month 28, and to the average of all follow-up measurements from Month 8 through Month 28. All such comparisons were adjusted for baseline log viral load.
Random effects models were used to describe the rate of decline of CD4+ cell count. To satisfy model assumptions, CD4+ data were square-root transformed. CD4+ data were censored (discarded) following initiation of antiretroviral therapy. Likewise, to prevent bias from regression to the mean effects, slope is estimated using CD4+ data from Month 4 onward, discarding baseline CD4+ cell counts. All random effects models were adjusted for baseline log HIV-RNA and tropism category as fixed effects and included individual random effects for both slope and intercept. The effect of D/M tropism type on slope was estimated using an interaction term for time (in years) and tropism category.
Comparisons between tropism groups with respect to baseline characteristics were made with linear regression or the Cochran-Mantel-Henzel test as appropriate, stratifying for clinical center.
Results
Of the 1090 treatment-naïve LTM subjects, 359 met the entry criteria for analysis of CRT. CRT assays were successfully performed in 314 specimens; 18 persons did not have available baseline plasma specimens and CRT assays were unsuccessful in 27 other persons. D/M-tropic virus was detected in 32 samples (10%) and R5-tropic virus was detected in 282 (90%) samples; no sample had pure X4-tropic virus.
The baseline characteristics of the subjects for whom baseline CRT results were available are provided in Table 1. Patients with D/M CRT were more likely to be Latino/a (25% vs 8%, p <0.01). Otherwise, the demographic characteristics including age, gender, HIV risk factor, prior AIDS diagnoses, body mass index, chronic infection by hepatitis B or hepatitis C and duration of known HIV infection of patients with D/M versus R5 CRT were similar. The mean CD4+ cell count was lower in patients with D/M versus R5 CRT (617±143 vs. 694±222 cells/μL; p=0.05) but the mean viral load (4.3±0.7 vs. 4.1±0.6 log10 HIV-1 RNA copies/mL, p=0.059) did not differ.
Table I.
Baseline Characteristics by Tropism Status
| Characteristic | R5 CRT | D/M CRT | p |
|---|---|---|---|
| N | 282 (90%) | 32 (10%) | |
| Female | 61 (21.6%) | 10 (31.3%) | 0.41 |
| Race | 0.02 | ||
| Latino/a | 22 (7.8%) | 8 (25%) | <0.01 |
| Black | 122 (43.3%) | 15 (46.9%) | 0.96 |
| White | 125 (44.3%) | 9 (28.1%) | 0.14 |
| Other | 13 (4.6%) | 0 (0%) | 0.26 |
| IDU | 51 (18.1%) | 4 (12.5%) | 0.40 |
| Male same-sex contact (% of males) | 173 (78.3%) | 15 (68.2%) | 0.90 |
| Previous OI/OM | 11 (3.9%) | 1 (3.1%) | 0.96 |
| Age (years) | 38.3 | 37.2 | 0.42 |
| Body mass index (kg/m2) | 26.8 | 28.5 | 0.22 |
| CD4 cells/μL, mean (STD) | 694 (222) | 617 (143) | 0.05 |
| CD4 cells/μL, median (25%–75%) | 635 (526,810) | 571 (520,670) | 0.11 |
| HIV RNA, mean log10 copies/mL (STD) | 4.1 (0.6) | 4.3 (0.7) | 0.06 |
| HIV RNA, median log10 copies/mL (25%–75%) | 4.1 (3.7,4.5) | 4.4 (3.8,4.7) | 0.09 |
| Months since first documentation of HIV | 47.3 | 52.9 | 0.61 |
| Hepatitis B surface antigen positive | 14 (5%) | 2 (6.3%) | 0.83 |
| Hepatitis C antibody positive | 49 (17.4%) | 5 (15.6%) | 0.72 |
| Median Follow-up (Months) | 50.2 | 48.8 | 0.61 |
As appropriate, all results are presented as N (%) or mean (SD) values unless otherwise specified OI = opportunistic infection; OM = opportunistic malignancy
P values are from Cochran-Mantel-Henzel or linear regression as appropriate, adjusting for clinical center. Median values compared using the Wilcoxon test without stratification.
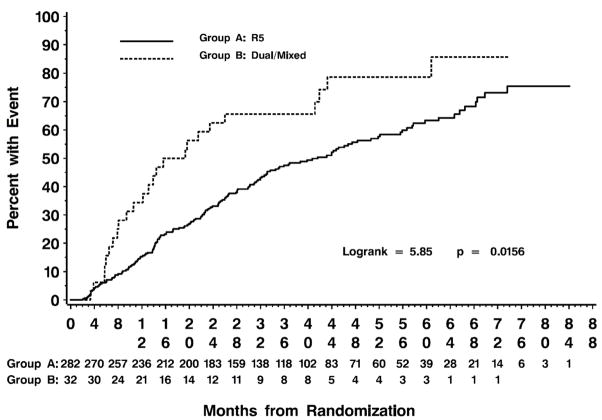
During the median follow-up of 50 months, 186 patients reached the primary combined endpoint [i.e., a CD4+ cell count ≤350 cells/μL (n=112), initiation of antiretroviral therapy (n=66) or death (n=8)]. After stratifying for tertile of baseline CD4+, compared with persons from whom R5-tropic virus was detected, persons with D/M-tropic virus reached the primary endpoint more rapidly (logrank test p = 0.0156; Figure 1).
Figure 1.
Rate of progression to composite endpoint of CD4+ ≤350, initiation of antiretroviral therapy or death for patients with R5 or D/M-tropic virus at baseline. Results are stratified for tertile of baseline CD4+ (≤ 547, 548–733 and ≥ 734 cells/μL).
The rates of progression to the individual endpoints of initiation of antiretroviral therapy, CD4+ cell decrease to ≤350 cells/μL as well as the combined endpoint were greater in patients with D/M-tropic virus (Table 2). Proportional hazards regression analyses that included the baseline viral load and CD4+ cell count demonstrated that the relative risk of progression to the composite endpoint was 2.15 (p = 0.002, 95% CI [1.32, 3.50]) for patients with D/M-tropic virus. In addition, the relative risk for progression was 2.07 per 1.0 log10 higher viral load (p <0.001, 95% CI [1.58, 2.69]) and 0.87 per 50 cell/μL higher CD4+ cell count (p <0.001, 95% CI [0.83, 0.91]). The effect of D/M CRT was also significant and of similar magnitude in separate regression analyses of time to initiation of antiretroviral therapy or CD4+ cell count ≤350 as separate endpoints censored for treatment initiation. Similar effects were obtained in other proportional hazards models that included gender, ethnicity, risk factors for acquisition of HIV infection, prior infection by hepatitis B or C, and the duration of known prior HIV infection (data not shown).
Table 2.
Proportional Hazards Regression of Time to Endpoints
| R5 CRT | D/M CRT | CRT [D/M Vs. R5] | CD4+ [per 50 cells/μL] | HIV-1 RNA [per log10/mL] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Events | Rate* | Events | Rate | RR+ [95% CI] | p | RR [95% CI] | p | RR [95% CI] | p |
| Initiation of antiretroviral therapy | 110 | 11.39 | 20 | 24.05 | 2.03 [1.19,3.45] | 0.009 | 0.90 [0.86,0.95] | <0.001 | 2.17 [1.60,2.93] | <0.001 |
| CD4+ cell count ≤ 350 cells/mL** | 95 | 11.11 | 17 | 25.91 | 2.40 [1.28,4.51] | 0.006 | 0.82 [0.77,0.88] | <0.001 | 2.25 [1.57,3.23] | <0.001 |
| Death | 10 | 1.07 | 1 | 1.24 | 1.01 [0.12,8.69] | 0.990 | --- | -- | --- | --- |
| Combined endpoint# | 161 | 20.17 | 25 | 38.88 | 2.15 [1.32,3.50] | 0.002 | 0.87 [0.83,0.91] | <0.001 | 2.07 [1.58,2.69] | <0.001 |
Rate per 100 patient years of follow-up
+ Relative risk adjustment for the multivariate proportional hazards model adjusted for baseline CD4+ cell count, log10 baseline HIV-1 RNA copies/mL. Stratified by clinical center
Censored for initiation of ART
CD4+ ≤ 350 cells/μL, initiation of therapy or death (numbers of events for constituent events do not sum to the total number of combined endpoints as individuals may experience more than one constituent event, but the combined endpoint counts only first occurrence of the combined event).
To further test the relationship between baseline CRT and outcomes, we repeated our analyses after excluding patients who initiated therapy within the first 8 or 12 months after enrollment into LTM. Proportional hazards regression analyses continued to show a robust relationship between D/M CRT and progression to the composite outcome or to the component outcomes of initiation of antiretroviral therapy or a decrease in the CD4+ cell count to ≤350 cells/μL (Relative Risk = 2.17–2.64, p <0.01 all analyses). In addition, we assessed the effect of CRT on progression to the composite outcome after stratifying the population by tertile of baseline CD4+ cell count. As shown in Table 3, the relative risk of progression to the composite outcome was greater for D/M versus R5-tropic virus in all three CD4+ tertiles, although statistical significance was demonstrated only for the middle tertile, relative risk = 5.06 [95% CI, 1.94–13.2, p <0.01]. Finally, we performed proportional hazards analyses in which the endpoint was time to progression to ≤200 CD4+ cells rather than ≤350 CD4+ cells. In these analyses, for persons with D/M vs R5 CRT, the relative risk of progression to the composite endpoint was 1.93 (95% CI 1.17, 3.18, p = 0.010) and to the separate endpoint of a CD4+ cell count of ≤200 cells/μL was 3.50 (95% CI 0.93,13.08; p=0.063).
Table 3.
| R5 CRT | D/M CRT | D/M vs R5 CRT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline CD4 Tertile | N | Events | Rate# | N | Events | Rate | RR+ | 95% CI | P-value |
| ≤547 cells/μL | 91 | 69 | 38.5 | 14 | 12 | 63.5 | 1.59 | [0.70,3.61] | 0.27 |
| 548–733 cells/μL | 91 | 52 | 19.5 | 14 | 10 | 33.8 | 5.06 | [1.94,13.2] | <0.01 |
| ≥ 734 cells/μL | 100 | 40 | 11.4 | 4 | 3 | 19 | 1.16 | [0.24,5.66] | 0.85 |
| All subjects | 282 | 161 | 20.2 | 32 | 25 | 38.9 | 2.15 | [1.32,3.50] | <0.01 |
CD4+ ≤350 cells/μL, initiation of therapy or death
Relative risk and associated p-value is based on proportional hazards regression models for each CD4+ tertile, stratified by clinical center and adjusted for baseline CD4+ cell count and log10 baseline HIV-1 RNA copies/mL.
Rates are per 100 person-years.
To better understand the relationship between CRT and disease progression, we assessed absolute changes in the CD4+ cell count and the rate of CD4+ cell loss in patients with D/M versus R5 CRT at baseline. The absolute change in CD4+ cell count from Month 4 to Month 16 tended to be greater in persons with D/M CRT after controlling for differences in baseline log viral load and censoring for the initiation of antiretroviral therapy but was not statistically significant (coefficient = −80.2 cells/μL; 95%CI = [−183,23], p=0.13). Similar non-statistically significant relationships were between CRT and the change of CD4+ cell count at month 24 or for the average of all available CD4+ cell counts through month 24.
Random effects models were used to analyze the slope of the decline of square-root (sqrt) transformed CD4+ cell counts, adjusting for baseline viral load and presence of D/M CRT as fixed effects with random effects for intercept and slope (Table 4). The interaction term between time and presence of D/M CRT indicates an additional loss of −1.64 sqrt (CD4+ cells/μL) per year for patients with D/M CRT over the reference slope of −0.79 sqrt (CD4+ cells/μL) per year for patients with R5 CRT (p<0.01). Measuring results on the square root scale implies that the rate of decline varies with CD4+ cell count. For example, for two participants with an initial CD4+ count of 750 cells/μL, our data predict a loss of 96 cells/μL in the first year for a patient with D/M CRT versus a loss of 55 cells/μL for a patient with R5 CRT. However, for two patients with an initial CD4+ of 350 cells/μL, the one year losses would be 66 cells/μL for a patient with D/M CRT versus 38 cells/μL for a patient with R5 CRT. In transforming from the square root scale to the linear scale, the absolute decline will always be relative to the current CD4+, with the size of predicted decline tending towards zero as CD4+ declines. Adjusting this model for additional baseline covariates (race, sex, and transmission risk category) did not qualitatively change the results.
Table 4.
Random Effects Model for Slope of CD4+ Cell Decline (square root transformed)*
| Parameter | Estimate | Std Err | 95% CI | P-value |
|---|---|---|---|---|
| Baseline Log RNA | −1.61 | 0.77 | [−3.12,−0.09] | 0.04 |
| Tropism [D/M Vs. R5] | −1.03 | 0.10 | [−1.22,−0.85] | <0.01 |
| CD4+ Slope (cells/μL/yr) | −0.79 | 0.36 | [−1.49,−0.09] | 0.03 |
| Interaction Tropism* Slope** | −1.64 | 0.36 | [−2.35,−0.94] | <0.01 |
Individual random effects included for intercept and slope. Fixed effect intercept term not shown
The interaction term between time and presence of D/M-tropic virus represents the difference in slope of square-root transformed CD4+ between participants with D/M-tropic virus measured against those with R5-tropic virus represents an additional decline of −1.64 sqrt (cells/μL) per year over the reference slope of −0.79 square root (cells/μL) per year.
Finally, we assessed the association between CRT in change in log10 viral load from baseline, adjusting for baseline log10 viral load. We found that the D/M-group experienced a statistically significant increase in viral load from baseline to month 12 of 0.36 log10 copies ([0.01,0.71], p=0.04), this result remained significant when adjusting for baseline demographics (sex, race, and transmission risk category). There was no observed statistical difference between the groups in change to Month 24 or change from baseline to the average of all follow-up measurements to Month 24. This may reflect a loss of power as many D/M CRT patients initiated therapy prior to Month 24.
Discussion
We studied an evaluable population of 314 persons with chronic untreated HIV-1 infection who did not meet indications for immediately initiating antiretroviral therapy (median CD4+ cell count = 629). Among these subjects, persons in whom D/M-tropic virus was detected progressed more rapidly to a composite endpoint of CD4+ cell count ≤350, initiation of antiretroviral treatment, or death than did persons in whom only R5 tropic virus was detected. Similarly, persons with D/M CRT more often progressed to the component endpoints of initiation of therapy or CD4+ decline to ≤350 cells/μL. Although persons with D/M CRT had slightly fewer CD4+ cells and were more likely to be Latino/a, the groups were otherwise similar.
The impact of D/M CRT on progression to the composite and individual endpoints wassimilar to that of a one-log10 increase in the HIV RNA copy number and greater than that of having 50 fewer CD4+ cells/μL. These effects were stable across several proportional hazards regression models that controlled for the baseline CD4+ cell count, viral load, HIV risk factors, demographic factors and other laboratory variables. Furthermore, random effects models indicated that persons with D/M CRT had a greater rate of CD4+ decline as determined by analyses of the slope of square-root transformed CD4+ cell counts, adjusted for baseline viral load and presence of D/M CRT. Finally, persons with D/M CRT demonstrated statistically significant increase in viral load from baseline to month 12.
The frequency of D/M vs R5 CRT in our study, wherein 10% of the population had D/M-tropic virus and 90% had R5-tropic virus and no person had pure X4-tropic virus is similar to that of previous reports in treatment-naïve patients wherein the proportion of patients with R5 CRT is 80–91%, D/M CRT is 9–20% and pure X4 CRT is less than 1%26;28;30–34. While the mean CD4+ cell count was 77 cells/μL lower in persons with D/M rather than R5 CRT (617 vs. 694 cells/μL, p = 0.05), there was no significant difference in the baseline viral loads. In addition, persons with a history of injection drug use were no more likely to have D/M-tropic virus whereas Latino/a patients were more likely to have D/M-tropic virus. Some13;26;31;32 but not all16;28 cross-sectional studies in treatment naïve patients have shown that D/M-tropic or syncitium-inducing virus is associated with lower CD4+ cell counts and higher viral load. Similarly, while some studies found that persons with a history of injection drug use were more likely to have X4 tropic virus28, other studies have not found this association with viral X4 tropism26 or the SI phenotype16. Finally, one group found that D/M-tropic virus was associated with a decreased likelihood of being Caucasian31.
In contrast to previous studies of the relationship between viral coreceptor tropism and the subsequent course of HIV infection disease progression, we evaluated an ethnically and behaviorally diverse population of chronically infected, truly treatment-naïve adults with relatively early stage HIV-1 infection who had a median of 629 CD4+ cells/μL at study entry and who were known to be infected for over 4 years. Previously, Waters et al. found that among 401 treatment-naïve subjects with a median baseline CD4+ of approximately 300 cells/μL, persons with D/M-tropic virus had a greater CD4+ cell decline than did persons with no evidence of X4 tropism30. Analysis was censored in 74% of the subjects due to the initiation of antiretroviral therapy during the 18 month observation period, which may expose the result to bias. In a second study of 68 participants from the Multicenter AIDS Cohort Study, progression to emergence of X4 CRT was associated with more frequent progression to AIDS. In addition, this study found that abrupt declines in the total T cell count (CD3 inflection) were often preceded by the emergence of X4 CRT29. In a separate one-year study of 296 recent HIV-1 seroconverters, persons with baseline X4 CRT lost more CD4+ cells and had greater increases in plasma HIV RNA copy number than did persons infected by R5 CRT28. Finally, among HIV-infected children with hemophilia, D/M CRT predicted more rapid CD4+ cell decreases and progression to a clinical AIDS event13. Of note, the median CD4+ cell count was 200 for hemophiliac patients with D/M CRT versus 449 for patients with R5 CRT13. In addition, half of the study population had previously received monotherapy with a nucleoside reverse transcriptase inhibitor.
Our results are consistent with and extend earlier studies that found that the presence of SI virus was an independent predictor of more rapid CD4+ cell loss and/or HIV disease progression35–41. In addition, we found that patients with D/M CRT had greater viral load increases during the first year of follow-up. These results may be of increased relevance with the recent approval of a CCR5 antagonist for use in treatment-experienced HIV-infected patients, as approximately 65% of patients with virological breakthrough on these agents demonstrate emergence of D/M CRT42;43. Although short-term data suggest that detectable D/M CRT wanes without untoward clinical consequences when use of a CCR5 antagonist is discontinued44;45, there is as yet little long-term follow-up of these patients.
The mechanisms by which CXCR4 utilizing virus is associated with increased rates of disease progression are not well understood. Our observation that persons with D/M had a more rapid loss of CD4+ cells is also consistent with observations that the effect of SI virus on disease progression is largely mediated through accelerated loss of CD4+ cells16;38. Other investigators have shown that CXCR4-utilizing virus is more cytopathic than their CCR5 predecessors46;47, and that thymocytes and naïve CD4+ T cells may be particularly susceptible to X4 and SI viruses due to high levels of CXCR4 expression by these cells48–53. Alternatively, it is possible that the emergence of X4 or SI virus is the consequence rather than the cause of immunologic failure.
The strengths of this study include the demographic diversity, prospective data collection and long duration of follow-up. Among the potential weaknesses of this study are the observations that month to month changes in CRT may occur in 3% of treatment naïve individuals54 and that over a longer period of observation oscillation between CCR5-tropism and mixed-tropism may occur in 23% of patients29. However, while such oscillations may lead to false categorizations of patients as having R5 or X4 CRT, this misallocation would tend to weaken rather than strengthen the association between baseline D/M CRT with disease progression. In addition, we did not obtain data regarding host genotypic data such as the presence of the CCR5Δ32 polymorphism or the SDF1-3A allele, both of which modify the rates of HIV disease progression and are associated with differences in the prevalence of D/M-tropic virus and affect the impact of D/M-tropic virus on HIV disease progression27;33;55. In regards to CCR5Δ32, since persons with this polymorphism generally have a slower rate of HIV disease progression55, failure to control for the effect of CCR5Δ32 would likely weaken the effect of D/M CRT rather than falsely strengthen the association of D/M-tropic virus with increased rates of disease progression as observed in our study. Finally, results with the original version of the Trofile assay used in this study may not apply to results obtained with new enhanced version of this assay. While the original version detects X4 subpopulations comprising 5–10% of circulating virus, the newer enhanced version is able to detect subpopulations that comprise as little as 0.1–0.3% X4 virus21;56. It remains to be determined whether inclusion of patients with such very low levels of X4 virus would diminish the prognostic significance found in this study.
In summary, this work confirms previous reports that patients harboring HIV with the SI phenotype35–41 or D/M tropism13 have more rapid rates of HIV disease progression and rate of CD4+ cells loss. These results extend prior findings by demonstrating the relationship between tropism and disease progression in patients with higher CD4+ cell counts than previously studied. Finally, these results suggest that determination of HIV co-receptor tropism may identify a subpopulation of treatment-naïve patients who might warrant earlier initiation of antiretroviral therapy based on their risk of more rapid disease progression. However, the clinical and cost-effectiveness of such strategies will require careful analysis of test costs, the prevalence of viruses that use CXCR4 for cell entry, and the marginal clinical utility of earlier versus later initiation of antiretroviral therapy for persons with greater than 350 CD4+ cells.
Acknowledgments
Author participation: All authors gave final approval for this manuscript. Matthew B. Goetz contributed to the conception of the study, interpretation of the data and drafting the article. Robert Leduc contributed to the conception and design of the study, analysis and interpretation of data, and drafting the article. Jay R. Kostman, Ann M. Labriola, and Roberta Luskin-Hawk, contributed to the conception and design of the study and revising the article for critical intellectual content. Yolanda Lie, Jodi Weidler, Eoin Coakley and Michael Bates contributed to acquisition of the data, interpretation of the data, critical revisions of the intellectual content of the manuscript. In addition, the authors acknowledge the statistical and analytical assistance provided by Nicole Wyman.
Sources of funding: NIAID grants U01 AI042170, U01 AI046362 and U01 AI068641 to the CPCRA and SBIR R44AI050321 to Monogram Biosciences.
Footnotes
These data have been presented previously in part at the 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention. July 22–25, 2007; Sydney, Australia; Abstract WEPDB07 and 47th Interscience Conference on Antimicrobial Agents and Chemotherapy. September 17 - 20, 2007; Chicago IL; Abstract H-1027
Reference List
- 1.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-infected adults and adolescents. February 9, 2008.
- 2.Lyles RH, Munoz A, Yamashita TE, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis. 2000;181:872–80. doi: 10.1086/315339. [DOI] [PubMed] [Google Scholar]
- 3.Learmont JC, Geczy AF, Mills J, et al. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N Engl J Med. 1999;340:1715–22. doi: 10.1056/NEJM199906033402203. [DOI] [PubMed] [Google Scholar]
- 4.Alexander L, Weiskopf E, Greenough TC, et al. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J Virol. 2000;74:4361–76. doi: 10.1128/jvi.74.9.4361-4376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grovit-Ferbas K, Ferbas J, Gudeman V, et al. Potential contributions of viral envelope and host genetic factors in a human immunodeficiency virus type 1-infected long-term survivor. J Virol. 1998;72:8650–8658. doi: 10.1128/jvi.72.11.8650-8658.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benito JM, Lopez M, Soriano V. The role of CD8+ T-cell response in HIV infection. AIDS Rev. 2004;6:79–88. [PubMed] [Google Scholar]
- 7.Ioannidis JP, Rosenberg PS, Goedert JJ, et al. Effects of CCR5-Delta32 CCR2-64I SDF-1 3′A alleles on HIV-1 disease progression: An international meta-analysis of individual-patient data. Ann Intern Med. 2001;135:782–95. doi: 10.7326/0003-4819-135-9-200111060-00008. [DOI] [PubMed] [Google Scholar]
- 8.Mulherin SA, O’Brien TR, Ioannidis JP, et al. Effects of CCR5-Delta32 and CCR2-64I alleles on HIV-1 disease progression: the protection varies with duration of infection. AIDS. 2003;17:377–87. doi: 10.1097/01.aids.0000050783.28043.3e. [DOI] [PubMed] [Google Scholar]
- 9.Sufka SA, Ferrari G, Gryszowka VE, et al. Prolonged CD4+ cell/virus load discordance during treatment with protease inhibitor-based highly active antiretroviral therapy: immune response and viral control. J Infect Dis. 2003;187:1027–37. doi: 10.1086/368359. [DOI] [PubMed] [Google Scholar]
- 10.Carrington M, O’Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–51. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 11.Differences in CD4 cell counts at seroconversion and decline among 5739 HIV-1-infected individuals with well-estimated dates of seroconversion. J Acquir Immune Defic Syndr. 2003;34:76–83. doi: 10.1097/00126334-200309010-00012. [DOI] [PubMed] [Google Scholar]
- 12.Barbour JD, Hecht FM, Wrin T, et al. Higher CD4+ T cell counts associated with low viral pol replication capacity among treatment-naive adults in early HIV-1 infection. J Infect Dis. 2004;190:251–56. doi: 10.1086/422036. [DOI] [PubMed] [Google Scholar]
- 13.Daar ES, Kesler KL, Petropoulos CJ, et al. Baseline HIV type 1 coreceptor tropism predicts disease progression. Clin Infect Dis. 2007;45:643–49. doi: 10.1086/520650. [DOI] [PubMed] [Google Scholar]
- 14.Schuitemaker H, Koot M, Kootstra NA, et al. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–60. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger EA, Doms RW, Fenyo EM, et al. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 16.Spijkerman IJ, Koot M, Prins M, et al. Lower prevalence and incidence of HIV-1 syncytium-inducing phenotype among injecting drug users compared with homosexual men. AIDS. 1995;9:1085–92. doi: 10.1097/00002030-199509000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Spijkerman I, de WF, Langendam M, et al. Emergence of syncytium-inducing human immunodeficiency virus type 1 variants coincides with a transient increase in viral RNA level and is an independent predictor for progression to AIDS. J Infect Dis. 1998;178:397–403. doi: 10.1086/515627. [DOI] [PubMed] [Google Scholar]
- 18.Coakley E, Petropoulos CJ, Whitcomb JM. Assessing chemokine co-receptor usage in HIV. Curr Opin Infect Dis. 2005;18:9–15. doi: 10.1097/00001432-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Trouplin V, Salvatori F, Cappello F, et al. Determination of coreceptor usage of human immunodeficiency virus type 1 from patient plasma samples by using a recombinant phenotypic assay. J Virol. 2001;75:251–59. doi: 10.1128/JVI.75.1.251-259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skrabal K, Low AJ, Dong W, et al. Determining human immunodeficiency virus coreceptor use in a clinical setting: degree of correlation between two phenotypic assays and a bioinformatic model. J Clin Microbiol. 2007;45:279–84. doi: 10.1128/JCM.01118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitcomb JM, Huang W, Fransen S, et al. Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism. Antimicrob Agents Chemother. 2007;51:566–75. doi: 10.1128/AAC.00853-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resch W, Hoffman N, Swanstrom R. Improved success of phenotype prediction of the human immunodeficiency virus type 1 from envelope variable loop 3 sequence using neural networks. Virology. 2001;288:51–62. doi: 10.1006/viro.2001.1087. [DOI] [PubMed] [Google Scholar]
- 23.Pillai S, Good B, Richman D, et al. A new perspective on V3 phenotype prediction. AIDS Res Hum Retroviruses. 2003;19:145–49. doi: 10.1089/088922203762688658. [DOI] [PubMed] [Google Scholar]
- 24.Jensen MA, Li FS, van ’t Wout AB, et al. Improved coreceptor usage prediction and genotypic monitoring of R5-to-X4 transition by motif analysis of human immunodeficiency virus type 1 env V3 loop sequences. J Virol. 2003;77:13376–88. doi: 10.1128/JVI.77.24.13376-13388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen MA, van ’t Wout AB. Predicting HIV-1 coreceptor usage with sequence analysis. AIDS Rev. 2003;5:104–12. [PubMed] [Google Scholar]
- 26.Brumme ZL, Goodrich J, Mayer HB, et al. Molecular and clinical epidemiology of CXCR4-using HIV-1 in a large population of antiretroviral-naive individuals. J Infect Dis. 2005;192:466–74. doi: 10.1086/431519. [DOI] [PubMed] [Google Scholar]
- 27.Daar ES, Lynn HS, Donfield SM, et al. Stromal Cell-Derived Factor-1 Genotype and Coreceptor Tropism HIV Type 1 Disease Progression. J Infect Dis. 2005;192:1597–605. doi: 10.1086/496893. [DOI] [PubMed] [Google Scholar]
- 28.De Mendoza C, Rodriguez C, Garcia F, et al. Prevalence of X4 tropic viruses in patients recently infected with HIV-1 and lack of association with transmission of drug resistance. J Antimicrob Chemother. 2007;59:698–704. doi: 10.1093/jac/dkm012. [DOI] [PubMed] [Google Scholar]
- 29.Shepherd JC, Jacobson LP, Qiao W, et al. Emergence and Persistence of CXCR4-Tropic HIV-1 in a Population of Men from the Multicenter AIDS Cohort Study. J Infect Dis. 2008;198:1104–12. doi: 10.1086/591623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waters LJ, Mandalia S, Wildfire A, Gazzard B, Moyle G. CXCR4/Mixed-Tropic HIV-1 is Associated with More Rapid CD4 Cell Decline Compared with CCR5-Tropic Virus in Antiretroviral-Naive Individuals. Intersci Conf Antimicrob Agents Chemother. 46 Abstract H-1667. 2006. Ref Type: Journal (Full) [Google Scholar]
- 31.Demarest J, Bonny T, Vavor C, et al. HIV-1 Co-Receptor Tropism in Treatment Naive and Experienced Subjects. Intersci Conf Antimicrob Agents Chemother. 2004;44:H-1136. [Google Scholar]
- 32.Moyle GJ, Wildfire A, Mandalia S, et al. Epidemiology and predictive factors for chemokine receptor use in HIV-1 infection. J Infect Dis. 2005;191:866–72. doi: 10.1086/428096. [DOI] [PubMed] [Google Scholar]
- 33.Hunt PW, Harrigan PR, Huang W, et al. Prevalence of CXCR4 tropism among antiretroviral-treated HIV-1-infected patients with detectable viremia. J Infect Dis. 2006;194:926–30. doi: 10.1086/507312. [DOI] [PubMed] [Google Scholar]
- 34.Kitrinos K, Irlbeck D, Bonny T, Frusciante K, Thorpe D, Mickalites R, Demarest J. HIV-1 co-receptor tropism changes over time in drug naive patients in the absence of antiretroviral therapy. International AIDS Conference XVI, MOPE0006. 2006 Ref Type: Journal (Full) [Google Scholar]
- 35.Koot M, Keet IP, Vos AH, et al. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–88. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 36.Sheppard HW, Lang W, Ascher MS, et al. The characterization of non-progressors: long-term HIV-1 infection with stable CD4+ T-cell levels. AIDS. 1993;7:1159–66. [PubMed] [Google Scholar]
- 37.Connor RI, Mohri H, Cao Y, et al. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 1993;67:1772–77. doi: 10.1128/jvi.67.4.1772-1777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richman DD, Bozzette SA. The impact of the syncytium-inducing phenotype of human immunodeficiency virus on disease progression. J Infect Dis. 1994;169:968–74. doi: 10.1093/infdis/169.5.968. [DOI] [PubMed] [Google Scholar]
- 39.Daar ES, Chernyavskiy T, Zhao JQ, et al. Sequential determination of viral load and phenotype in human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 1995;11:3–9. doi: 10.1089/aid.1995.11.3. [DOI] [PubMed] [Google Scholar]
- 40.Connor RI, Sheridan KE, Ceradini D, et al. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1--infected individuals. J Exp Med. 1997;185:621–28. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes MD, Johnson VA, Hirsch MS, et al. Monitoring plasma HIV-1 RNA levels in addition to CD4+ lymphocyte count improves assessment of antiretroviral therapeutic response. ACTG 241 Protocol Virology Substudy Team. Ann Intern Med. 1997;126:929–38. doi: 10.7326/0003-4819-126-12-199706150-00001. [DOI] [PubMed] [Google Scholar]
- 42.Nelson M, Fatkenheuer G, Konourina I, Lazzarin A, Clumeck N, Horban A, Tawadrous M, Sullivan J, Mayer H, van der Ryst E. Efficacy and Safety of Maraviroc plus Optimized Background Therapy in Viremic, ART-experienced Patients Infected with CCR5-tropic HIV-1 in Europe, Australia, and North America: 24-Week Results. Conf Retroviruses Opportunistic Infect. 14 Abstract 104aLB. 2007. Ref Type: Journal (Full) [Google Scholar]
- 43.Lalezari J, Goodrich J, DeJesus E, Lampiris H, Gulick R, Saag M, Ridgway C, McHale M, van der Ryst E, Mayer H. Efficacy and Safety of Maraviroc plus Optimized Background Therapy in Viremic ART-experienced Patients Infected with CCR5-tropic HIV-1: 24-Week Results of a Phase 2b/3 Study in the US and Canada. Conf Retroviruses Opportunistic Infect. 14 Abstract 104bLB. 2007. Ref Type: Journal (Full) [Google Scholar]
- 44.Westby M, Lewis M, Whitcomb J, et al. Emergence of CXCR4-using human immunodeficiency virus type 1 (HIV-1) variants in a minority of HIV-1-infected patients following treatment with the CCR5 antagonist maraviroc is from a pretreatment CXCR4-using virus reservoir. J Virol. 2006;80:4909–20. doi: 10.1128/JVI.80.10.4909-4920.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayer H, van der Ryst E, Saag M, Clotet B, Fatkenheuer G, Clumeck N, Turner K, Goodrich JM. Safety and Efficacy of MARAVIROC, a Novel CCR5 Antagonist, When Used in Combination with Optimized Background Therapy for the Treatment of Antiretroviral-Experienced Subjects Infected with Dual/Mixed-Tropic HIV-1: 24-Week Results of a Phase 2b Exploratory Trial. International AIDS Conference. 16 Abstract THLB0215. 8-15-2006. Ref Type: Journal (Full) [Google Scholar]
- 46.Kreisberg JF, Kwa D, Schramm B, et al. Cytopathicity of human immunodeficiency virus type 1 primary isolates depends on coreceptor usage and not patient disease status. J Virol. 2001;75:8842–47. doi: 10.1128/JVI.75.18.8842-8847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grivel JC, Penn ML, Eckstein DA, et al. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J Virol. 2000;74:5347–51. doi: 10.1128/jvi.74.11.5347-5351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bleul CC, Wu L, Hoxie JA, et al. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997;94:1925–30. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blaak H, van’t Wout AB, Brouwer M, et al. In vivo HIV-1 infection of CD45RA(+)CD4(+) T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4(+) T cell decline. Proc Natl Acad Sci U S A. 2000;97:1269–74. doi: 10.1073/pnas.97.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Correa R, Munoz-Fernandez MA. Viral phenotype affects the thymic production of new T cells in HIV-1-infected children. AIDS. 2001;15:1959–63. doi: 10.1097/00002030-200110190-00007. [DOI] [PubMed] [Google Scholar]
- 51.Schmitt N, Chene L, Boutolleau D, et al. Positive regulation of CXCR4 expression and signaling by interleukin-7 in CD4+ mature thymocytes correlates with their capacity to favor human immunodeficiency X4 virus replication. J Virol. 2003;77:5784–93. doi: 10.1128/JVI.77.10.5784-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor JR, Jr, Kimbrell KC, Scoggins R, et al. Expression and function of chemokine receptors on human thymocytes: implications for infection by human immunodeficiency virus type 1. J Virol. 2001;75:8752–60. doi: 10.1128/JVI.75.18.8752-8760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunt P, Martin J, Bates M, Huang W, Spudich S, Price R, Williamson D, Sinclair E, Roh R, Deeks S. CXCR4-tropic Viruses Are Common among Antiretroviral Treated Patients with Detectable Viremia and Associated with Lower Treatment-mediated CD4 Gains. Conf Retroviruses Opportunistic Infect. 13 Abstract 43. 2006. Ref Type: Journal (Full) [Google Scholar]
- 54.Heera J, Saag M, Ive P, Whitcomb J, Lewis M, McFadyen L, Goodrich J, Mayer H, van der Ryst E, Westby M. Virological Correlates Associated with Treatment Failure at Week 48 in the Phase 3 Study of Maraviroc in Treatment-naive Patients. Conf Retroviruses Opportunistic Infect. 15 Abstract 40LB. 2-4-2008. Ref Type: Journal (Full) [Google Scholar]
- 55.O’Brien SJ, Nelson GW. Human genes that limit AIDS. Nat Genet. 2004;36:565–74. doi: 10.1038/ng1369. [DOI] [PubMed] [Google Scholar]
- 56.Reeves JD, Han D, Liu Y, et al. Enhancements to the TrofileTM HIV Co-receptor Tropism Assay Enable Improved Detection of CXCR4-using Subpopulations [H-1026]. Presented at: 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 17, 2007; Chicago, IL. [Google Scholar]