Abstract
This study investigated the age effect on antioxidant adaptation to muscle disuse. Adult and old rats were randomized into 4 groups: weight bearing (control), 3 days of hind-limb unloading (HU), 7 days of HU, and 14 days of HU. Activities of Cu-Zn superoxide dismutase (SOD), catalase, and glutathione (GSH), as well as GSH peroxidase levels were measured in the soleus. Neither disuse nor aging changed the activity of Cu-Zn SOD. The old rats had greater GSH peroxidase activity, whereas the activity of catalase had a compensatory increase with disuse, independent of age. Reduced GSH level and total glutathione (tGSH) level had age-related change with disuse. In old rats, the GSH and tGSH levels were lower with disuse, whereas the levels remained stable with disuse in adult rats. The depletion of intracellular GSH and tGSH levels of muscles from aged animals with disuse may make aged muscles more susceptible to oxidative damage.
Keywords: Antioxidant, Aging, Disuse
Individuals who are restricted to bed rest due to severe trauma (e.g., brain injury or complicated fracture) or serious disease (e.g., renal disease) are prone to experience muscle atrophy and weakness (1). This disuse-induced muscle dysfunction is thought to be related to the intracellular oxidative stress of the muscles (2-4). Oxidative stress develops when the production of oxidants exceeds the scavenger capacity of the antioxidant system. Support for the disuse-induced oxidative stress is found in investigations using young animals. These studies report increased oxidant production (5-8), antioxidant adaptations (5,9,10), and the accumulation of oxidatively modified proteins (9) with muscle disuse.
The aging process is associated with increased oxidative stress. Numerous studies have shown that muscles from aged animals exhibit enhanced oxidative stress, characterized by lipid peroxidation, protein oxidative modification, and DNA damage (11-13). Muscle cells are able to adapt and respond to this increased oxidative stress by altering the expression of antioxidant enzymes. Several studies have demonstrated that the activity of antioxidant enzymes are increased in the aged muscles (11,13-16).
Although the antioxidant systems have the ability to adapt in the presence of increased oxidative stress, the adaptation may be influenced by the age of the animals. For example, the study of Leeuwenburgh and colleagues (15) found that exercise training increased the activity of antioxidant enzymes in muscles from young rats, but not in the muscles from aged rats. Other age-dependent responses are also observed in muscles following periods of disuse. Siu and colleagues (17) found that the level of heat shock protein 27, an antiapoptotic protein, increased in muscles from young animals following disuse but not in aged animals. Studies by Thompson and colleagues (18) found that hind-limb unloading deteriorated the muscle function in both adult and old animals. However, this muscle function deterioration was more pronounced in old animals. In addition, they also showed that the ability of aged muscles to maintain the ratio of force to fiber size during hind-limb unloading was compromised (19). It is unknown whether adaptations of the antioxidant systems to muscle disuse are age-dependent and contribute to the different responses observed in muscle function.
The aim of this study was to investigate the influence of age on the ability of skeletal muscle tissue antioxidants to adapt to disuse. Based on the age-related responses in muscle function to disuse and to the already enhanced antioxidant capacity in aged muscles, we hypothesized that the adaptation of antioxidant systems to muscle disuse is age-dependent. The soleus muscle was chosen to be investigated in this study because it (a) is composed predominately of type I fibers, (b) is an antigravity, weight-bearing muscle, and (c) shows physiological and biochemical adaptations with unloading (5,18,20). The novel finding of this study was that, unlike the muscles from adult rats (13 months), which can maintain the intracellular glutathione (GSH) level at the control level after 14 days of muscle disuse, the GSH level in the muscles from aged rats (26 months) is reduced dramatically with disuse. This compromised antioxidant capacity of the aged muscles may be associated with the age-related responses of muscle functions with muscle disuse.
Methods
Animals and Hind-Limb Unloading
Fifty-six male Fischer 344 rats aged 13 months (100% strain survival) (n = 28) and 26 months (25% strain survival) (n = 28) were purchased from the Minneapolis Veterans Administration Aged Rodent Colony. These rats were randomized into four groups: normal weight bearing (control), hind-limb unloading for 3 days (3d HU), hind-limb unloading for 7 days (7d HU), and hind-limb unloading for 14 days (14d HU). The hind-limb unloading intervention was achieved by attaching the tail of the rat to a swivel mounted at the top of the cage. The height of the suspension was adjusted to prevent the hind limbs from contacting the floor. This arrangement permits animals to walk around with their forelimbs while hind limbs are unloaded. All the animals were housed in a research animal facility and were checked daily for any abnormal response to suspension. The protocol of this study was approved by the University of Minnesota Institutional Animal Care and Use Committee.
Tissue Preparation
The rats were anesthetized with pentobarbital sodium (35 mg/kg body weight) after the intervention. Soleus muscles were harvested, weighed, and immediately frozen in liquid nitrogen. The frozen soleus muscles were stored in a −80°C freezer until homogenization. To measure enzyme activities of catalase and GSH peroxidase, soleus muscles were homogenized in buffer containing 20 mM 3-(4-morpholino) propane sulfonic acid (MOPS), 62 mM sucrose, and 0.1 mM EDTA (pH 7.2). The supernatant that contained the extracted protein was collected after centrifuging at 12,000 g for 25 minutes. Protein concentration was then measured by the Bradford method. For measurement of GSH content, soleus muscles were homogenized in 1% picric acid. The supernatant was collected after centrifuging at 12,941 g for 30 minutes (21,22).
Enzyme Activity Measurement
Catalase activity
Catalase activity was determined spectrophotometrically (23) by measuring the breakdown of hydrogen peroxide at a wavelength of 240 nm for 5 minutes at 30°C. Briefly, assay medium containing 0.34% hydrogen peroxide, 50 mM potassium phosphate monobasic, and 50 mM sodium phosphate dibasic (pH 7.0) was added to the samples to initiate the reaction as previously described (21,22).
GSH peroxidase activity
GSH peroxidase activity was determined spectrophotometrically (24) by measuring the oxidation of NADPH at a wavelength of 340 nm, 30°C for 5 minutes. Briefly, samples were incubated in an assay medium containing 50 mM Tris-EDTA buffer, 1 mM reduced GSH, GSH reductase at 5 U/mL, and 0.15 mM NADPH for 15 minutes. The reaction was then initiated by adding t-butyl hydroperoxide as previously described (21,22).
Cu-Zn superoxide dismutase activity
Cu-Zn superoxide dismutase (SOD) activity was determined using spectrophotometric assay (Bioxytech, Portland, OR).
Level of total glutathione, reduced GSH, and reduced to oxidized GSH ratio
The GSH level was determined based on the standard curve, which was generated from the known concentrations of GSH and the formation of 2-nitro-5-thiobenzoic acid measured spectrophotometrically at a wavelength of 412 nm (25) using previously described procedures (21). Briefly, the reaction was initiated by adding GSH reductase to the medium containing 125 mM phosphate–EDTA buffer, 0.3 mM NADPH, 6 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), and different amounts of reduced GSH. To measure oxidized glutathione (GSSG), 4-vinyl pyridine was added to the sample and incubated for 1 hour. This incubation conjugated the reduced GSH in the sample; therefore, only oxidized GSH could reduce to GSH. The GSH level was determined by standard curve, and the GSSG level was calculated. The intracellular total glutathione level (tGSH) was indicated by GSH+GSSH. The ratio of reduced to oxidized glutathione (GSH/GSSG), which represented total tissue oxidative stress, was then determined.
Statistics
Data were presented as mean ± standard error of the mean. Two-way analysis of variance (ANOVA) was used to determine the effect of aging and hind-limb unloading on the antioxidant system in the soleus muscle from rats. Tukey's honest significant difference test was used as a post hoc test when the main effect of aging or hind-limb unloading reached significance. A significant difference was considered achieved when p < .05.
Results
Activity of Cu-Zn SOD
Cu-Zn SOD is an isoform of SOD that exists in the cytosol and converts superoxide anions to hydrogen peroxide. Neither hind-limb unloading nor aging changed the activity of Cu-Zn SOD (range: 18.06–29.27 U/mg protein). The activity of Cu-Zn SOD in the soleus muscle from both adult and old rats remained stable with hind-limb unloading.
Activity of GSH Peroxidase
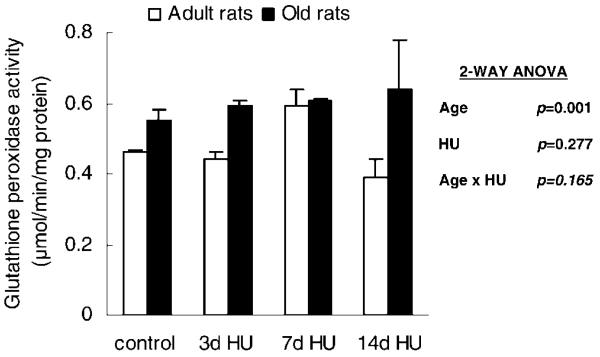
Hind-limb unloading did not change the activity of the antioxidative enzyme, GSH peroxidase. However, the activity of GSH peroxidase was significantly affected by age. The soleus muscle from old rats had greater GSH peroxidase activity than did the muscle from adult rats (Figure 1). The soleus muscle from old rats had 28% greater GSH peroxidase activity compared to the value seen in the adult muscle.
Figure 1.
Activity of glutathione peroxidase from rats with weight bearing (control), 3 days of hind-limb unloading (3d HU), 7 days of hind-limb unloading (7d HU), and 14 days of hind-limb unloading (14d HU). Values are mean ± standard error of the mean. The effect of age was independent of hind-limb unloading. ANOVA = analysis of variance.
Activity of Catalase
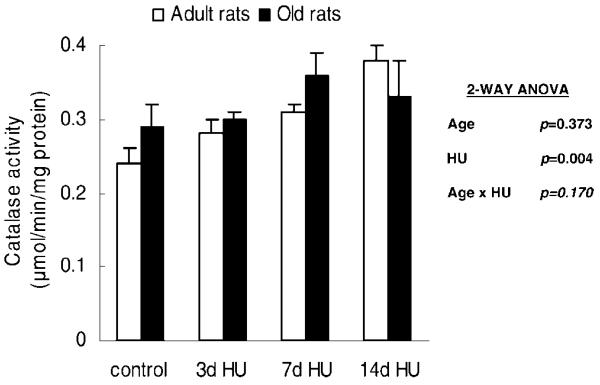
Catalase, an antioxidant enzyme that degrades hydrogen peroxide to water, reduces the H2O2 concentration of the cells. Hind-limb unloading altered the activity of catalase in the soleus muscles from rats, independent of age (Age × HU, p = .170). Catalase activities of the 7d HU and 14d HU rats were 22% and 30% greater, respectively, than the rats with weight bearing (control group) (Figure 2).
Figure 2.
Catalase activity from rats with weight bearing (control), 3 days of hind-limb unloading (3d HU), 7 days of hind-limb unloading (7d HU), and 14 days of hind-limb unloading (14d HU). Values are mean ± standard error of the mean. The effect of hind-limb unloading was independent of age. Catalase activities of the rats with 7d HU and 14d HU were higher than those of the rats with weight bearing (control). ANOVA = analysis of variance.
GSH Level
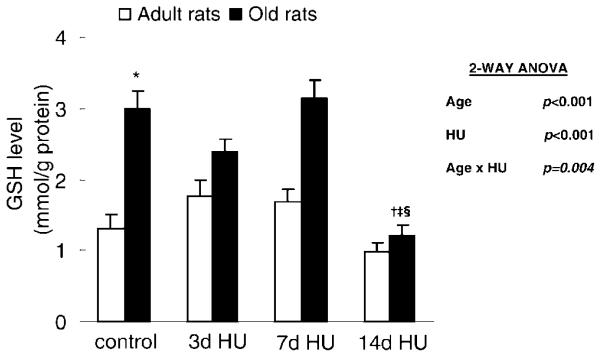
Hind-limb unloading affected the GSH level in the soleus muscle; however, the effect depended on the age of the rats (Age × HU, p = .004) (Figure 3). In adult rats, the GSH level of soleus muscles remained stable with hind-limb unloading. In old rats, the GSH level of muscle with 14 days of hind-limb unloading was 1.21 ± 0.15 mmol/g protein, which was significantly lower than the level of muscle with weight bearing and both 3 days and 7 days of hind-limb unloading (3.00 ± 0.24, 2.39 ± 1.67, and 3.14 ± 0.24 mmol/g protein, respectively).
Figure 3.
Glutathione (GSH) level from rats with weight bearing (control), 3 days of hind-limb unloading (3d HU), 7 days of hind-limb unloading (7d HU), and 14 days of hind-limb unloading (14d HU). Values are mean ± standard error of the mean. The effect of hind-limb unloading was dependent on age. *Significantly different from adult control. †Significantly different from old control. ‡Significantly different from old 3d HU rats. §Significantly different from old 7d HU rats. ANOVA = analysis of variance.
In addition to disuse, aging also affected the GSH level. The GSH levels of soleus muscles were 130% greater in the old control rats compared to the level in the adult control rats (p < .001).
tGSH Level
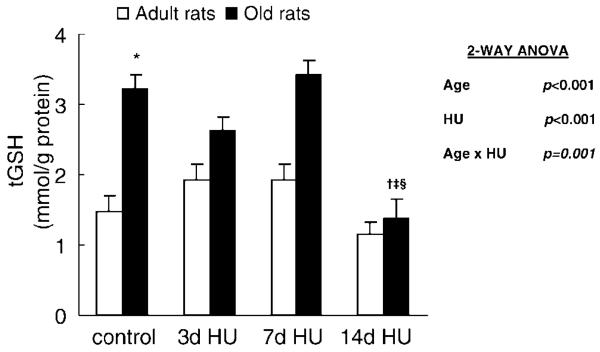
tGSH level was the sum of GSH and oxidized glutathione in the muscle tissue. Hind-limb unloading affected the tGSH level in the soleus muscle; however, the effect depended on the age of the rats (Age × HU, p = .001) (Figure 4). In adult rats, the tGSH level of soleus muscles remained stable with hind-limb unloading. In old rats, the tGSH level of muscle with 14 days of hind-limb unloading was 1.37 ± 0.27 mmol/g protein, which was significantly lower than the tGSH level of muscle with weight bearing and both 3 days and 7 days of hind-limb unloading (3.29 ± 0.20, 2.62 ± 0.19, and 3.24 ± 0.20 mmol/g protein, respectively).
Figure 4.
Total glutathione level (tGSH) from rats with weight bearing (control), 3 days of hind-limb unloading (3d HU), 7 days of hind-limb unloading (7d HU), and 14 days of hind-limb unloading (14d HU). Values are mean ± standard error of the mean. The effect of hind-limb unloading was dependent on age. *Significantly different from adult control. †Significantly different from old control. ‡Significantly different from old 3d HU rats. §Significantly different from old 7d HU rats. ANOVA = analysis of variance.
In addition to disuse, aging also affected the tGSH level. The tGSH levels of soleus muscles were 147% greater in the old control rats compared to the adult control rats (p < .001).
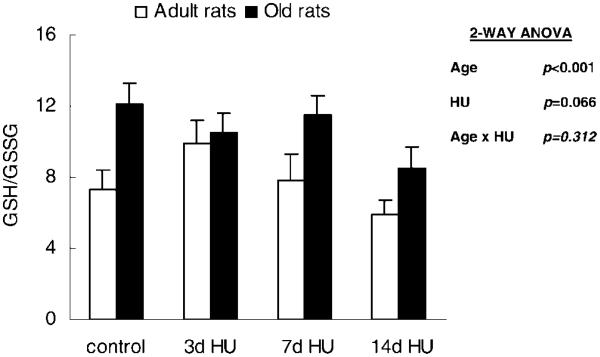
GSH/GSSG
GSH/GSSG tended to decrease with hind-limb unloading, although this change did not reach significance (p = .066). However, this ratio was significantly affected by age. The soleus muscle from old rats had a 38% greater GSH/GSSG (Figure 5).
Figure 5.
The reduced to oxidized glutathione ratio (GSH/GSSG) from rats with weight bearing (control), 3 days of hind-limb unloading (3d HU), 7 days of hind-limb unloading (7d HU), and 14 days of hind-limb unloading (14d HU). Values are mean ± standard error of the mean. The effect of age was independent of hind-limb unloading. ANOVA = analysis of variance.
Discussion
The aim of this study was to investigate the influence of age on the adaptative ability of the antioxidant systems in the soleus muscle, a muscle composed of predominantly type I fibers, to disuse. We hypothesized that the adaptation of antioxidant systems to muscle disuse is age-dependent. In general, our results show that the antioxidant systems of the soleus muscles from both adult and aged rats did adapt to disuse (hind-limb unloading). Specifically, we found that the age of the rats influences the response of GSH and tGSH levels in the soleus muscle to hind-limb unloading. In adult rats, the levels of GSH and tGSH of muscles under 14 days of hind-limb unloading were not different from the levels seen in the muscles with normal weight bearing. However, the levels of GSH and tGSH in muscles from aged rats under 14 days of hind-limb unloading were approximately 60% lower than the levels seen in the muscles with normal weight bearing.
Age-Related Adaptation of Antioxidant Systems
Aging is associated with increased oxidative stress. Many studies show the age-related increase of lipid peroxidation, protein oxidative modification, and DNA damage (11–13). Similarly, the results of the current study suggest that the soleus muscles from aged rats have greater oxidative stress than muscles from adult rats. We find the tGSH level, the GSH level, and GSH peroxidase activity of the aged muscles were 72%, 70%, and 28% higher, respectively, than the values seen in the adult muscles. Although the changes in the antioxidants reveal an enhanced oxidative environment of the cells, the changes demonstrate a positive adaptation of the antioxidant system with aging. This adaptation is positive to the cells because by increasing the content and/or activity of antioxidants, the impact (damage) of the increased intracellular oxidative stress with aging is minimized. Indeed, this positive adaptation is believed to be an important factor to cell survival. The age-related positive adaptation in skeletal muscle is consistent with the work of Leeuwenburgh and colleagues (15) that demonstrated an age-related increase of the GSH level in the soleus muscles.
Activities of antioxidant enzymes, Cu-Zn SOD and catalase, were not different between the soleus muscles of adult and aged rats. The unchanged activity of Cu-Zn SOD and catalase in the soleus muscles with aging in our study is consistent with the findings of several studies that investigated the age related adaptation of antioxidant systems in this same tissue (16,26). In contrast, other studies have shown increased activity of Cu-Zn SOD and catalase (16,27). The different results of the enzyme adaptation with aging are likely due to the fiber type composition of the muscles that were investigated. For example, Hollander and colleagues (16) measured the age-related adaptation of the antioxidant systems in both type IIb and type I muscles. They found the activities of Cu-Zn SOD and catalase increased in type IIb muscles but remained unchanged in type I muscles with aging. Most likely, chronic oxidative stress can be better managed in type I fibers, which possess greater antioxidant capacity than do type II fibers (28).
Potential Mechanisms to Explain the Antioxidant Adaptation With Aging
The mechanisms to explain the age-related positive adaptation of antioxidant systems are unknown; however, the adaptation is likely associated with the chronic enhanced oxidative environment (stress). For instance, previous studies show that chronic moderate oxidative stress, such as exercise training and chronic smoking, induces a compensatory increase in GSH levels (29,30). The finding of the age-related positive adaptation of antioxidant systems in the current study suggests that muscle cells are under chronic oxidative stress during the aging process.
In the current study, we investigated the response of GSH, tGSH, activity of GSH peroxidase, and GSH/GSSG to muscle disuse in the soleus muscles from both adult and old rats because this antioxidant system is a key pathway to prevent protein damage with oxidative stress. GSH, a tripeptide (γ-glutamyl-cysteinyl-glycine) containing a sulf-hydryl (−SH) group, is a crucial peptide that protects cells from oxidants. This protective function of GSH is achieved by direct conjugation with radicals as well as electron donation in redox reactions. GSH is the substrate in redox reactions, and these reactions are catalyzed by GSH peroxidase. This redox reaction oxidizes GSH to GSSG while hydrogen peroxide and other peroxides are reduced. The generated GSSG is recycled to GSH catalyzed by the NADPH-dependent GSH reductase. Previous studies clearly demonstrate that the GSH to GSSG cycling is crucial in regulating the redox status in the cell, and the intracellular GSH level is related to the redox status of the cells (31,32). We found that muscles from old rats have a greater tGSH level, a greater GSH level, and a greater GSH peroxidase activity. In addition, the GSSG level in muscles of old rats is no different from that in muscles of adult rats. The results suggest that the GSH-mediated redox system is up-regulated with aging to maintain the redox balance of the muscle cells.
It is thought that chronic oxidative stress induces an increase of intracellular GSH by increasing the expression of the rate-limiting enzyme involved in the synthesis of GSH, γ-glutamylcysteine synthetase (GCS). The transcription factors, activator protein-1 (AP-1) and nuclear factor-κB (NF-κB), have been shown to be redox sensitive and modulate gene expression of GCS (30,33,34) leading to increased GSH levels. Thus, the increased GSH levels in muscles from aged rats in the current study could result from the increased activity of GCS.
Adaptation of Antioxidant Systems to Muscle Disuse
There are many studies investigating the effects of muscle disuse on the intracellular adaptation of skeletal muscles; however, the investigations are mainly done on muscles from young or adult rats (5-7,20,35-37). Few studies investigate the adaptative ability of the antioxidant systems in skeletal muscles to muscle disuse in aged animals (38). In general, muscle disuse induces greater oxidant production (5-7,36,37), accumulation of oxidative modified proteins (9), and antioxidant adaptations (5-7,36,37) in muscles from young and adult rats. Consistent with previous research, the results of this study show that hind-limb unloading increased oxidative stress of the muscle as indicated by the increased catalase activity. The increased catalase activity with muscle disuse is reported in other studies that have a muscle disuse duration similar to that of our study (5,9). The finding of the unchanged activity of GSH peroxidase with muscle disuse is also consistent with the studies that have a muscle disuse duration similar to that of our study (5,9). In contrast, two studies report an increase of Cu-Zn SOD activity (5,9), whereas our present data showed no change of Cu-Zn SOD activity with muscle disuse. The different results of the enzyme adaptation with disuse are likely due to different models of muscle disuse as well as different ages of the rats that were studied. For example, both Kondo and coworkers (5) and Selsby and Dodd (9) used limb immobilization by cast as the model whereas we used hind-limb unloading as the model of muscle disuse. In addition, different from the study by Kondo and coworkers, which investigated the antioxidant responses to disuse of young rats (15 weeks old), this current study investigated the responses of the adult and aged rats (13 months and 26 months, respectively).
The novel finding of the current study was the age-dependent response to muscle disuse. In general, the GSH level is maintained in soleus muscles from adult rats but is depleted in the muscles from aged rats with disuse. The GSH level in the muscles from aged rats decreased to 40% of the control value with 14 days of hind-limb unloading. The age-dependent response to muscle disuse is also observed in intracellular tGSH levels. The tGSH level of aged muscles decreased to 42% of the control value with 14 days of hind-limb unloading. These decreases in GSH and tGSH levels compromise the overall antioxidant defense capacity of the aged muscle. Thus, this response of aged muscles to disuse implies a negative adaptation to cell survival.
Potential Mechanisms to Explain the Age-Dependent GSH Adaptation With Disuse
The differential intracellular GSH and tGSH response of soleus muscle to disuse between adult and old rats implies that the positive increase in GSH levels during the aging process leaves the aged muscles with fewer reserves and less adaptation ability.
The finding of the dramatic decrease of GSH and tGSH levels in the aged muscles with 14 days of disuse suggests that muscles with 14 days of disuse have (a) greater GSH consumption or/and (b) lower GSH production compared to the muscles in control weight-bearing animals.
Greater GSH consumption
The greater GSH consumption is likely due to increased GSH utilization and efflux. GSH is utilized (a) to detoxify oxidants by direct conjugation (catalyzed by glutathione S-transferase [GST]) and (b) as an electron donator in a redox reaction (catalyzed by GSH peroxidase). In the redox reaction, GSH is recycled by GSH reductase; however, GSH is consumed in direct conjugation with oxidants. In addition, GSH conjugates, GSH, and GSSG can be transported out of cells by γ-glutamyl transferase (GGT), a membrane-bound enzyme that breaks the γ-peptide bond of the GSH and degrades it. When the redox balance of cells is disrupted, intracellular GSSG and GSH-protein adducts accumulate and the GSH level decreases. The increased GSSG and GSH-protein adducts could be transported out of the cell, thus decreasing the intracellular tGSH level (31). As a vicious cycle, the decreased GSH level reduces the activity of enzymes that catalyze the reducing reaction of GSSG and GSH-protein adducts (39).
Thus, it is possible that reactive oxygen species (ROS) production significantly increases in the aged muscles with 14 days of disuse and the increased GST and GGT activities catalyze the utilization and efflux of the GSH in the cells. This hypothesis needs to be further tested.
Lower GSH production
GSH production is affected by substrate availability and the synthesis rate. Cysteine is the limiting amino acid for GSH synthesis because the concentrations of glutamate and glycine are relatively high intracellularly. Factors that influence cysteine metabolism (such as insulin and growth factors) affect the intracellular GSH level. The synthesis of GSH is catalyzed consecutively by GCS and GSH synthetase. GCS expression and its activity are modulated by factors such as inflammation and oxidative stress at transcriptional, translational, and post-translational levels. As discussed earlier, the greater GSH level of muscles with aging is a compensatory adaptation to the oxidative stress.
Thus, the dramatic decrease of GSH and tGSH levels in aged muscles with 14 days of disuse could be due to (a) the limitation of cysteine availability of the cells or (b) the fact that GCS cannot further adapt to the increased oxidative stress in the aged muscles with 14 days of disuse. Further investigation is needed to test this hypothesis.
Summary
Hind-limb unloading induced adaptations of antioxidant systems in the soleus muscles from both adult and old rats. This adaptation, however, was different between adult and aged muscles. In adult muscles, the levels of GSH and tGSH were maintained at the control level. In contrast, the levels of GSH and tGSH in the aged muscle were significantly reduced by 14 days of hind-limb unloading. This dramatic decrease in the GSH levels in aged muscles may compromise the overall antioxidant capacity of the aged muscles. The compromised antioxidant capacity of the aged muscles with muscle disuse may predispose the proteins in the soleus muscle to damage. Future studies are needed to investigate the possible mechanisms of the impaired adaptation of GSH to disuse in the muscles of old animals.
Acknowledgments
This work was supported, in part, by National Institute on Aging grants AG-17768 and AG-21626 (L. V. Thompson).
We thank Sheng Zhong and Janice A Shoeman for technical assistance and Dr. Richard P Di Fabio for statistical consultation.
References
- 1.Yamashita-Goto K, Okuyama R, Honda M, et al. Maximal and submaximal forces of slow fibers in human soleus after bed rest. J Appl Physiol. 2001;91:417–424. doi: 10.1152/jappl.2001.91.1.417. [DOI] [PubMed] [Google Scholar]
- 2.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287:C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 3.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337–R344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- 4.Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102:2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- 5.Kondo H, Nakagaki I, Sasaki S, Hori S, Itokawa Y. Mechanism of oxidative stress in skeletal muscle atrophied by immobilization. Am J Physiol. 1993;265:E839–E844. doi: 10.1152/ajpendo.1993.265.6.E839. [DOI] [PubMed] [Google Scholar]
- 6.Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 7.Liu MJ, Li JX, Lee KM, Qin L, Chan KM. Oxidative stress after muscle damage from immobilization and remobilization occurs locally and systemically. Clin Orthop Relat Res. 2005;(434):246–250. doi: 10.1097/01.blo.0000150464.29883.ca. [DOI] [PubMed] [Google Scholar]
- 8.Arbogast S, Smith J, Matuszczak Y, et al. Bowman-birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J Appl Physiol. 2007;102:956–964. doi: 10.1152/japplphysiol.00538.2006. [DOI] [PubMed] [Google Scholar]
- 9.Selsby JT, Dodd SL. Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol. 2005;289:R134–R139. doi: 10.1152/ajpregu.00497.2004. [DOI] [PubMed] [Google Scholar]
- 10.Selsby JT, Rother S, Tsuda S, Pracash O, Quindry J, Dodd SL. Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J Appl Physiol. 2007;102:1702–1707. doi: 10.1152/japplphysiol.00722.2006. [DOI] [PubMed] [Google Scholar]
- 11.Gunduz F, Senturk UK, Kuru O, Aktekin B, Aktekin MR. The effect of one year's swimming exercise on oxidant stress and antioxidant capacity in aged rats. Physiol Res. 2004;53:171–176. [PubMed] [Google Scholar]
- 12.Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol. 1999;87:465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- 13.Gianni P, Jan KJ, Douglas MJ, Stuart PM, Tarnopolsky MA. Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp Gerontol. 2004;39:1391–1400. doi: 10.1016/j.exger.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Luhtala TA, Roecker EB, Pugh T, Feuers RJ, Weindruch R. Dietary restriction attenuates age-related increases in rat skeletal muscle antioxidant enzyme activities. J Gerontol. 1994;49:B231–B238. doi: 10.1093/geronj/49.5.b231. [DOI] [PubMed] [Google Scholar]
- 15.Leeuwenburgh C, Fiebig R, Chandwaney R, Ji LL. Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am J Physiol. 1994;267:R439–R445. doi: 10.1152/ajpregu.1994.267.2.R439. [DOI] [PubMed] [Google Scholar]
- 16.Hollander J, Bejma J, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression in skeletal muscle: fiber-specific effect of age. Mech Ageing Dev. 2000;116:33–45. doi: 10.1016/s0047-6374(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 17.Siu PM, Pistilli EE, Murlasits Z, Alway SE. Hindlimb unloading increases muscle content of cytosolic but not nuclear Id2 and p53 proteins in young adult and aged rats. J Appl Physiol. 2006;100:907–916. doi: 10.1152/japplphysiol.01012.2005. [DOI] [PubMed] [Google Scholar]
- 18.Thompson LV, Johnson SA, Shoeman JA. Single soleus muscle fiber function after hindlimb unweighting in adult and aged rats. J Appl Physiol. 1998;84:1937–1942. doi: 10.1152/jappl.1998.84.6.1937. [DOI] [PubMed] [Google Scholar]
- 19.Husom AD, Ferrington DA, Thompson LV. Age-related differences in the adaptive potential of type I skeletal muscle fibers. Exp Gerontol. 2005;40:227–235. doi: 10.1016/j.exger.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Vermaelen M, Marini JF, Chopard A, Benyamin Y, Mercier J, Astier C. Ubiquitin targeting of rat muscle proteins during short periods of unloading. Acta Physiol Scand. 2005;185:33–40. doi: 10.1111/j.1365-201X.2005.01446.x. [DOI] [PubMed] [Google Scholar]
- 21.Brown-Borg HM, Bode AM, Bartke A. Antioxidative mechanisms and plasma growth hormone levels: potential relationship in the aging process. Endocrine. 1999;11:41–48. doi: 10.1385/ENDO:11:1:41. [DOI] [PubMed] [Google Scholar]
- 22.Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp Gerontol. 2000;35:199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- 23.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 24.Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 25.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 26.Lambertucci RH, Levada-Pires AC, Rossoni LV, Curi R, Pithon-Curi TC. Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech Ageing Dev. 2007;128:267–275. doi: 10.1016/j.mad.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Ji LL, Dillon D, Wu E. Alteration of antioxidant enzymes with aging in rat skeletal muscle and liver. Am J Physiol. 1990;258:R918–R923. doi: 10.1152/ajpregu.1990.258.4.R918. [DOI] [PubMed] [Google Scholar]
- 28.Powers SK, Criswell D, Lawler J, et al. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol. 1994;266:R375–R380. doi: 10.1152/ajpregu.1994.266.2.R375. [DOI] [PubMed] [Google Scholar]
- 29.Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am J Physiol. 1999;277:L1067–L1088. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]
- 30.Sen CK. Glutathione homeostasis in response to exercise training and nutritional supplements. Mol Cell Biochem. 1999;196:31–42. [PubMed] [Google Scholar]
- 31.Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res Rev. 2005;4:288–314. doi: 10.1016/j.arr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 33.Lou H, Kaplowitz N. Glutathione depletion down-regulates tumor necrosis factor α-induced NF-κB activity via IκB kinase-dependent and -independent mechanisms. J Biol Chem. 2007;282:29470–29481. doi: 10.1074/jbc.M706145200. [DOI] [PubMed] [Google Scholar]
- 34.Droge W. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Philos Trans R Soc Lond B Biol Sci. 2005;360:2355–2372. doi: 10.1098/rstb.2005.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naito H, Powers SK, Demirel HA, Sugiura T, Dodd SL, Aoki J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol. 2000;88:359–363. doi: 10.1152/jappl.2000.88.1.359. [DOI] [PubMed] [Google Scholar]
- 36.Ikemoto M, Okamura Y, Kano M, et al. A relative high dose of vitamin E does not attenuate unweighting-induced oxidative stress and ubiquitination in rat skeletal muscle. J Physiol Anthropol Appl Human Sci. 2002;21:257–263. doi: 10.2114/jpa.21.257. [DOI] [PubMed] [Google Scholar]
- 37.Lawler JM, Song W, Kwak HB. Differential response of heat shock proteins to hindlimb unloading and reloading in the soleus. Muscle Nerve. 2006;33:200–207. doi: 10.1002/mus.20454. [DOI] [PubMed] [Google Scholar]
- 38.Zarzhevsky N, Menashe O, Carmeli E, Stein H, Reznick AZ. Capacity for recovery and possible mechanisms in immobilization atrophy of young and old animals. Ann N Y Acad Sci. 2001;928:212–225. doi: 10.1111/j.1749-6632.2001.tb05651.x. [DOI] [PubMed] [Google Scholar]
- 39.Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]